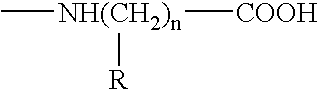Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
37 results about "Prostatic acid phosphatase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Prostatic acid phosphatase (PAP), also prostatic specific acid phosphatase (PSAP), is an enzyme produced by the prostate. It may be found in increased amounts in men who have prostate cancer or other diseases.
Composition and method for producing an immune response against tumor-related antigens
Disclosed are a novel prostatic acid phosphatase and corresponding coding region derived from mouse. Also disclosed is a method of producing an immune response against an autologous polypeptide tumor antigen by immunizing a subject with a xenogeneic polypeptide antigen, either alone, as part of a viral antigen construct, or as part of a pulsed dendritic cell preparation.
Owner:DENDREON PHARMA LLC
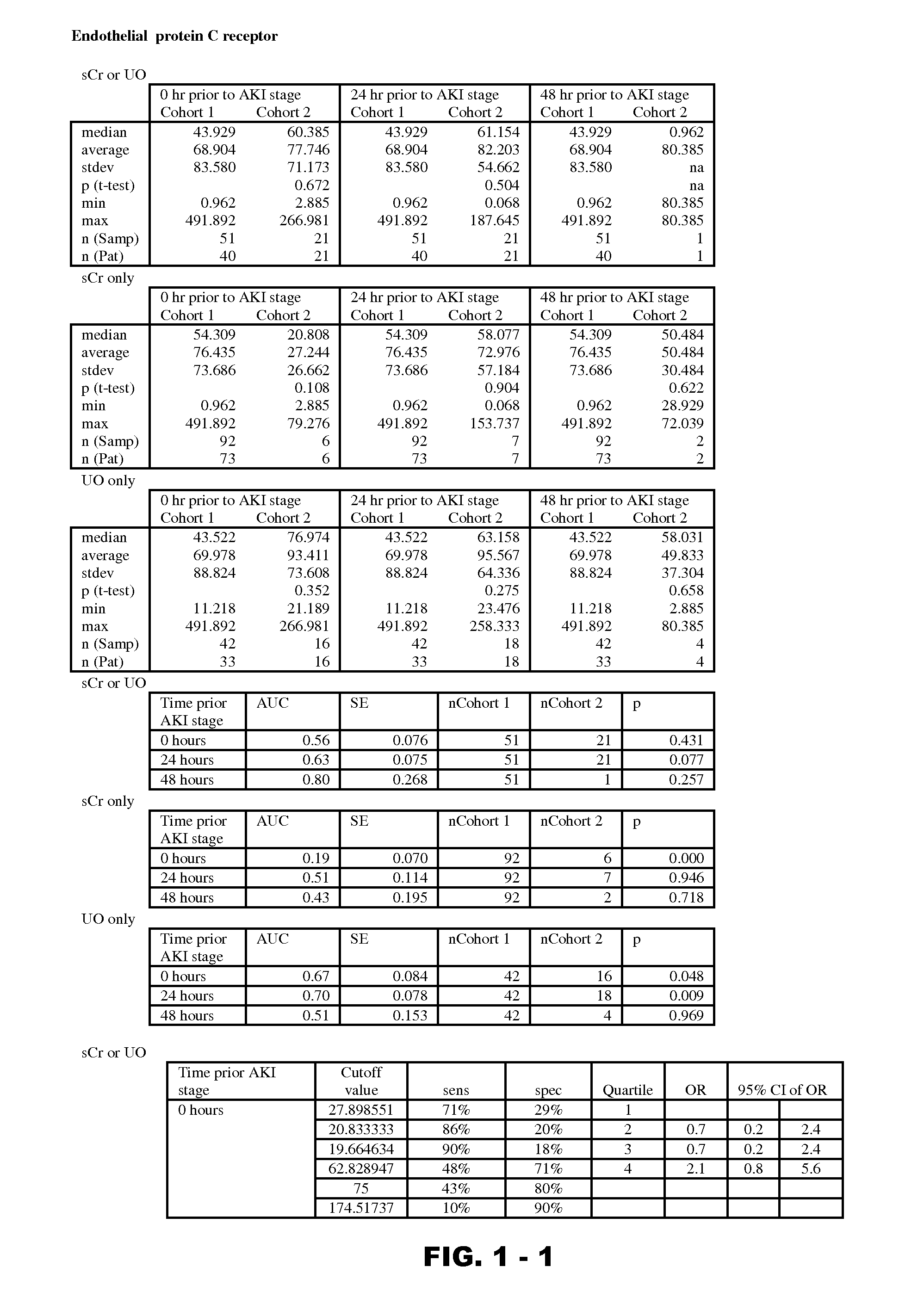
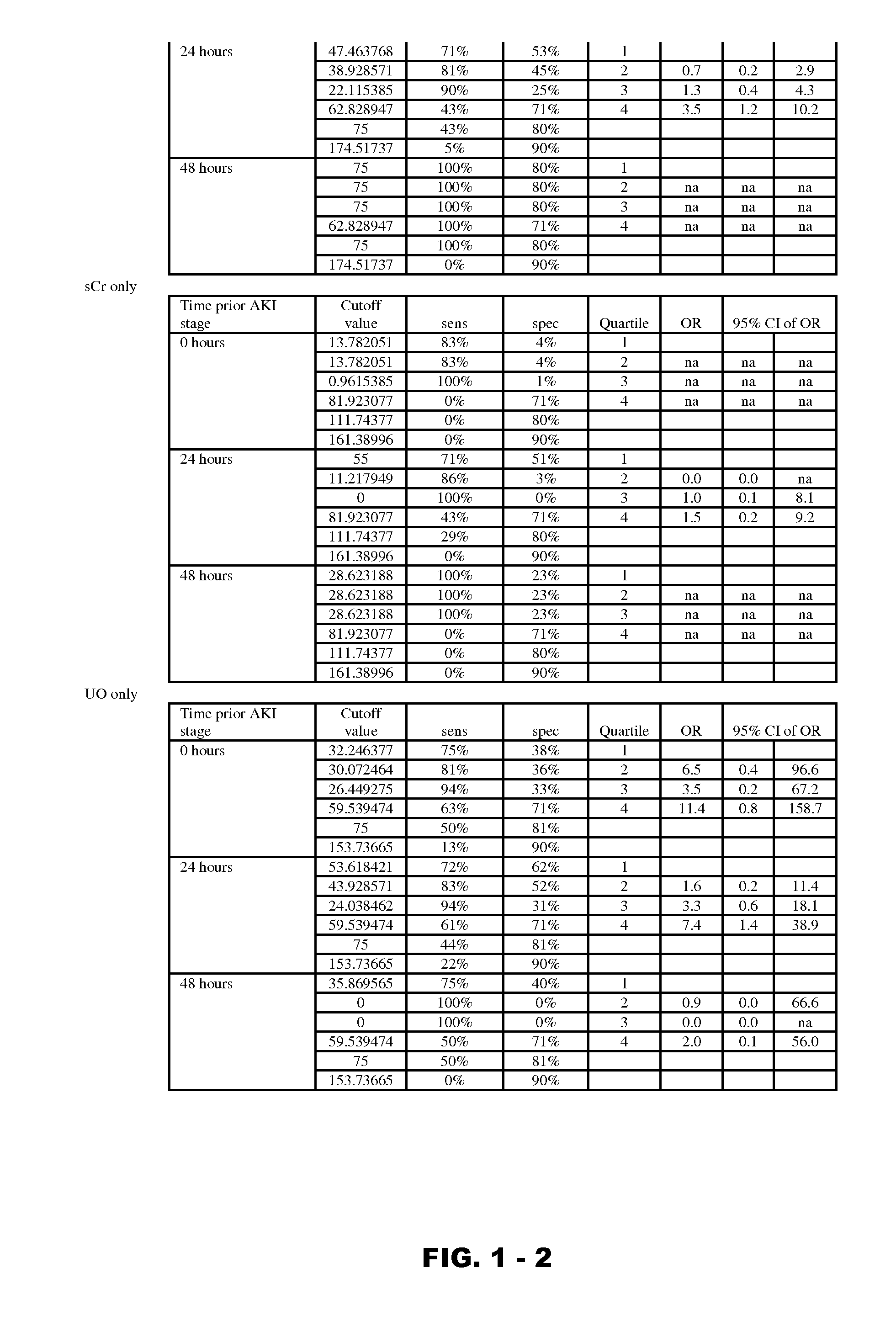
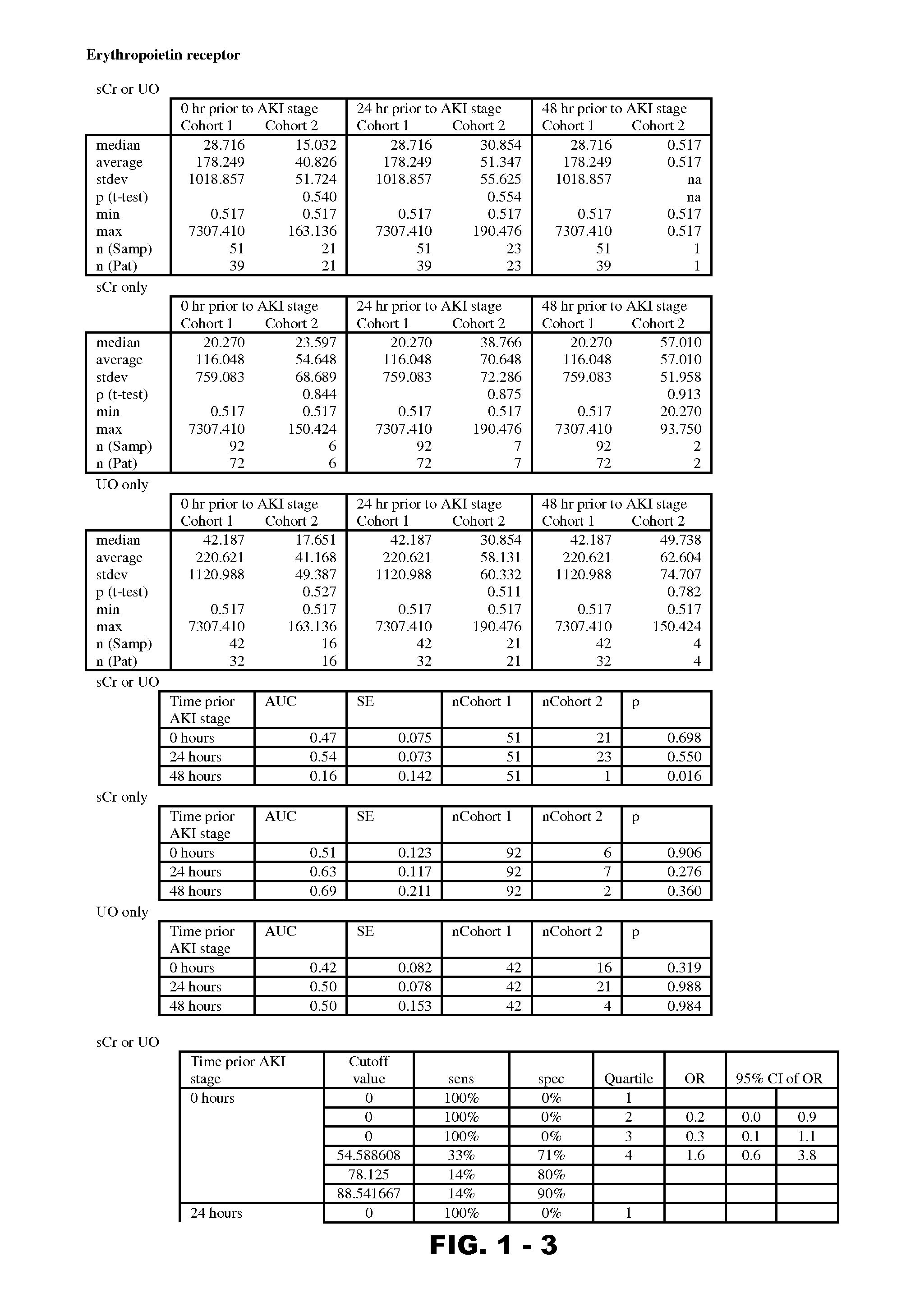
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
InactiveUS20120190051A1Eliminate needPeptide/protein ingredientsTransferrinsErythropoietin receptorFactor VIII vWF
Disclosed are methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, disclosed are assays that detect one or more markers selected from the group consisting of Prostatic acid phosphatase, Lactotransfenin, Soluble erythropoietin receptor, Von Willebrand factor, Soluble endothelial protein C receptor, and Beta-2-glycoprotein 1 as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
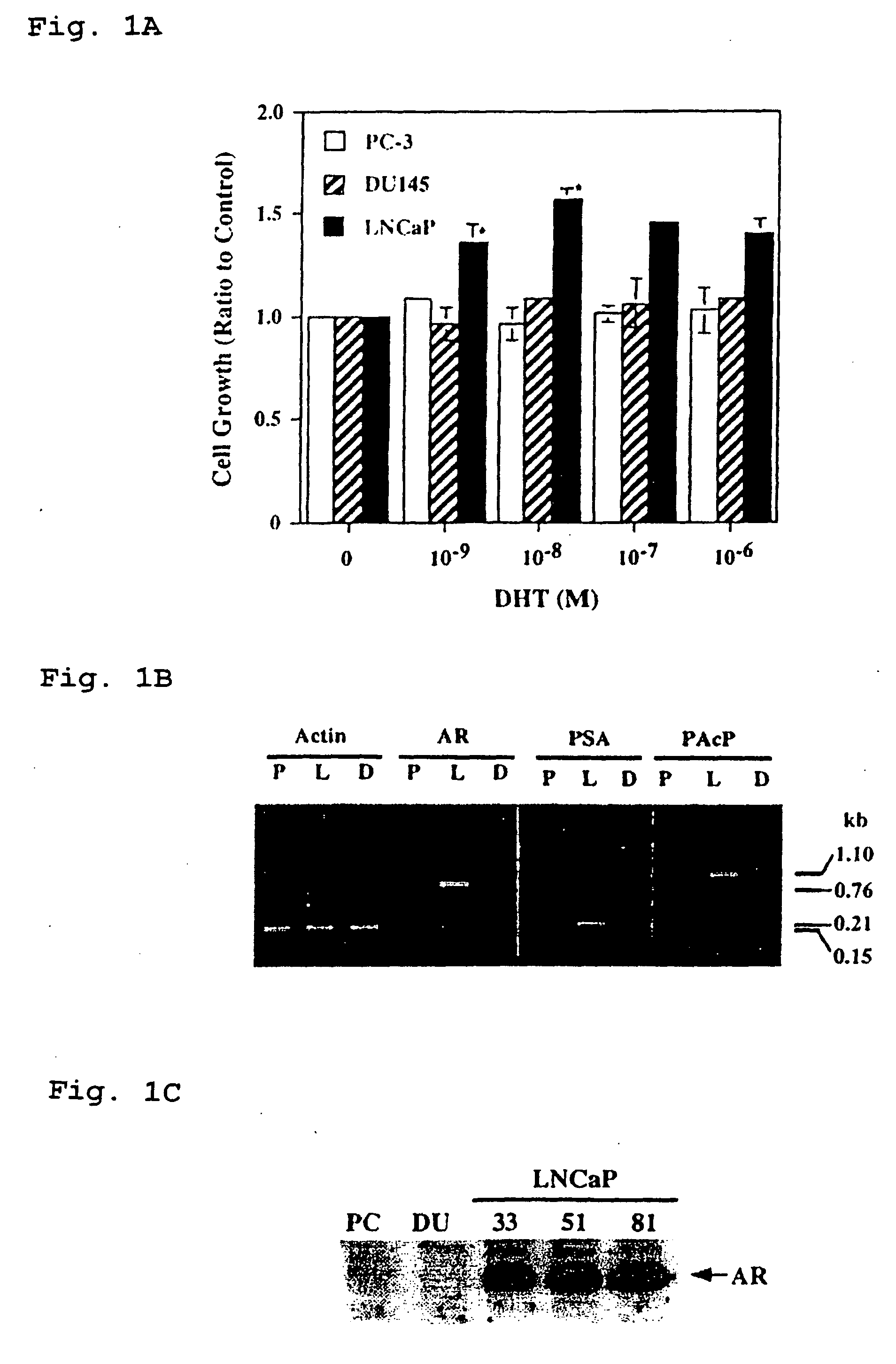
Therapeutic and diagnostic applications of prostatic acid phosphatase in prostate cancer
InactiveUS20060294615A1Good curative effectGood effectBiocideGenetic material ingredientsAndrogenMammal
Presented is a therapeutic method to treat prostate carcinomas in mammals comprising the administration of cellular PAcP protein. Also presented is a method to diagnose androgen-insensitive prostate carcinomas by determining the expression level of cellular PAcP in the prostate carcinomas, a decrease in expression being indicative of androgen-insensitivity. A promoter region that is specifically expressed in prostate tissue is presented, as is a xenograft animal model that mimics human prostate carcinomas in the expression of cellular PAcP.
Owner:BOARD OF RGT UNIV OF NEBRASKA
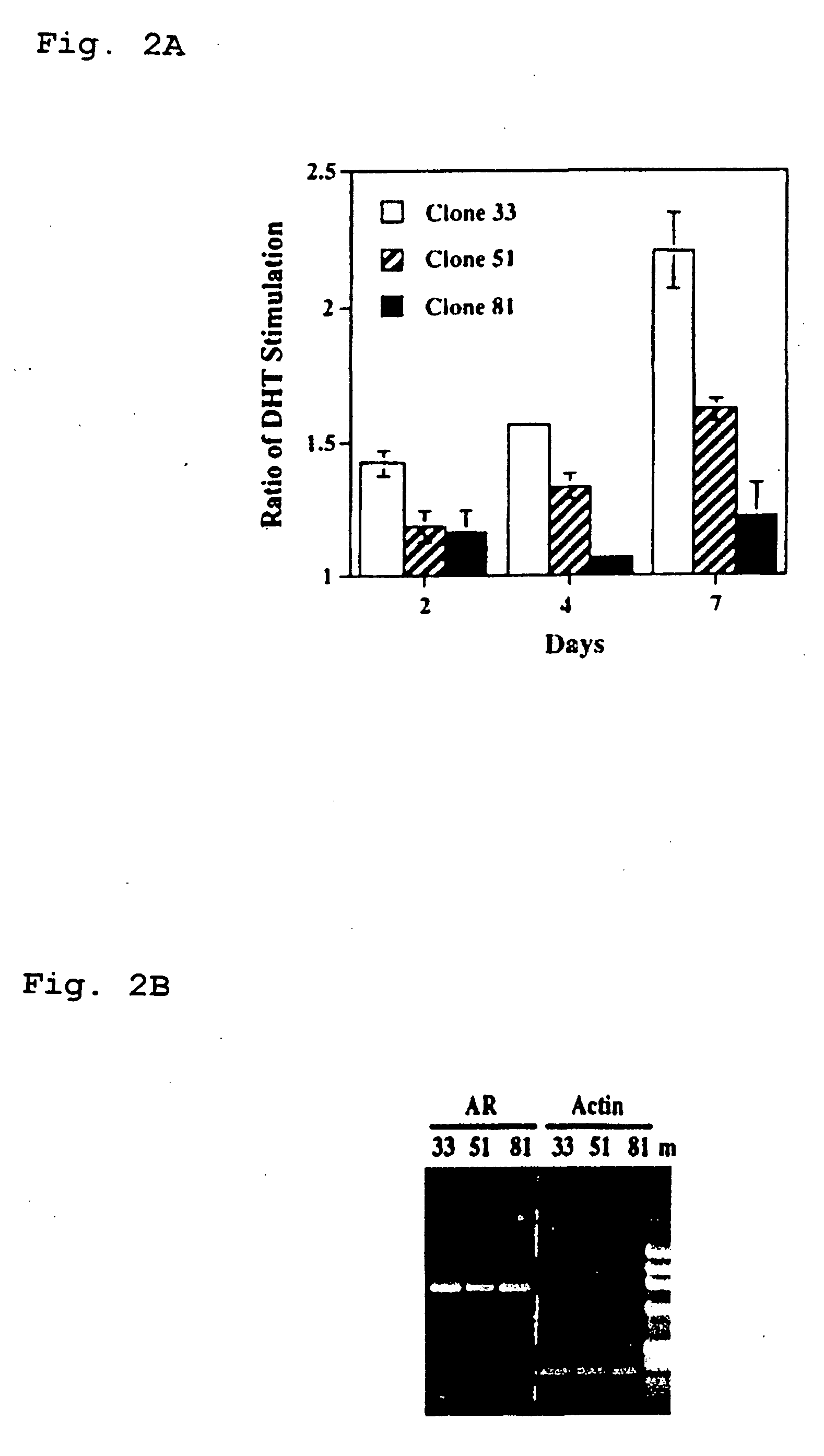
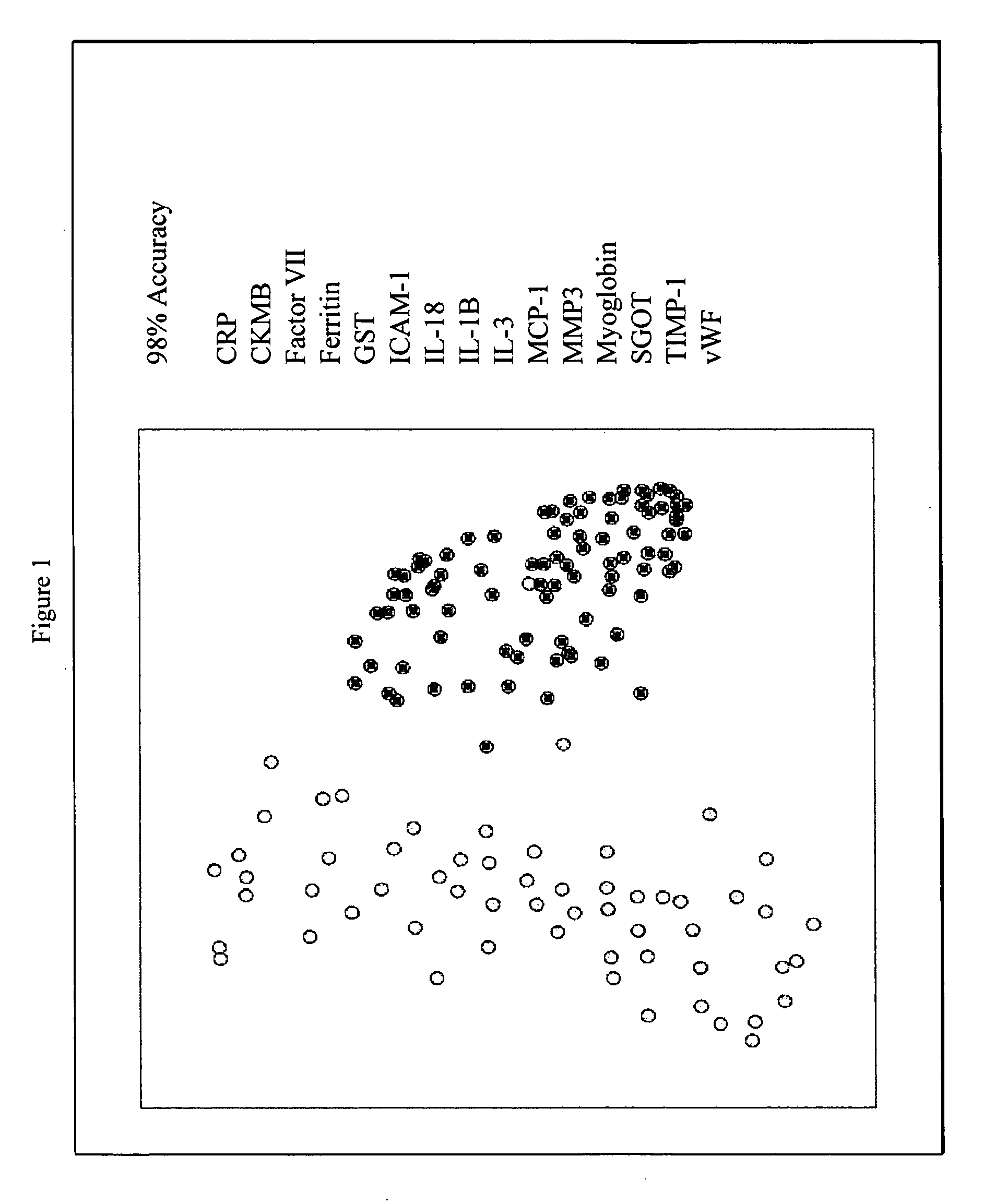
Methods and kits for the diagnosis of acute coronary syndrome
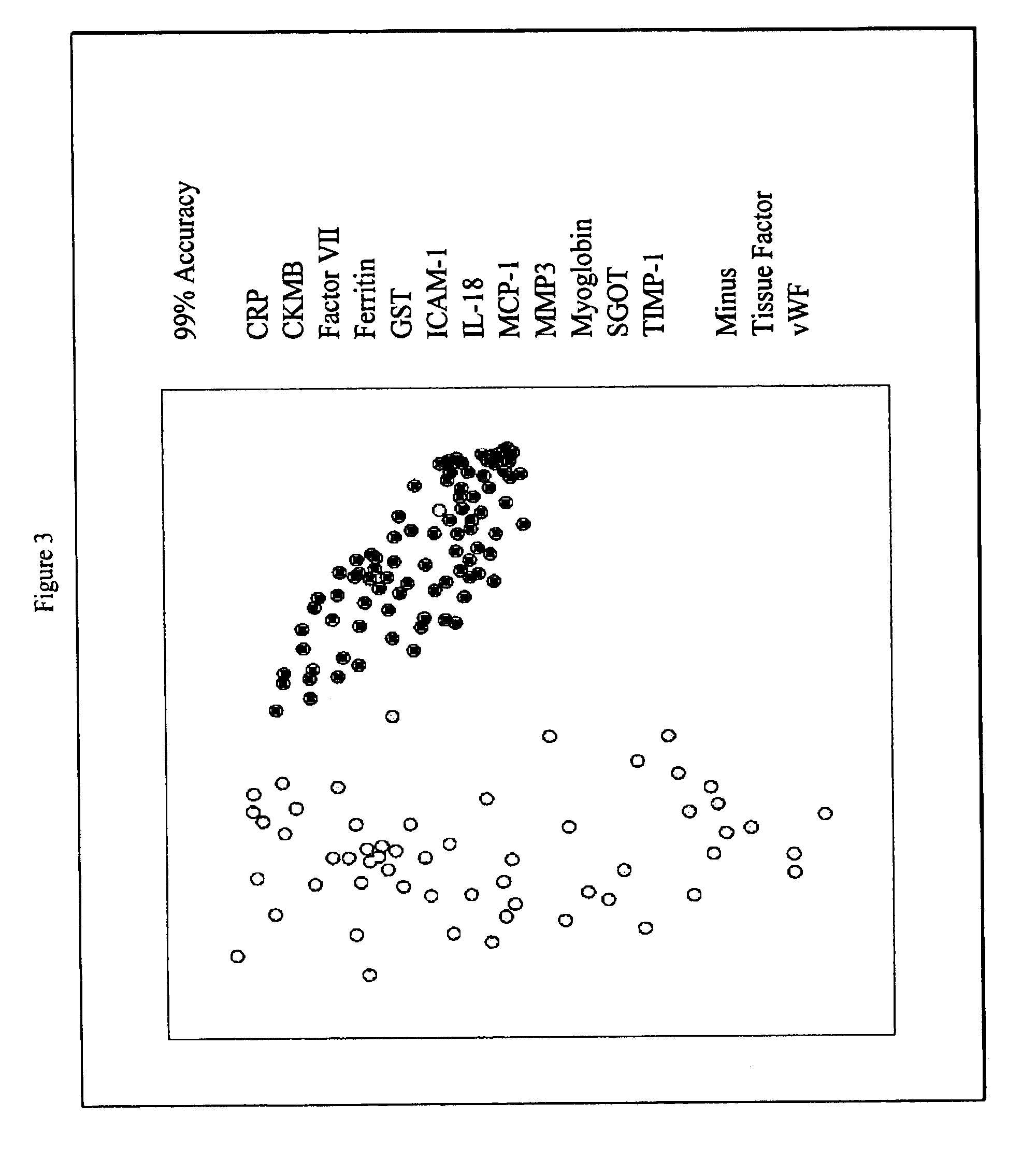
InactiveUS20070003981A1Quick checkAccurate diagnosisMicrobiological testing/measurementDisease diagnosisComplement 3Factor VII
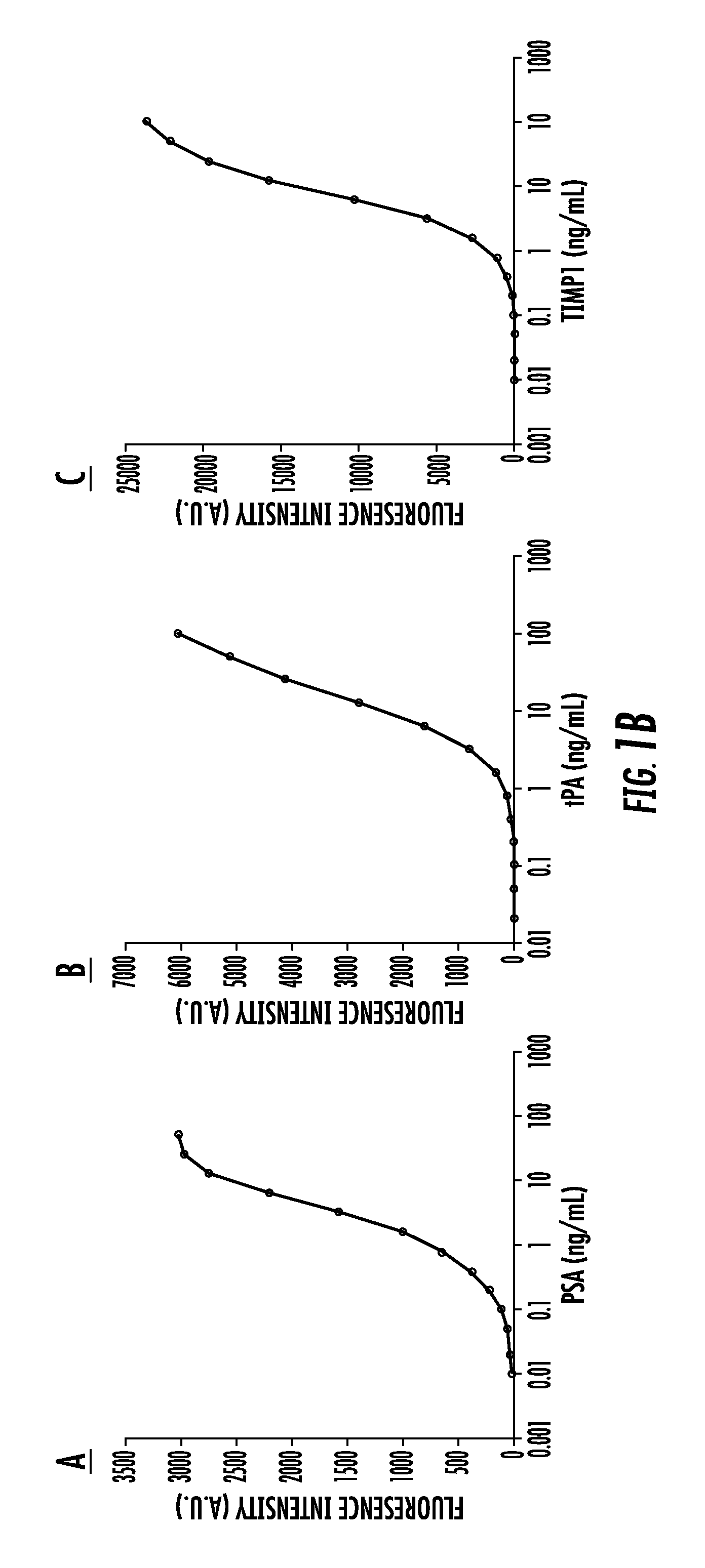
Provided are methods for the detection and diagnosis of acute coronary syndrome or ACS. The methods are based on the discovery that abnormal levels of selected analytes in sample fluid, typically blood samples, of patients who are at risk are supportive of a diagnosis of ACS. At least two new biomarkers for ACS are thus disclosed, MMP-3 and SGOT. Altogether the concentrations of twelve analytes provide a sensitive and selective picture of the patient's condition, namely, whether the patient is suffering a heart attack. Other important biomarkers for ACS are described, including but not limited to IL-18, Factor VII, ICAM-1, Creatine Kinase-MB, MCP-1, Myoglobin, C Reactive Protein, von Willebrand Factor, TIMP-1, Ferritin, Glutathione S-Transferase, Prostate Specific Antigen (free), IL-3, Tissue Factor, alpha-Fetoprotein, Prostatic Acid Phosphatase, Stem Cell Factor, MIP-1-beta, Carcinoembryonic Antigen, IL-13, TNF-alpha, IgE, Fatty Acid Binding Protein, ENA-78, IL-1-beta, Brain-Derived Nerotrophic Factor, Apolipoprotein A1, Serum Amyloid P, Growth Hormone, Beta-2 microglobulin, Lipoprotein (a), MMP-9, Thyroid Stimulating hormone, alpha-2 Macroglobulin, Complement 3, IL-7, Leptin, and IL-6. Kits containing reagents to assist in the analysis of fluid samples are also described.
Owner:RULES BASED MEDICINE
Tumor associated antigen peptides and use of same as anti-tumor vaccines
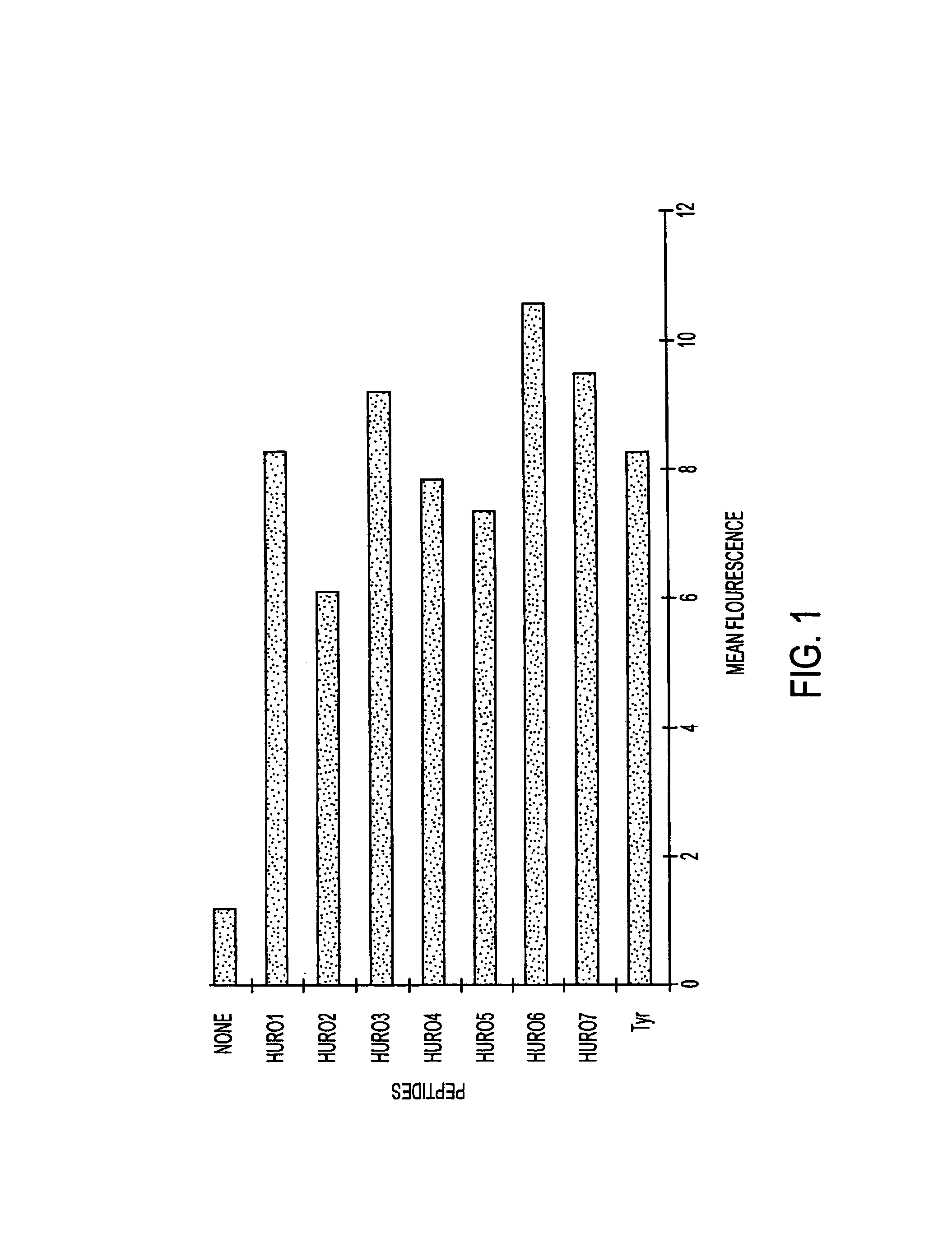
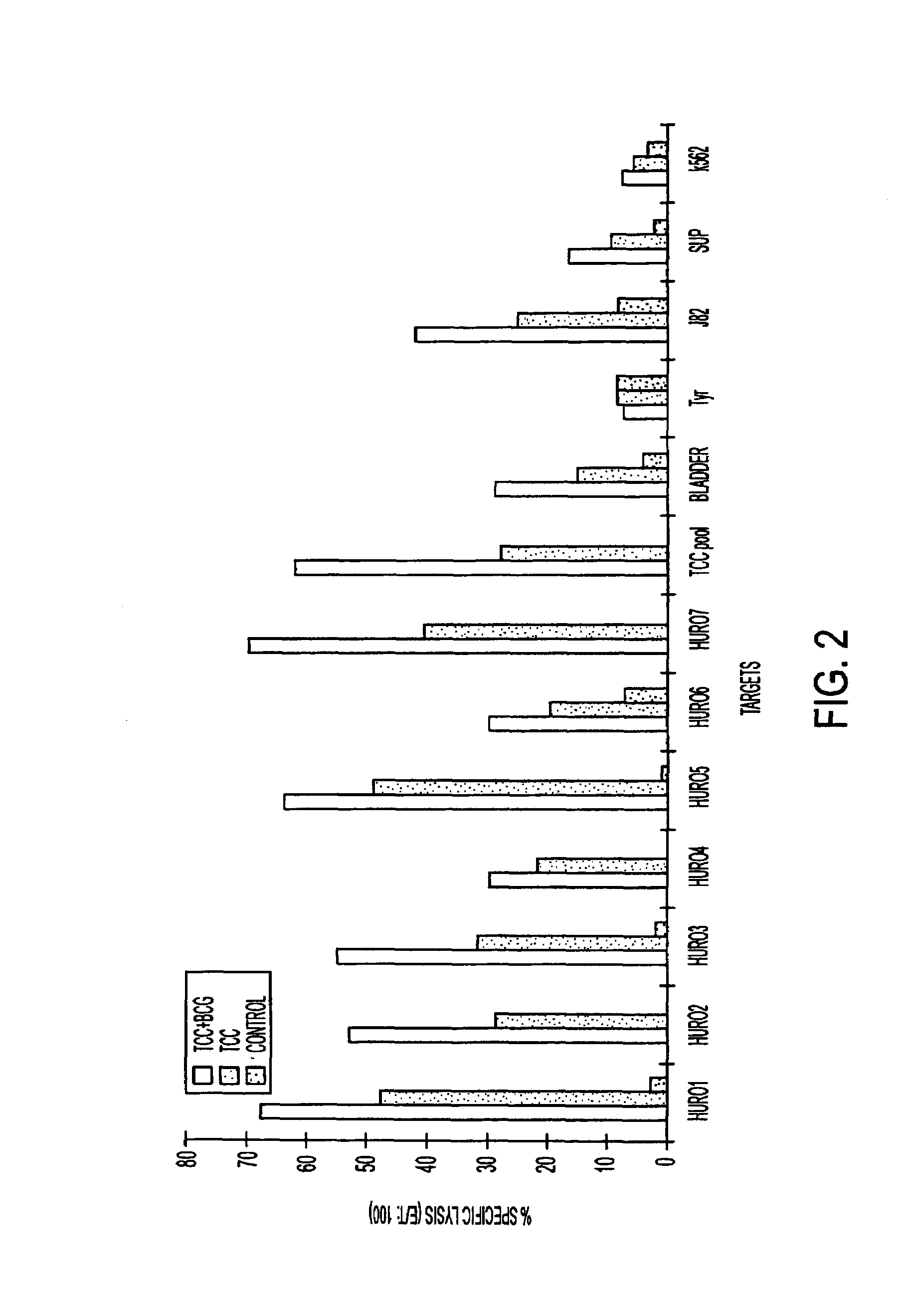
The present invention relates to tumor associated antigen (TAA) peptides and to use of same, of polynucleotides encoding same and of cells presenting same as anti-tumor vaccines. More particularly, the present invention relates to tumor associated antigen peptides derived from Uroplakin Ia, Ib, II and III, Prostate specific antigen (PSA), Prostate acid phosphatase (PAP) and Prostate specific membrane antigen (PSMA), BA-46 (Lactadherin), Mucin (MUC-1), and Teratocarcinoma-derived growth factor (CRIPTO-1) and the use of same as anti-tumor vaccines to prevent or cure bladder, prostate, breast or other cancers, carcinomas in particular. Most particularly, the present invention relates to tumor associated antigen peptides which are presentable to the immune system by HLA-A2 molecules.
Owner:YEDA RES & DEV CO LTD
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
InactiveUS9229010B2Peptide/protein ingredientsMammal material medical ingredientsFactor VIII vWFErythropoietin receptor
Disclosed are methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, disclosed are assays that detect one or more markers selected from the group consisting of Prostatic acid phosphatase, Lactotransfenin, Soluble erythropoietin receptor, Von Willebrand factor, Soluble endothelial protein C receptor, and Beta-2-glycoprotein 1 as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
Methods and Compositions for Treating Prostate Cancer Using DNA Vaccines
A DNA vaccine for the treatment of prostate cancer, comprising a plasmid vector comprising a nucleotide sequence encoding prostatic acid phosphatase (PAP) operably linked to a transcription regulatory element, wherein upon administration to a mammal a cytotoxic immune reaction against cells expressing PAP is induced. In preferred embodiment, the PAP encoded is a xenoantigen highly homologous to the autoantigen PAP of the mammal. Also disclosed are methods for inducing prostatitis, or inducing immune reaction to PAP, or treating prostate cancer in a mammal, using the DNA vaccine and pharmaceutical compositions comprising the vaccine. Preferably, xenoantigen vaccination is followed by boosting with autoantigen PAP from the same animal species as the mammal being treated.
Owner:WISCONSIN ALUMNI RES FOUND
Chemical luminescence immune assay determination reagent kit for prostate gland acid phosphatase and preparation method thereof
InactiveCN101368962AGuaranteed SensitivityReduce use costChemiluminescene/bioluminescenceMonoclonal antibodyProstatic acid phosphatase
The invention provides a chemiluminescence immunoassay test kit of prostatic acid phosphatase, which belongs to the clinical blood detecting and assaying technique field. The test kit includes: 1) prostatic acid phosphatase calibrating article; 2) solid-phase carrier which is coated by monoclonal antibody against prostatic acid phosphatase; 3) monoclonal antibody against prostatic acid phosphatase which is labeled by enzyme; 4) chemiluminescence zymolyte on which the enzyme reacts; 5) washing liquid. Furthermore, the invention also provides a preparing method of the test kit which includes steps as following: the calibrating article is prepared; the solid-phase carrier is coated by the monoclonal antibody against prostatic acid phosphatase; the monoclonal antibody against prostatic acid phosphatase is labeled by the enzyme; the calibrating article, the enzyme labeled antibody and the chemiluminescence zymolyte above can be filled and packed through separate packing; and the finished produce is prepared after assembly. With security and reliability, the test kit has the advantages that the sensitivity is high, and the specificity is strong.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Tumor associated antigen peptides and use of same as anti-tumor vaccines
The present invention relates to tumor associated antigen (TAA) peptides and to use of same, of polynucleotides encoding same and of cells presenting same as anti-tumor vaccines. More particularly, the present invention relates to tumor associated antigen peptides derived from Uroplakin Ia, Ib, II and III, Prostate specific antigen (PSA), Prostate acid phosphatase (PAP) and Prostate specific membrane antigen (PSMA), BA-46 (Lactadherin), Mucin (MUC-1), and Teratocarcinoma-derived growth factor (CRIPTO-1) and the use of same as anti-tumor vaccines to prevent or cure bladder, prostate, breast or other cancers, carcinomas in particular. Most particularly, the present invention relates to tumor associated antigen peptides which are presentable to the immune system by HLA-A2 molecules.
Owner:YEDA RES & DEV CO LTD
Biomarkers for distinguishing between aggressive prostate cancer and non-aggressive prostate cancer
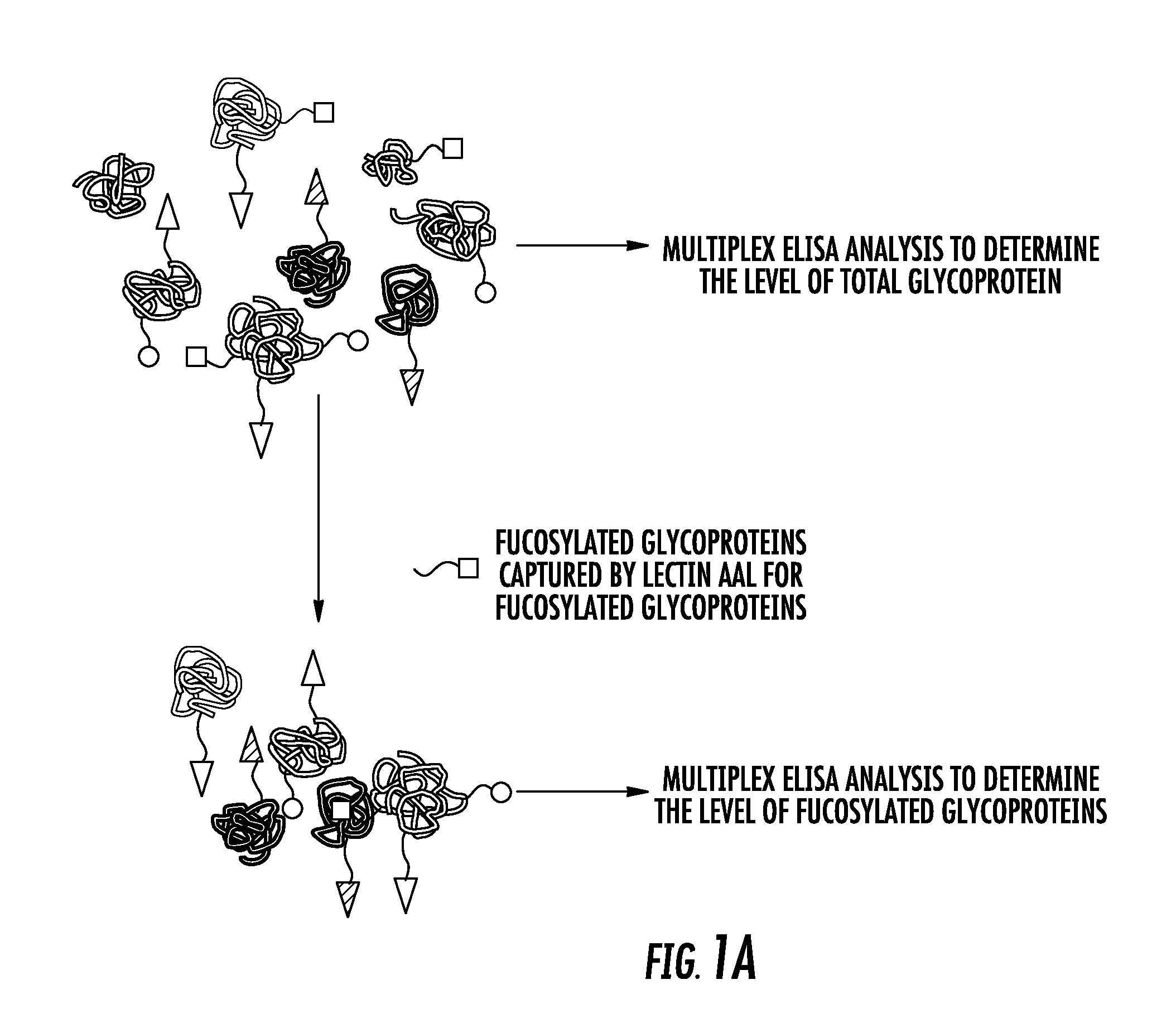
The present invention relates to the field of biomarkers. More specifically, the present invention relates to biomarkers useful in diagnosing aggressive prostate cancer. In one embodiment, a method for identifying patients as having or likely to have aggressive prostate cancer comprises the steps of (a) performing an assay on a biological sample obtained from the patient to detect fucosylated prostate specific antigen (PSA), transmembrane prostate androgen-induced protein, kallikrein-2, lipid phosphate phosphohydrolase 3, heparan-sulfate 6-O-sulfotransferase, prostatic acid phosphatase (PAP), nuclear RNA export factor 2, and protein POF1B; and (b) identifying the patient as having or likely to have aggressive prostate cancer if there is a statistically significant difference in the levels of fucosylated PSA, transmembrane prostate androgen-induced protein, kallikrein-2, lipid phosphate phosphohydrolase 3, heparan-sulfate 6-O-sulfotransferase, PAP, nuclear RNA export factor 2, and protein POF1B as compared to corresponding levels in a control sample that correlates to non-aggressive prostate cancer.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Serum Markers Predicting Clinical Response to Anti-TNF Alpha Antibodies in Patients with Psoriatic Arthritis
InactiveUS20120178100A1Sustained responseBioreactor/fermenter combinationsBiological substance pretreatmentsPsoriasis arthropathySerum protein
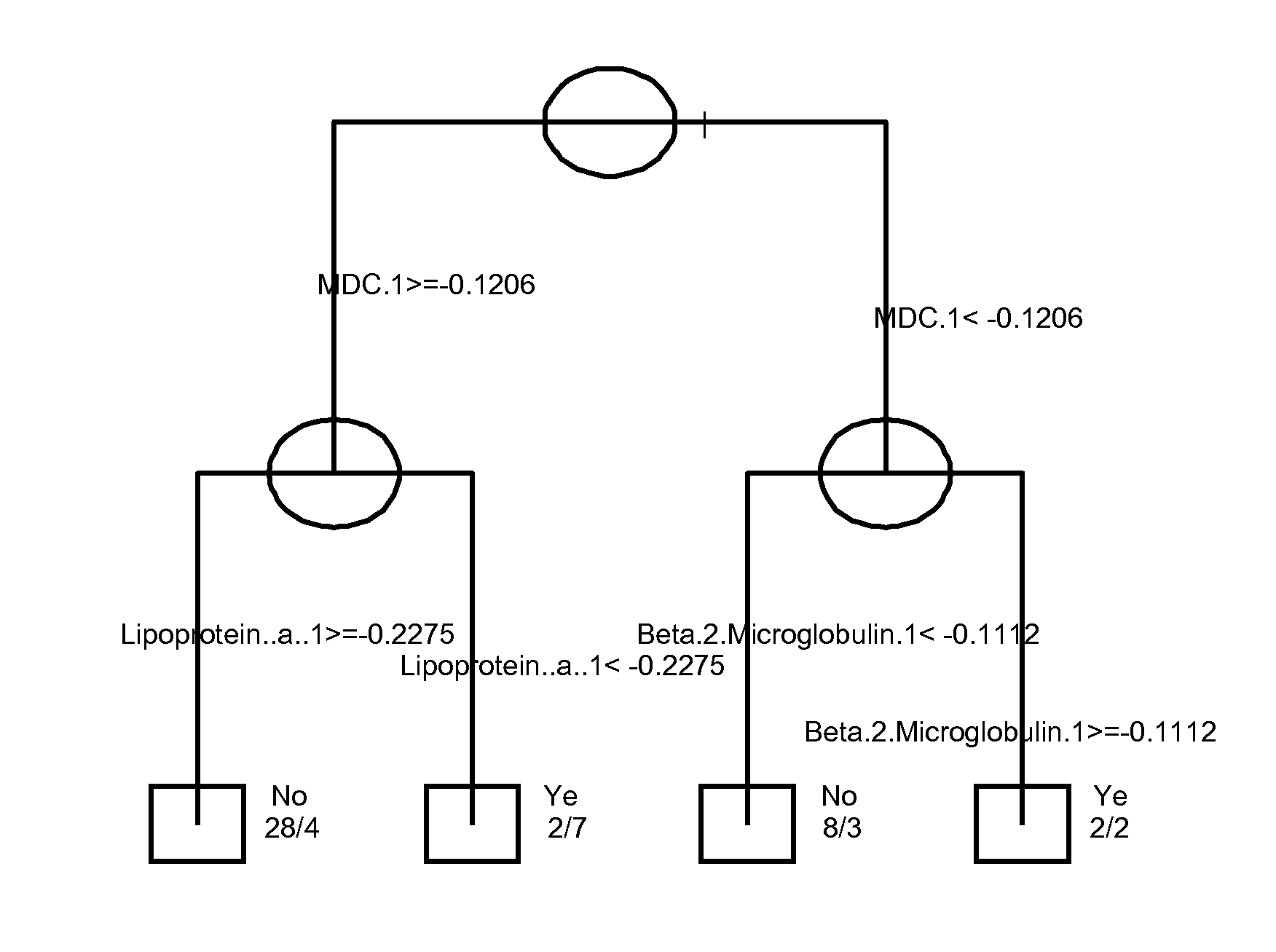
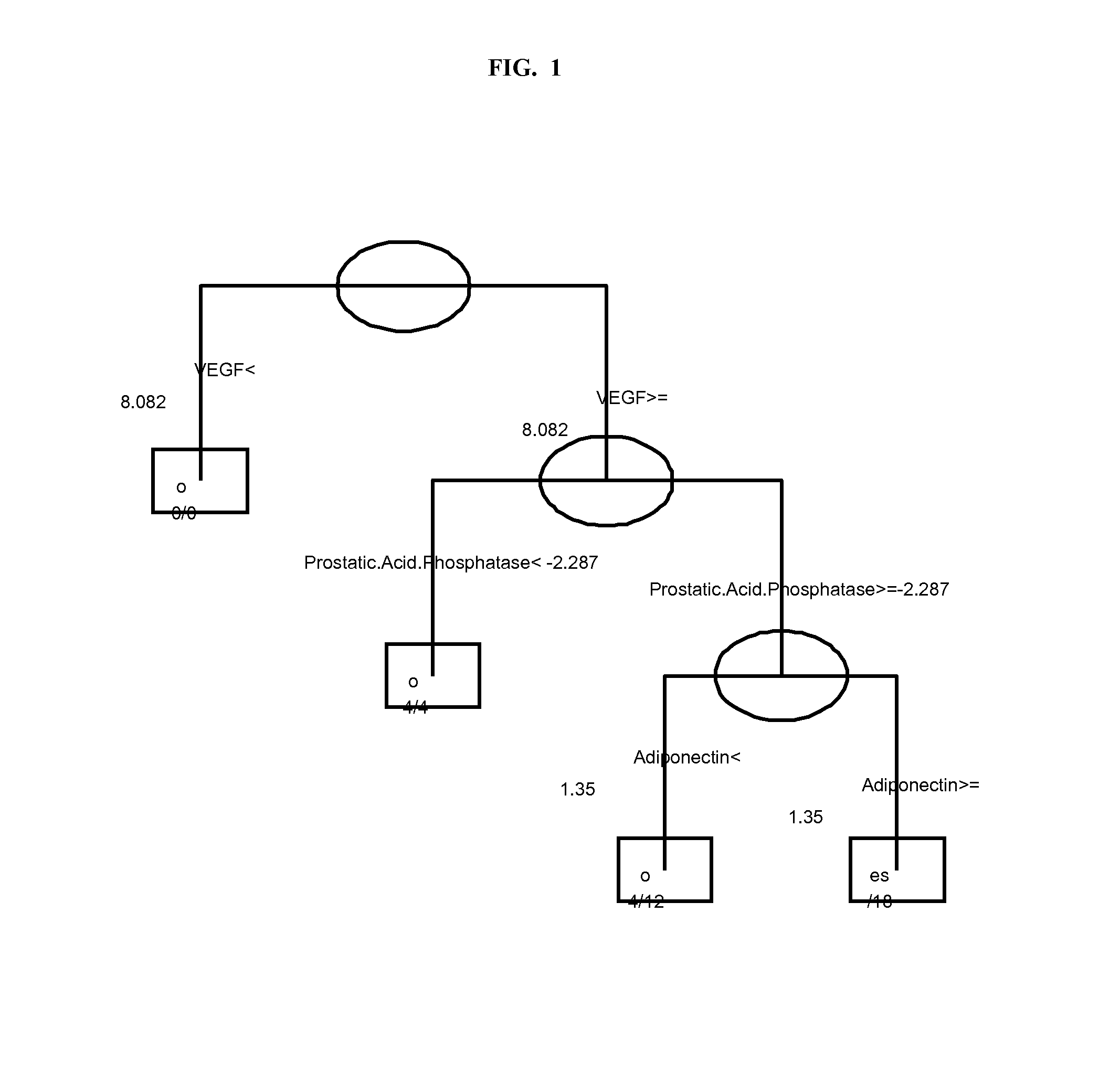
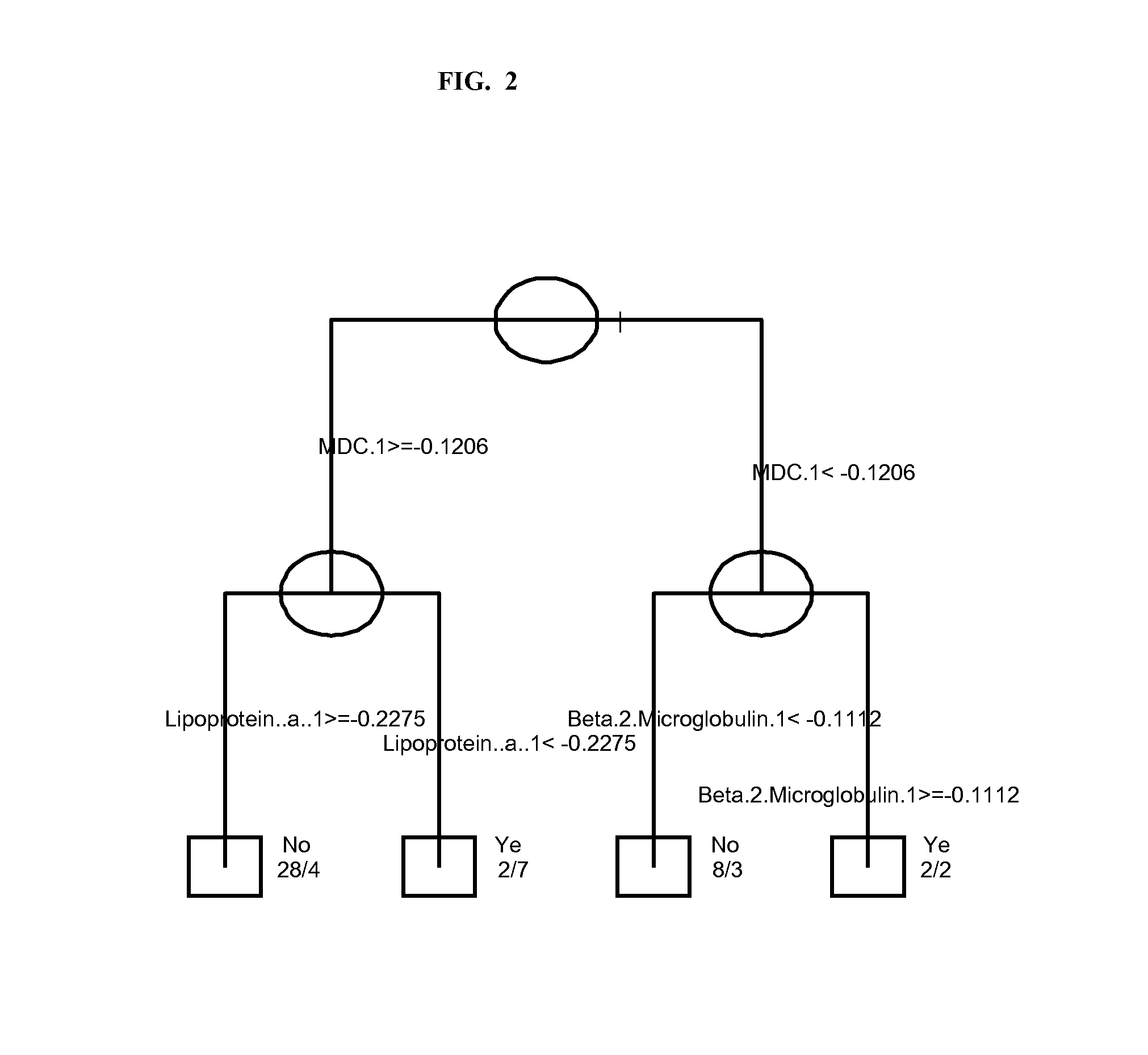
The invention provides tools for management of patients diagnosed with psoriatic arthritis, specifically, prior to the initiation of therapy with an anti-TNFα agent. The tools are specific markers and algorithms of predicting response to therapy based on standard clinical primary and secondary endpoints using serum marker concentrations. In one embodiment the baseline levels of VEGF, prostatic acid phosphatase, and adiponectin are used to predict the response at Week 14 after the initiation of therapy. In another embodiment, the change in a serum protein biomarker after 4 weeks of therapy is used such as MDC, lipoprotein a, and beta2-microglobulin.
Owner:CENTOCOR ORTHO BIOTECH
Promiscuous pap cd4 t cell epitopes
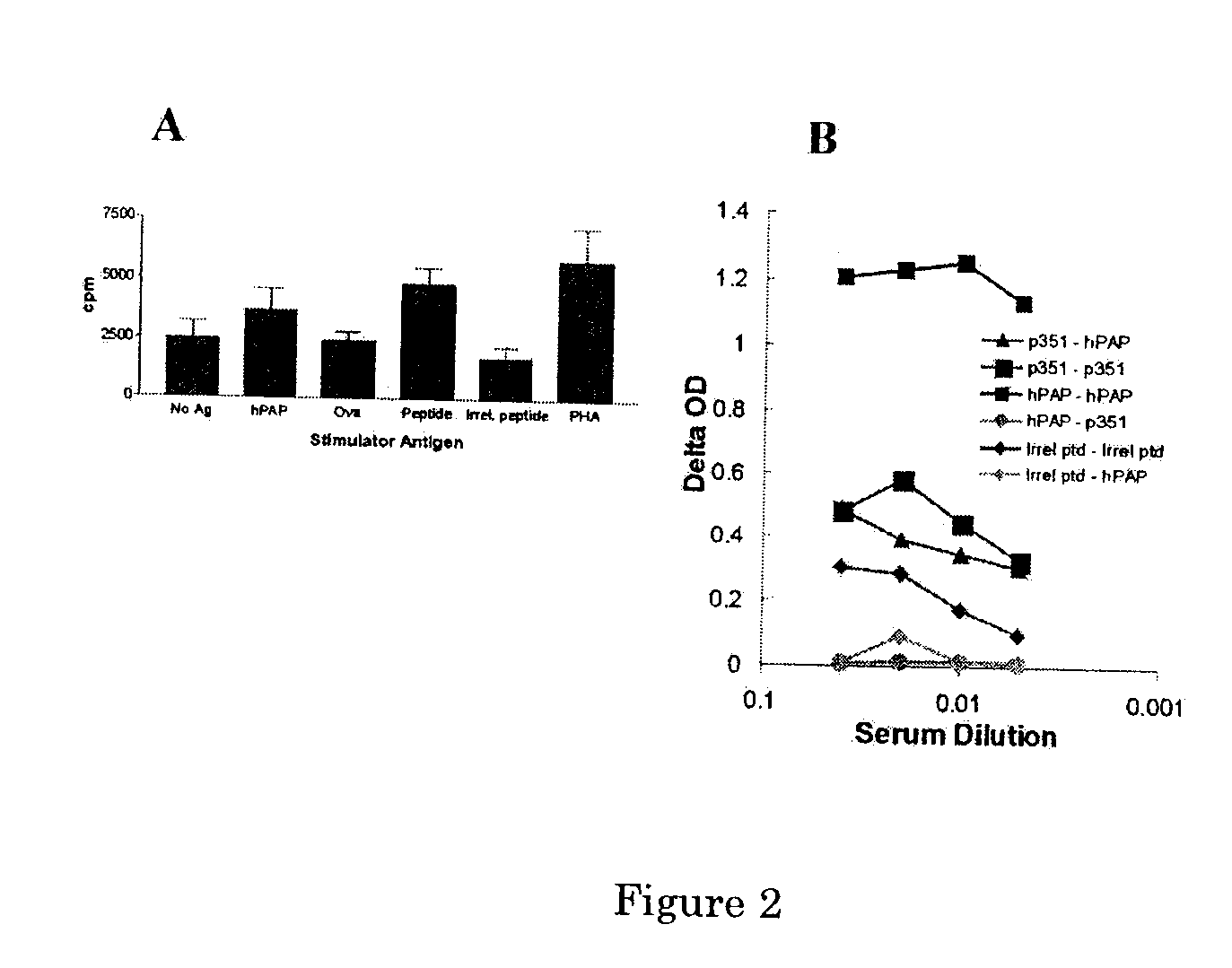
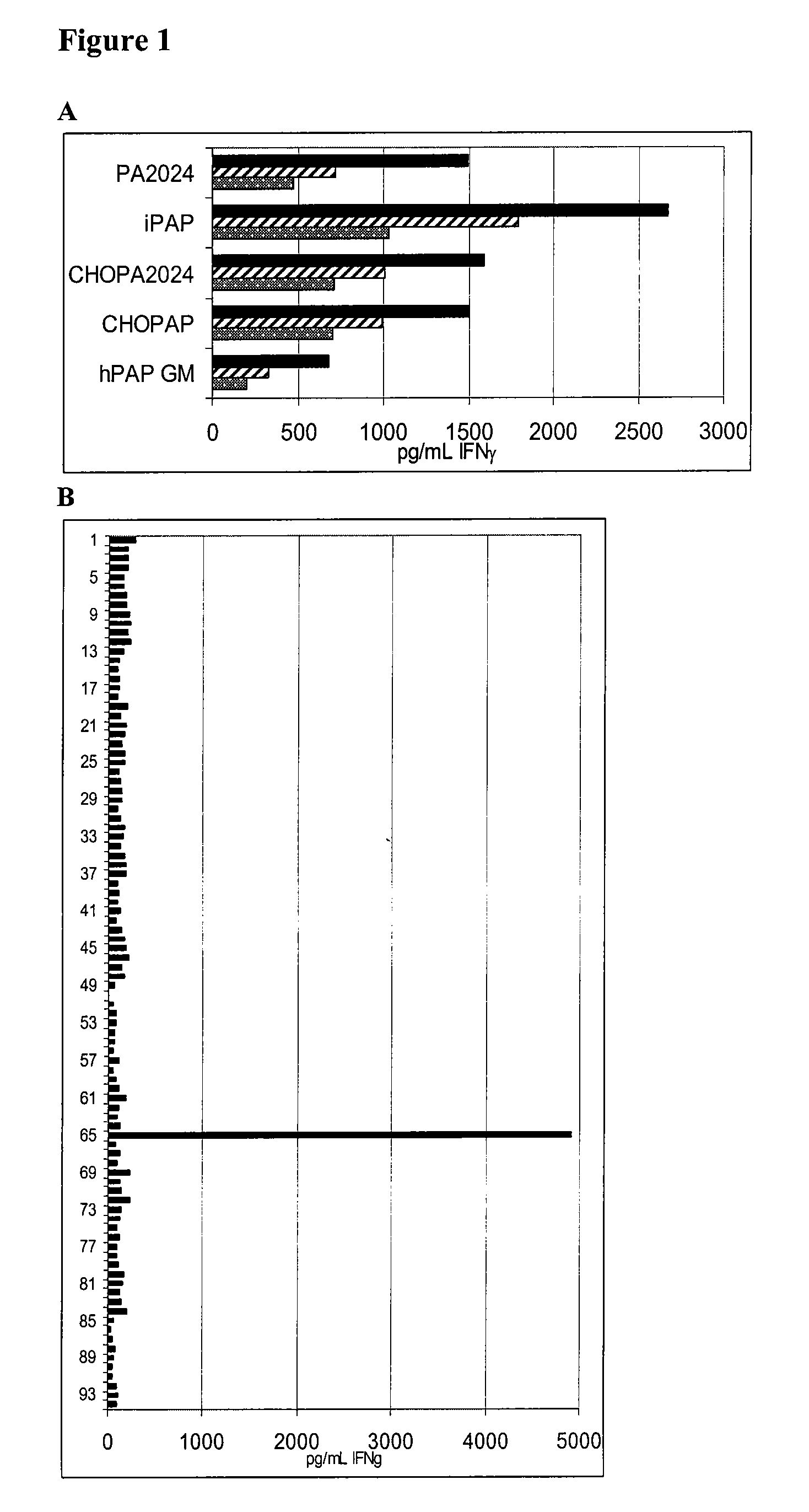
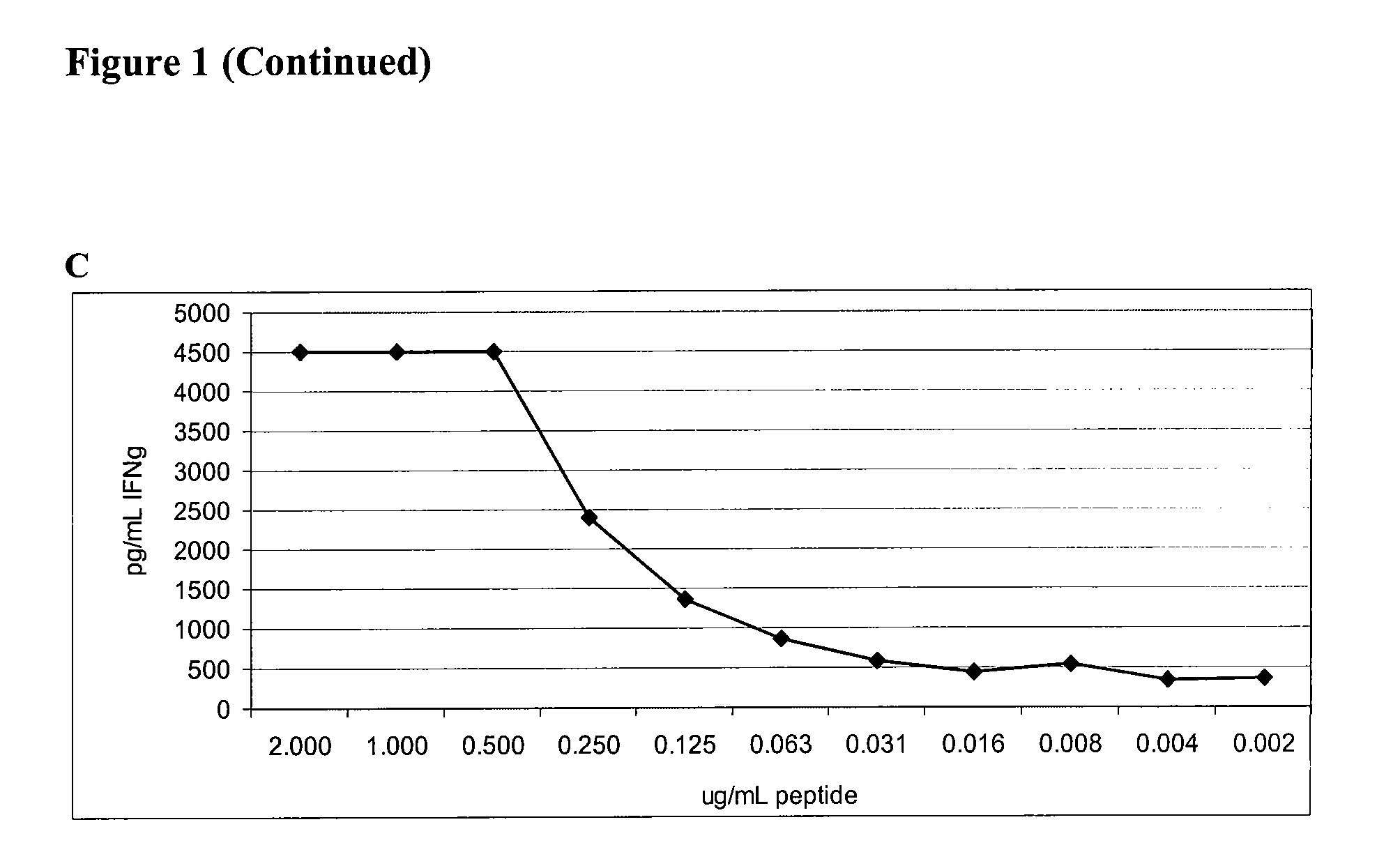
The present invention relates to the discovery of novel T cell epitopes of the human prostatic acid phosphatase (PAP) protein that is promiscuous for at least 15 different HLA-DR alleles. The invention also relates to compositions that contain one of the novel epitopes or a fusion peptide of such an epitope and a heterologous polypeptide. Further disclosed herein is the use of the epitopes or their fusion peptides, and compositions containing the epitopes or their fusion peptides.
Owner:DENDREON PHARMA LLC
Prostate-cancer-specific PAP (prostatic acid phosphatase)-GM-CSF (granulocyte-macrophage colony-stimulating factor)-IL-6 (interluekin-6) genetic recombinant fusion protein and preparation method thereof
The invention relates to preparation of a genetic recombinant protein, in particular to a prostate-cancer-specific PAP (prostatic acid phosphatase)-GM-CSF (granulocyte-macrophage colony-stimulating factor)-IL-6 (interluekin-6) genetic recombinant fusion protein and a preparation method thereof. The preparation method comprises steps of design and synthesis of primers, gene amplification and sequencing, construction of a recombinant vector pc DNA3.1-PAP-GM-CSF-IL-6, screening and identification of the recombinant vector pc DNA3.1-PAP-GM-CSF-IL-6, transfection of the recombinant vector pc DNA3.1-PAP-GM-CSF-IL-6 into an HEK293 cell line, as well as expression, separation, purification, concentration and determination of the PAP-GM-CSF-IL-6 recombinant fusion protein in the HEK293 cell line.
Owner:河北彤苓榕生物科技有限公司
Preparation method of PAP/GM-CSF (prostatic acid phosphatase/grain macrophage-colony stimulating factor)
ActiveCN110079539AHydrolasesAntibody mimetics/scaffoldsGranulocyte colony-stimulating factorNucleotide
The invention relates to a preparation method of PAP / GM-CSF (prostatic acid phosphatase / grain macrophage-colony stimulating factor), in particular to a nucleotide sequence for encoding the PAP / GM-CSF.The amino acid sequence is encoded as the amino acid sequence shown in SEQ ID No.:1; preferably, the nucleotide sequence is shown in SEQ ID NO.:2. The invention also provides an expression carrier for high-efficiency expression of PAP / GM-CSF, engineering cell and a preparation method. The constructed PAP / GM-CSF has the advantages that when the PAP / GM-CSF is used for expressing the engineering cell, the expression amount is high, the expression is stable, and the like; the purifying technology is stable and reliable, the yield rate is high, and the large-scale production is convenient.
Owner:SHANGHAI HUIMMUTECH BIOTECHNOLOGY CO LTD
TAT-PAP fusion protein as well as preparation method and application thereof
InactiveCN104211816AHigh activityLow costNervous disorderPeptide/protein ingredientsEscherichia coliFusion Protein Expression
The invention relates to a TAT-PAP fusion protein. The TAT-PAP fusion protein is formed by protein transduction domain PTD of a trans-activator of transcription of human I type immunodeficiency virus HIV-I and prostatic acid phosphatase PAP. A preparation method of the TAT-PAP fusion protein comprises the following steps: selecting a pTAT-HA expression carrier containing Nco I and Xho I restriction enzyme cutting site sequences at the two ends respectively; synthesizing a pUC57-PAP carrier; carrying out double digestion on the pTAT-HA expression carrier and the pUC57-PAP carrier, and recycling respectively; obtaining a pTAT-PAP recombinant expression plasmid carrier; transforming the pTAT-PAP recombinant expression plasmid carrier into escherichia coli DE3 competent cells; and inducing expression of the fusion protein. The TAT-PAP fusion protein has the advantages that not only is the original penetrating power of TAT remained, but also activity of a foreign protein PAP is hardly influenced, so that the TAT-PAP fusion protein has a broad prospect in clinical application of a macromolecule protein medicine on diabetic neuropathic pain.
Owner:XIAN MEDICAL UNIV
Bisphosphonate-prostatic acid phosphatase inhibitor conjugates to treat prostate cancer bone metastasis
InactiveUS20110065672A1Growth inhibitionReduce the overall heightBiocidePhosphorous compound active ingredientsMedicineProstatic acid phosphatase
The present invention concerns conjugate compounds comprising a bisphosphonate covalently bonded to a prostatic acid phosphatase inhibitor and compositions comprising such conjugates. Methods for treating and inhibiting prostate cancer bone metastases, and determining whether a conjugate is useful for such treatment are also provided. In some instances, the bisphosphonate is alendronate, and it is covalently bonded to either tartaric acid or glyceric acid.
Owner:MT SINAI SCHOOL OF MEDICINE
System and method for expressing and purifying recombinant protein with 3'-nucleotidase as tag and application of recombinant protein
ActiveCN102676566ASimple and fast operationHigh affinityDepsipeptidesPeptide preparation methodsBiotechnologyLysis
The invention relates to the technical field of biology, in particular to a system and a method for expressing and purifying recombinant protein with 3'-nucleotidase as a tag and application of the recombinant protein. The system is characterized in by comprising a 3'-nucleotidase nucleotide sequence and a protein over-expression vector, a 3'-nucleotidase nucleotide sequence added with a protease recognition site, a protease nucleotide sequence matched with the protease recognition site, a lysis solution containing at least one of Li<+> and Ca<2+> ions, an eluent of a chelating agent containing at least one of Li<+> and Ca<2+> ions, and PAP (Prostatic Acid Phosphatase) agarose gel. The system disclosed by the invention is a simple, high-efficiency and economic system for purifying a target recombinant protein.
Owner:海宁市袁花镇工业投资有限公司
Alkaloid compound separated from pumpkin seeds and application thereof
InactiveCN107840810AGood inhibitory effectInhibit benign prostatic hyperplasiaCarbamic acid derivatives preparationOrganic compound preparationPumpkin seedMedicine
The invention discloses an alkaloid compound separated from pumpkin seeds and an application thereof, which belong to the technical field of food and health products. The compound is extracted from pumpkin seeds and used for treating prostatic hyperplasia, the compound has obvious inhibition effect for three indexes of prostate wet weight, prostate index and prostate acidic phosphatase (PAP) whileeffect dosage is 250 mg / kg, has obvious inhibition effect for three indexes while effect dosage is 500 mg / kg, and has important application potential.
Owner:徐一达
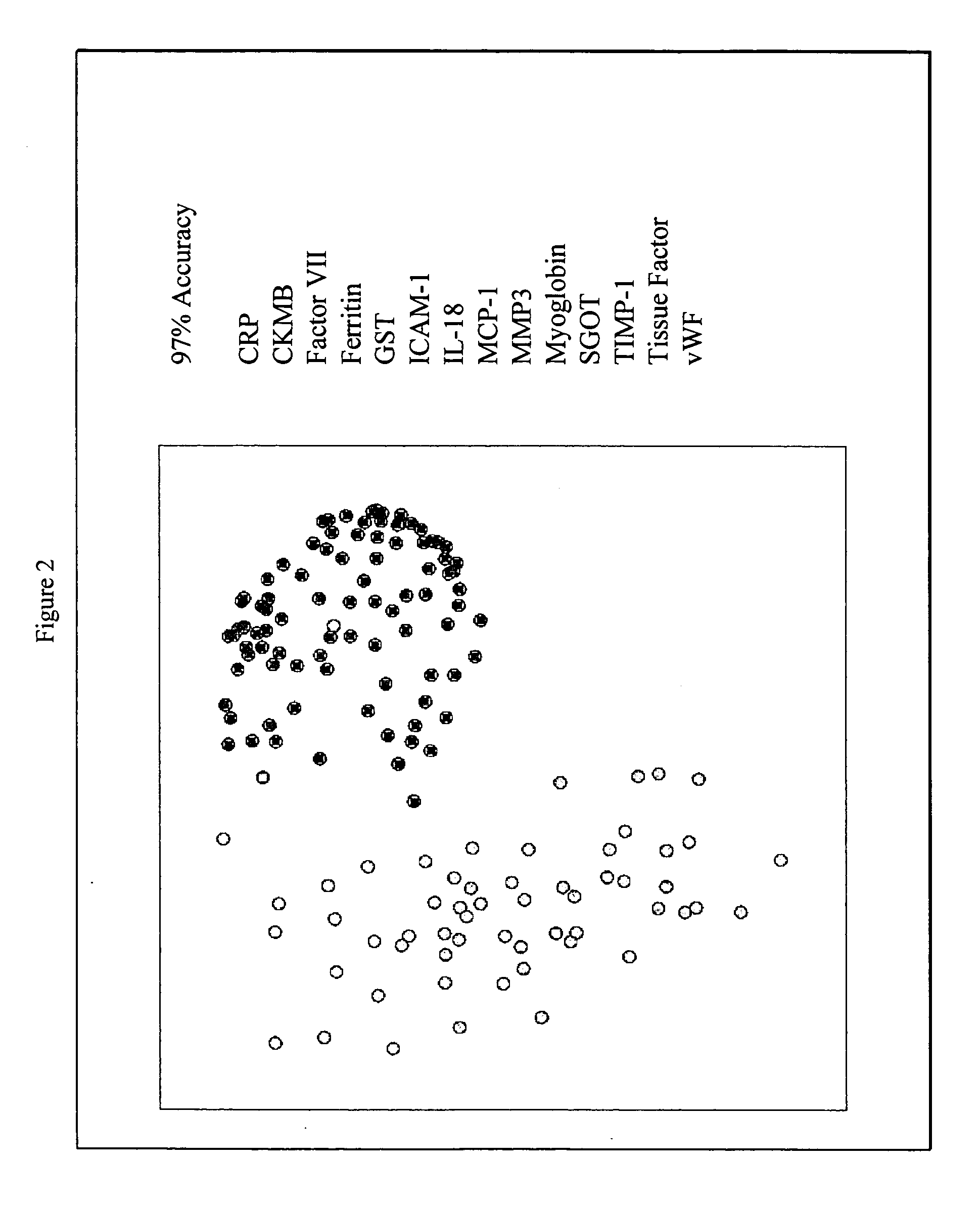
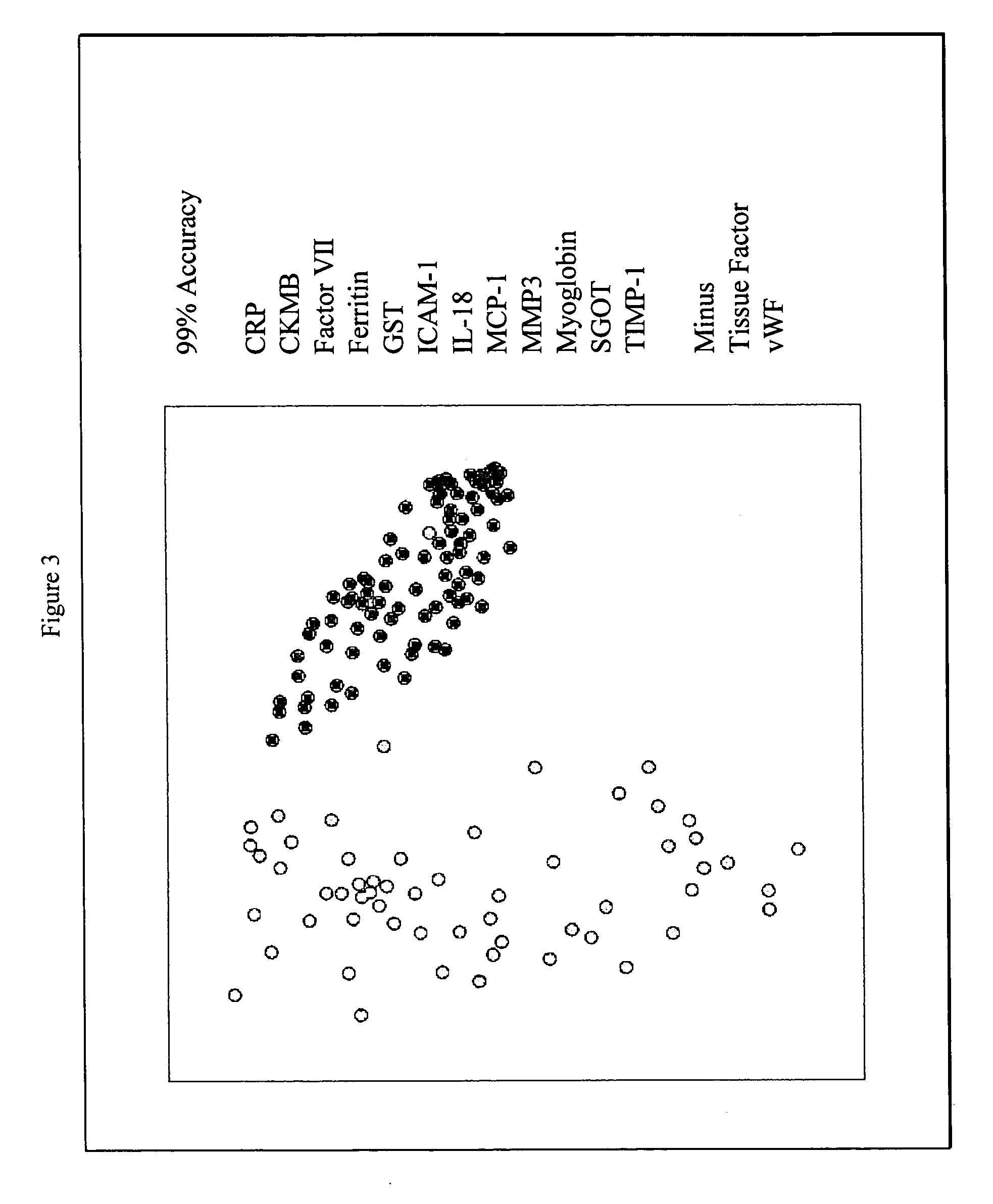
Methods for diagnosis of acute coronary syndrome
InactiveUS20090215077A1Quick checkAccurate diagnosisMicrobiological testing/measurementDisease diagnosisComplement 3Factor VII
Owner:RULES BASED MEDICINE
System and method for expressing and purifying recombinant protein with 3'-nucleotidase as tag and application of recombinant protein
ActiveCN102676566BSimple and fast operationHigh affinityPeptide preparation methodsDepsipeptidesBiotechnologyLysis
The invention relates to the technical field of biology, in particular to a system and a method for expressing and purifying recombinant protein with 3'-nucleotidase as a tag and application of the recombinant protein. The system is characterized in by comprising a 3'-nucleotidase nucleotide sequence and a protein over-expression vector, a 3'-nucleotidase nucleotide sequence added with a protease recognition site, a protease nucleotide sequence matched with the protease recognition site, a lysis solution containing at least one of Li<+> and Ca<2+> ions, an eluent of a chelating agent containing at least one of Li<+> and Ca<2+> ions, and PAP (Prostatic Acid Phosphatase) agarose gel. The system disclosed by the invention is a simple, high-efficiency and economic system for purifying a target recombinant protein.
Owner:海宁市袁花镇工业投资有限公司
Promiscuous pap cd4 t cell epitopes
The present invention relates to the discovery of novel T cell epitopes of the human prostatic acid phosphatase (PAP) protein that is promiscuous for at least 15 different HLA-DR alleles. The invention also relates to compositions that contain one of the novel epitopes or a fusion peptide of such an epitope and a heterologous polypeptide. Further disclosed herein is the use of the epitopes or their fusion peptides, and compositions containing the epitopes or their fusion peptides.
Owner:DENDREON PHARMA LLC
Protein assay method specific to TRACP-5b (tartrate resistant acid phosphatase 5b)
ActiveUS10214592B2Efficiently detect and quantify TRACP-5bPeptide/protein ingredientsHydrolasesAntigenTartrate-Resistant Acid Phosphatase 5b
Owner:NITTO BOSEIKI CO LTD
Phosphatidic acid phosphatase gene and use thereof
ActiveUS20120309950A1Improve abilitiesImprove productivityFungiSugar derivativesNucleotideMicrobiology
The present invention provides a novel phosphatidic acid phosphatase gene. The object of the present invention can be solved by providing a nucleotide sequence set forth in SEQ ID NO: 1 or SEQ ID NO: 7, SEQ ID NO: 4 or SEQ ID NO: 9, or SEQ ID NO: 5 or SEQ ID NO: 10; and an amino acid sequence set forth in SEQ ID NO: 2 or SEQ ID NO: 6.
Owner:SUNTORY HLDG LTD
Phosphatidic acid phosphatase gene
The present invention provides a novel phosphatidic acid phosphatase gene. The object of the present invention can be solved by providing a nucleic acid comprising the nucleotide sequence set forth in SEQ ID NO: 1, SEQ ID NO: 4, or SEQ ID NO: 5; a protein comprising the amino acid sequence set forth in SEQ ID NO: 2; and mutants thereof.
Owner:SUNTORY HLDG LTD
Method For Testing A Compound For A Therapeutic Effect And A Diagnostic Method
A method for testing compound for a therapeutic effect utilizing a non-human animal or cell having disruption in the prostatic acid phosphatase gene resulting in a decrease or absence in the activity or the level of prostatic acid phosphatase. The compound may be used for treating disorders related to unbalanced phosphatidylinositol phosphate cascade or signaling pathway. Diagnostic methods and methods for treating the disorders with therapeutic compounds or by gene therapy are also disclosed.
Owner:CHEMPATH
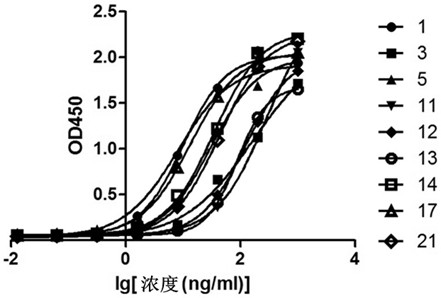
Anti-prostatic acid phosphatase antibody and use thereof
The present invention belongs to the field of biomedicine, and more particularly, the present invention provides a sequence of an anti-prostatic acid phosphatase (PAP) antibody and use thereof. The anti-PAP antibody disclosed in the present invention has higher levels of PAP or prostatic acid phosphatase-granulocyte-macrophage colony-stimulating factor fusion protein (Fusion Protein of Prostatic acid phosphatase-Granulocyte-macrophage colony-stimulating factor, PAP-GM-CSF). The affinity can be used for the purification of PAP or PAP-GM-CSF. At the same time, it can also be used for the diagnosis and treatment of prostate cancer.
Owner:SHANGHAI HUIMMUTECH BIOTECHNOLOGY CO LTD
Anti-prostate acid phosphatase antibody and application thereof
ActiveCN114349864AHydrolasesAntibody mimetics/scaffoldsAntiendomysial antibodiesProstatic acid phosphatase
The invention belongs to the field of biological medicine, and particularly provides a sequence of a prostate acid phosphatase (PAP) resisting antibody and application of the prostate acid phosphatase (PAP) resisting antibody. The anti-PAP antibody disclosed by the invention has relatively high affinity with PAP or a fusion protein of a prostate acid phosphatase-granulocyte-macrophage colony stimulating factor (PAP-GM-CSF), and can be used for purifying the PAP or the PAP-GM-CSF (Prostate acid phosphatase-granulocyte-macrophage colony stimulating factor), so that the anti-PAP antibody disclosed by the invention can be used for purifying the PAP or the PAP-GM-CSF (Prostate acid phosphatase-granulocyte-macrophage colony stimulating factor) and can be used for purifying the PAP or the PAP-GM-CSF (Prostate acid phosphatase-granulocyte-macrophage colony stimulating factor). Meanwhile, the kit can also be used for diagnosis and treatment of prostatic cancer.
Owner:SHANGHAI HUIMMUTECH BIOTECHNOLOGY CO LTD
Phosphatidic acid phosphatase gene and use thereof
ActiveUS9453212B2Improve abilitiesImprove productivityFungiSugar derivativesPhosphatidate phosphataseNuMA Protein
The present invention provides phosphatidic acid phosphatase cDNAs and recombinant vectors comprising nucleic acids encoding proteins having phosphatidic acid phosphatase activity wherein 100 amino acids at the N-terminal region and DXDX(T / V) catalytic site motif are conserved in the protein.
Owner:SUNTORY HLDG LTD
Prostate cancer therapeutics and diagnostics based on conformers of prostatic cid phosphatase
InactiveUS20060088893A1Maintain androgen sensitivityAnimal cellsCompound screeningCancer cellAndrogen
The signals that determine important characteristics of prostate cancer, such as loss of dependence on androgens are described. A key protein, prostatic acid phosphatase, is shown to fold in different ways in response to cellular signals, and the conformer “mix” of this protein in prostatic cells may represent a fundamental difference between normal cells and cancer cells.
Owner:RGT UNIV OF CALIFORNIA
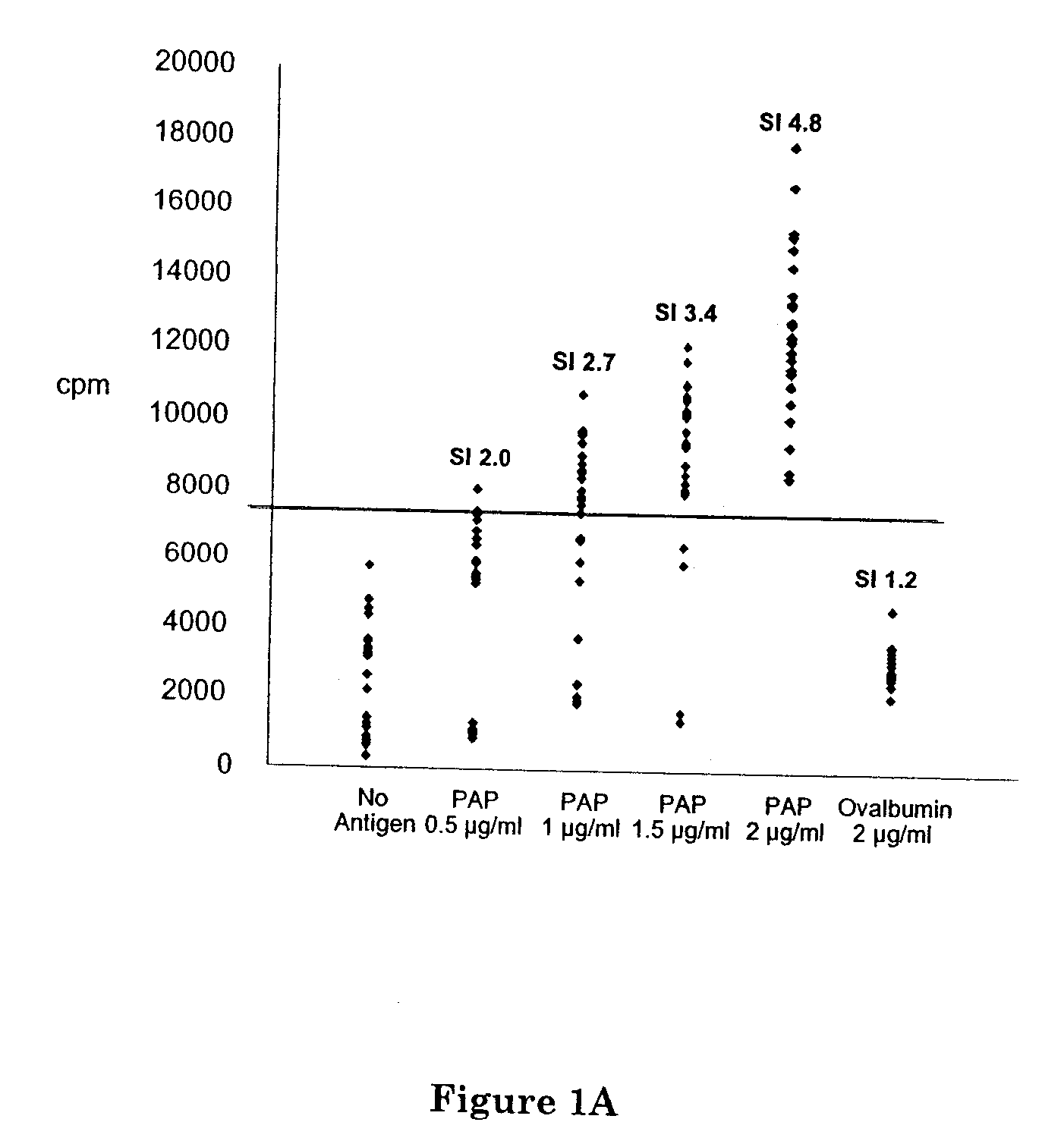
Prostatic Acid Phosphatase (Pap) Materials and Methods of Use Thereof in the Prophylactic and Therapeutic Treatment of Prostate Cancer
A nucleic acid molecule comprising at least one nucleotide sequence encoding SEQ ID NO: 14, 15, 19, 41, or a sequence that is at least about 95% identical thereto; a composition comprising same and a method of administering same to induce an immune response; a polypeptide consisting of SEQ ID NO: 14, 15, 19, 41, or a sequence that is at least about 95% identical thereto; a composition comprising same and a method of administering same to induce an immune response; a composition comprising APC, which have been exposed to the polypeptide, and a method of administering same to treat prostate cancer; a composition comprising T-cells, which are specific for an epitope in a polypeptide consisting of SEQ ID NO: 14, 15, 19, or 41 and a method of administering same to treat prostate cancer; a composition comprising an anti-idiotypic antibody having an internal image of an epitope of a polypeptide consisting of SEQ ID NO: 14, 15, 19, or 41 and a method of administering same to treat prostate cancer; and an immortal B-cell line that produces an anti-idiotypic monoclonal antibody having an internal image of an epitope of a polypeptide consisting of SEQ ID NO: 14, 15, 19, or 41.
Owner:UNIV OF MARYLAND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com