Gene expression cassette, lentiviral vector and application of lentiviral vector in treatment of beta thalassemia
A gene expression cassette and lentiviral packaging technology, applied in the field of biomedicine, can solve problems such as damage to HSC function and differentiation, and achieve the effects of enhanced therapeutic effect, huge economic benefits and social benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] vector construction
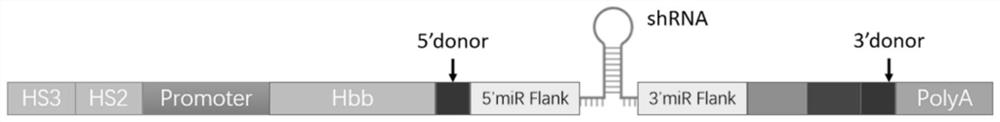
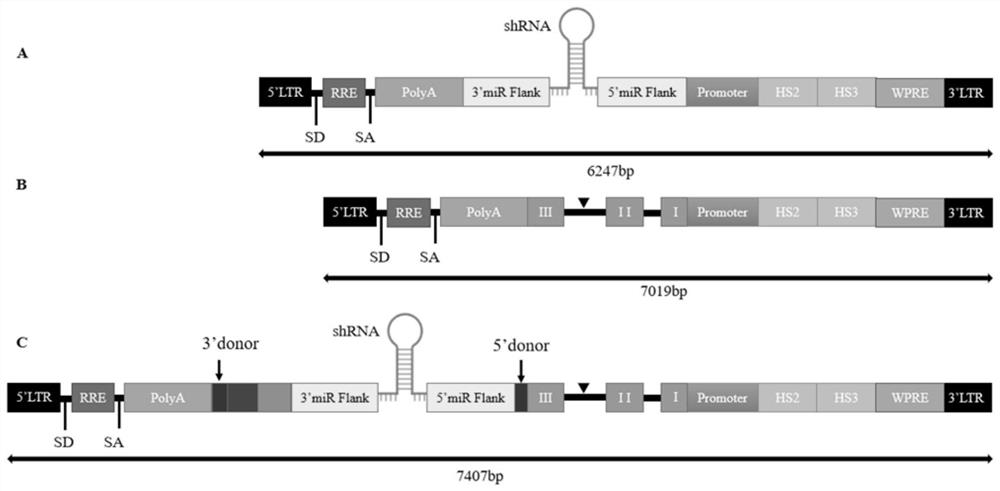
[0088] The HS3-HS2-promoter DNA sequence, miR E5 (E5-shRNAmir) DNA sequence and intron-miR E5 DNA sequence were synthesized by Guangzhou Aiji Biotechnology Co., Ltd. respectively; and ligated into the lentiviral vector. The main sequences involved in the present invention are shown in the following table:
[0089] name length sequence HS3 1202bp SEQ ID NO: 6 HS2 1411bp SEQ ID NO: 7 βglobin promoter 265bp SEQ ID NO: 3 shRNAmir 316bp SEQ ID NO: 8 PolyA 395bp SEQ ID NO: 4 β-globin gene 1052bp SEQ ID NO: 1 Intron-shRNAmir 356bp SEQ ID NO: 2 pCDH-miR E5-HS2-HS3 9597bp SEQ ID NO: 9 pCDH-βglobin-HS2-HS3 10369bp SEQ ID NO: 10 pCDH-inron-miR E5-βglobin-HS2-HS3 10757bp SEQ ID NO: 5 βglobin protein 147aa SEQ ID NO: 11
[0090] The sequence of E5-shRNA is: passenger strand (gcgcgatcgagtgttgaataa), guide strand (ttatcaacactcgatcgcgc).
Embodiment 2
[0092] The four-plasmid system is used for lentiviral packaging, and the specific steps are as follows:
[0093] (1) The four-plasmid system respectively expresses gag / pol, Rev, VSV-G required for lentiviral vector packaging and the E5-shRNAmir expression vector constructed by the present invention: the four plasmids are transiently transfected into 293T cells, and the DNA content is 2 μg / mL;
[0094] (2) Mix the above plasmid with PEI transfection reagent, add it to a certain volume of serum-free DMEM, mix it and let it stand for 15 minutes, then add the above mixture into the T75 culture flask covered with 293T cells, lightly Mix gently, at 37°C, 5% CO 2 Cultivate in a cell incubator for 6 hours;
[0095] (3) Replace the fresh medium after 6 hours, continue the culture, and add 10 mM sodium butyrate solution, and collect the culture supernatant of the lentivirus after 72 hours for purification and detection.
[0096] (4) Use the lentivirus titer (HIV P24) ELISA detection...
Embodiment 3
[0098] E5-shRNAmir lentivirus infection of MEL cells
[0099] 1. Mouse murine erythroleukemia (MEL cells), cultured in complete medium (1640+10% FBS+1% P / S), subcultured every 2-3 days at a ratio of 1:5 to 1:10.
[0100] 2. Collect the cells by centrifugation at 200g for 5-10min, resuspend the cells in complete medium containing 200nM infection enhancer B, and incubate in the incubator for 2h.
[0101] After 3.2 hours, collect the cells by centrifugation at 200g for 5-10 minutes, resuspend the cells with complete medium, and adjust the density to 5×10 6 individual / mL, inoculate 100uL (5×10 5 per well) into a 48-well plate.
[0102] 4. Add an appropriate amount of lentivirus to make the MOI 15, then add infection enhancer A to make the final concentration of infection enhancer A 10uM, and then gently mix the cells.
[0103] 5. Place the cells in an incubator overnight.
[0104] 6. Wash the overnight cultured cells twice with PBS.
[0105] 7. Then resuspend the cells with c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com