Beta-globin recombinant lentiviral vector and application thereof
A technology of recombinant lentivirus and globin, which is applied in the field of molecular biology, can solve the problems of high production cost of vectors and low titer of lentivirus packaging, and achieve the effects of reducing potential carcinogenic risk, low production cost and high packaging titer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: vector construction
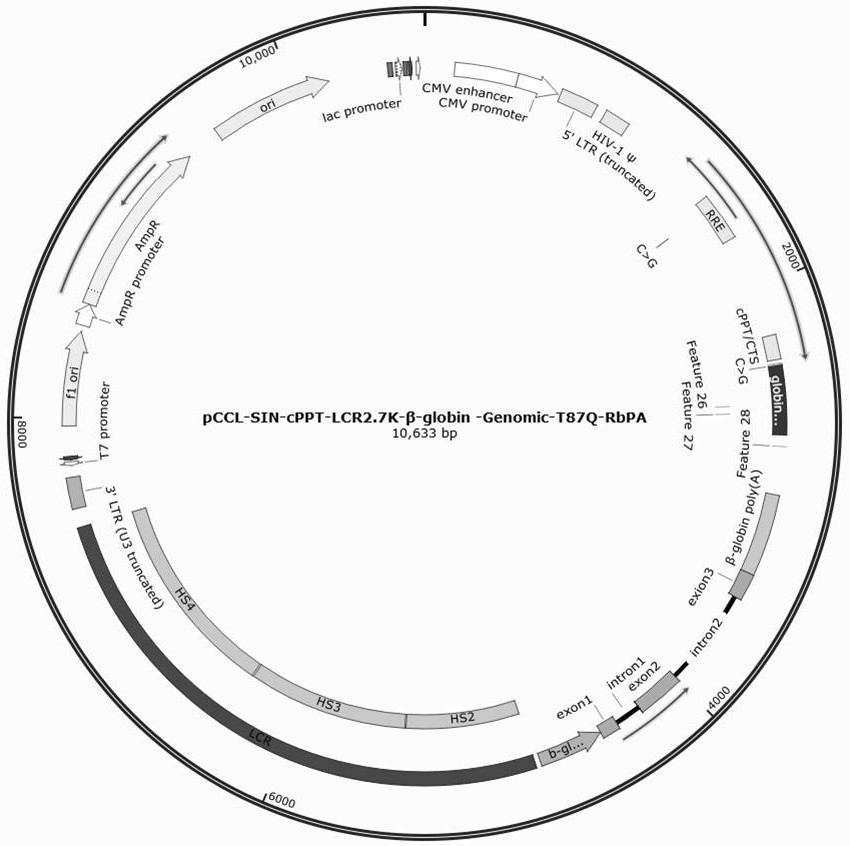
[0039]1. Construction of lentiviral vector pCCL-SIN-cPPT-LCR2.7K-β-globin-Genomic-T87Q-RbPA, including vector pCCL-SIN-cPPT-MCS-RbPA (SEQ ID No: 1), 2.7kb β- LCR regulatory sequence (SEQ ID No:2), β-globin promoter sequence (SEQ ID No:3), genomic sequence encoding β-globin (SEQ ID No:4), β-globin polyA sequence (SEQ ID No: 5) and β-globin enhancer sequence (SEQ ID No: 6).
[0040] 1.1 Digest the pBSK vector plasmid with restriction endonucleases ClaI and XhoI at 36.5-37.5°C for 0.8-1.2h, and cut the gel to recover the pBSK vector fragment after agarose electrophoresis;
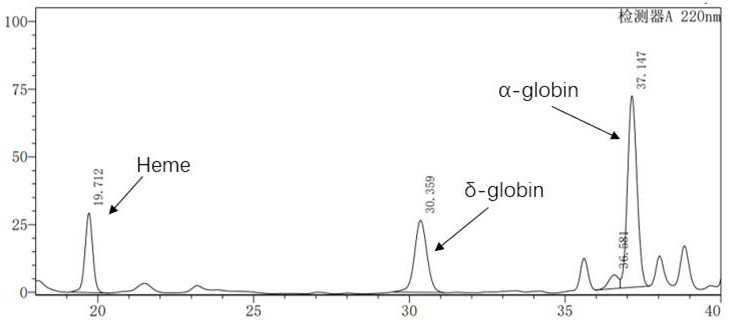
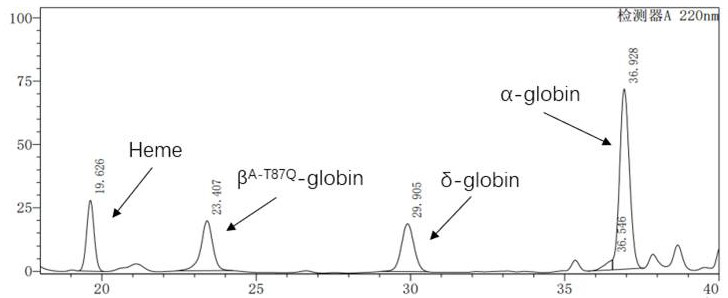
[0041] will express Hbβ A-T87Q The genome sequence of the expression cassette is divided into two parts, β-globin1 and β-globin2, and PCR is carried out respectively, wherein the primer sequence PCR1 used for the β-globin1 gene fragment is SEQ ID No: 7, PCR2 is SEQ ID No: 8, and the β-globin2 gene The primer sequence PCR3 used for the fragment is SEQ ID No: 9, and ...
Embodiment 2
[0075] Example 2: Production and purification of lentivirus
[0076]2.1 Inoculate the successfully constructed pCCL-SIN-cPPT-LCR2.7K-β-globin-Genomic-T87Q-RbPA plasmid and lentiviral packaging kit plasmid Mix in a ratio of 1:3 in a 10-layer cell factory the day before transfection For HEK293T cells, the fresh DMEM medium was replaced 6 hours after transfection, and the supernatant was collected 72 hours later for chromatographic purification;
[0077] 2.2 Purification adopts tangential flow filtration-chromatography system, uses core700 chromatography, Q ImpRes chromatography purification process, and purifies lentivirus. The specific purification process is as follows:
[0078] ① Benzonase treatment (nuclease digestion): Treat with 25U / mL Benzonase at 37°C for 1 hour to remove residual plasmid DNA and genome released from lysed cells during transfection;
[0079] ② MF (clarification): The virus harvest solution is filtered (0.45 μm) to remove insoluble particles such as HEK2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com