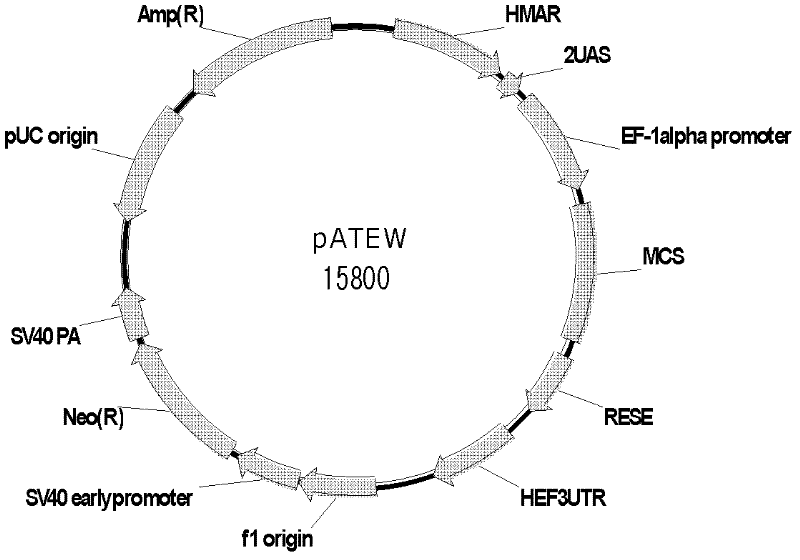
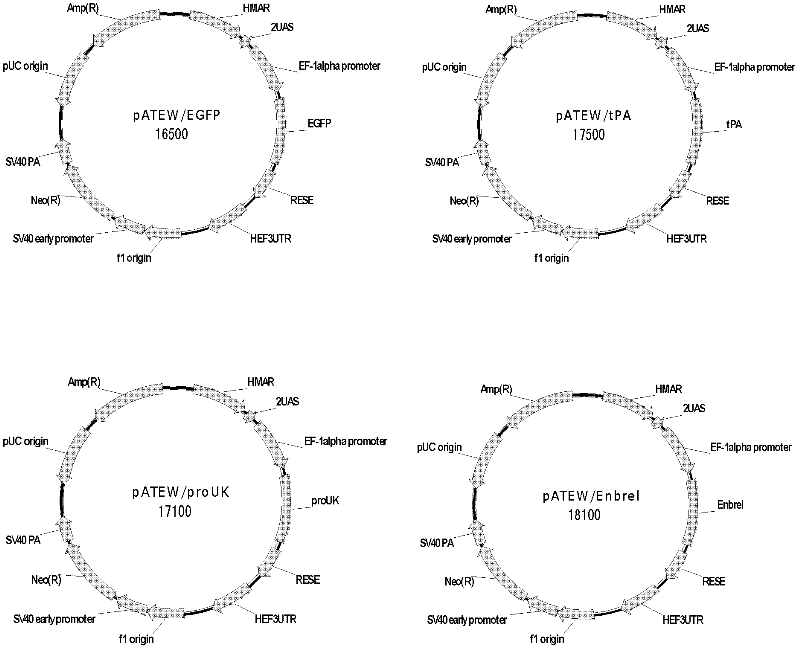
Vector for mediating high-efficiency expression of exogenous gene in cells of mammal and use thereof
A technology of exogenous gene and high-efficiency expression, applied to cells modified by introducing foreign genetic material, using vectors to introduce foreign genetic material, recombinant DNA technology, etc., can solve problems such as gene silencing effect, achieve overcoming position effect, increase transcription Intensive, expressive effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Cloning of the MAR sequence of embodiment 1 people β-globin
[0048] Genomic DNA was extracted from HEK293 cells. According to the human β-globin MAR sequence reported by GenBank, which contains a segment of Alu sequence that should be a repetitive sequence in the human genome, the segmented cloning method was used to clone 1-2804bp by PCR. The synthetic method synthesizes 2804-2998bp, and then splicing the two to obtain a human β-globin MAR sequence with a length of 2998bp. Synthesize HUMAR1 and HUMAR2 primers to amplify 2804bp fragments. The amplification conditions are: denaturation at 94°C for 4min, 1min at 94°C, 1min at 68°C, 40sec at 72°C for 2min, 40sec at 72°C, extension at 72°C for 10min, and cooling at 4°C. The DNA polymerase was LA Taq DNA polymerase.
[0049] The sequence of primer HUMAR1 is shown in SEQ ID No.7.
[0050] The sequence of the primer HUMAR2 is shown in SEQ ID No.8.
Embodiment 2
[0051] Example 2 Cloning of hEF-1α Gene Transcription Regulatory Elements
[0052] The following primers were designed according to the sequence reported by Genbank:
[0053] The sequence of the primer HuEF1 is shown in SEQ ID No.9.
[0054] The sequence of the primer HuEF2 is shown in SEQ ID No.10.
[0055] The sequence of the primer HuEF3 is shown in SEQ ID No.11.
[0056] The sequence of the primer HuEF4 is shown in SEQ ID No.12.
[0057] Use primers HuEF1 and HuEF2 to amplify the 4093bp nucleotide sequence of hEF-1α5' including the promoter and intron 1, and the amplified product contains NheI and MluI restriction sites; primers HuEF3 and HuEF4 amplify 4018bp hEF-1α3 The nucleotide sequence at the 'end, the amplified product contains XbaI and BspEI restriction sites. LA Taq DNA polymerase from Treasure Biotech was used. Amplification conditions were 94°C for 5 min, 94°C for 30 sec, 60°C for 30 sec, 72°C for 4 min, 30 cycles, and 72°C for 10 min. The amplified product ...
Embodiment 3
[0058] Cloning of Example 3 Artificial Transcription Factor Binding Site Nucleotide Sequence
[0059] For the convenience of cloning, PacI, AgeI, HpaI restriction site, use these two primers to amplify the artificial transcription factor binding site sequence containing 5 GaL4 binding sites from the plasmid PG5luc (Promega product). Use TAKARA high-fidelity Pybest enzyme to amplify, and recover a fragment of about 150bp. Ligated into pcDNA3.1(+) / MCS vector through PacI and AgeI, connected into pcDNA3.1(+) / UAS through AgeI and HpaI, and named pcDNA3.1(+) / 2UAS after correct sequencing. Cloning of Example 4 Post-transcriptional Regulatory Element WPRE Sequence
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com