Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
87results about How to "Reduction in hospitalization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Drug adherence monitoring system
InactiveUS20070224128A1Low costImprove efficacyCompounds screening/testingGeneral/multifunctional contrast agentsDrug adherenceEnvironmental health
The present invention provides novel methods for monitoring subject adherence in taking prescribed drugs by detecting markers in exhaled breath after a subject takes the prescribed drug. In particular, the present invention provides novel methods for making additives that are combined with the drug(s). Upon biological breakdown of the drug / additive formulation in a subject's body, markers resulting directly from the biological breakdown of the additives are detected in exhaled breath using sensor technology. In certain embodiments of the invention, the drug adherence monitoring systems and methods include a reporting system capable of tracking subject compliance (either remotely or proximately) and of providing necessary alerts to the subject, caregiver, healthcare provider, and the like.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
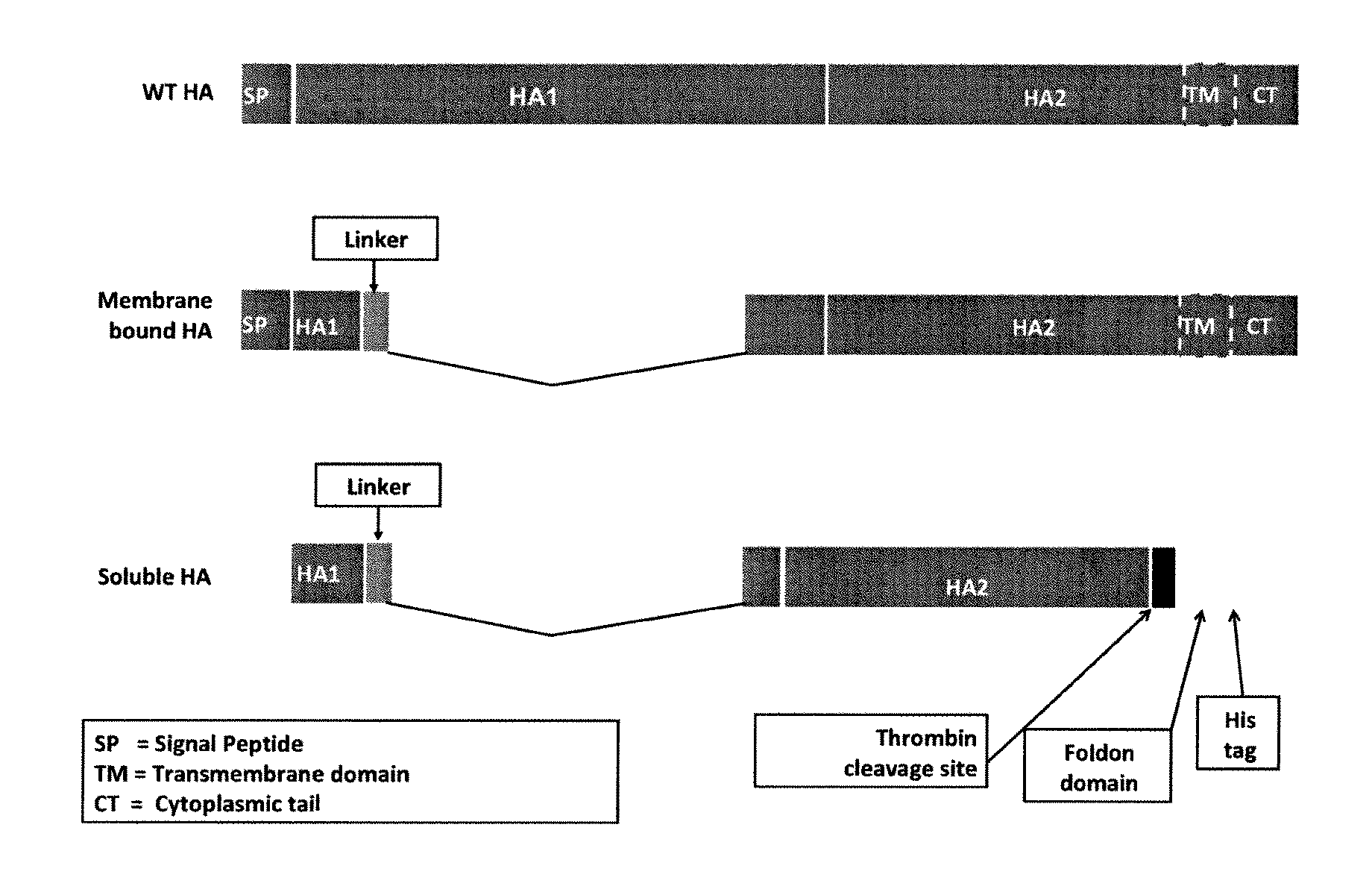
Influenza virus vaccines and uses thereof
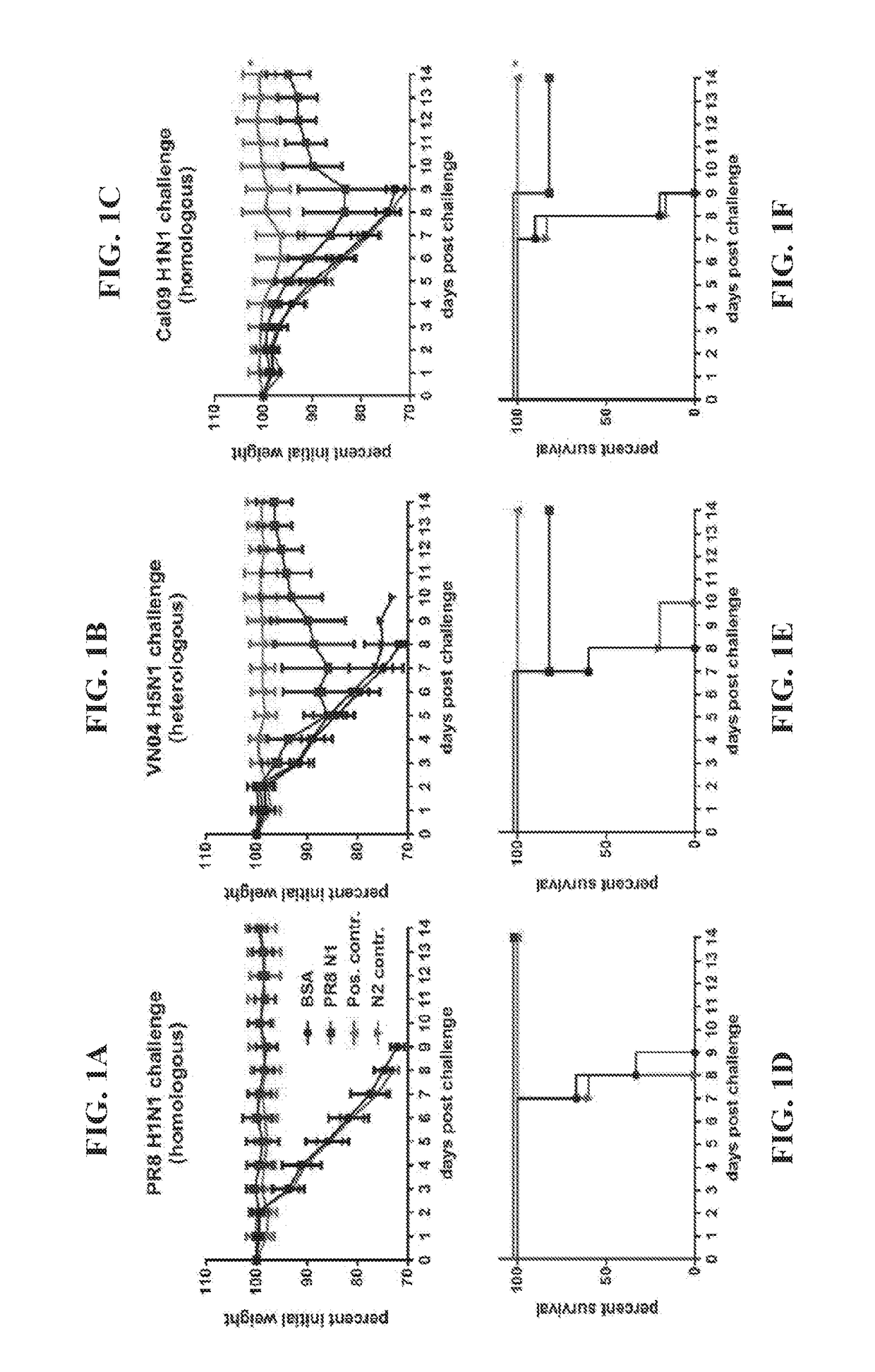
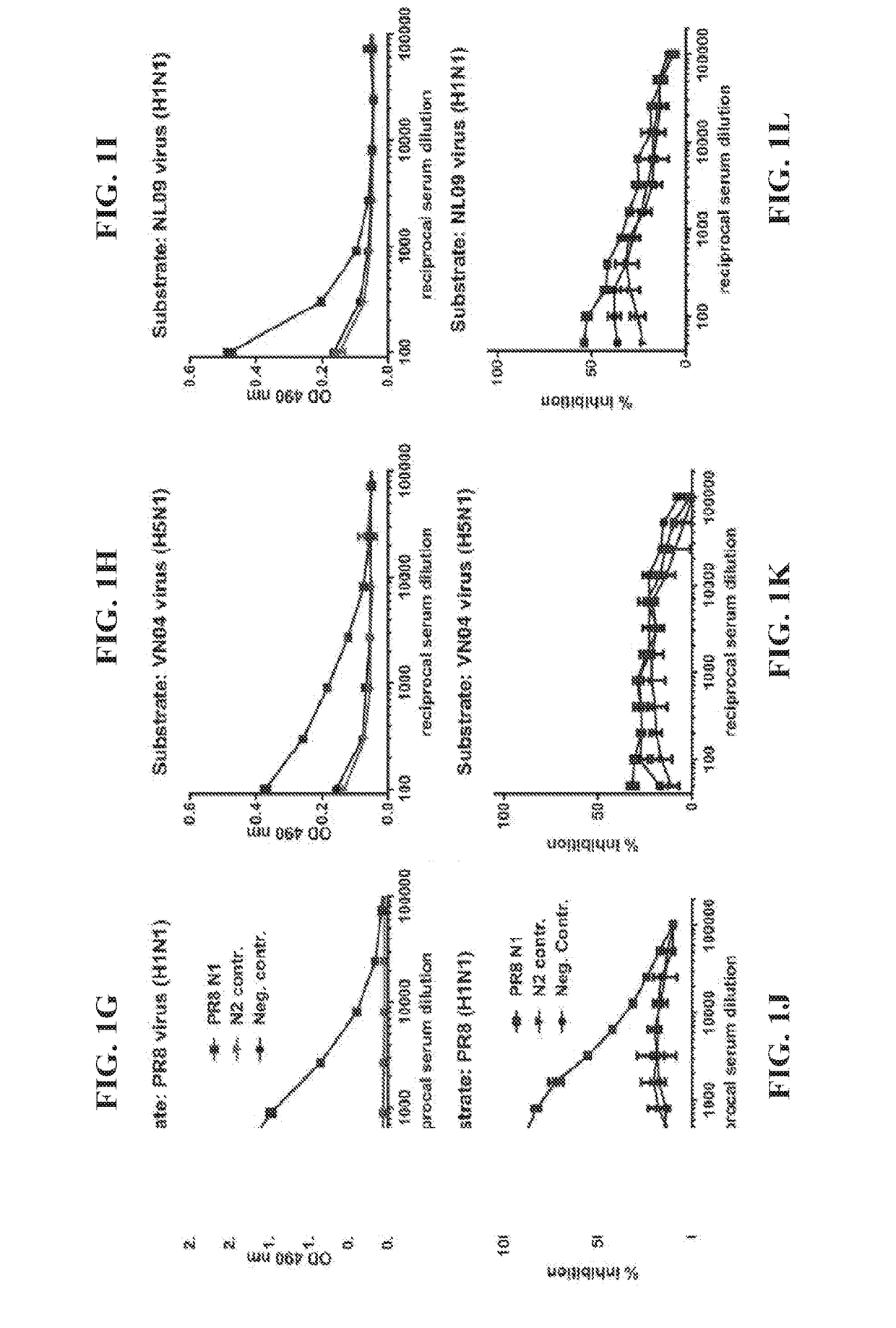
ActiveUS20100297174A1Reduce severityImprove survivalSsRNA viruses negative-sensePeptide/protein ingredientsHemagglutininInfluenza virus vaccine
Owner:MT SINAI SCHOOL OF MEDICINE
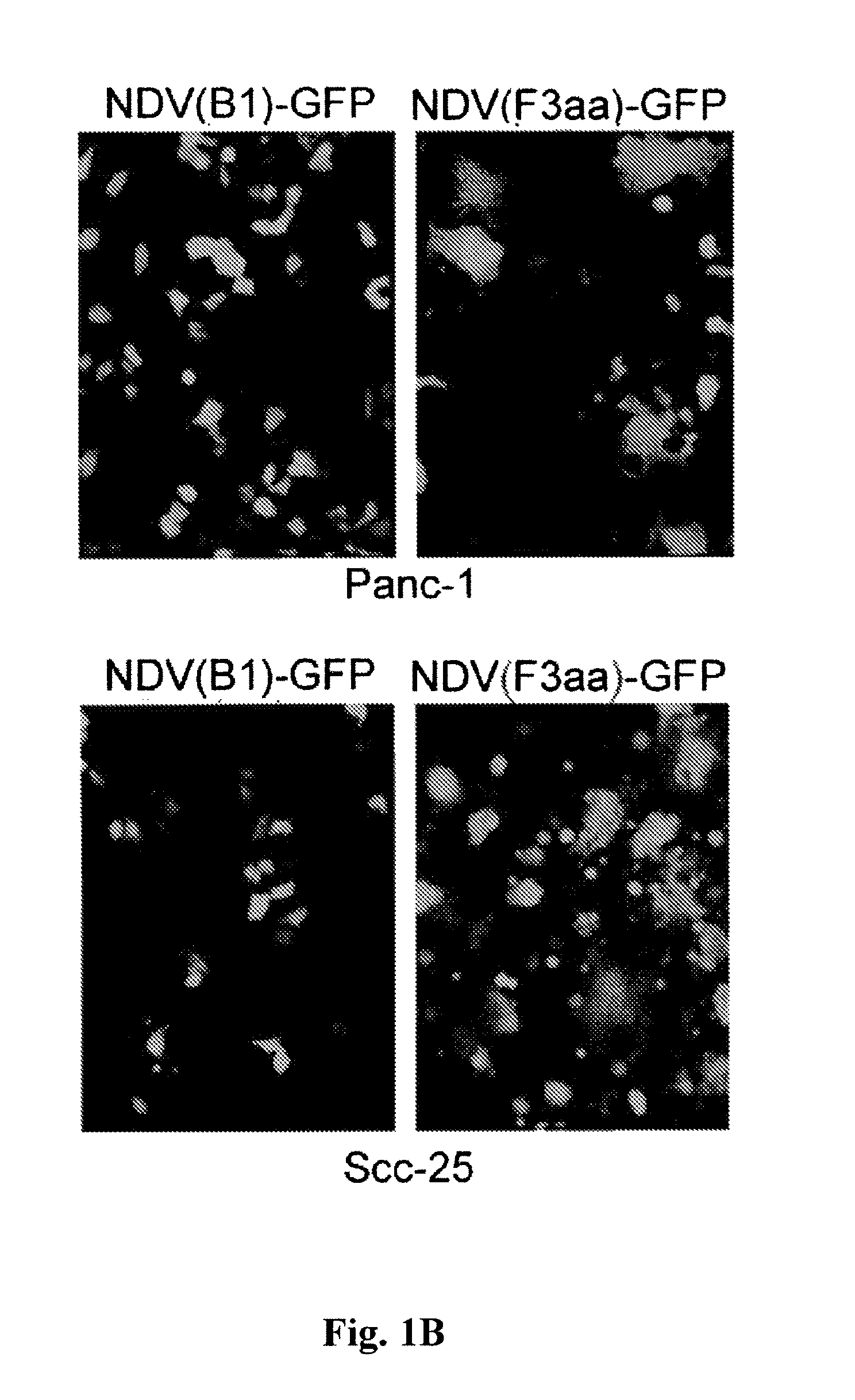
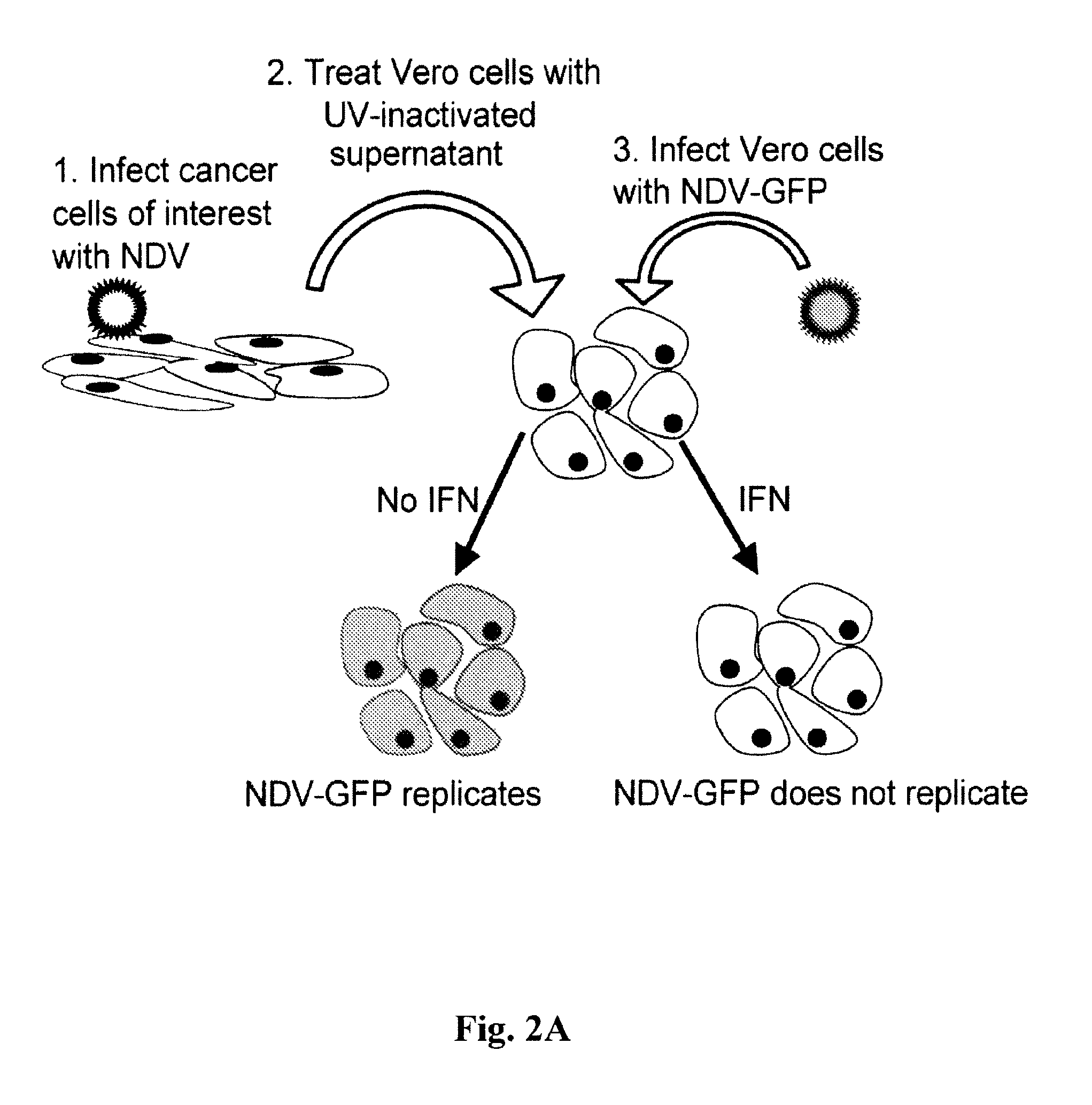
Chimeric Newcastle disease viruses and uses thereof
ActiveUS8591881B2Prevents progression and worseningReduce severitySsRNA viruses negative-senseBiocideNewcastle disease virus NDVAntagonist
Described herein are chimeric Newcastle disease viruses engineered to express a heterologous interferon antagonist and compositions comprising such viruses. The chimeric Newcastle disease viruses and compositions are useful in the treatment of cancer.
Owner:MT SINAI SCHOOL OF MEDICINE +1
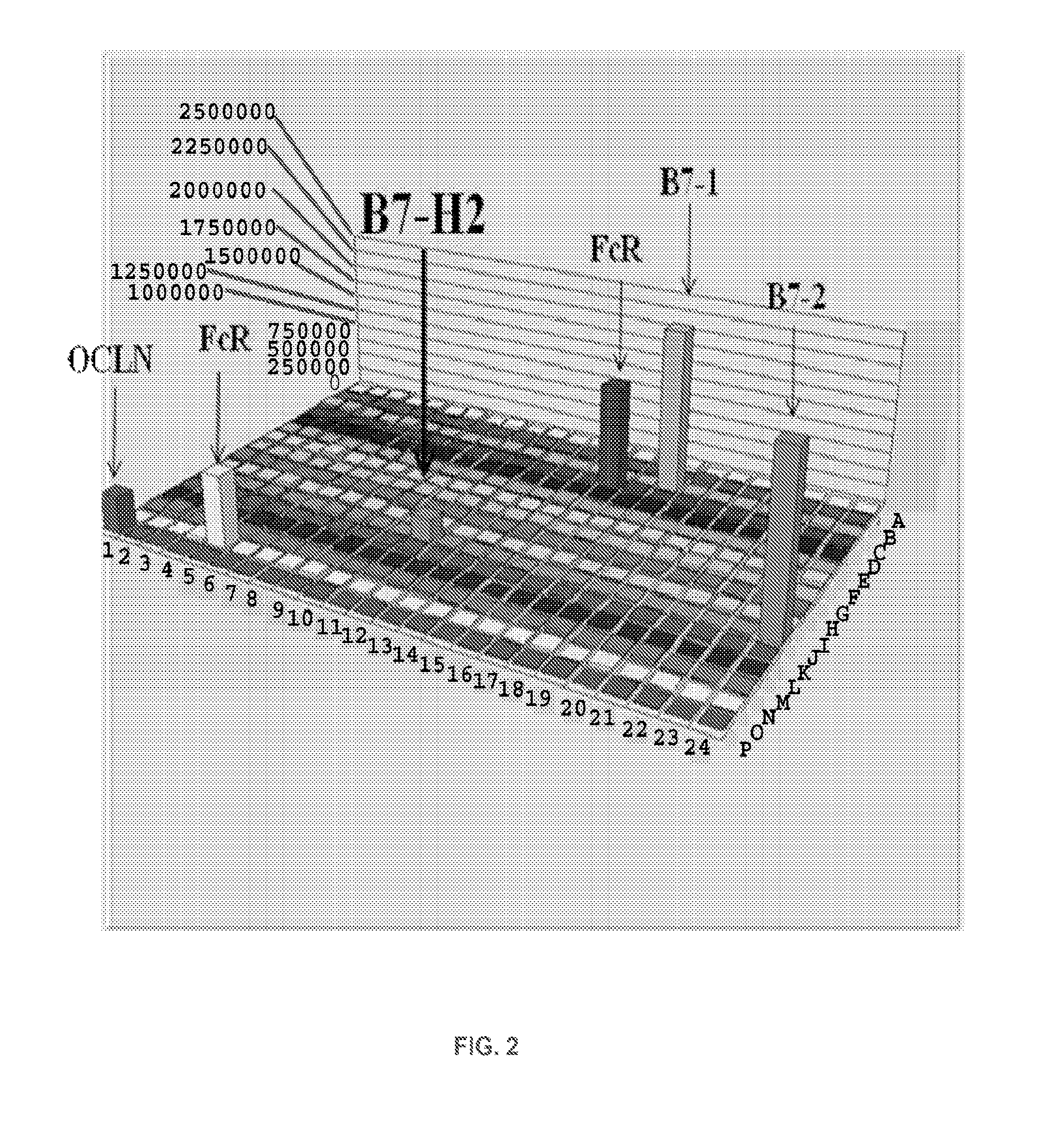
Methods of modulating immune function
ActiveUS20120219559A1Modulate its functionAdjust immune functionBiocidePeptide/protein ingredientsCTLA-4Autoimmune disease
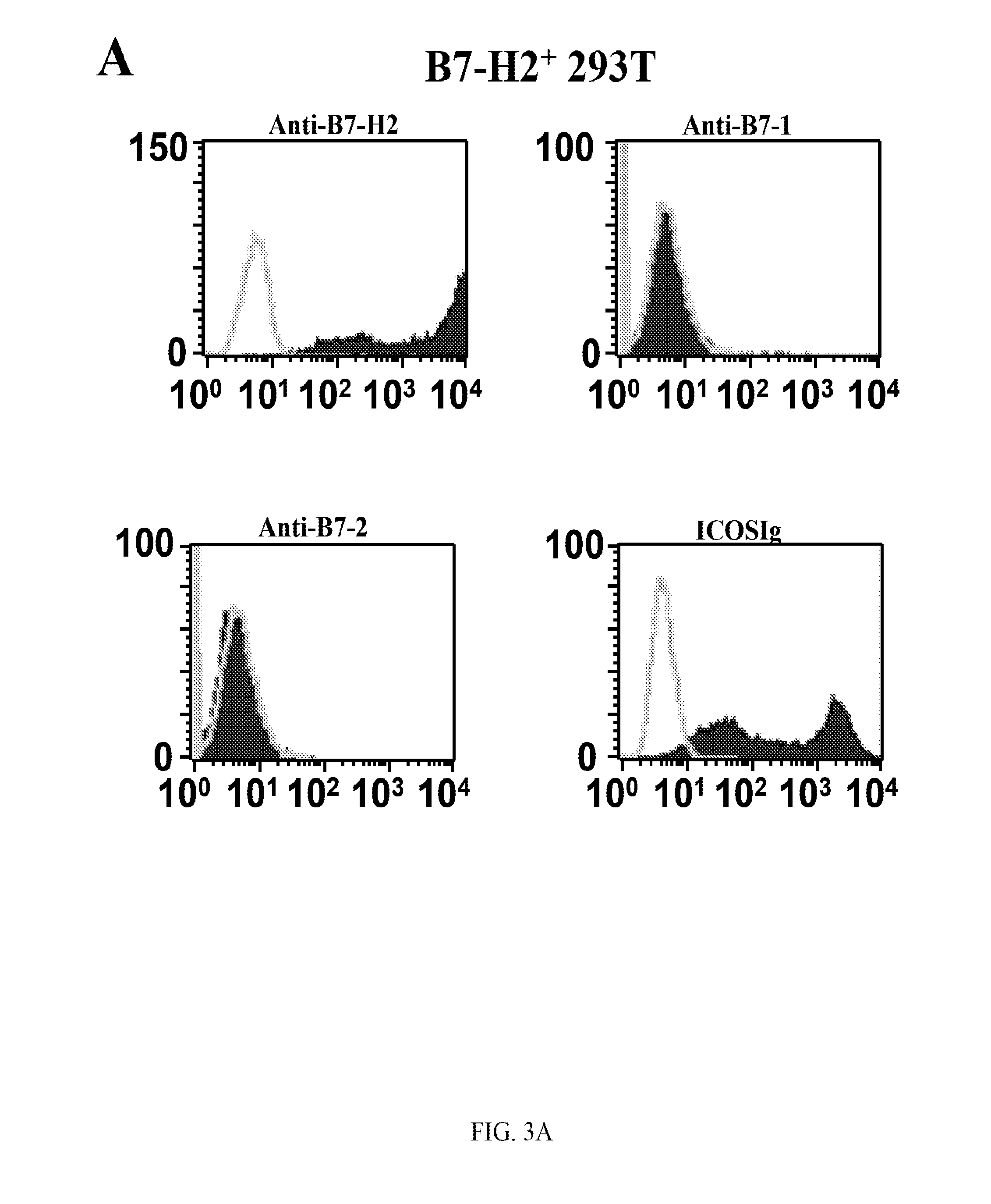
Presented herein are therapeutic agents that modulate one or more immune functions and uses of such therapeutic agents in the prevention, treatment and management of diseases. In one aspect, the therapeutic agents modulate one or more signal transduction pathways induced by the binding of B7-H7 to B7-H7CR, or the binding of B7-H2 to either ICOS, CD28, or CTLA-4. In another aspect, the therapeutic agents modulate the binding of B7-H7 to B7-H7CR, or the binding of B7-H2 to either ICOS, CD28, or CTLA-4. The therapeutic agents can be used in the prevention, treatment and / or management of diseases in which it might be useful to modulate one or more immune functions (e.g., cancer, infectious disease, autoimmune disease, and transplantation rejection). In another aspect, presented herein are methods for identifying receptor-ligand interactions.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Medication Identification, Tracking And Adherence Management
InactiveUS20160147976A1Improve adhesionEasily dispatchData processing applicationsDrug and medicationsGraphicsUser device
A method and a wellness adherence tracking system (WATS) for tracking wellness adherence of a healthcare recipient are provided. An identifier code or an existing code is positioned on a medical implement, for example, a medication bin, a parenteral device, a fitness device, etc. The WATS accessible on a user device scans, decodes, and validates the identifier code, and obtains medical information associated with the medical implement and / or an activity, for example, an exercise activity, a diet activity, etc., associated with the medical implement from the decoded and validated identifier code. The WATS renders the medical information and multiple wellness adherence options on a graphical user interface and receives inputs for the wellness adherence options from the user device. The WATS logs the received inputs in association with the wellness adherence criteria in the user device and / or one or more databases to track the wellness adherence of the healthcare recipient.
Owner:RXADVANCE
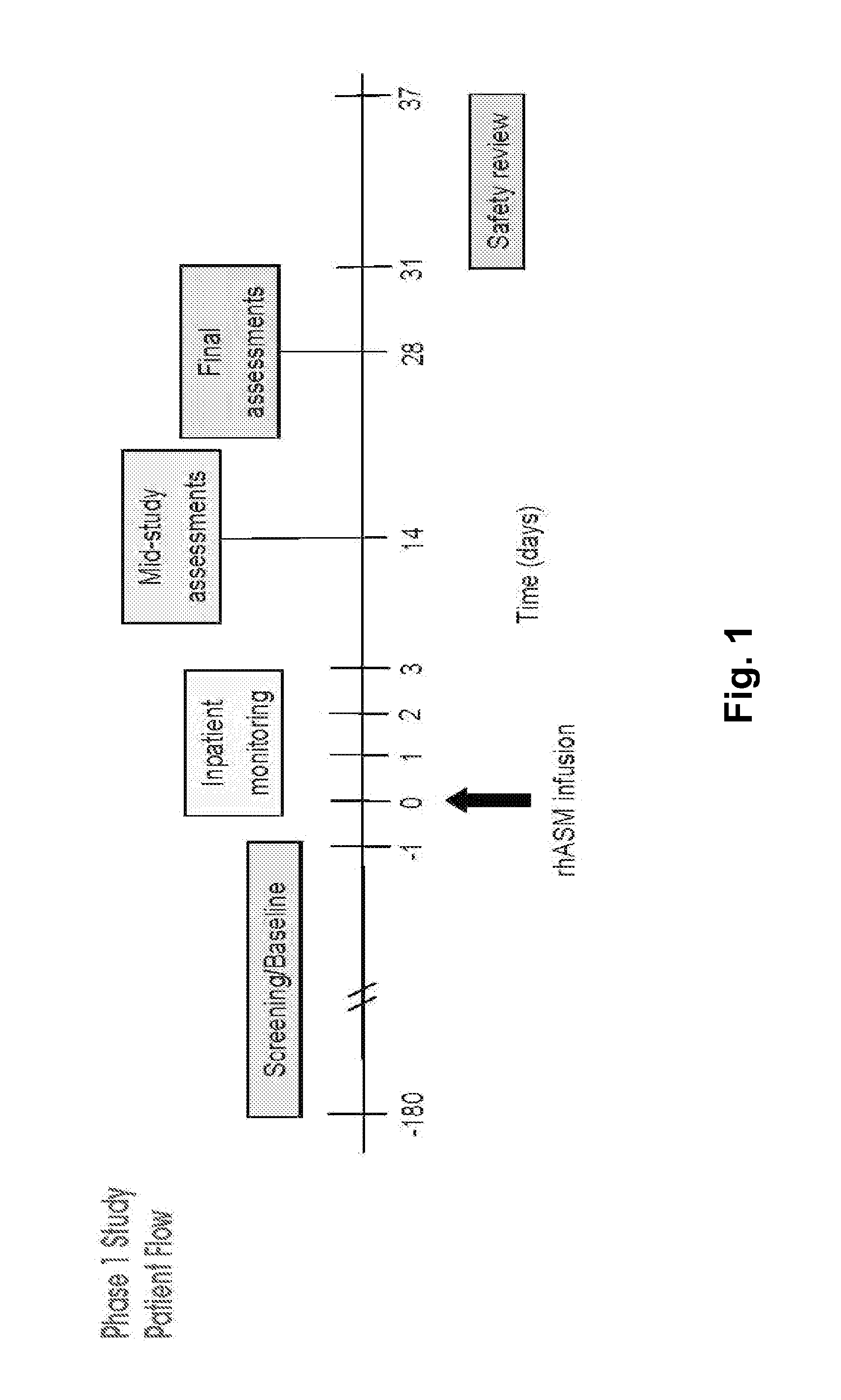
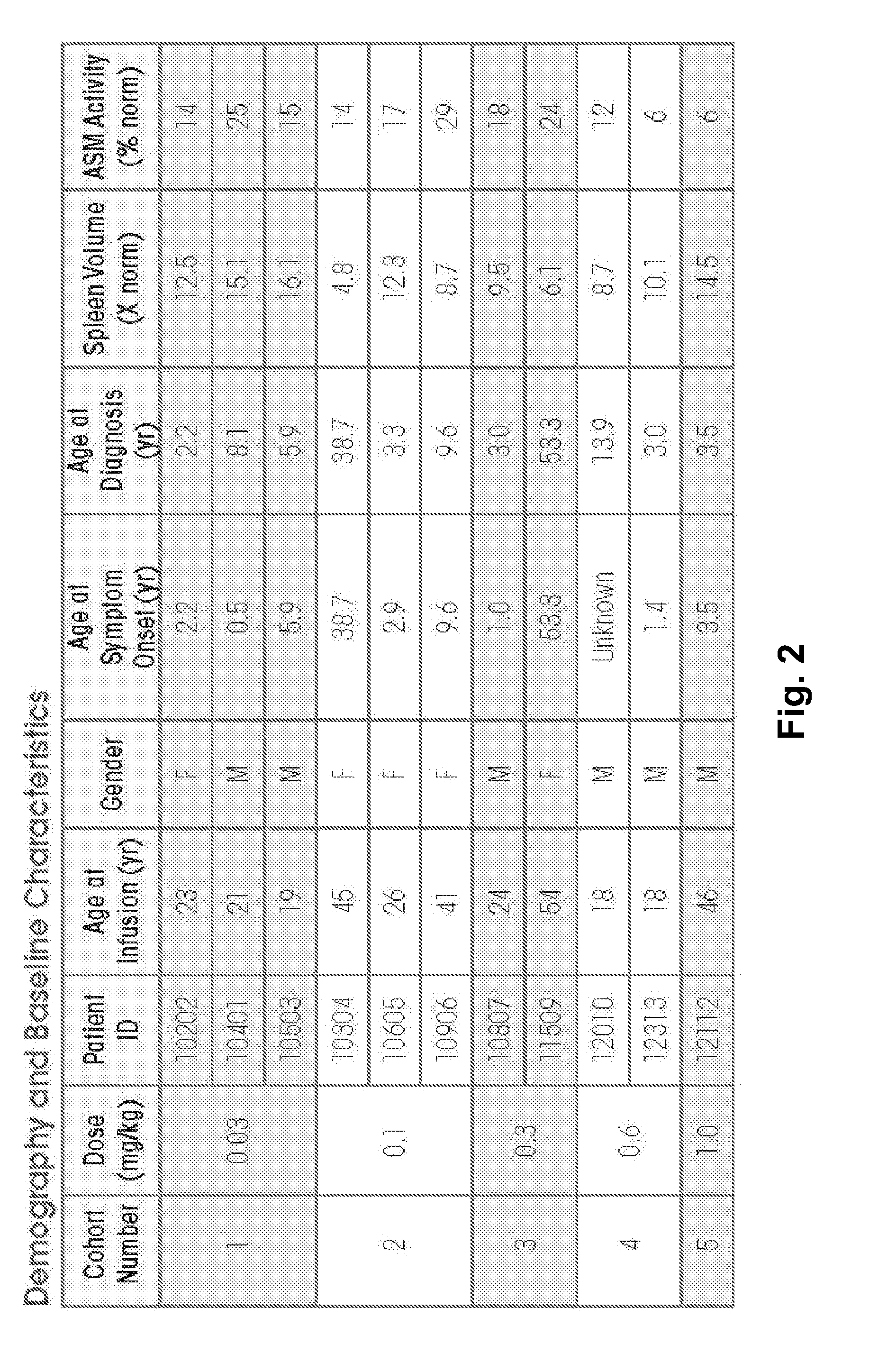
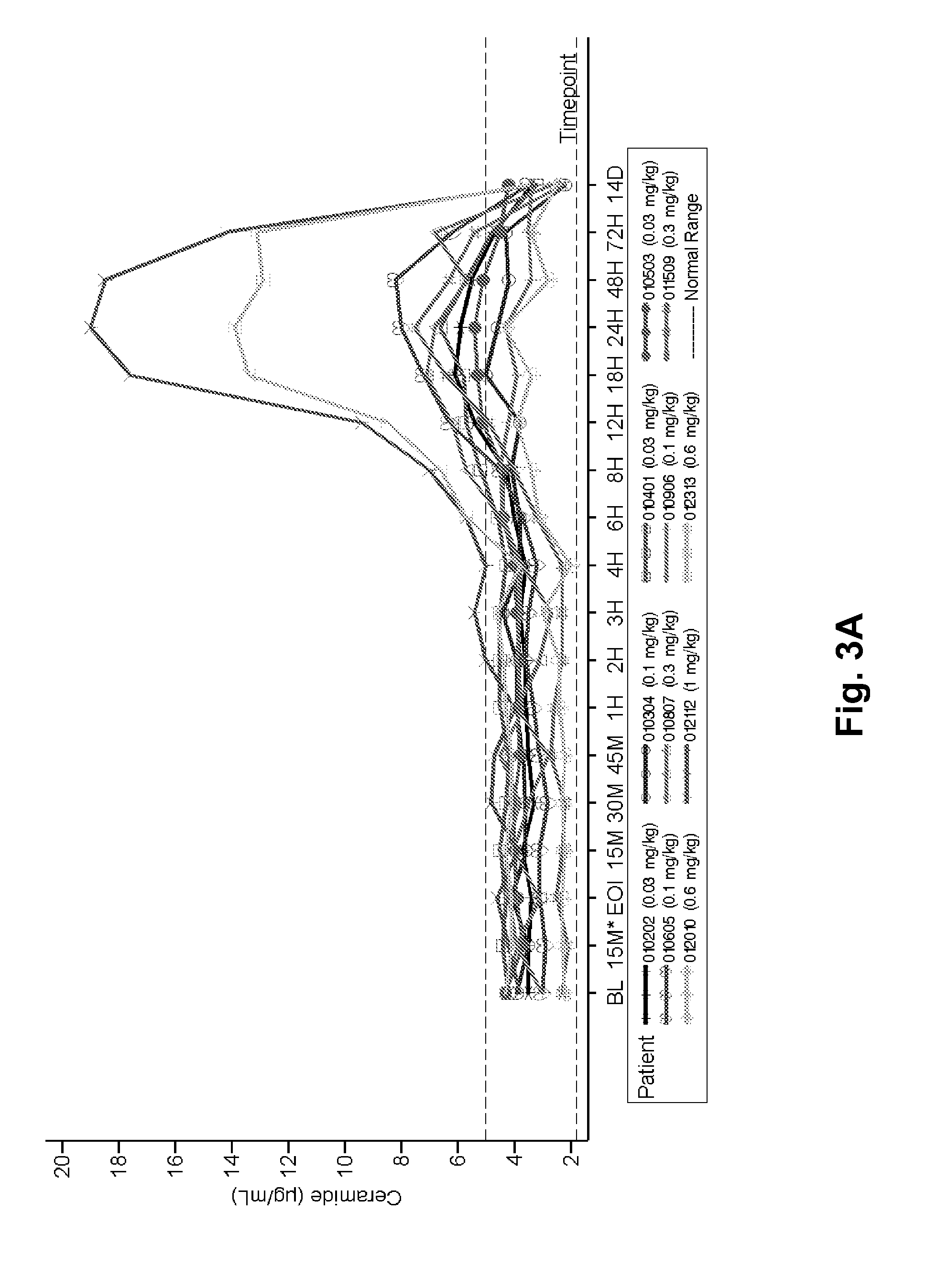
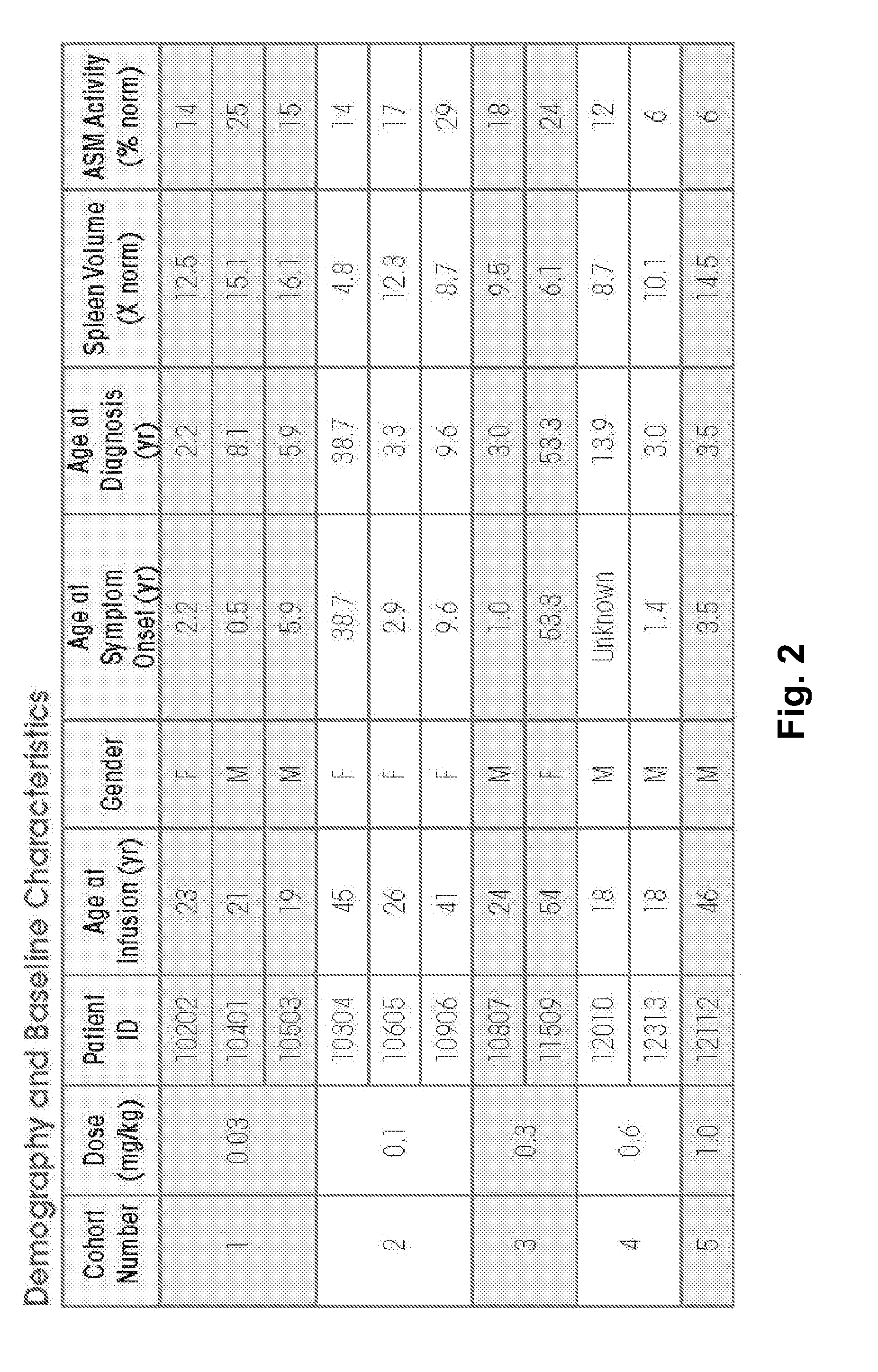
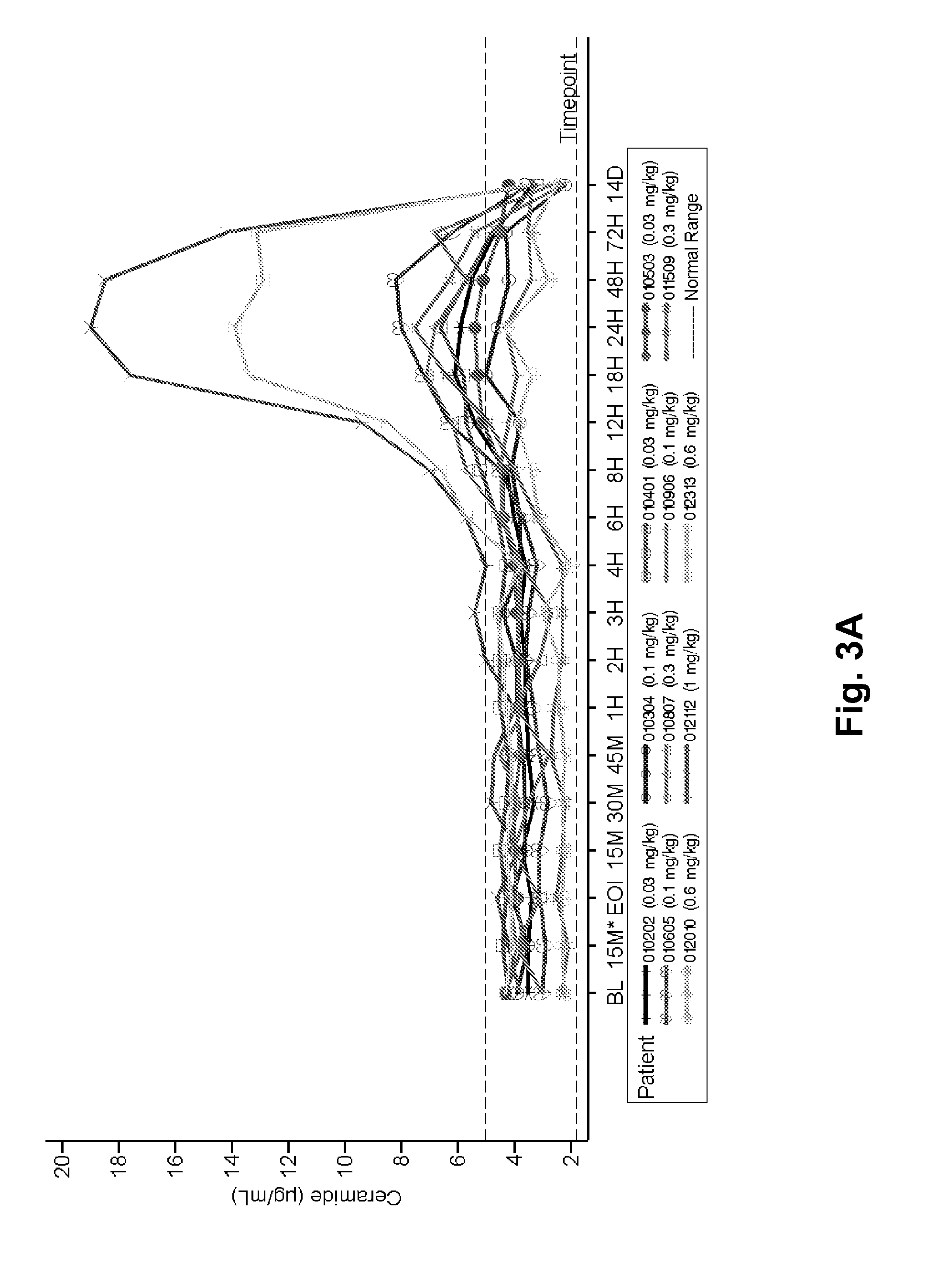
Dose escalation enzyme replacement therapy for treating acid sphingomyelinase deficiency
ActiveUS20110052559A1Avoid rapid degradationLess side effectsNervous disorderHydrolasesNeurological manifestationDose escalation
The invention relates to dose escalation enzyme replacement therapy using acid sphingomyelinase (ASM) for the treatment of human subjects having acid sphingomyelinase deficiency (ASMD), and, in particular, patients with non-neurological manifestations of Niemann-Pick Disease (NPD), and in certain embodiments, NPD type B.
Owner:MT SINAI SCHOOL OF MEDICINE +1
Markers of pre-term labor
InactiveUS20060166242A1High positiveHigh negative predictive valueMicrobiological testing/measurementBiological testingPremature labourBioinformatics
Owner:MOUNT SINAI HOSPITAL
Influenza virus vaccines and uses thereof
ActiveUS20140328875A1Highly potent and broadly neutralizing antibodiesStimulate immune responseSsRNA viruses negative-senseBacteriaHemagglutininInfluenza virus vaccine
Provided herein are chimeric influenza hemagglutinin (HA) polypeptides, compositions comprising the same, vaccines comprising the same, and methods of their use.
Owner:MT SINAI SCHOOL OF MEDICINE
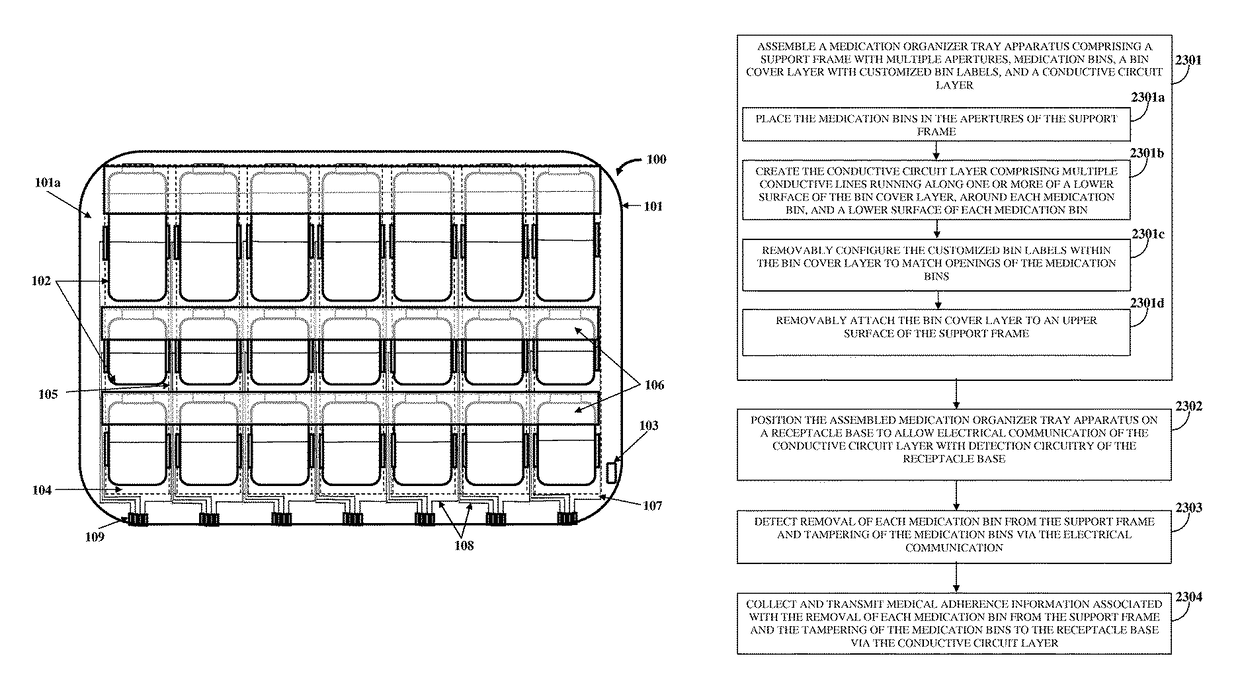
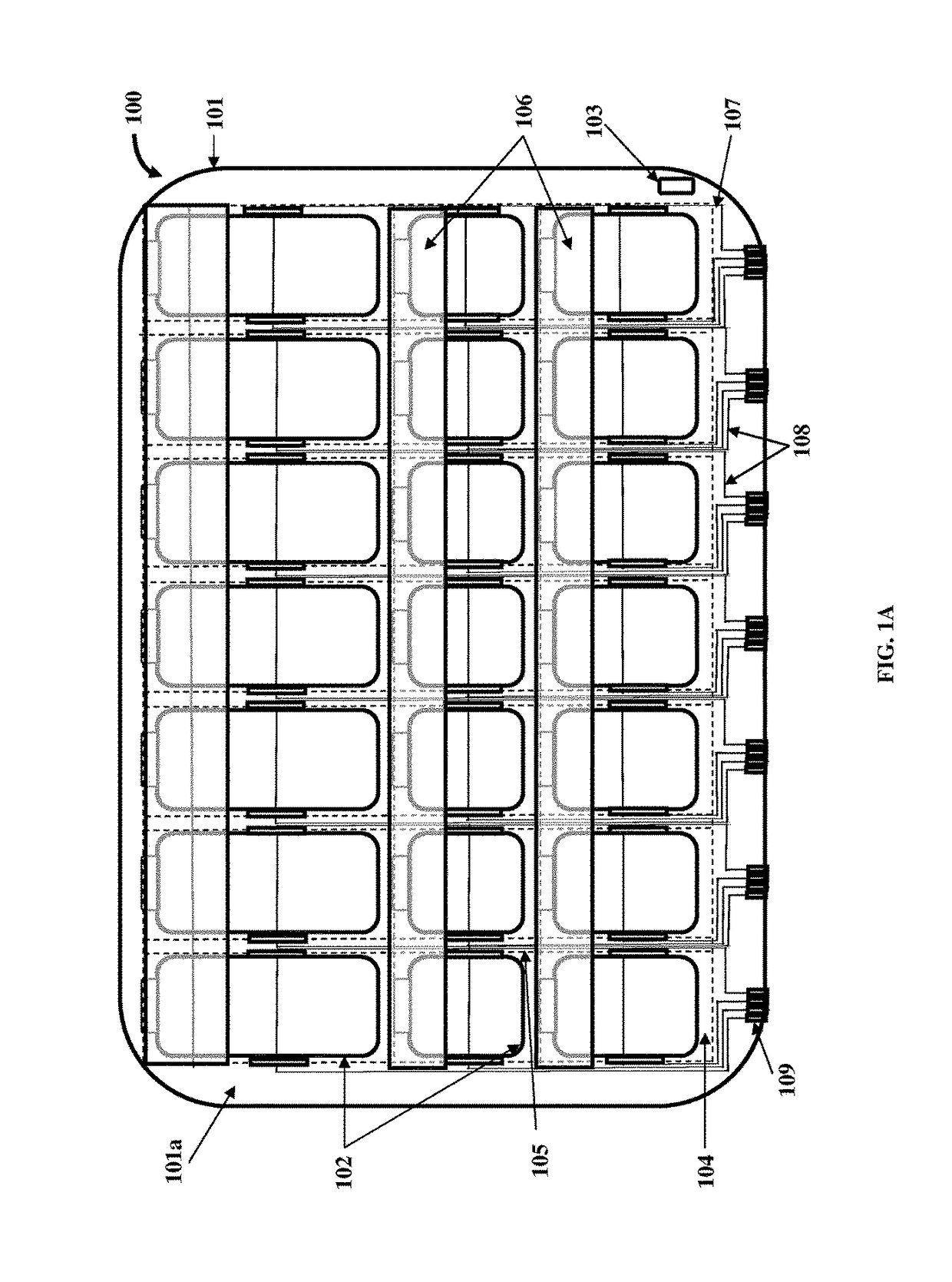
Medication Organizer Tray Apparatus
ActiveUS20160143807A1Improve adhesionOrganize effectivelyDrug and medicationsPharmaceutical containersMedication adherenceBiomedical engineering
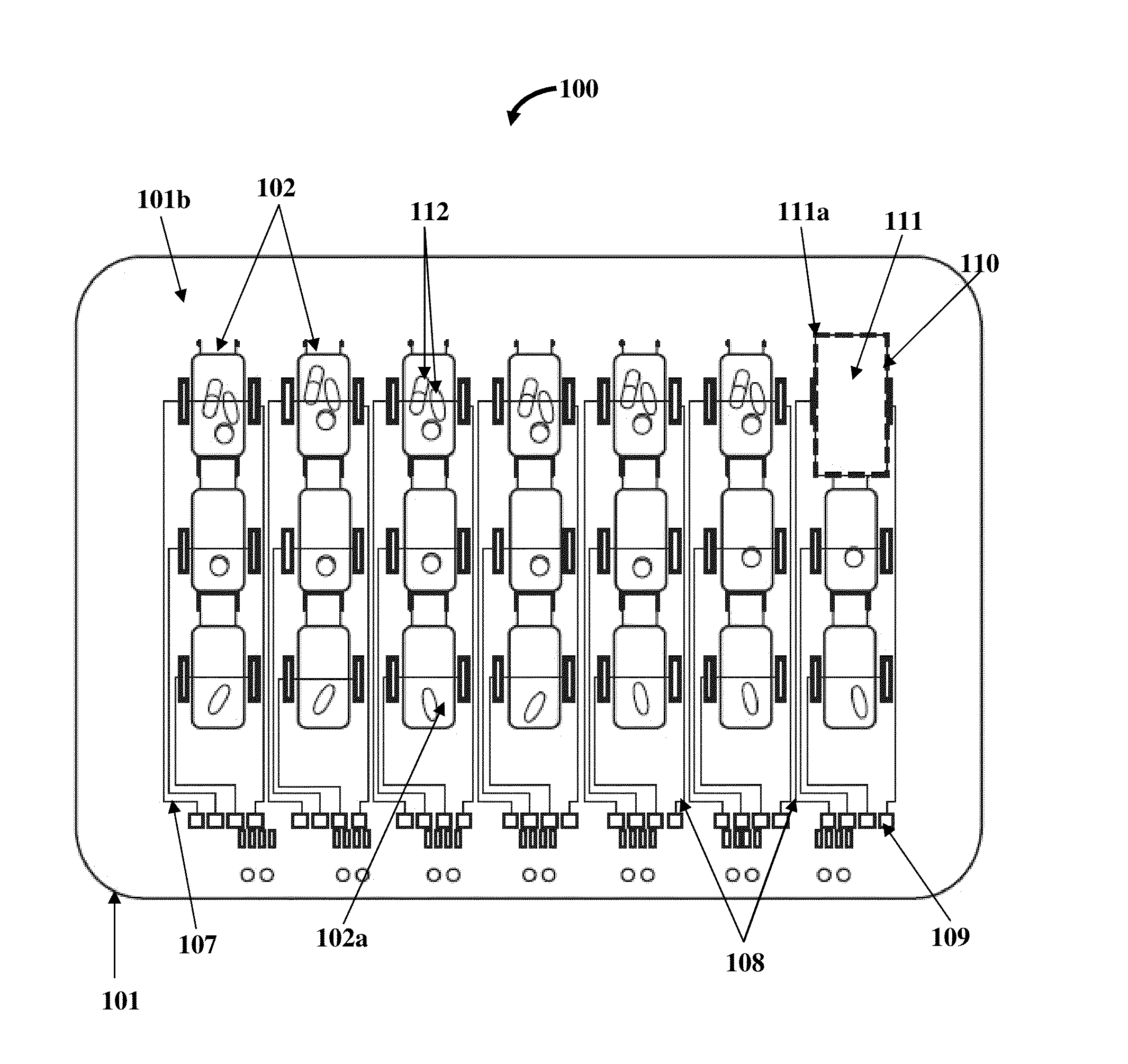
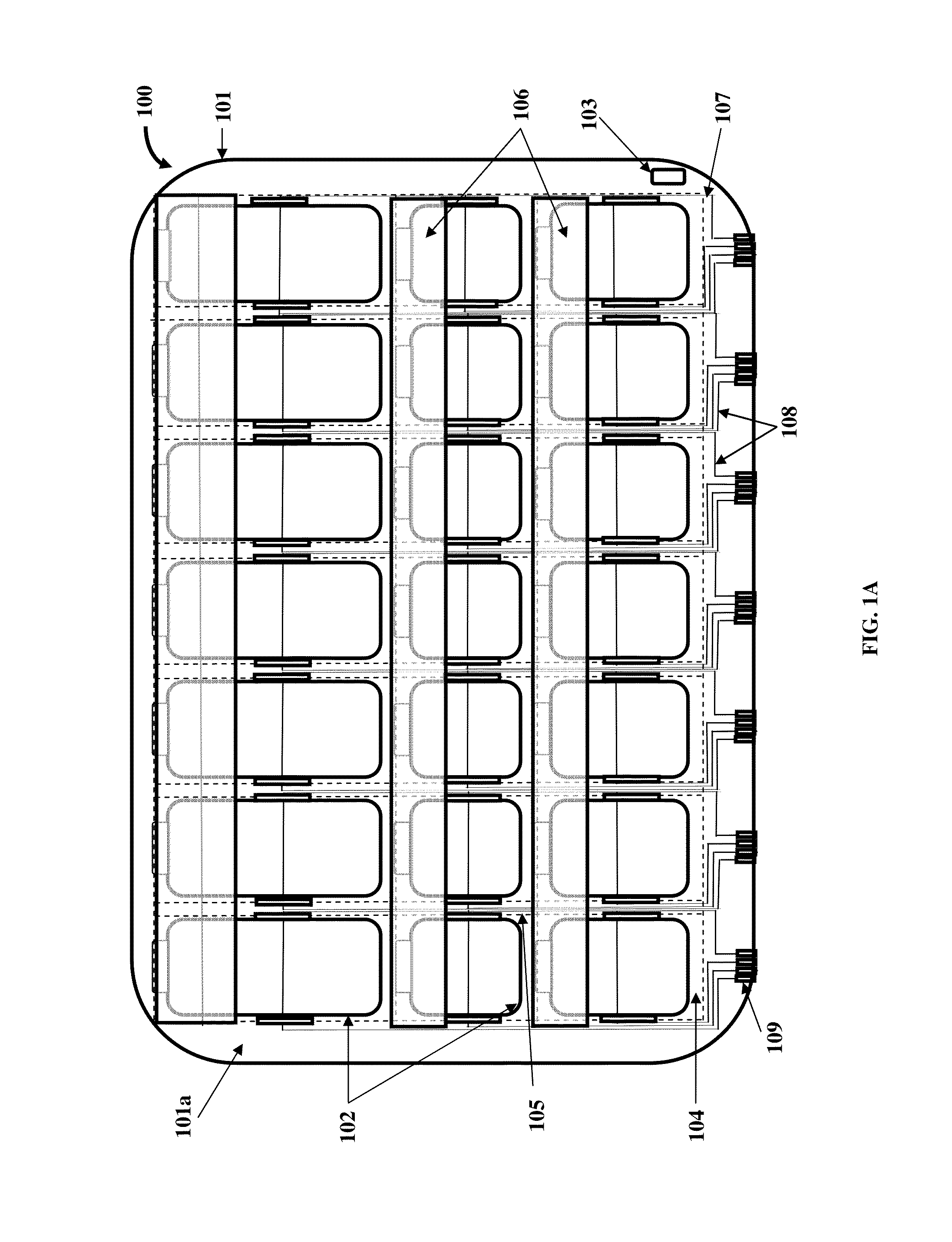
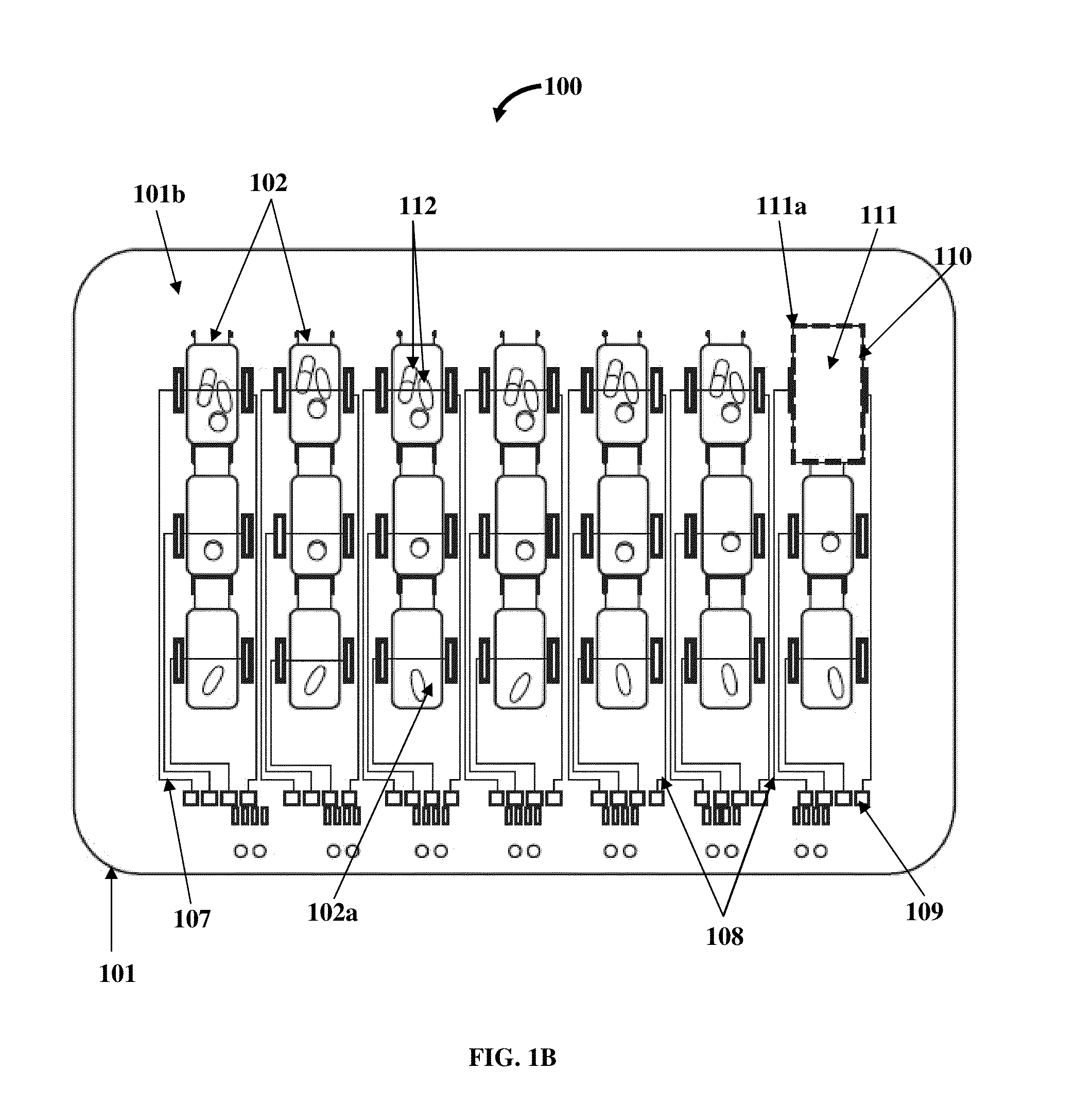
A method and a medication organizer tray apparatus for organizing medications and collecting medication adherence information are provided. The medication organizer tray apparatus includes a support frame, multiple medication bins accommodating multiple medications, a bin cover layer with multiple customized bin labels, and a conductive circuit layer. The support frame includes multiple apertures positioned at predefined intervals from each other for placement of the medication bins. The customized bin labels having medical information printed thereon seal openings of the medication bins. The conductive circuit layer includes conductive lines running along one or more of a lower surface of the bin cover layer, around each medication bin, and a lower surface of each medication bin. The conductive circuit layer electrically communicates with a receptacle base to enable detection of removal of each medication bin from the support frame and detection of tampering of the medication bins.
Owner:RXADVANCE
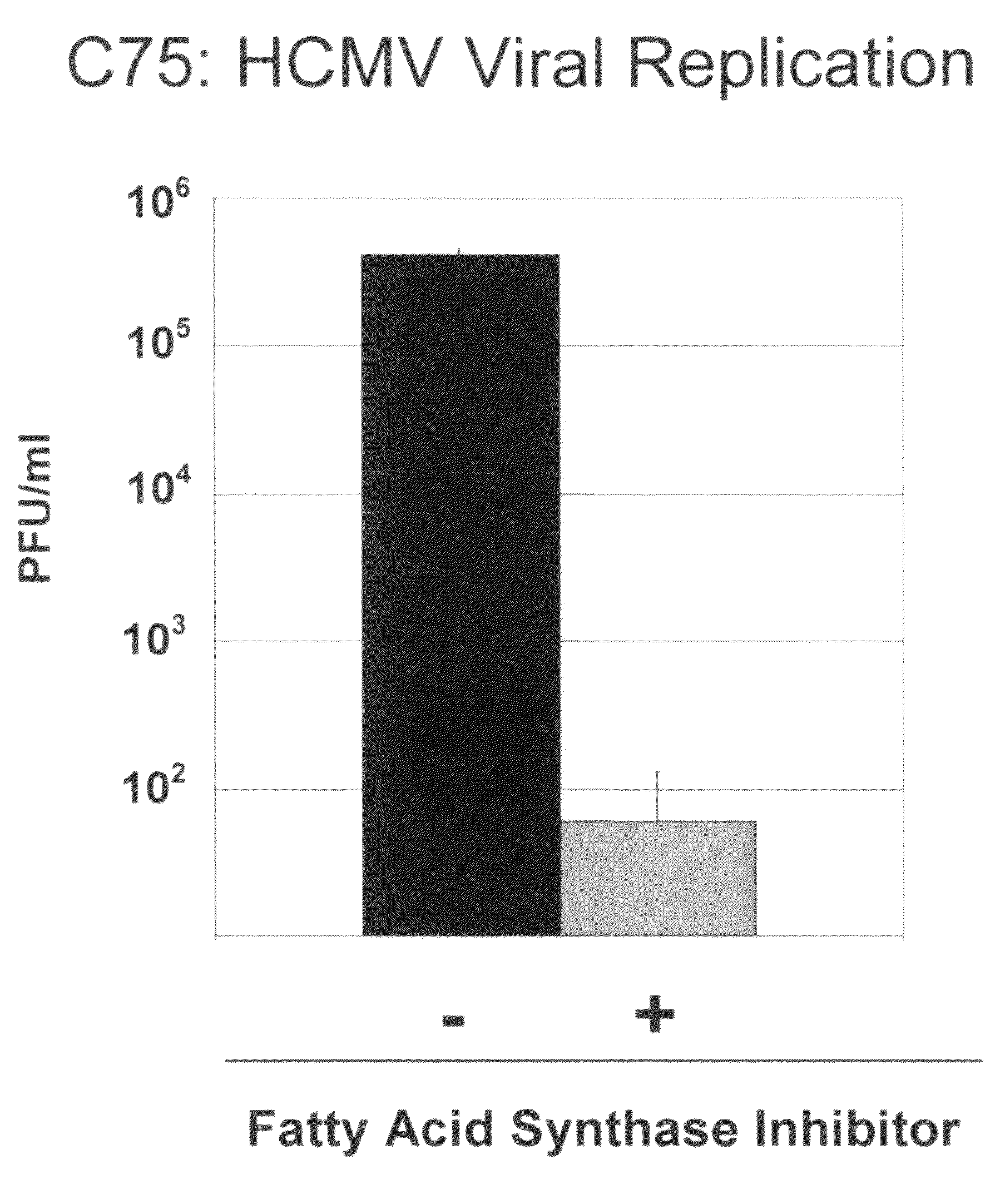
Treatment of viral infections by modulation of host cell metabolic pathways
InactiveUS8158677B2Reduce severityImprove survivalBiocideDigestive systemCritical control pointMetabolite
Owner:THE TRUSTEES FOR PRINCETON UNIV
Dose escalation enzyme replacement therapy for treating acid sphingomyelinase deficiency
ActiveUS8349319B2Avoid rapid degradationLess side effectsNervous disorderHydrolasesNeurological manifestationAcid sphingomyelinase
Owner:MT SINAI SCHOOL OF MEDICINE +1
Influenza virus vaccines and uses thereof
ActiveUS9051359B2Elimination of glycosylationReduce severitySsRNA viruses negative-sensePeptide/protein ingredientsHemagglutininInfluenza virus vaccine
Provided herein are influenza hemagglutinin stem domain polypeptides, compositions comprising the same, vaccines comprising the same and methods of their use.
Owner:MT SINAI SCHOOL OF MEDICINE
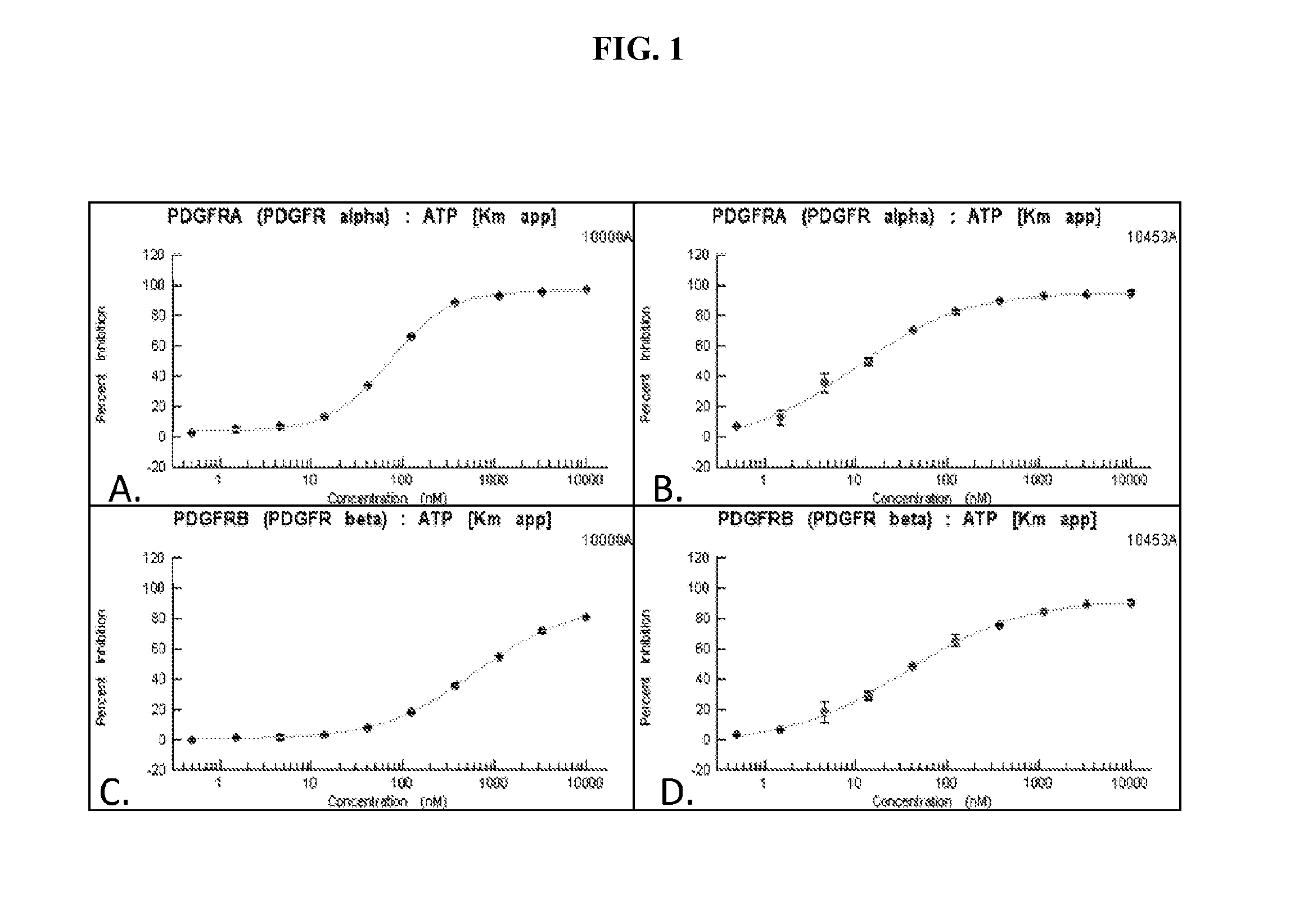
Spray dry formulations
ActiveUS20160235742A1Improve athletic abilityReduction in hospitalizationOrganic active ingredientsPowder deliveryDiseaseSpray dried
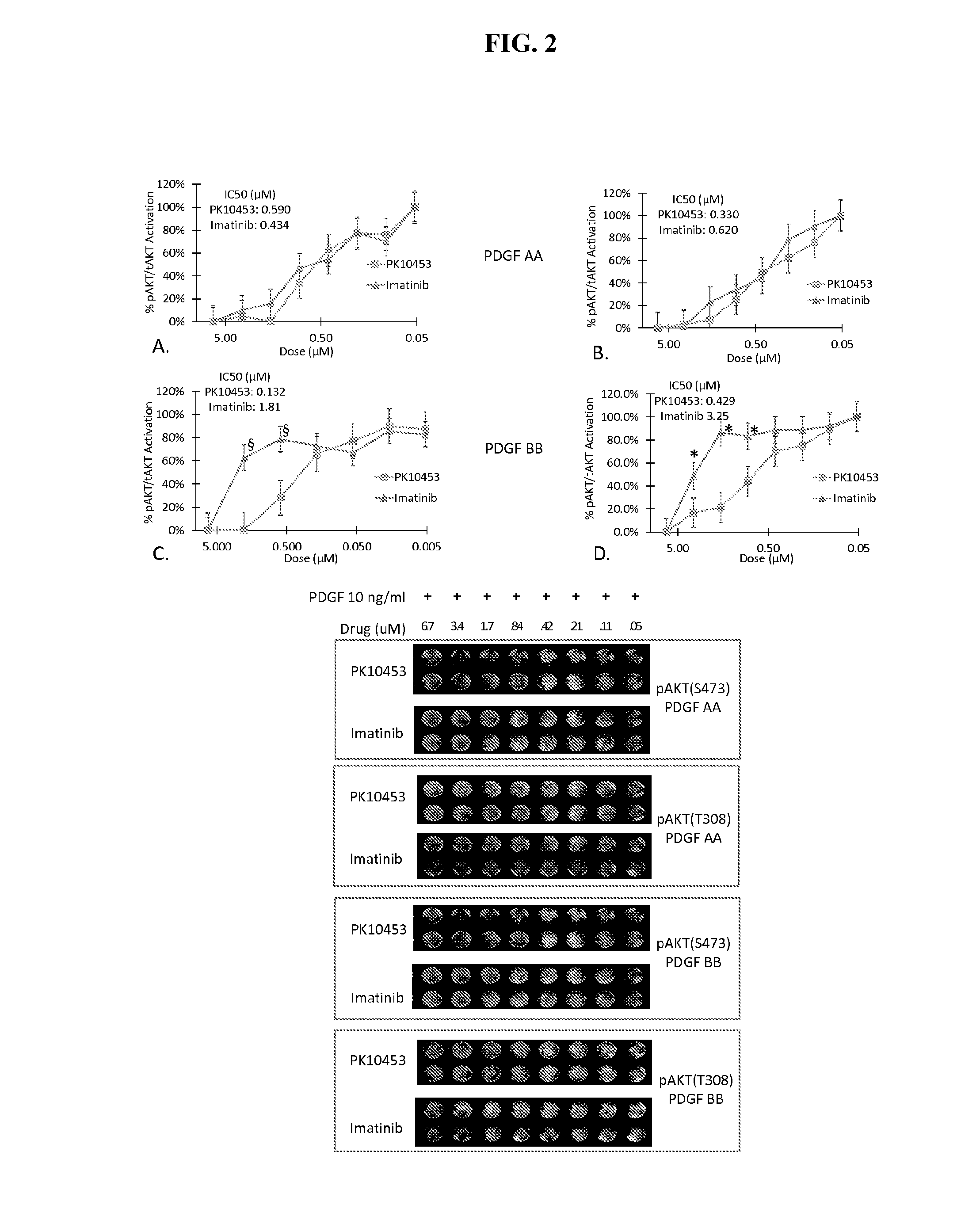
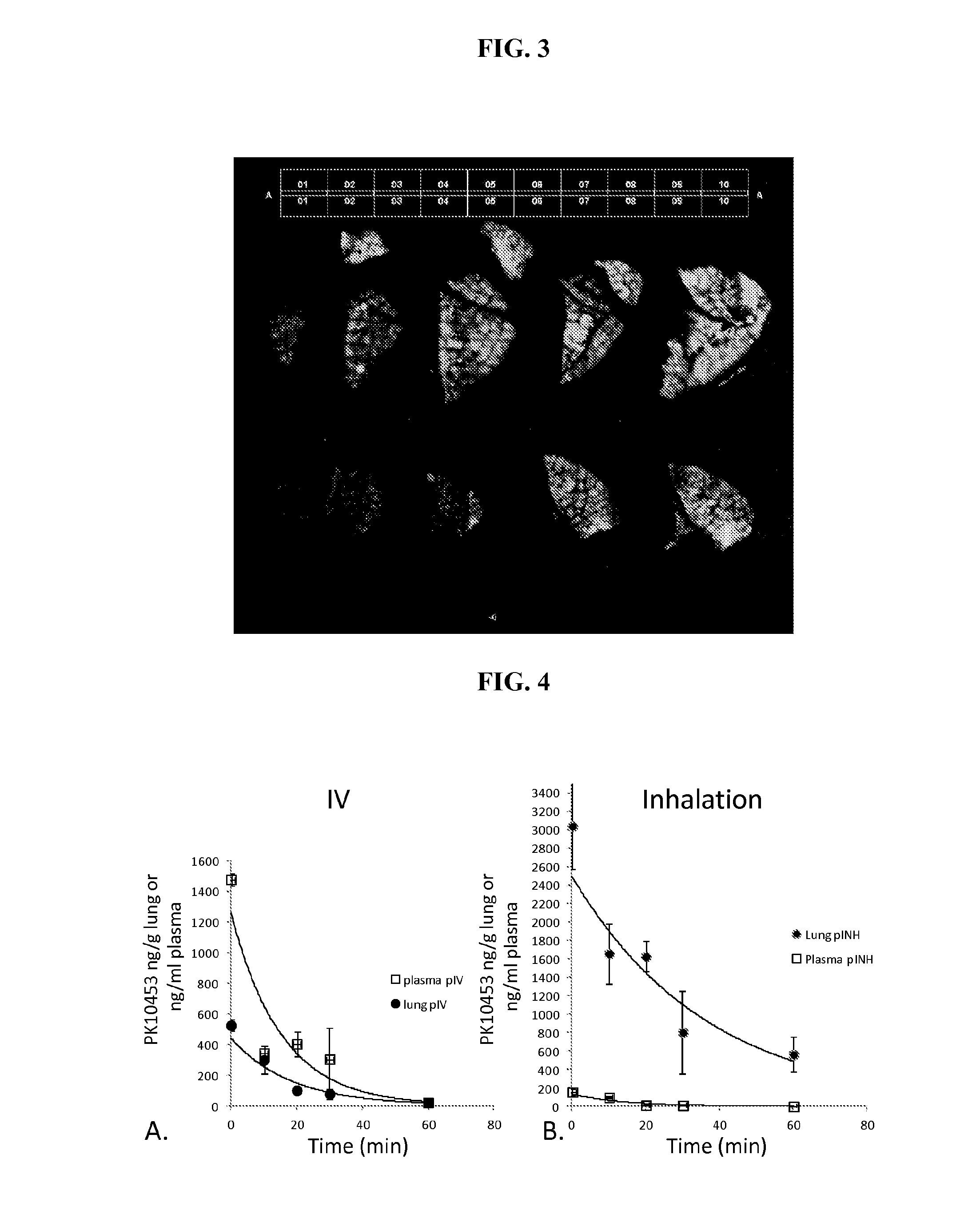
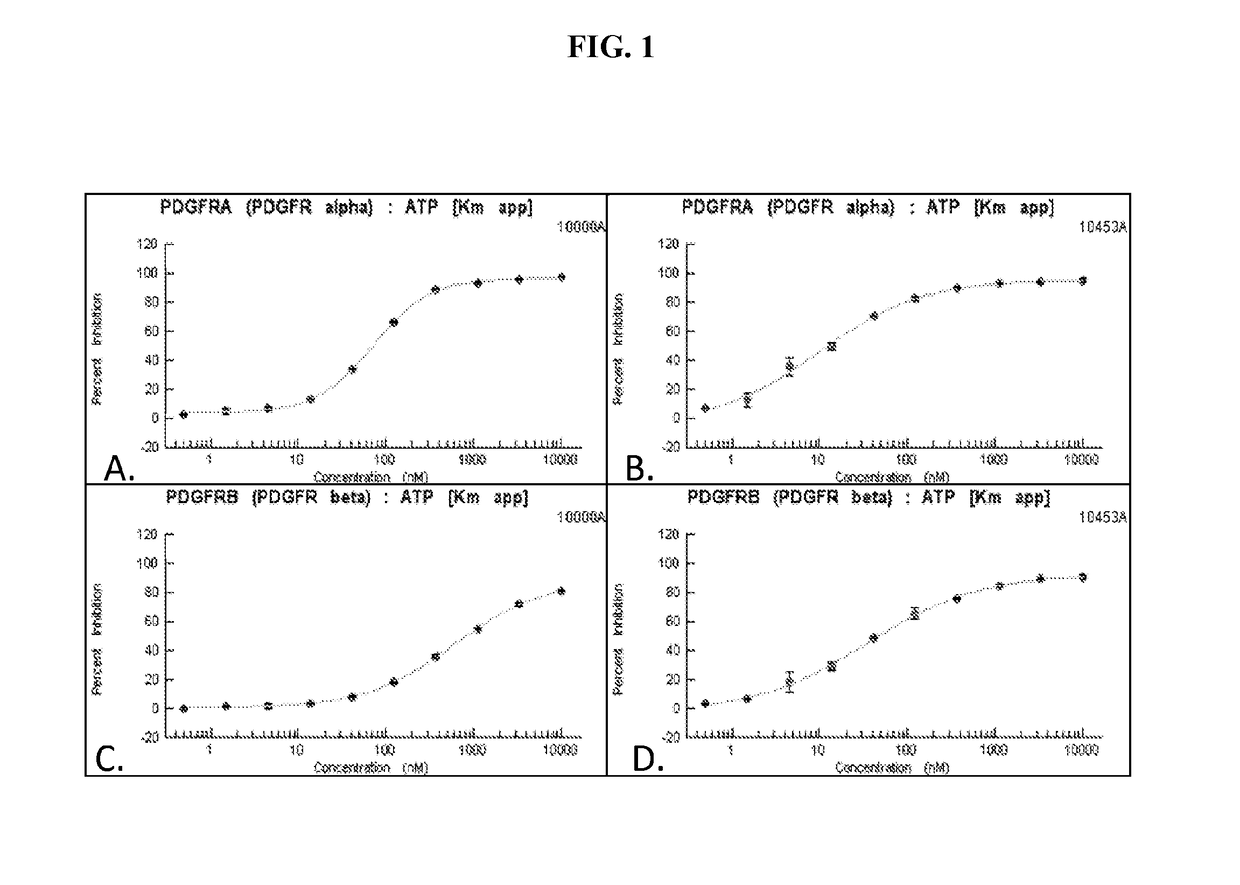
Disclosed herein are compounds, compositions, and methods for preventing and treating proliferative diseases associated with aberrant receptor tyrosine kinase (RTK) activity. The therapeutic indications described herein more specifically relate to the non-selective inhibition of RTKs associated with vascular and pulmonary disorders.
Owner:GILEAD SCI INC +1
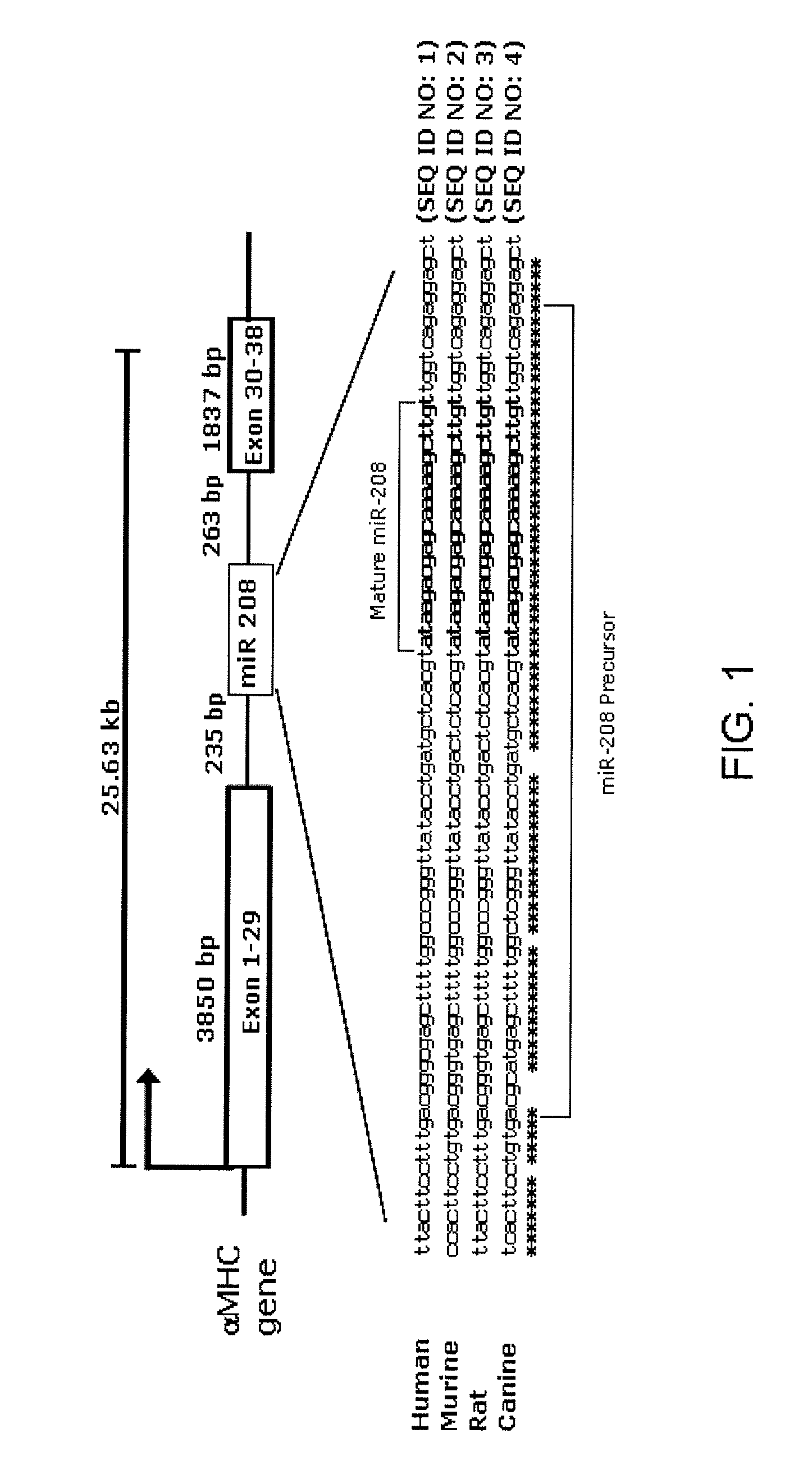
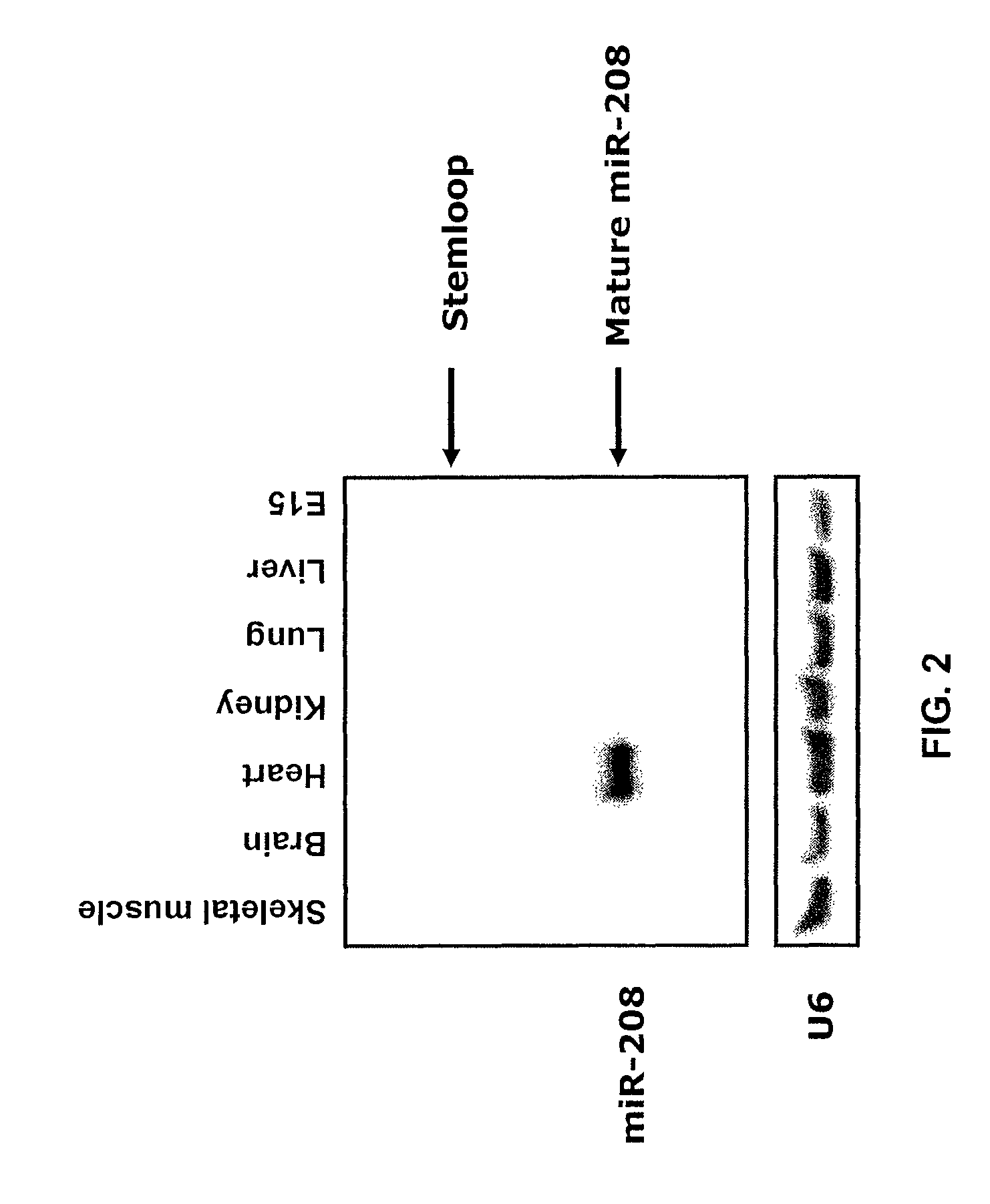
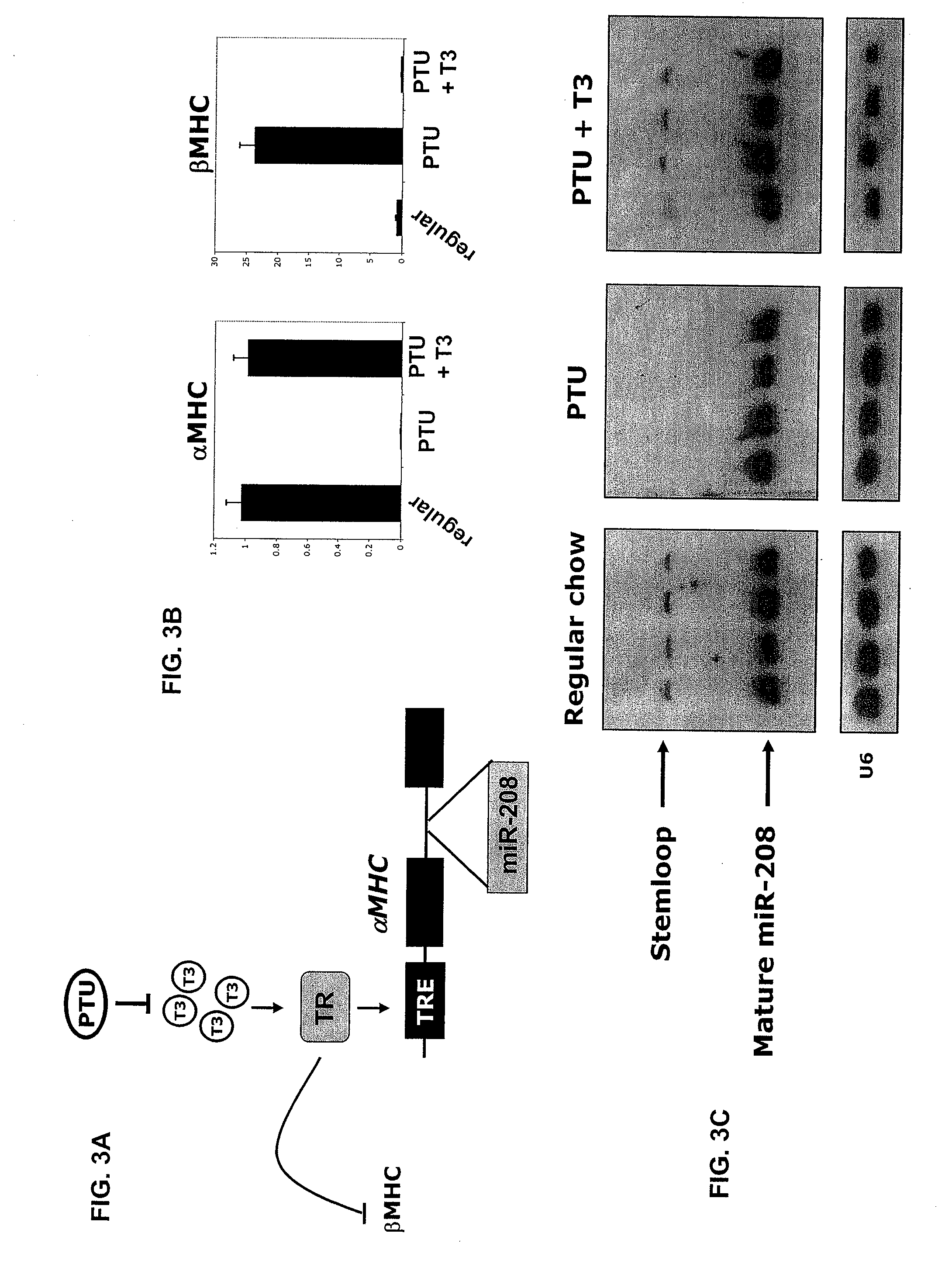
Identification of a micro-rna that activates expression of beta-myosin heavy chain
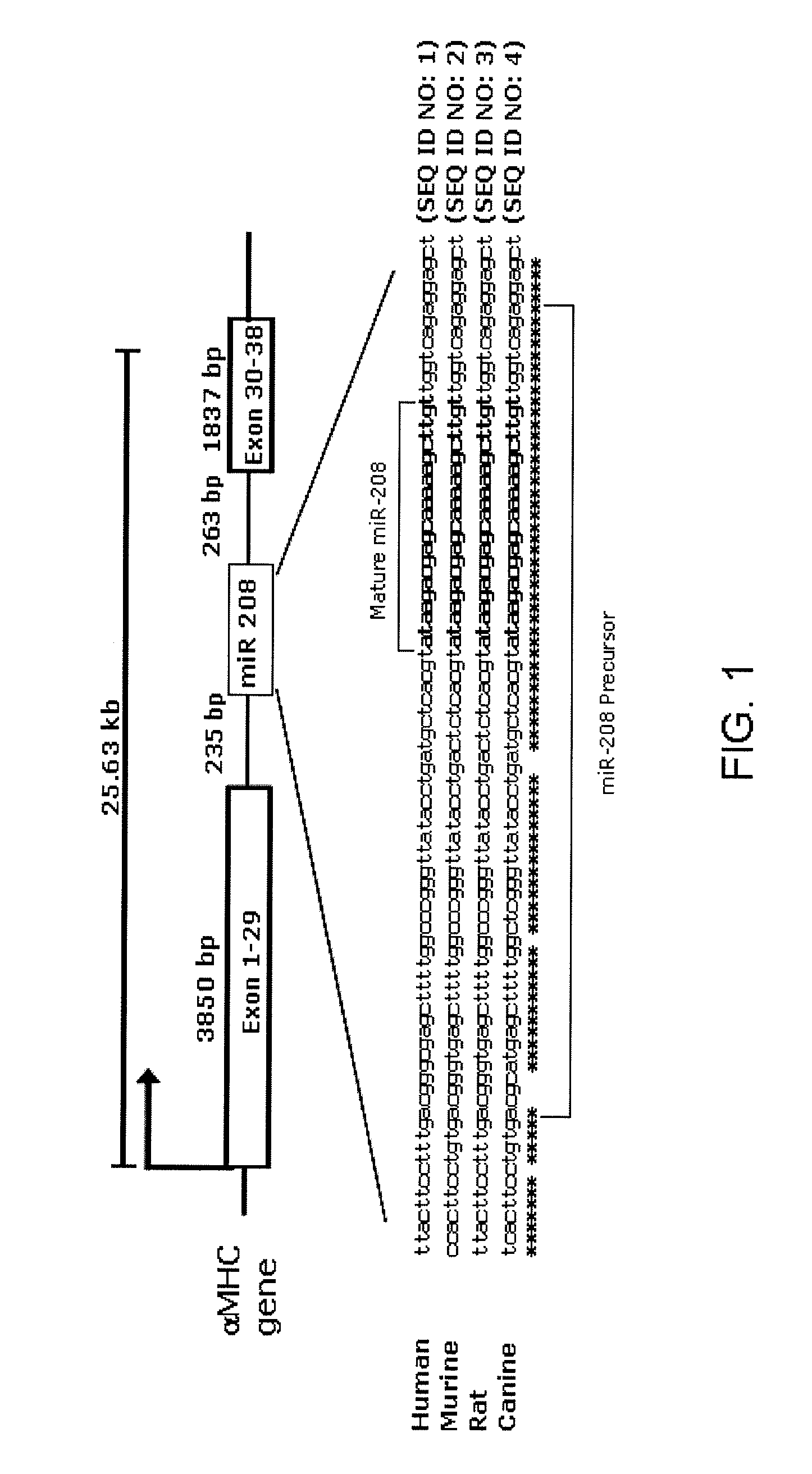
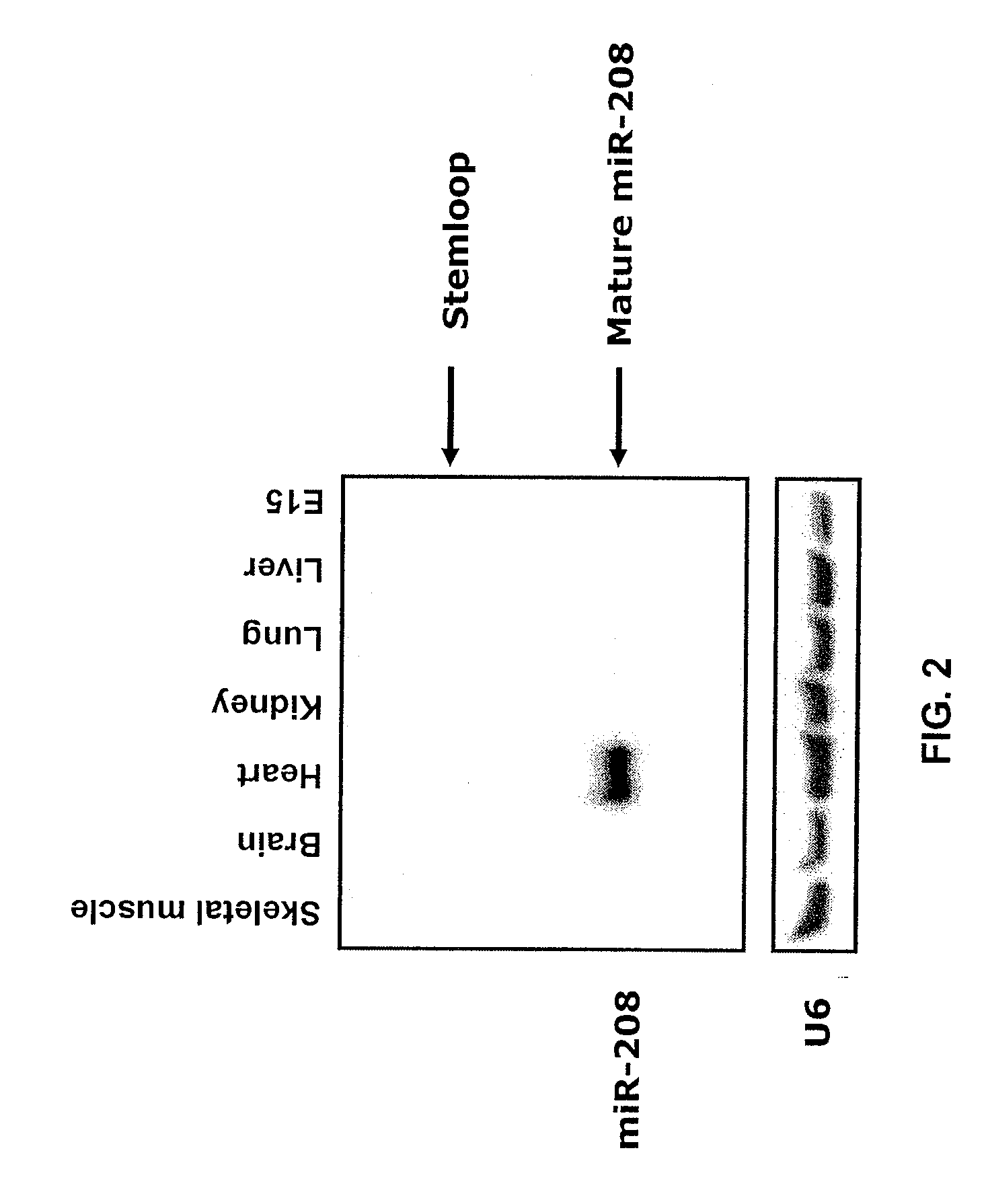
InactiveUS20090180957A1Decrease mortality and morbidityImprovement of symptomBiocideOrganic active ingredientsMusculoskeletal impairmentEccentric hypertrophy
The present invention relates to the identification of a microRNA, miR-208, that induces the expression of β-myosin heavy chain (β-MHC) and represses fast skeletal muscle contractile protein genes. Inhibition of this function is proposed as a treatment for cardiac fibrosis, hypertrophy and / or heart failure, and augmentation of this function can be used to repress slow fiber genes and activate fast fiber genes in the treatment of musculoskeletal disorders.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Devices for determining the relative spatial change in subsurface resistivities across frequencies in tissue
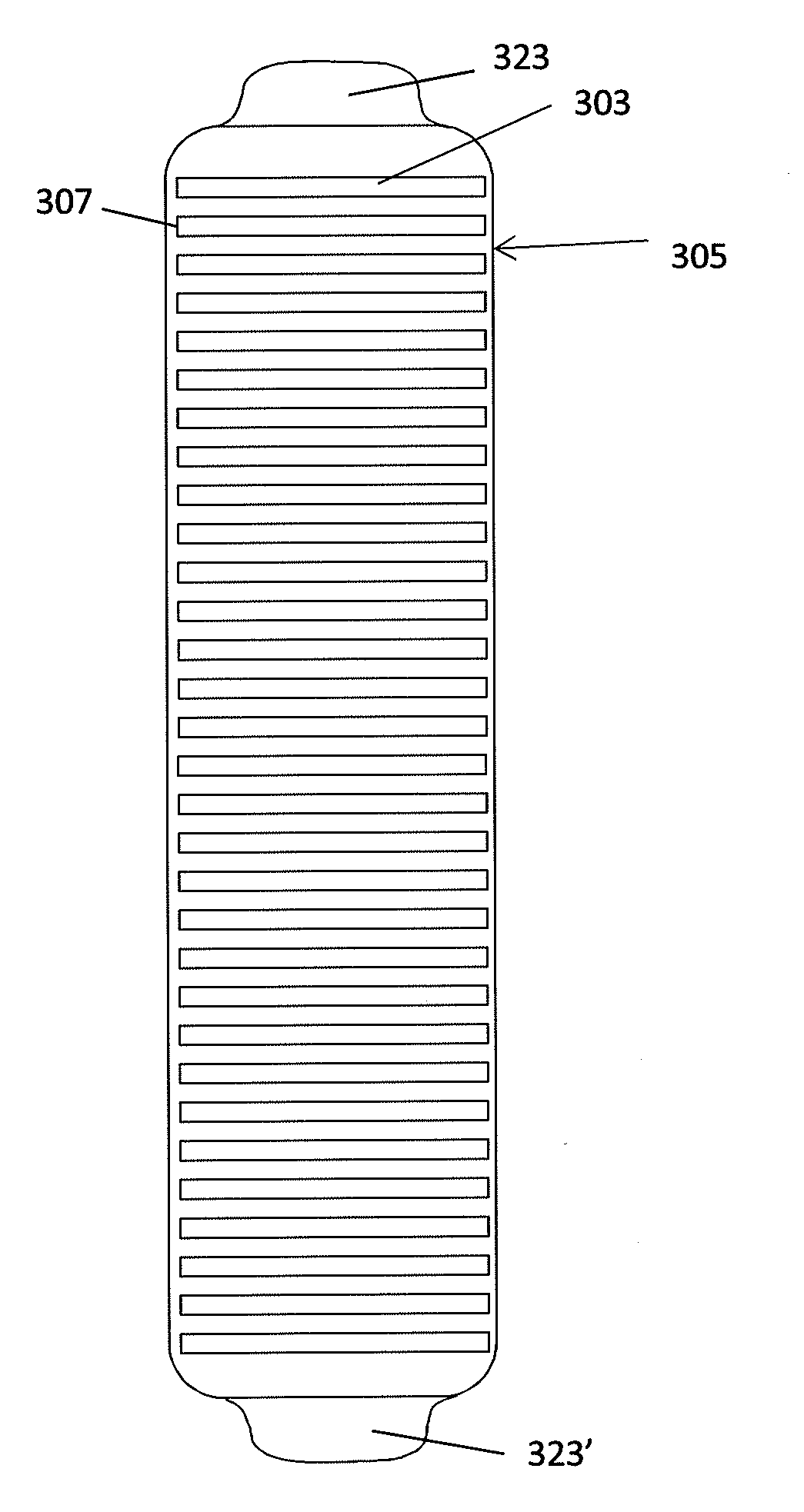
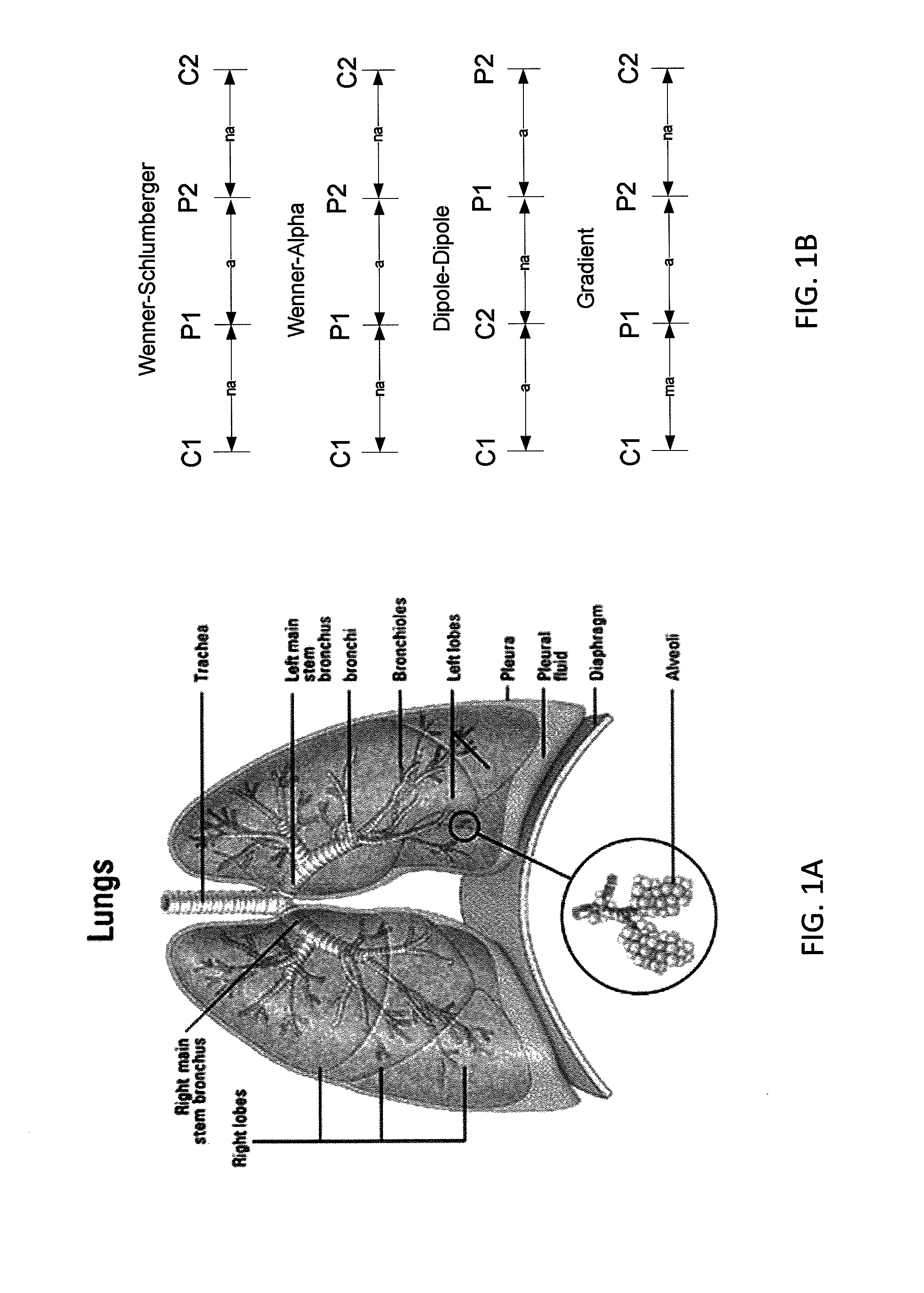
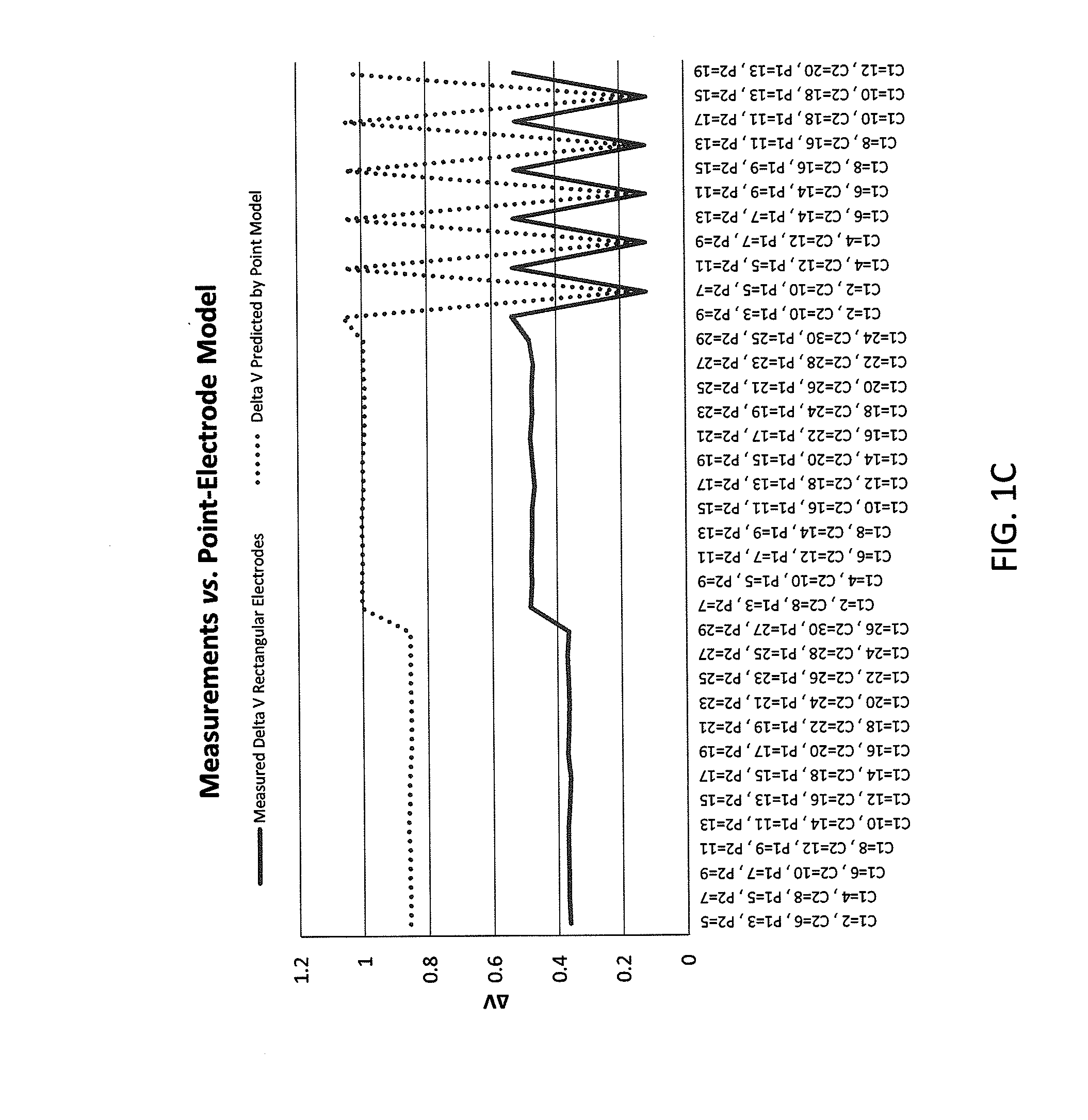
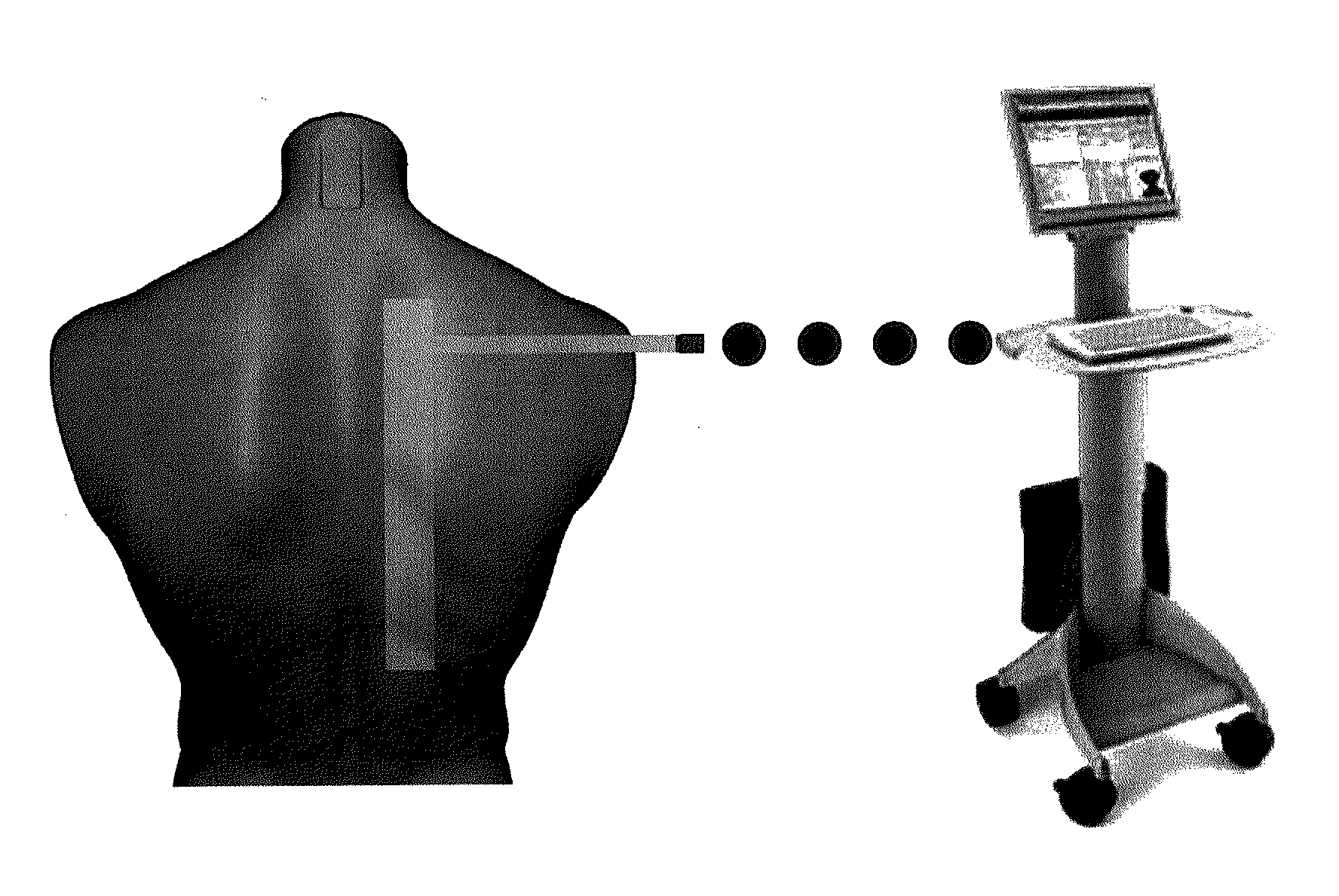
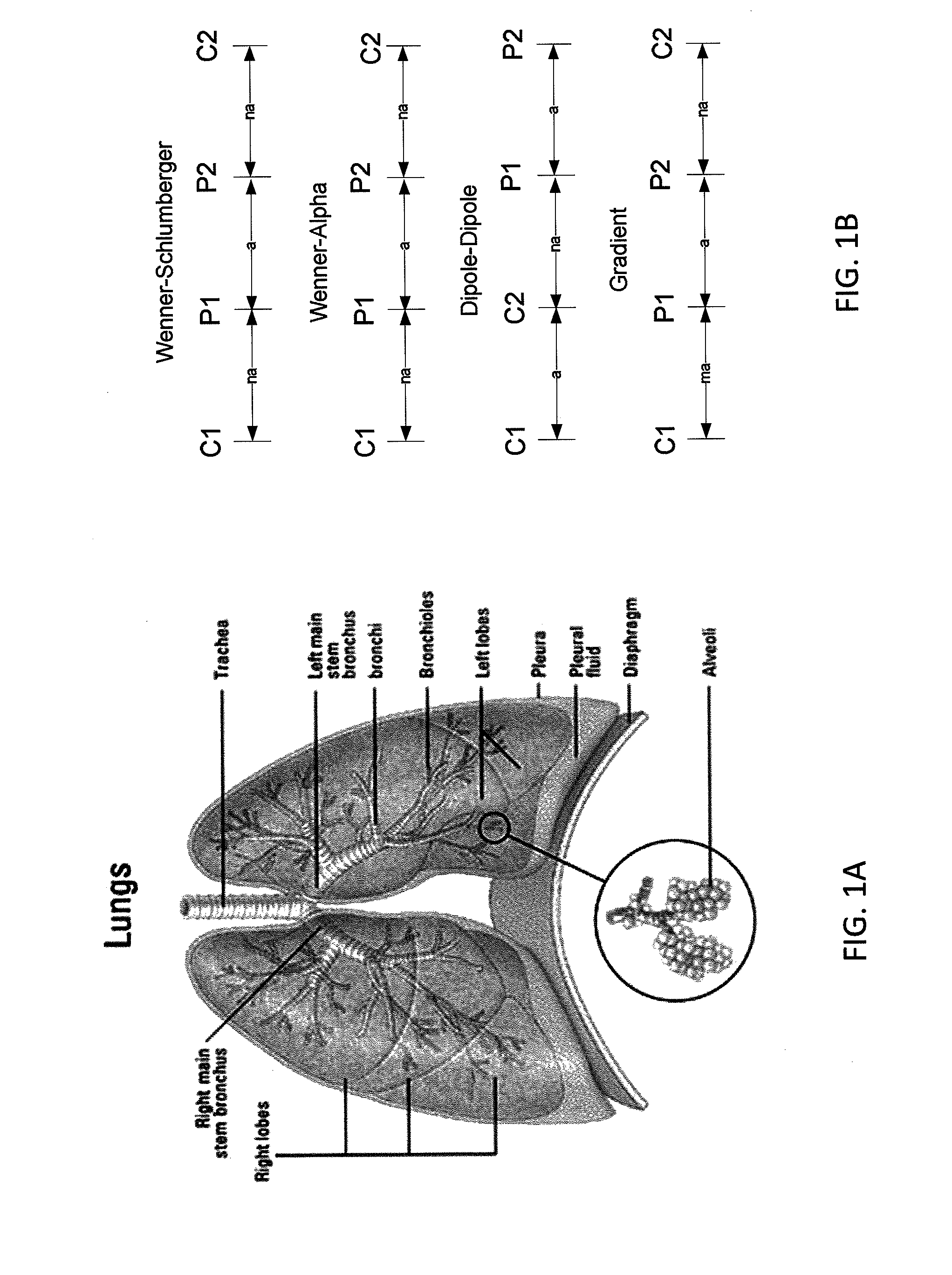
InactiveUS20130165760A1Improve medical managementDelay progressBioelectric signal measurementRespiratory organ evaluationSurface resistivityElectric resistivity
Sensors for non-invasively determining tissue wetness / hydration based on relative changes in subsurface resistivities in tissue below the sensor when applied to a human body across different frequencies. A sensor including arrays of current-injecting and voltage-sensing electrodes may be placed on a subject's back to determine lung wetness. Sensors may be used as part of a systems and method for determining tissue water content, systems and methods for determining lung wetness, or the like. Sensors for determining relative changes in subsurface resistivities across frequencies and systems include arrays of electrodes used to determine relative changes in subsurface resistivities across frequencies may include pairs of current-injecting and voltage sensing electrodes.
Owner:IMPEDIMED
Influenza virus vaccines and uses thereof
ActiveUS20150297712A1Elimination of glycosylationReduce severitySsRNA viruses negative-sensePeptide/protein ingredientsHemagglutininInfluenza virus vaccine
Provided herein are influenza hemagglutinin stem domain polypeptides, compositions comprising the same, vaccines comprising the same and methods of their use.
Owner:MT SINAI SCHOOL OF MEDICINE
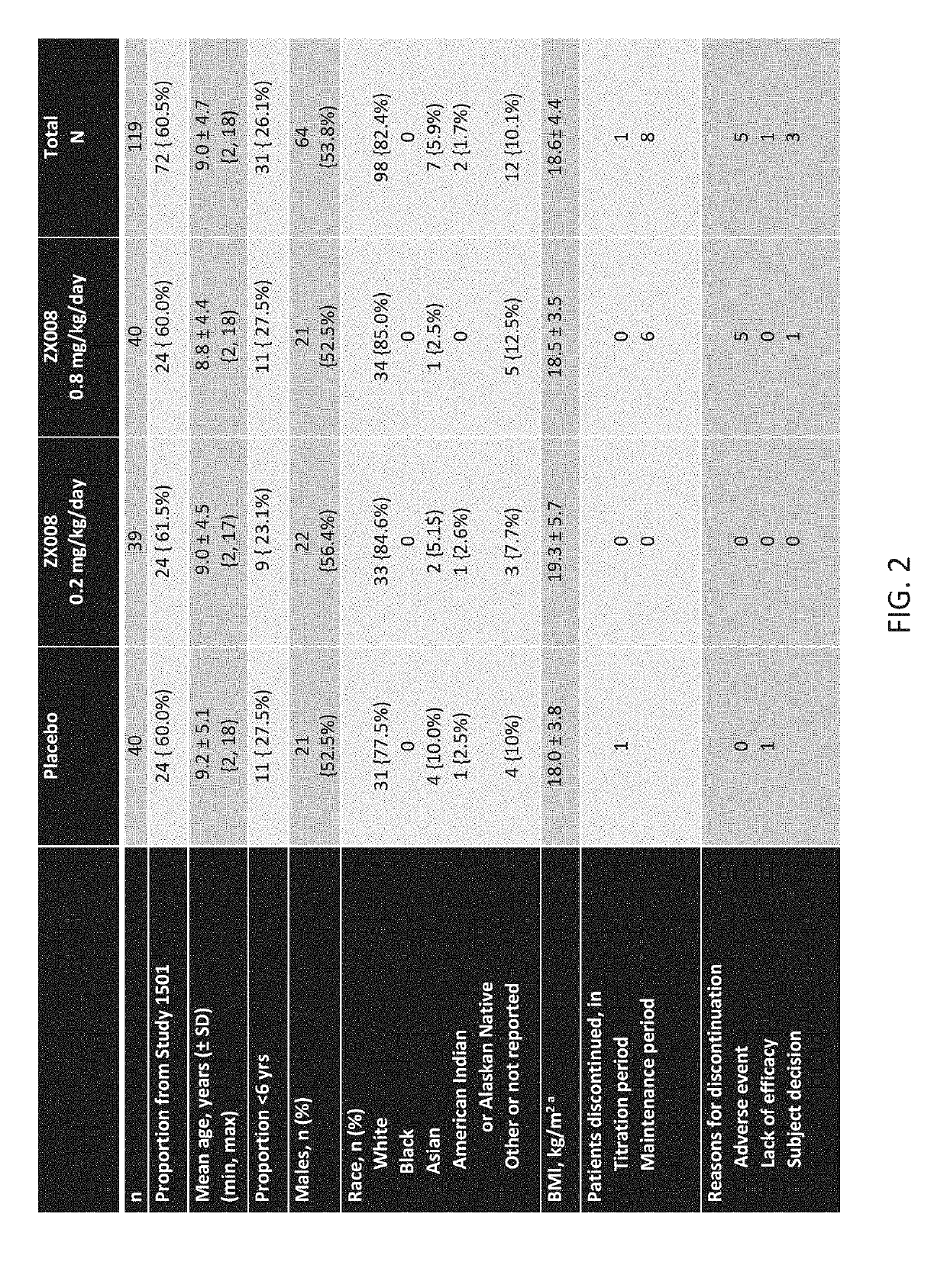
Method of reduction in convulsive seizure frequency
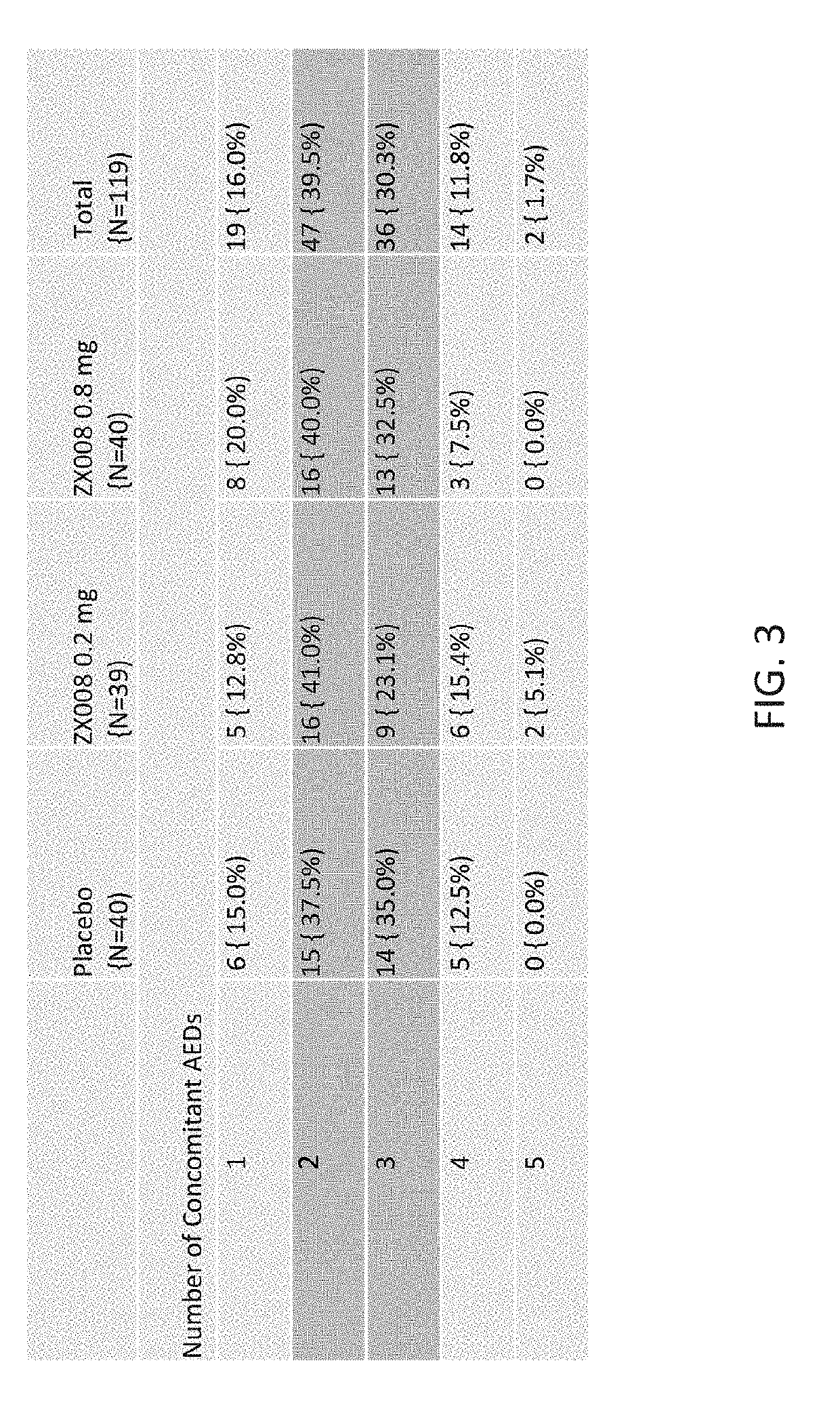
InactiveUS20190247333A1Reduce convulsive seizure frequencyEliminate seizureOrganic active ingredientsNervous disorderFenfluramineHuman patient
A method of reducing convulsive seizure frequency in a human patient diagnosed with Dravet syndrome, comprising administering to the patient a therapeutically effective dose of fenfluramine or a pharmaceutically acceptable salt, base, acid or amine thereof, and repeating the administering over a period of days until the patient exhibits a significant reduction (e.g., 40% or greater) from baseline in convulsive seizure frequency. In some embodiments of the method, convulsive seizures are completely eliminated for 10 days or more, 20 days or more, 30 days or more, 50 days or more, 100 days or more.
Owner:ZOGENIX INT
Compositions and methods related to heart failure
InactiveUS20060014828A1Reduce in quantityShorten the construction periodBiocideNervous disorderDiseaseType B Natriuretic Peptide
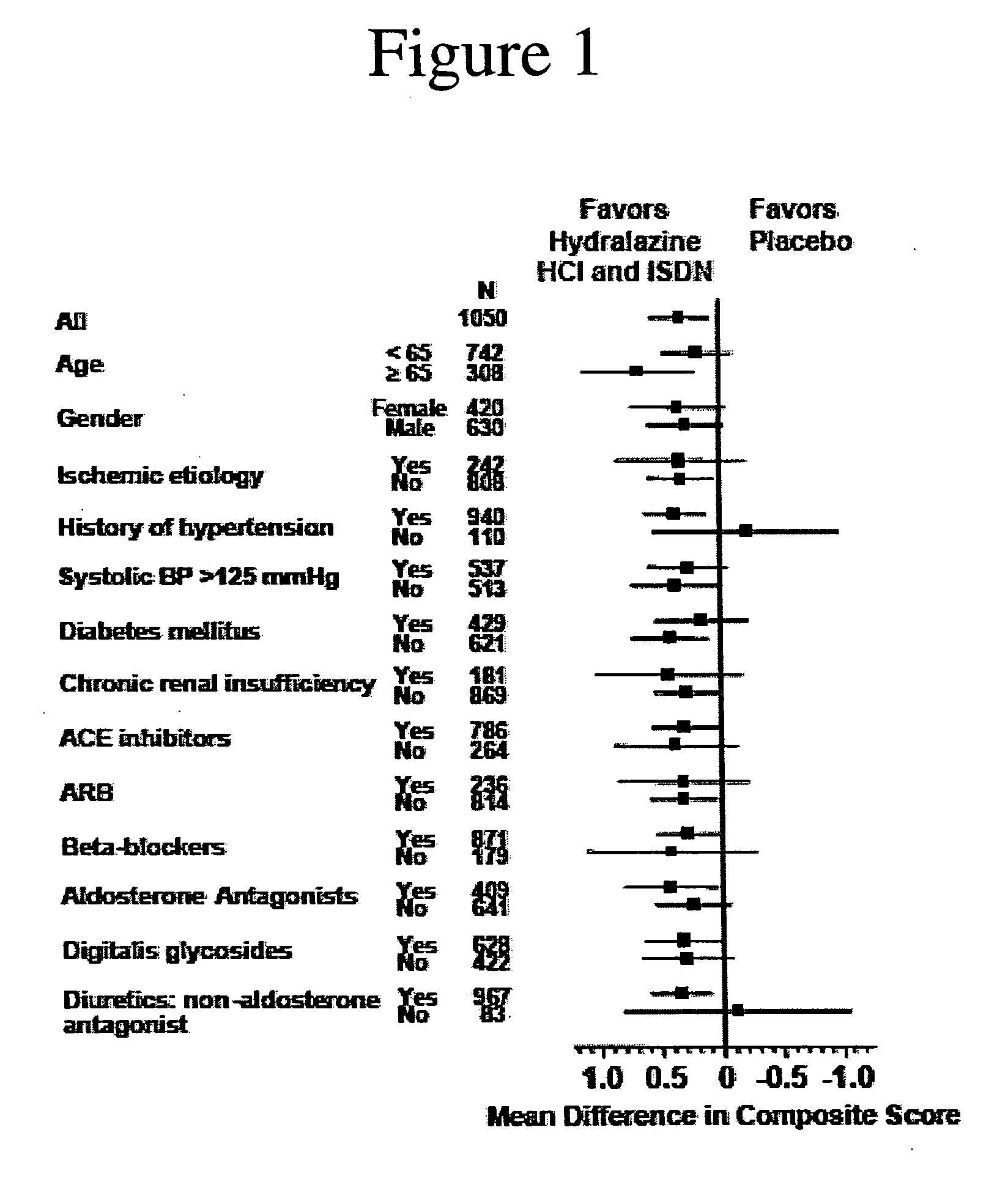
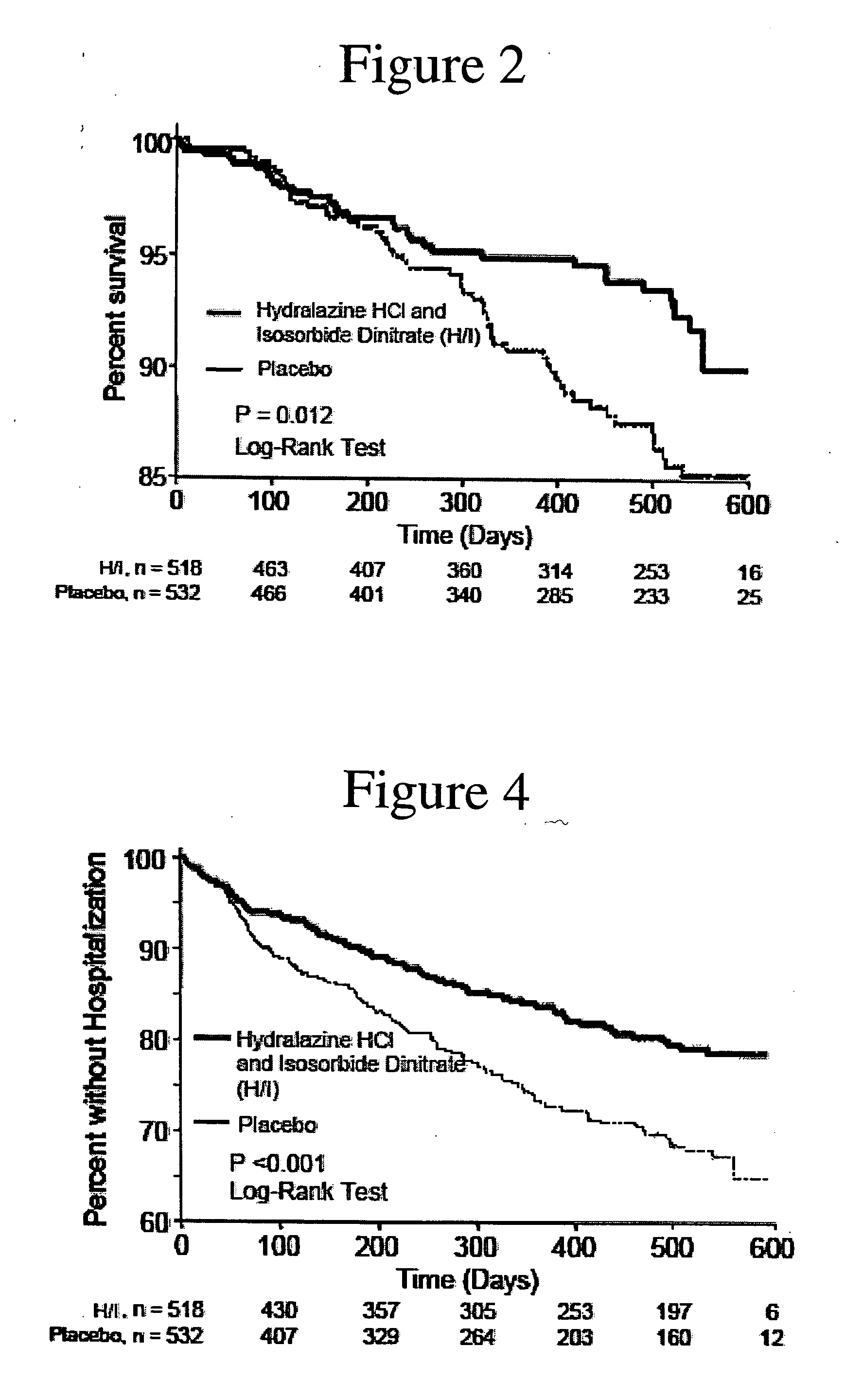
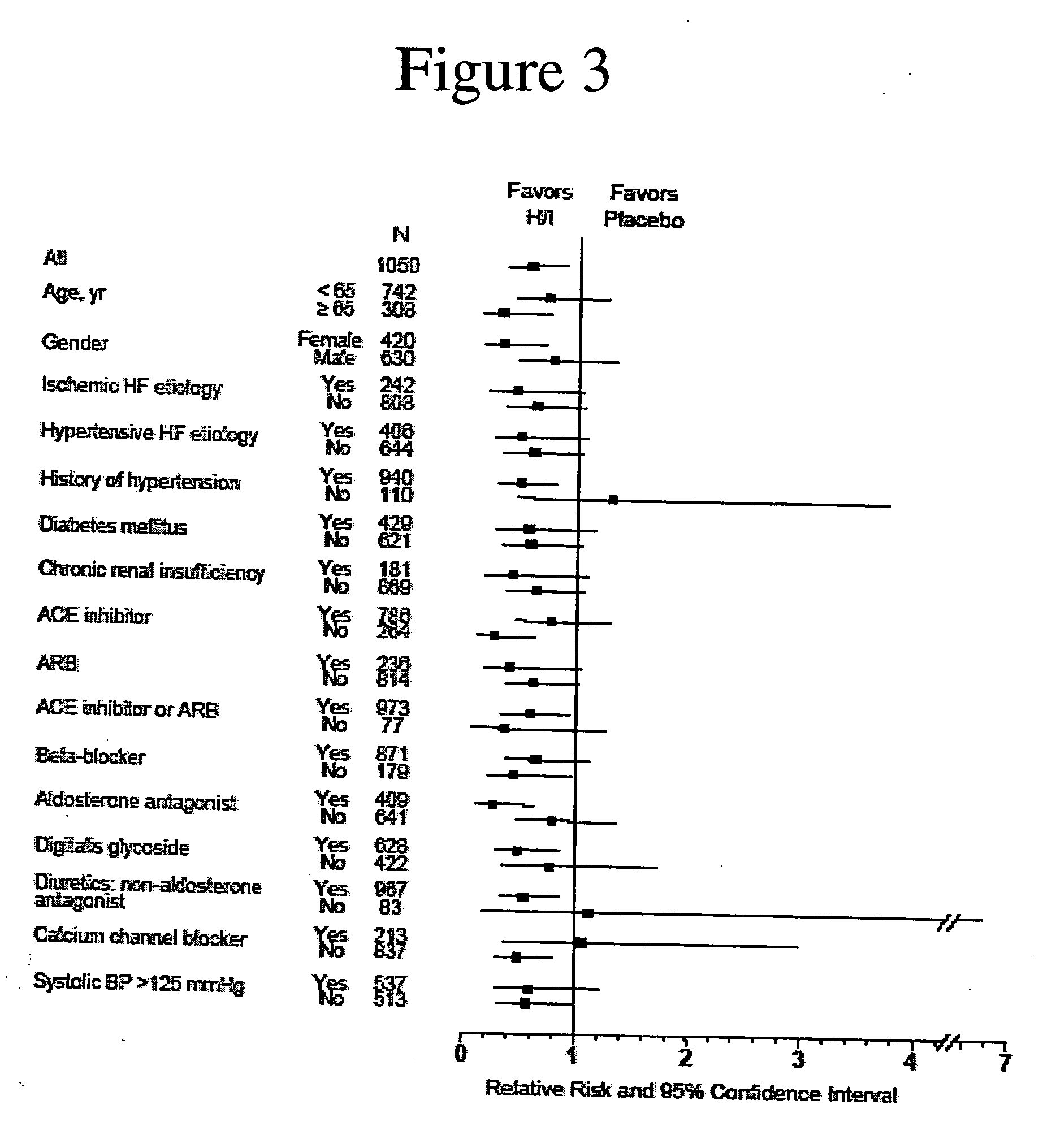
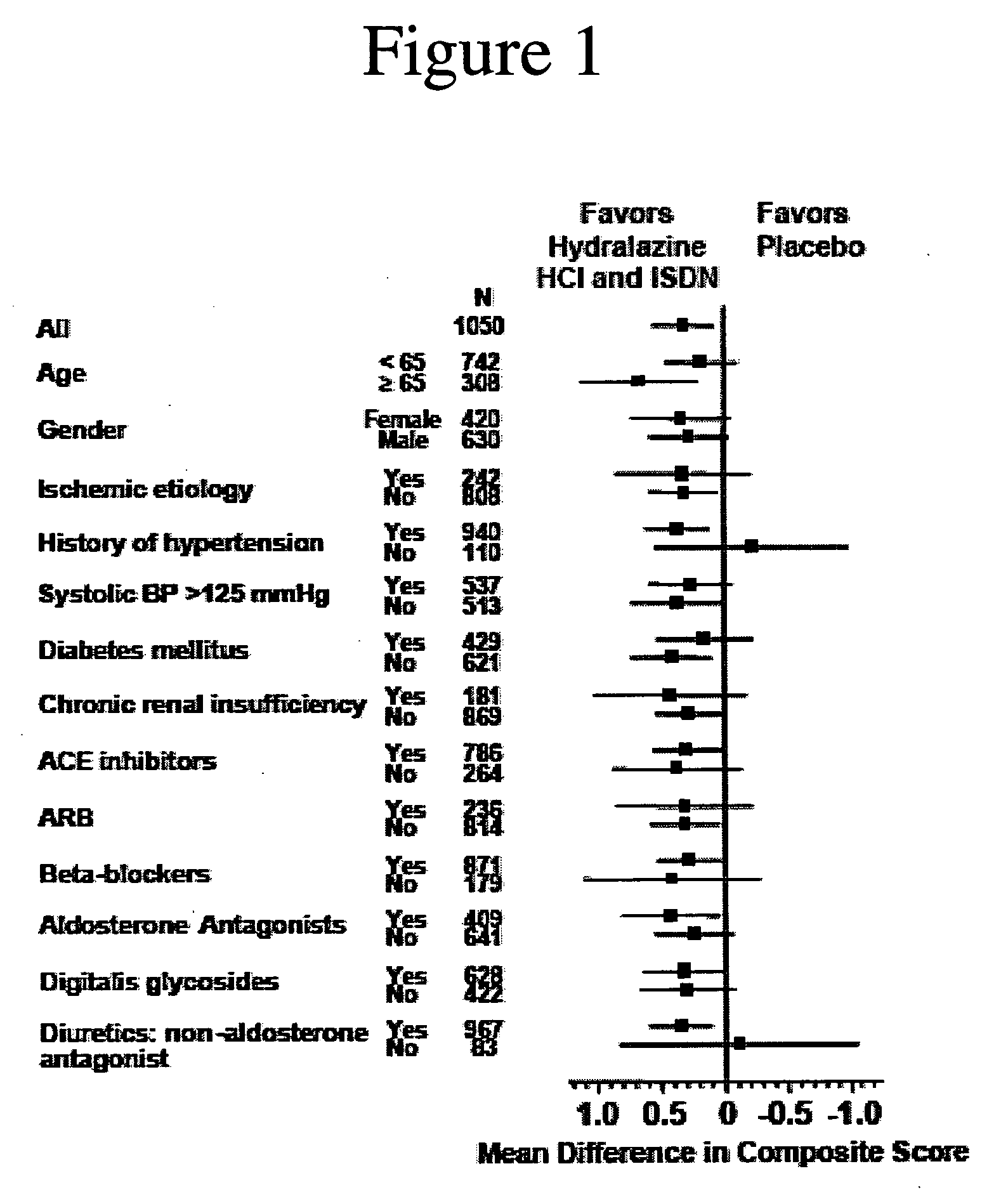
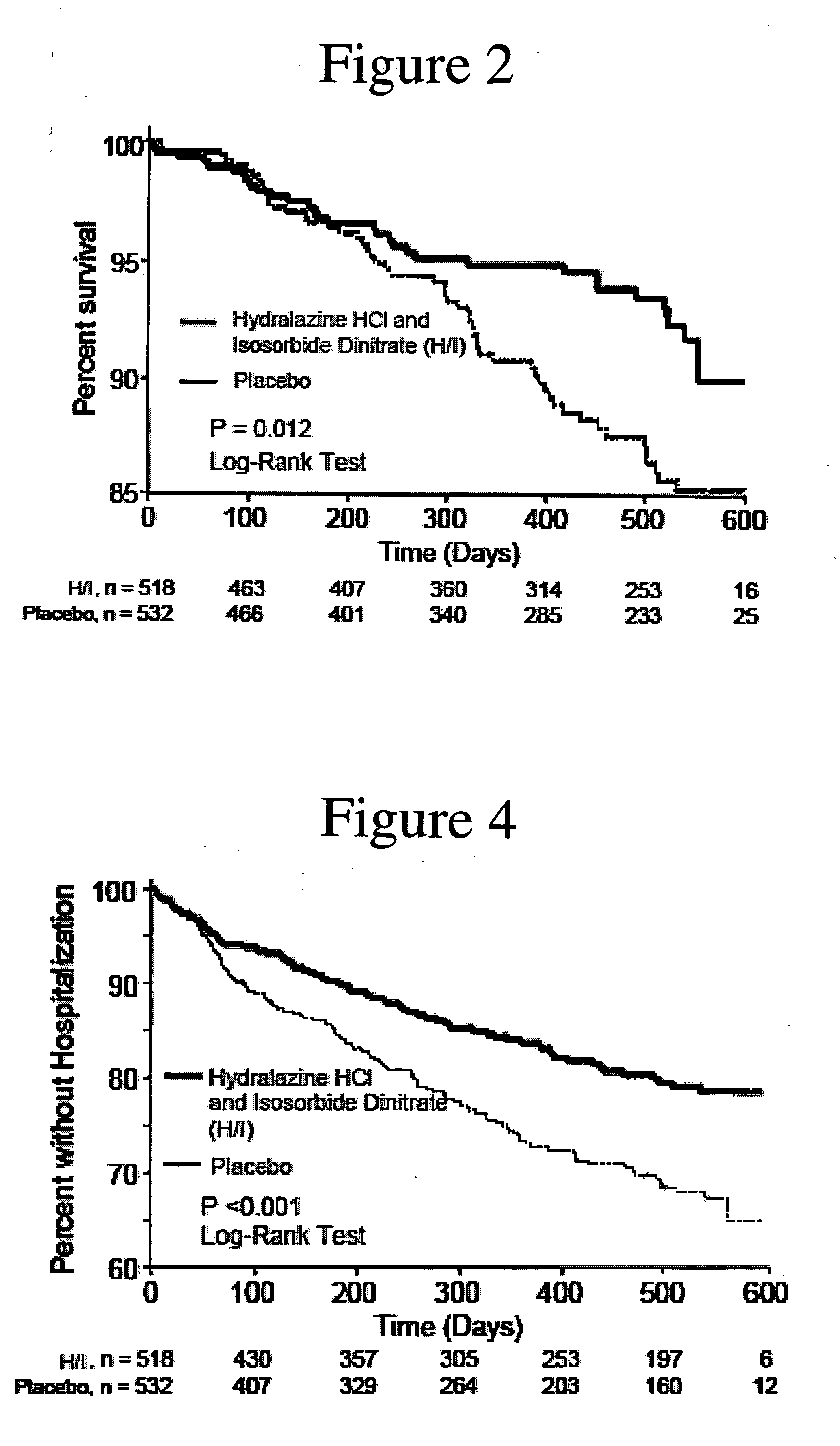
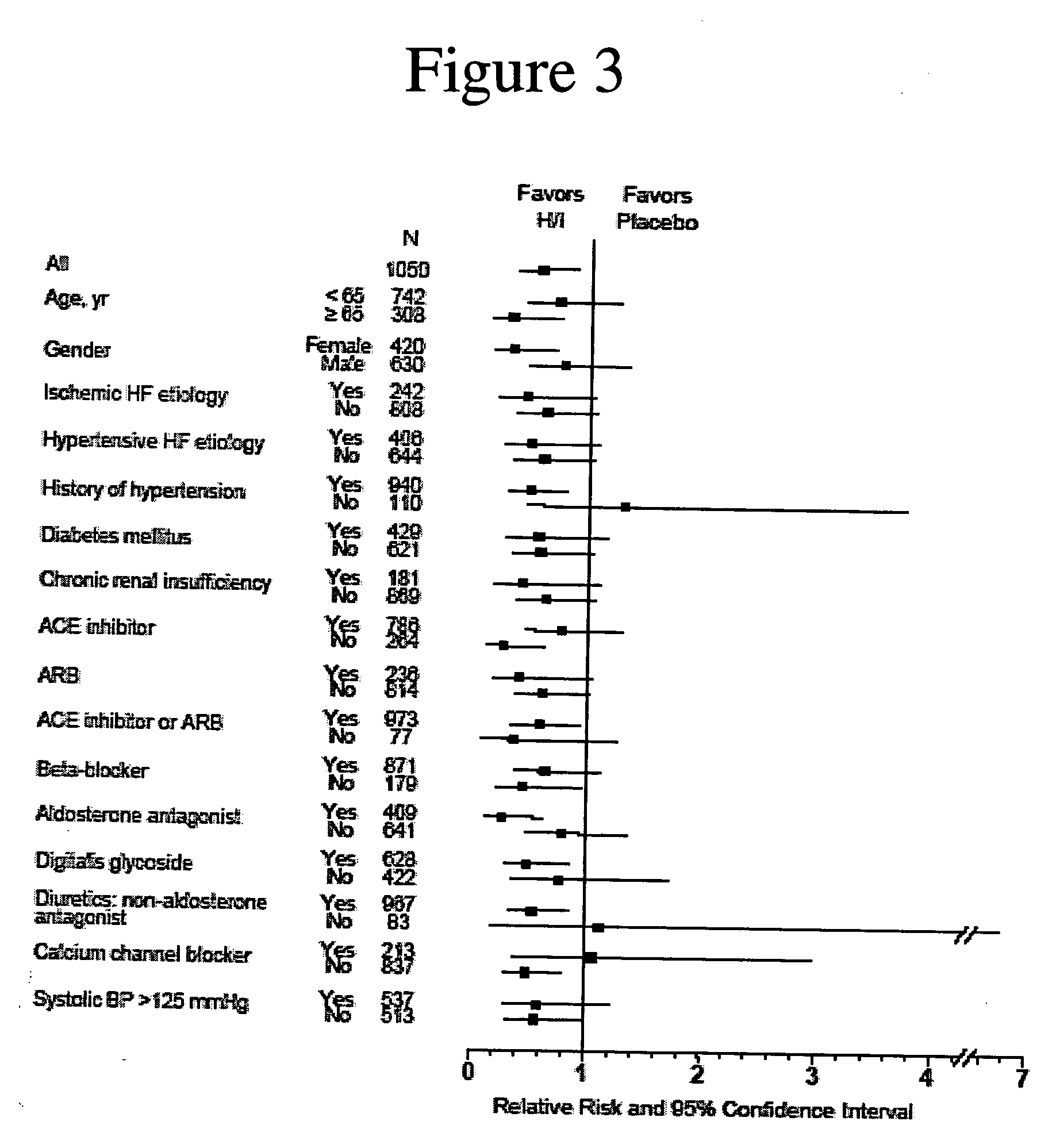
The invention provides methods for (a) prolonging time to hospitalization for heart failure; (b) prolonging time to first hospitalization for heart failure; (c) reducing the total number of days a patient with heart failure spends in the hospital for heart failure for a single hospital stay (i.e., reducing the duration of a single hospital stay for a patient with heart failure); (d) reducing the total number of days a patient spends in the hospital for heart failure for multiple hospital stays (i.e., two or more hospital stays); (e) reducing the number of hospital admissions for heart failure; (f) reducing mortality and reducing hospitalizations for heart failure (e.g., the total number of days in the hospital and / or the number of hospital visits); (g) increasing the left ventricular ejection fraction in a heart failure patient; (h) treating a sexual dysfunction (e.g., erectile dysfunction and female sexual dysfunction) (j) treating a headache in a heart failure patient by administering a non-steroidal antiinflammatory compound (i.e., NSAIDs); (k) treating a heart failure patient who has a history of hypertension (but who is not currently diagnosed with hypertension); (l) improving the quality of life in a heart failure patient based on the Minnesota Living with heart failure questionnaire; (m) decreasing the levels of B-type natriuretic peptide; (n) treating hypertension in a heart failure patient; (o) lowering blood pressure in a heart failure patient; (p) treating labile hypertension; (q) treating idiopathic hypertension; (r) increasing patient compliance with medication dosing in a heart failure patient; (s) treating hypertension in a patient with a dilated heart; (t) treating ischemic disease and / or coronary artery disease; and (u) reducing cardiomegaly in a patient in need thereof comprising administering to the patient a therapeutically effective amount of (i) a hydralazine compound or pharmaceutically acceptable salt thereof, (ii) isosorbide dinitrate and / or isosorbide mononitrate, and (iii) optionally at least one compound selected from the group consisting of angiotensin converting enzyme inhibitors, β-adrenergic antagonists, angiotensin II antagonists, aldosterone antagonists, cardiac glucosides (digitalis), and diuretic compounds.
Owner:NITROMED
Portable Blood Count Monitor
InactiveUS20140270458A1Easy to set upSimple methodBiological testingMicroscopic object acquisitionMedicineWhite blood cell
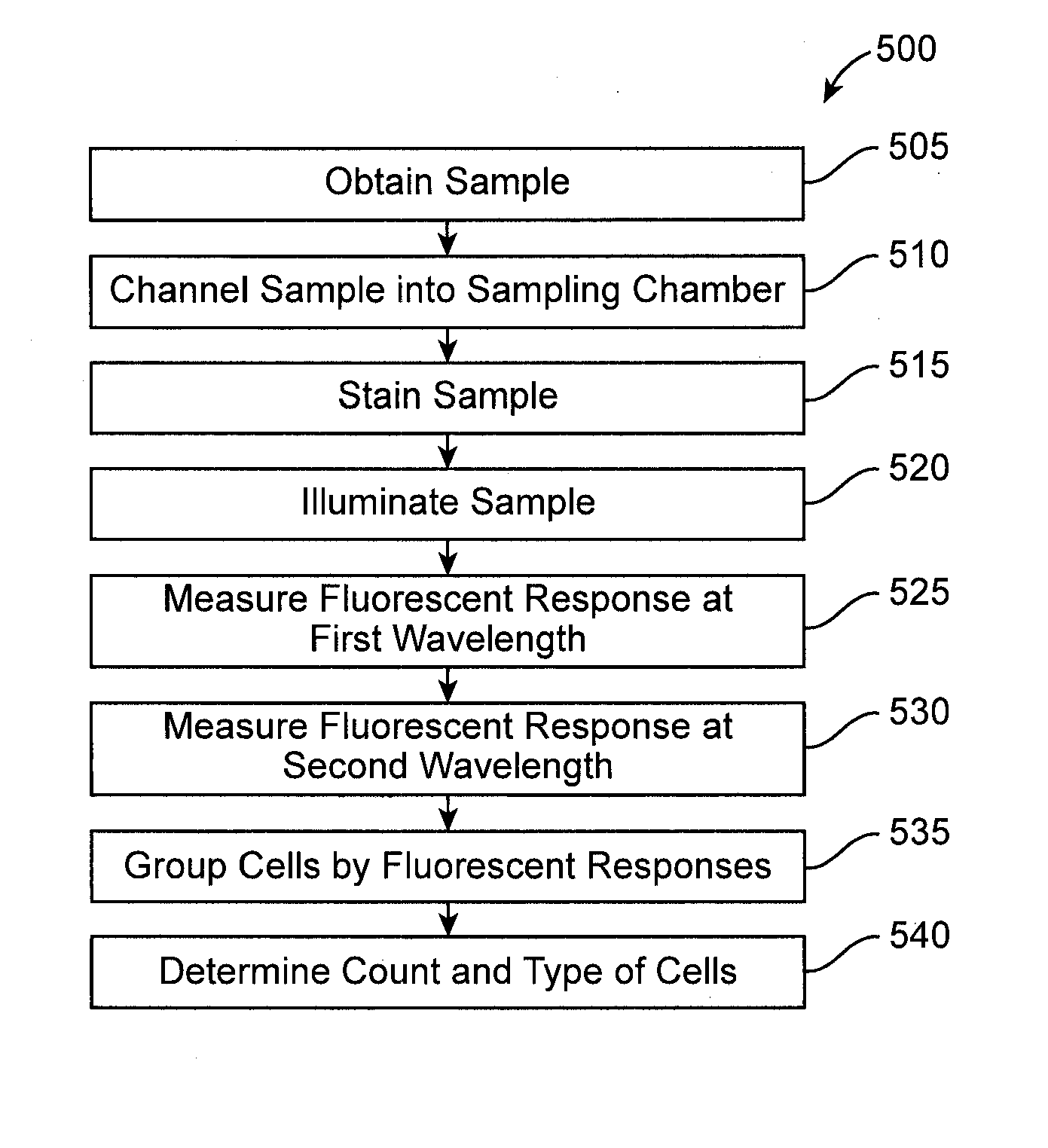
Devices, systems, and methods are disclosed for determining the number and type of blood cells in a blood sample. The blood sample is collected and held in a slide. In the slide, the blood sample is separated and channeled into at least two sampling chambers, one for red blood cells, another for white blood cells, and optionally yet another for platelets. The sampling chambers have wetting agents, lysing agents, staining agents, or the like therein to mix with the blood and facilitate cell count. The slide is placed in a portable slide analyzer where the sampling chambers are illuminated and images of the sampling chambers are taken. These images are converted into electronic form and sent by a communications module of the slide analyzer to a remote external location where the images are analyzed to determine the number and type of blood cells in the blood sample.
Owner:RGT UNIV OF CALIFORNIA +1
Method of reducing seizure type experienced by a dravet patient
InactiveUS20190091174A1Reduce convulsive seizure frequencyEliminate seizureNervous disorderHydroxy compound active ingredientsFenfluramineHuman patient
A method of reducing a particular type of seizure in a human patient diagnosed with Dravet syndrome, by administering to the patient a therapeutically effective dose of fenfluramine or a pharmaceutically acceptable salt, base, acid or amine thereof, and repeating the administering over a period of days until the patient exhibits a reduction from baseline in seizures of a particular type. The reduction may be of one, two or three specific types of seizures.
Owner:ZOGENIX INT
Identification of a micro-RNA that activates expression of β-myosin heavy chain
InactiveUS8304397B2Inhibit progressHigh expressionBiocideGenetic material ingredientsCardiac fibrosisFiber
The present invention relates to the identification of a microRNA, miR-208, that induces the expression of β-myosin heavy chain (β-MHC) and represses fast skeletal muscle contractile protein genes. Inhibition of this function is proposed as a treatment for cardiac fibrosis, hypertrophy and / or heart failure, and augmentation of this function can be used to repress slow fiber genes and activate fast fiber genes in the treatment of musculoskeletal disorders.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Vaccines for use in the prophylaxis and treatment of influenza virus disease
ActiveUS9701723B2Reduce severityShorten the construction periodSsRNA viruses negative-senseBacteriaHemagglutininDisease
Provided herein are polypeptides comprising portions of the influenza virus hemagglutinin, compositions comprising such polypeptides that can be used as immunogens in vaccines and methods of their use to generate an immune response against multiple influenza subtypes in a subject.
Owner:MT SINAI SCHOOL OF MEDICINE
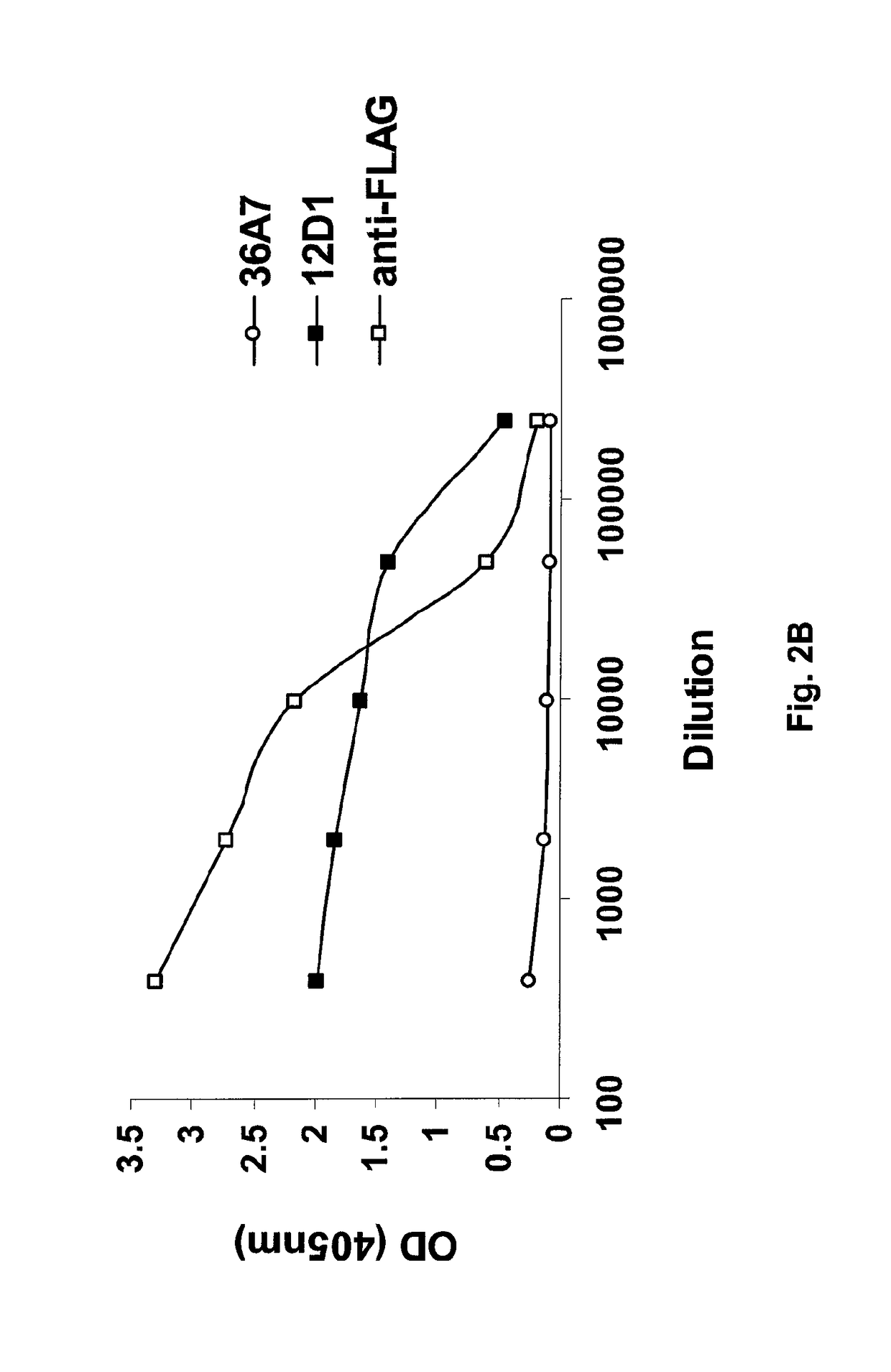
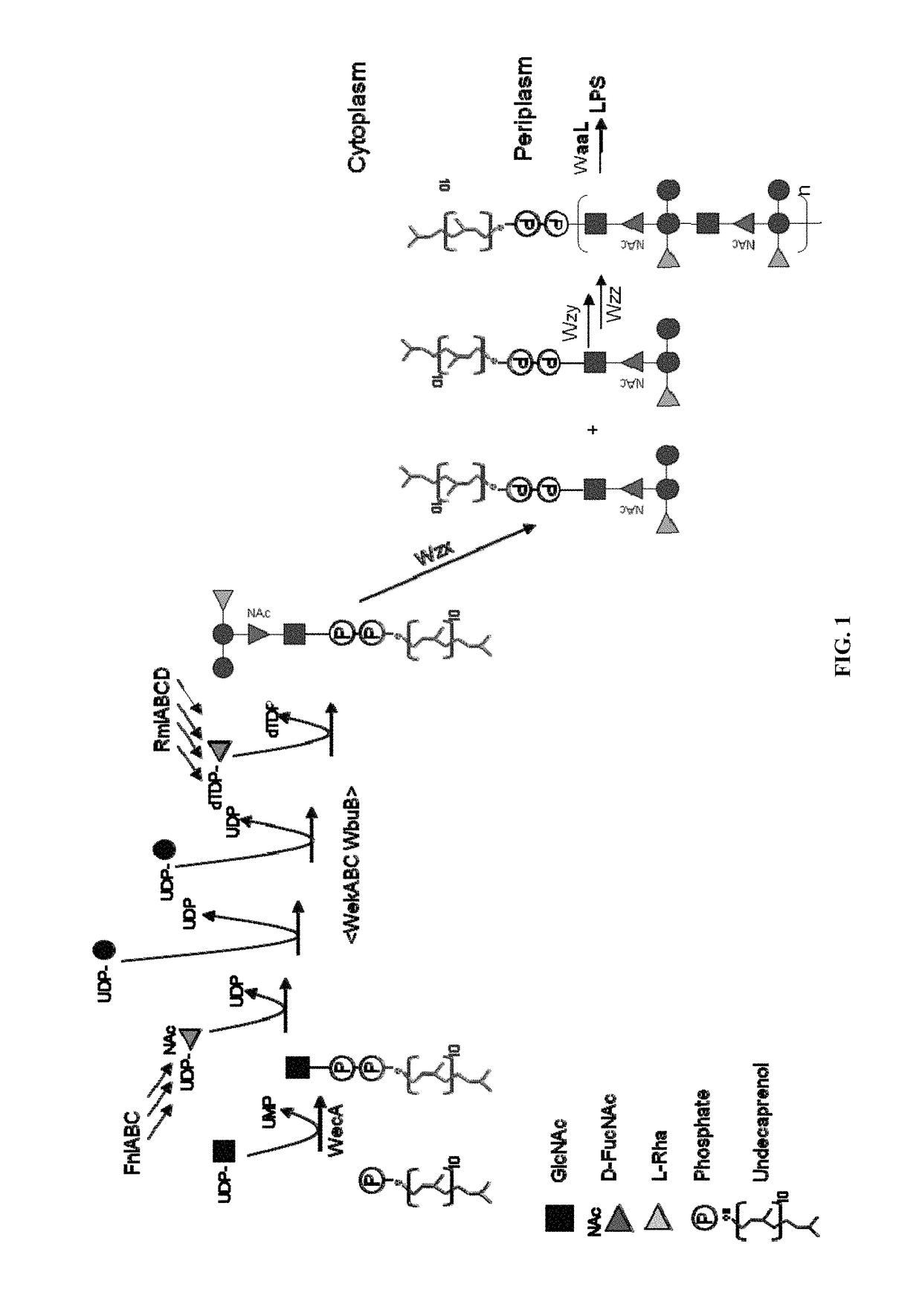
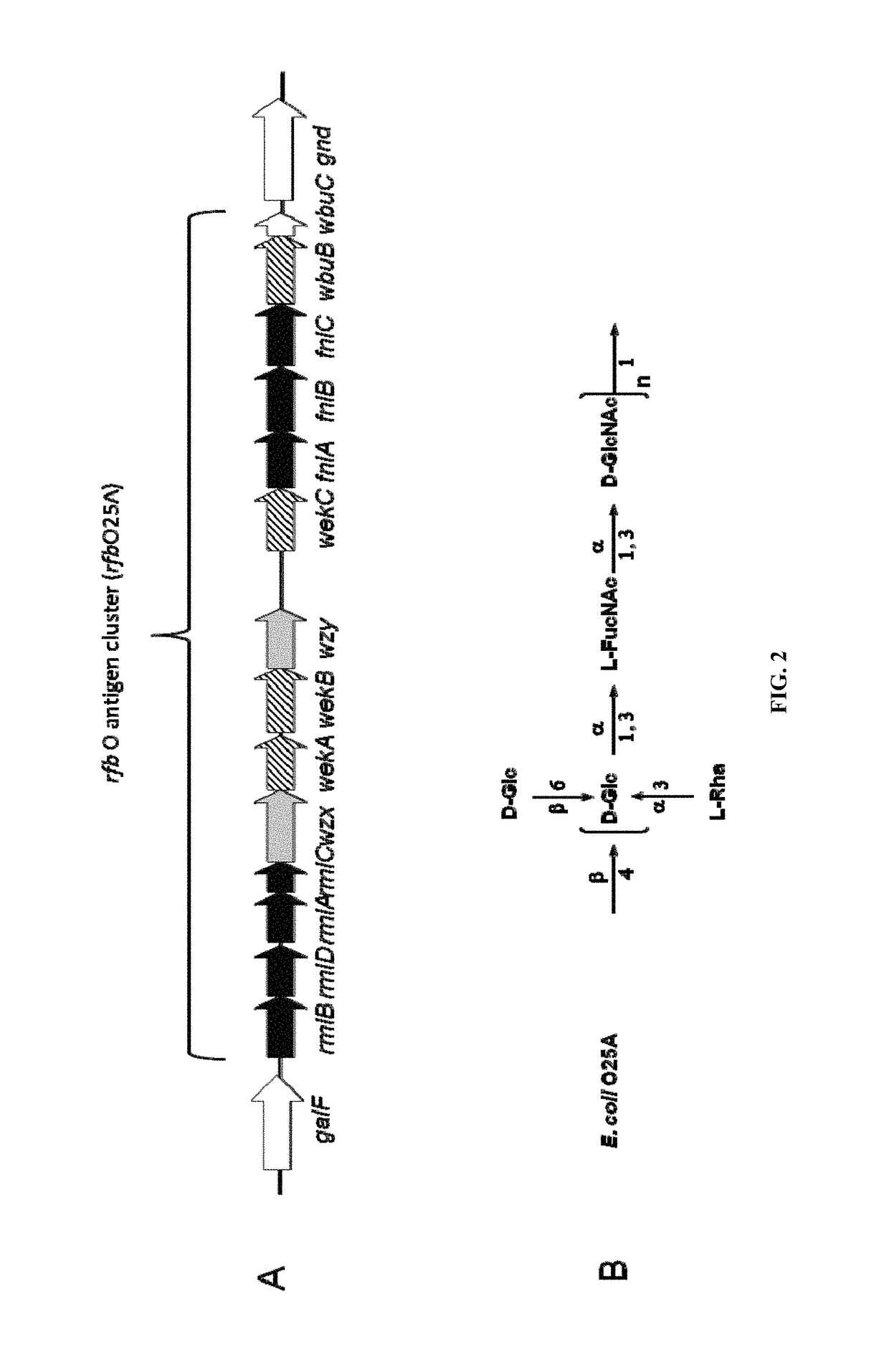
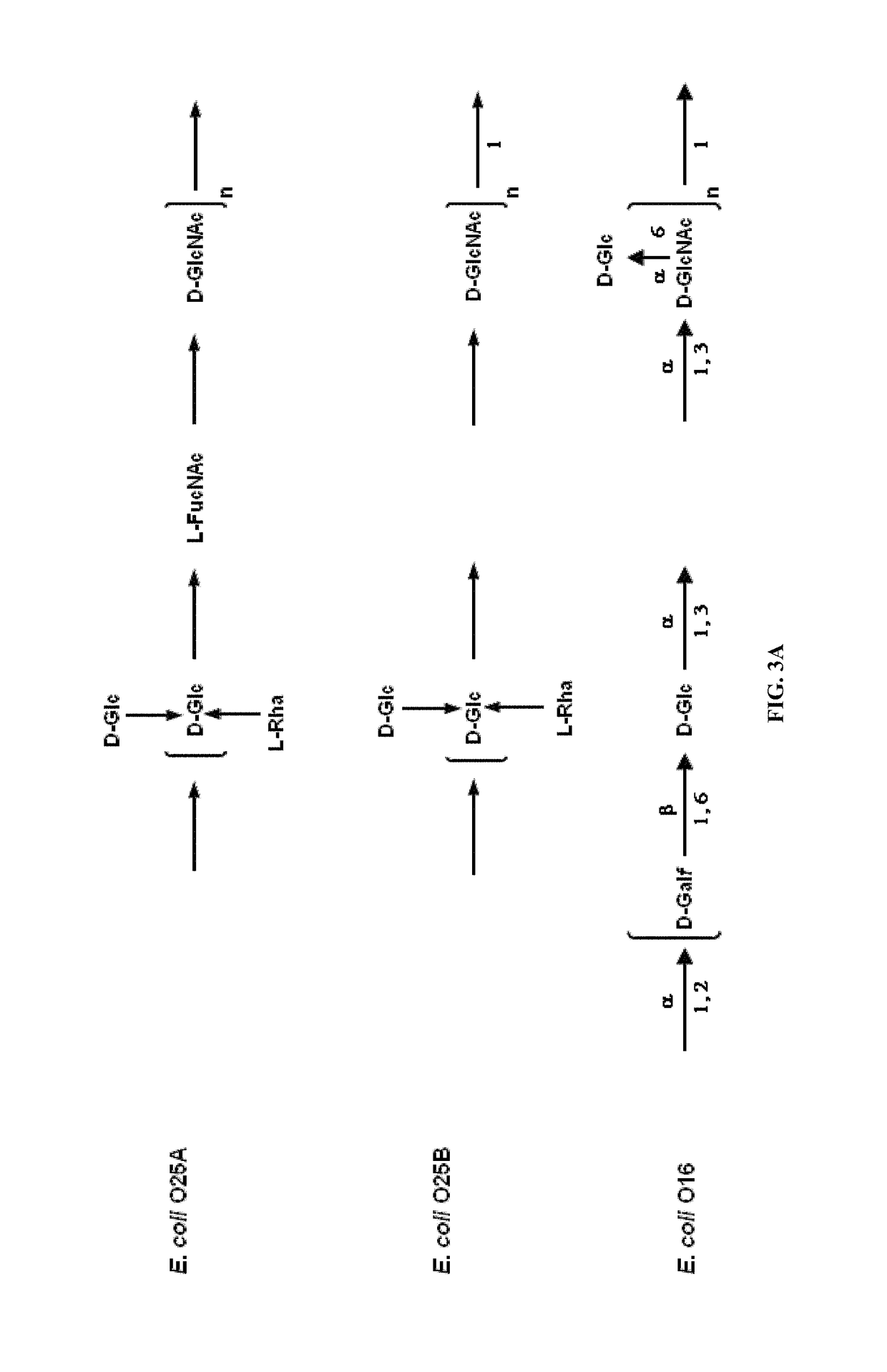
Polysaccharide and uses thereof
ActiveUS9700612B2Reduce severityInhibit progressAntibacterial agentsBacterial antigen ingredientsCarrier proteinPolysaccharide
Provided herein is a novel E. coli O polysaccharide, O25B. Also provided herein are prokaryotic host cells comprising enzymes (e.g., glycosyltransferases) used in O25B production. The host cells provided herein produce O25B bioconjugates, wherein said bioconjugates comprise O25B linked to a carrier protein. Further provided herein are compositions, e.g., pharmaceutical compositions, comprising O25B and / or bioconjugates comprising O25B. Such compositions can be used as vaccines against infection with ExPEC, and may further comprise one or more additional bioconjugates.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Methods for determining the relative spatial change in subsurface resistivities across frequencies in tissue
ActiveUS20130165761A1Accurate and reliable interpretationAccurate diagnosisBioelectric signal measurementRespiratory organ evaluationPower flowMedicine
Non-invasive devices and systems to determine tissue wetness / hydration based on relative changes in subsurface resistivities in tissue below an electrode array applied to a human body across different frequencies. For example, these a sensor including arrays of current-injecting and voltage-sensing electrodes may be placed on a subject's back to determine lung wetness. Systems and methods for determining tissue water content, systems and methods for determining lung wetness, sensors for determining relative changes in subsurface resistivities across frequencies and systems and methods to determine which arrays of electrodes in a sensor to use to determine relative changes in subsurface resistivities across frequencies are all described.
Owner:IMPEDIMED
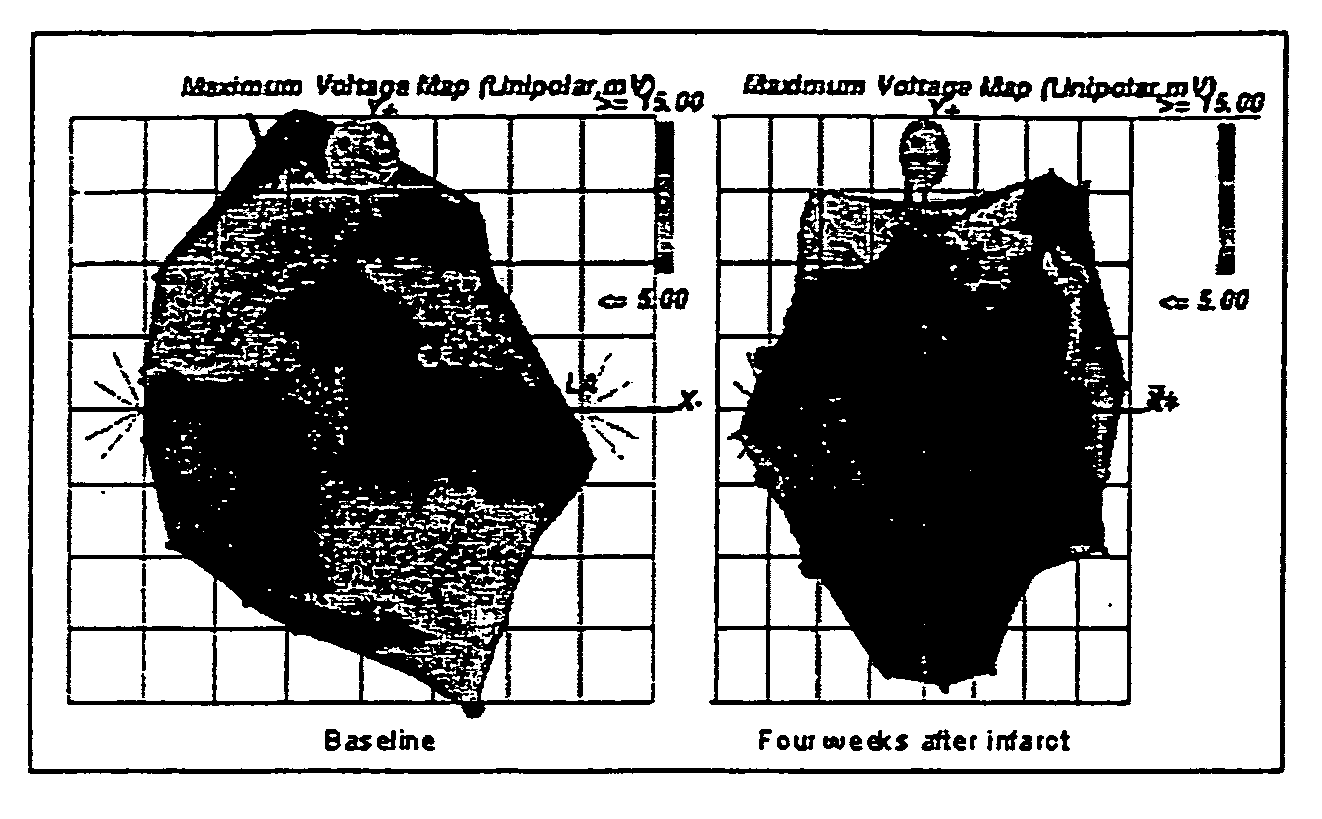
Cellular cardiomyoplasty as supportive therapy in patients with heart disease
ActiveUS20060276685A1Improve the quality of lifeImprove heart functionMammal material medical ingredientsSkeletal/connective tissue cellsDiseaseHeart transplantation
The present invention provides a system for treating heart disease as a “bridge to recovery” using cellular cardiomyoplasty. The system is particularly useful in selecting and treating patients with damaged myocardium due coronary artery disease, myocardial infarction, congestive heart failure, and ischemia. Based on various clinical criteria, a patient is selected and optionally treated using cellular cardiomyoplasty to improve the patient's cardiac function. The cardiomyoplasty may be combined with other treatments such as medications or left ventricular assist devices. Preferably, the cellular cardiomyoplasty eliminates the need for invasive surgery such as by-pass grafting or cardiac transplantation. The invention also provides kits for use in selecting and treating patients using the inventive method.
Owner:MYTOGEN
Medication organizer tray apparatus
ActiveUS9757305B2Improve adhesionOrganize effectivelyDrug and medicationsPharmaceutical containersMedication adherenceBiomedical engineering
A method and a medication organizer tray apparatus for organizing medications and collecting medication adherence information are provided. The medication organizer tray apparatus includes a support frame, multiple medication bins accommodating multiple medications, a bin cover layer with multiple customized bin labels, and a conductive circuit layer. The support frame includes multiple apertures positioned at predefined intervals from each other for placement of the medication bins. The customized bin labels having medical information printed thereon seal openings of the medication bins. The conductive circuit layer includes conductive lines running along one or more of a lower surface of the bin cover layer, around each medication bin, and a lower surface of each medication bin. The conductive circuit layer electrically communicates with a receptacle base to enable detection of removal of each medication bin from the support frame and detection of tampering of the medication bins.
Owner:RXADVANCE
Influenza virus vaccination regimens
ActiveUS20180008696A1Reduce severityImprove survivalSsRNA viruses negative-senseViral antigen ingredientsHemagglutininRegimen
Provided herein are immunization regimens for inducing an immune response (e.g., an antibody response) against influenza virus. In specific aspects, the immunization regimens involve the administration of a chimeric hemagglutinin (HA), a headless HA or another influenza virus stem domain based construct (e.g., the HA stem domain or a fragment thereof) to a subject. In certain aspects, the immunization regimens also involve the administration of an influenza virus neuraminidase immunogen.
Owner:MT SINAI SCHOOL OF MEDICINE
Methods for reducing hospitalizations related to heart failure
InactiveUS20060014829A1Reduce in quantityShorten the construction periodBiocideNervous disorderDigitalisMortality rate
The invention provides methods for (a) prolonging time to hospitalization for heart failure; (b) prolonging time to first hospitalization for heart failure; (c) reducing the total number of days a patient with heart failure spends in the hospital for heart failure for a single hospital stay (i.e., reducing the duration of a single hospital stay for a patient with heart failure); (d) reducing the total number of days a patient spends in the hospital for heart failure for multiple hospital stays; (e) reducing the number of hospital admissions for heart failure; and (f) reducing mortality and reducing hospitalizations for heart failure (e.g., the total number of days in the hospital and / or the number of hospital visits) in a patient in need thereof comprising administering to the patient a therapeutically effective amount of (i) a hydralazine compound or pharmaceutically acceptable salt thereof, (ii) isosorbide dinitrate and / or isosorbide mononitrate, and (iii) optionally at least one compound selected from the group consisting of angiotensin converting enzyme inhibitors, β-adrenergic antagonists, angiotensin II antagonists, aldosterone antagonists, cardiac glucosides (digitalis), and diuretic compounds.
Owner:NITROMED
Spray-dry formulations
ActiveUS9925184B2Improve athletic abilityFunction increasePowder deliveryOrganic active ingredientsDiseaseCancer research
Disclosed herein are compounds, compositions, and methods for preventing and treating proliferative diseases associated with aberrant receptor tyrosine kinase (RTK) activity. The therapeutic indications described herein more specifically relate to the non-selective inhibition of RTKs associated with vascular and pulmonary disorders.
Owner:GILEAD SCI INC +1
Novel paramyxovirus and uses thereof
Described herein are isolated paramyxovirus, a morbillivirus (FmoPV), isolated nucleic acids encoding the genome of FmoPV, isolated amino acid sequences of FmoPV proteins, antibodies to FmoPV and its proteins, and uses thereof. In certain embodiments, the modified FmoPV is a feline morbillivirus. Also described herein is a recombinant FmoPV comprising a modified FmoPV gene or gene segments and the use of such a virus. The recombinant FmoPV may be used in the prevention and / or treatment of diseases related to FmoPV or as a delivery vector. Also described herein is a diagnostic assay for the FmoPV. In certain embodiments, the FmoPV causes kidney disease. In certain embodiments, the kidney disease is in felines. In certain embodiments, the kidney disease is tubulointerstitial nephritis (“TIN”). Also described herein is a quantitative assay for the detection of the FmoPV, natural or artificial variants, analogs, or derivatives thereof. In certain embodiments, the quantitative assay is reverse transcription and polymerase chain reaction (RT-PCR). Also described herein is a vaccine and a kit containing the vaccine for the prevention and treatment of FmoPV infection. Described herein is a diagnostic kit that comprises nucleic acid molecules for the detection of the FmoPV.
Owner:VERSITECH LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com