Vectors, mutant viruses and methods for generating mutant viruses
a technology of mutant viruses and vectors, applied in the field of nucleic acid vectors, can solve the problems of inability of a broad spectrum first generation oncolytic virus to replicate in or provide an delay in generating recombinant hsv, and inability to provide effective treatment for all tumour types, etc., to achieve the effect of convenient identification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of Plasmid RL1.dIRES-GFP
General Approach
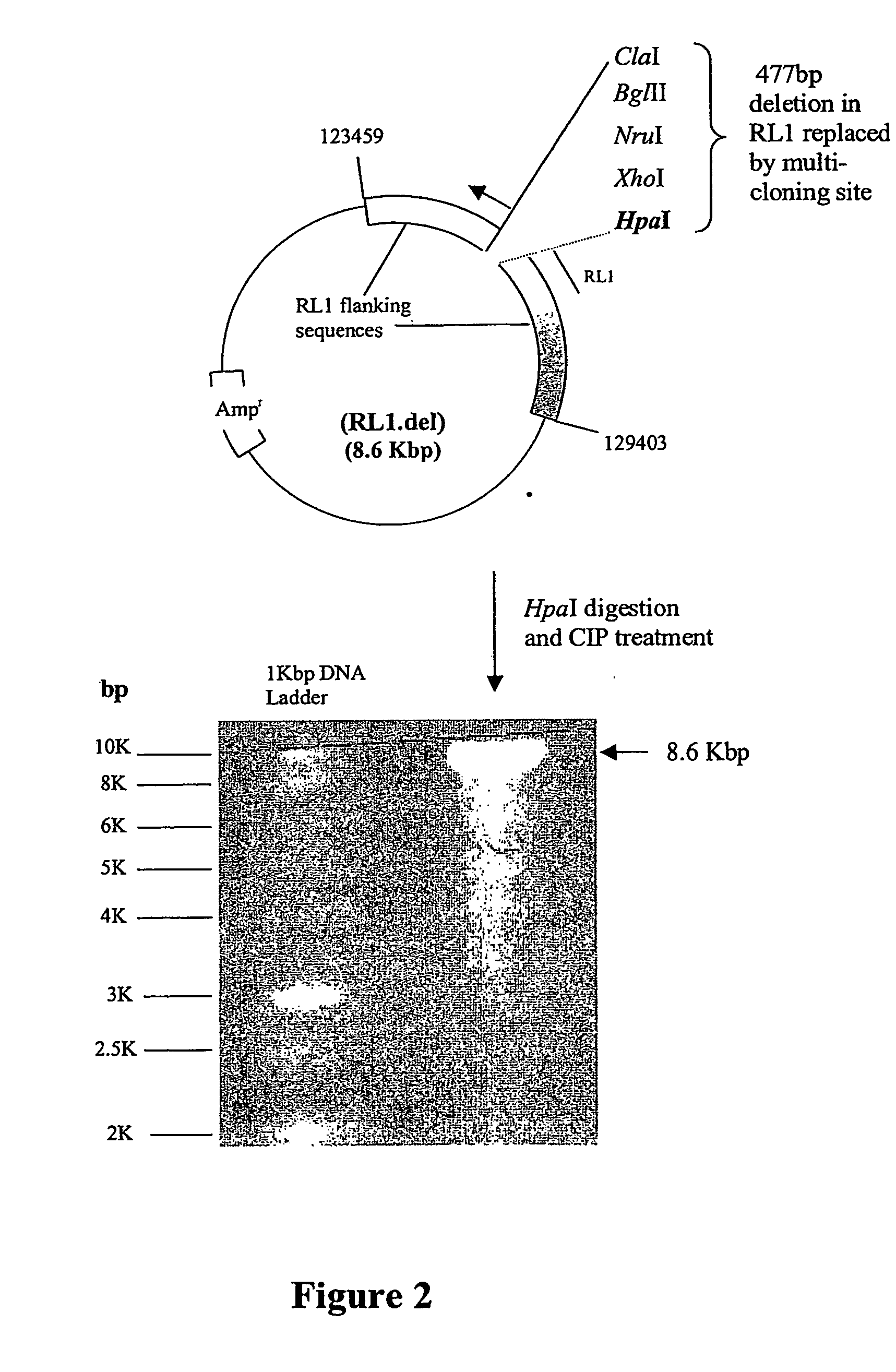
[0255] Plasmid RL1.dIRES-GFP was generated in three stages, illustrated in FIG. 1.
[0256] 1. The DNA sequences containing the CMV IE promoter (pCMV), the NAT gene, the internal ribosome entry site (IRES), the GFP reporter gene and the SV40 polyadenylation sequences were excised from pNAT-IRES-GFP using NsiI and SspI and purified.
[0257] 2. The purified pCMV-NAT-IRES-GFP-PolyA DNA fragment was cloned into RL1.del to form a new plasmid designated RL1.dCMV-NAT-GFP.
[0258] 3. The pCMV-NAT DNA sequences of RL1.dCMV-NAT-GFP were excised using XhoI and the remainder of the plasmid re-ligated to form a novel plasmid designated RL1.dIRES-GFP. This novel plasmid contained a multi-cloning site (all sites shown are unique) upstream of an IRES, the GFP gene and the SV40 polyA sequences all within the HSV-1 RL1 flanking sequences. Recombinant ICP34.5 null HSV-1, expressing a gene of interest in the RL1 locus, can be generated by cloning the ...
example 2
Generation of ICP34.5 Null HSV-1 Expressing a Gene Product of Interest and GFP Using Plasmid RL1.dIRES-GFP
General Approach
[0270] Generation of ICP34.5 null HSV-1 expressing a gene product of interest requires insertion of nucleotide sequence encoding the gene product (polypeptide) of interest, and often a desired promoter, at the MCS of RL1.dIRES.GFP followed by co-transfection of BHK cells with the linearised plasmid, containing the gene of interest, and HSV DNA. Following homologous recombination viral plaques expressing GFP are identified. FIG. 7 illustrates the method steps involved.
[0271] Referring to FIG. 7A plasmid DNA, containing the gene of interest and the desired promoter (X), is digested with restriction endonucleases to release the promoter / gene fragment.
[0272] The promoter / gene fragment is purified and cloned into the multi-cloning site (MCS) of RL1.dIRES.GFP forming a shuttle vector suitable for generating oncolytic HSV-1 (FIG. 7B). This vector contains HSV-1 seq...
example 3
Construction of HSV1716 / CMV-NTR / GFP
General Approach
[0282] HSV1716 / CMV-NTR / GFP was generated by cloning a 1.6 Kbp BamHI fragment from pPS94910, consisting of the E. coli nitroreductase (NTR) gene downstream of the CMV IE promoter (pCMV), into the MCS of the RL1.dIRES-GFP smart cassette, in the forward orientation with respect to the GFP gene in RL1.dIRES-GFP (FIG. 8). The resultant plasmid, named RL1.dCMV-NTR-GFP, was then linearised and recombinant virus generated and purified as described above. The plasmid pPS949 (referred to as ‘pxLNC-ntr’ in Ref 10) containing the NTR gene downstream of the CMV IE promoter (pCMV-NTR) in a pLNCX (Clontech) backbone, was a kind gift from Professor Lawrence Young, University of Birmingham, UK.
Materials and Methods
[0283] 4×1 μg of pPS949 was digested with 10 units of BamHI (Promega), in a suitable volume of 10× buffer (Promega) and nuclease free water (Promega), at 37° C. for 16 hrs. The reaction mixture was electrophoresed in a 1% agarose gel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tm | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com