Preventive or therapeutic agent for herpesvirus-related disease
A herpes virus and virus agent technology, applied in antiviral agents, medical preparations containing active ingredients, organic active ingredients, etc., can solve problems such as unclear combined effects, achieve excellent therapeutic effects, high safety, and reduce adverse effects The effect of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Example 1 Helicase-primer inhibitory activity
[0109] Baculovirus (Dr. Nigel D.Stow, Medical Research Council, provided by UK) for expression of UL5, UL52, and UL8 proteins constituting the helicase-priming enzyme complex of HSV-1, by papers such as Crute The method described in (JBC, 1991, Vol.266, P21252-21256) prepared the recombinant HSV-1 helicase-prime enzyme complex. Detection of DNA-dependent ATPase activity, which is the enzymatic activity of the HSV-1 helicase-primerase complex, was carried out according to the method described in Crute et al. (JBC, 1991, Vol. 266, P4484-4488). In summary, 520ng of HSV-1 helicase-prime enzyme complex was reacted at 30°C for 30 minutes in a reaction solution containing 20 μg / mL of DNA from heat-denatured bovine sperm and 2mM ATP, and the ATPase activity was passed. ATP is hydrolyzed into ADP and monophosphoric acid, adding malachite green reagent (0.03% malachite green, 0.1% ammonium molybdate, 4.8% sulfuric acid) equal to th...
Embodiment 2
[0112] Embodiment 2 HSV-1 skin infection mouse model (test in vivo)
[0113] The in vivo activity of the pharmaceutical composition of the present invention was tested using a mouse model of HSV-1 skin infection using the method according to H. Machida et al. (Antiviral Res. 1992 17 133-143). Brush the skin of HR-1 hairless mice (female, 7 weeks old) under ether anesthesia vertically and horizontally with an injection needle several times, and drop the virus liquid (HSV-1 WT-51 strain 1.5×10 4 PFU / 15μL) to make it saturated, so that it can be infected with HSV-1.
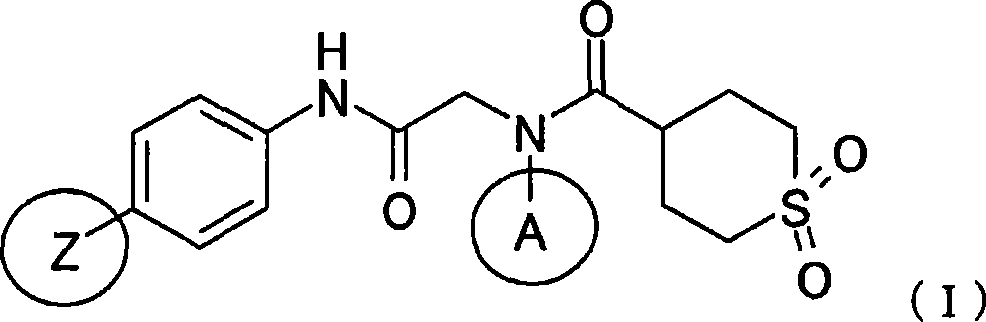
[0114] The compounds to be tested are: using the methylcellulose suspension of VCV as a polymerase inhibitor, and in addition, using the compound of Production Example 2 described later, that is, N-(4-methylphenyl)-N-(2-{ [4-(1,3-oxazol-4-yl)phenyl]amino}-2-oxoethyl)1,1-dioxotetrahydro-2H-thiopyran-4-carboxamide methyl cellulose The plain suspension was used as a helicase-priming enzyme inhibitor, and the dosage in...
manufacture example
[0132] Production examples of the compound (I) as a helicase-prime enzyme inhibitor of the present invention are shown below. In addition, many raw material compounds used in the following reactions are disclosed in patent documents (International Publication No. 02 / 38554 pamphlet) and the like, and can be easily obtained by methods described in these known documents. Production examples of novel compounds among starting compounds are shown as reference examples.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com