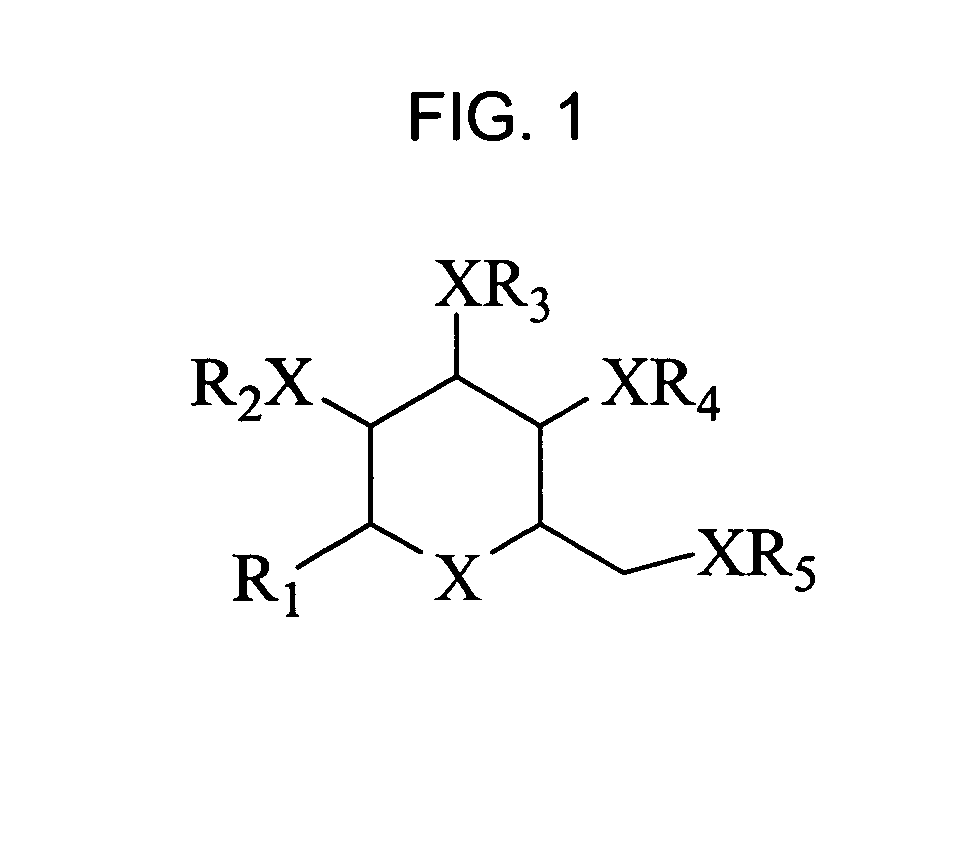
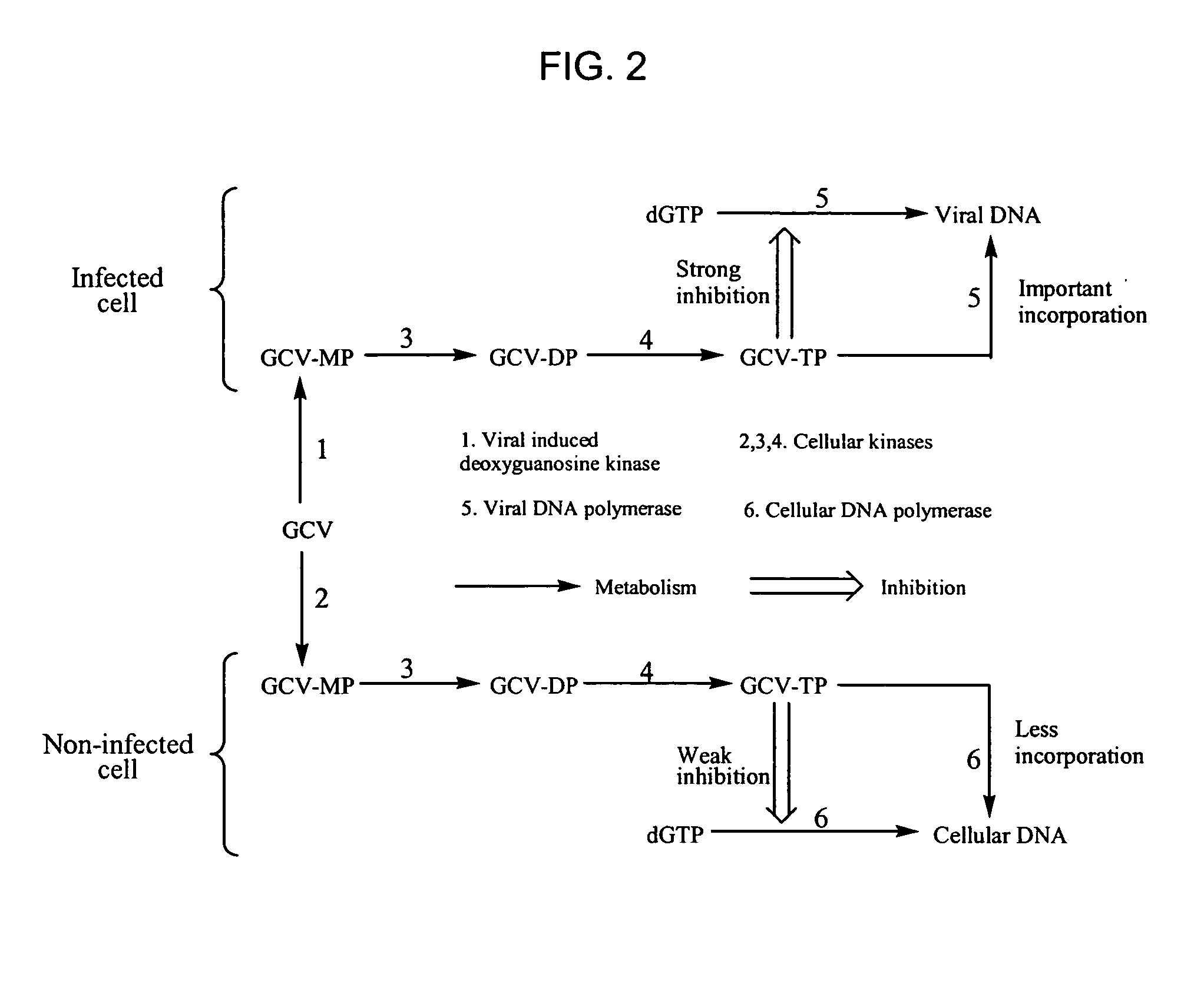
Bicyclic carbohydrate compounds useful in the treatment of infections caused by herpesviridae
a carbohydrate compound and herpes virus technology, applied in the field of compounds active against viral diseases, can solve the problems of unable to clear the virus, cannot eliminate the virus from the body, and doubt the significance of transmission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of the Compounds of Formula A
[0026] The compounds were synthesized as follows:
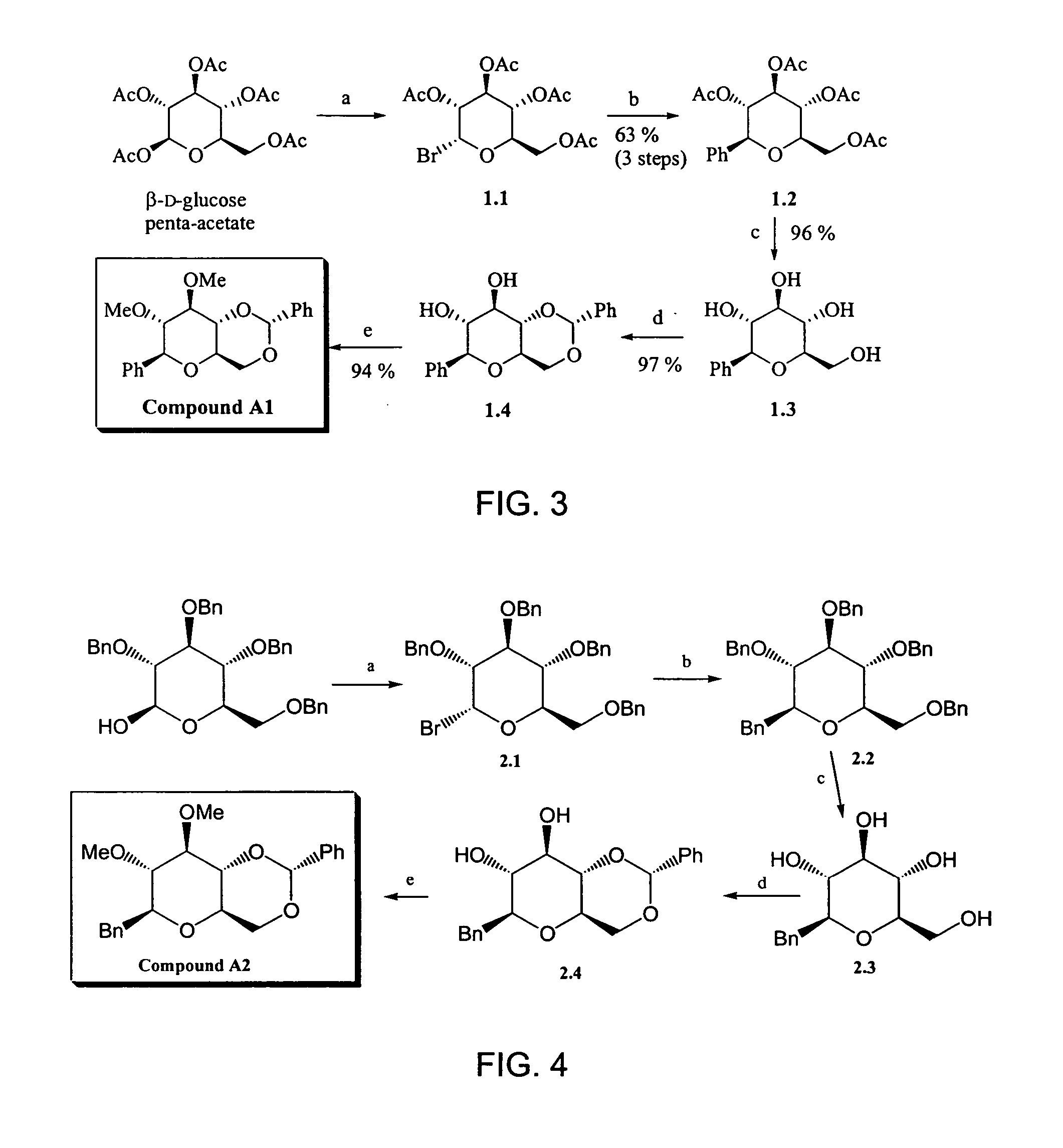
1. Synthesis of Compound A1
[0027] The scheme of the synthesis of Compound A1 is illustrated in FIG. 3.
Synthesis of Compound 1.1
[0028] To (β)-D-glucose penta-acetate (24.6 g, 63.0 mmol) was added a solution of hydrogen bromide in acetic acid (33 wt %, 100 ml). A dark brown color immediately appears. The reaction mixture was stirred at room temperature for 30 minutes under argon atmosphere. Subsequently the solvent was removed by azeotropic distillation in vacuo with toluene (4×50 ml), yielding a green-brown solid Compound 1.1. The crude product was used in the next reaction step without further purification.
[0029] Formula: C14H19O9Br
[0030] Molecular weight: 411.20
[0031] Rf: 0.46 (cyclohexane / ethyl acetate 1:1)
[0032] IR (KBr): 2962, 2360, 2342, 1748, 1435, 1369, 1218, 1162, 1112, 1079, 1042, 911, 752, 668, 601, 563 cm−1
[0033] ES-MS: 433=[410+Na]+, 435=[412+Na+]
[0034]1H-NMR (500 MHz, CDC...
example 2
Bioactivity of the Compounds of Formula A
[0253] The compounds were screened against various pathogenic viruses and more specific the human cytomegalovirus (CMV). For determination of the antiviral activity, expressed in IC50, against 2 CMV-strains (Davis and AD-169), human embryonic lung fibroblast (HEL) cells grown in 96-well microplates were infected with 20 PFU virus / well. After 2 hours of incubation at 37° C., the infected cells were replenished with 0.1 ml of medium containing serial dilutions of the test compound. On day 7 the plaques were counted microscopically after staining the cells with Giemsa's solution. The minimum antiviral concentration was expressed as the dose required to inhibit virus-induced plaque formation by 50%.
[0254] The results of the screening of the new compounds against the human cytomegalovirus (CMV) are presented in Table 3.
TABLE 3Antiviral activity of the compoundsIC50(μg / ml)aCMVAD-169DavidCompoundStrainStrainCompound A12.72.0Compound A25.020.0Com...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com