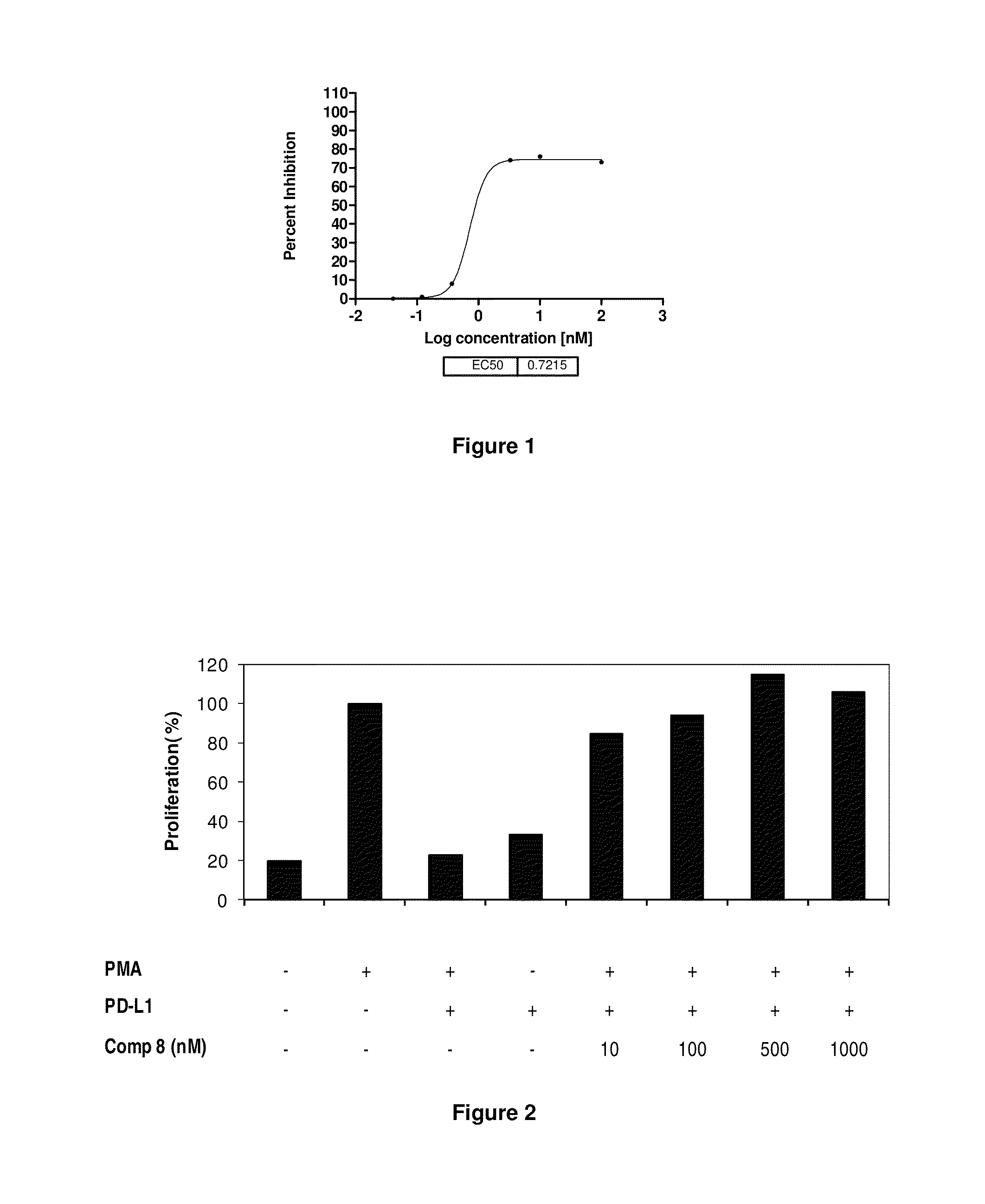
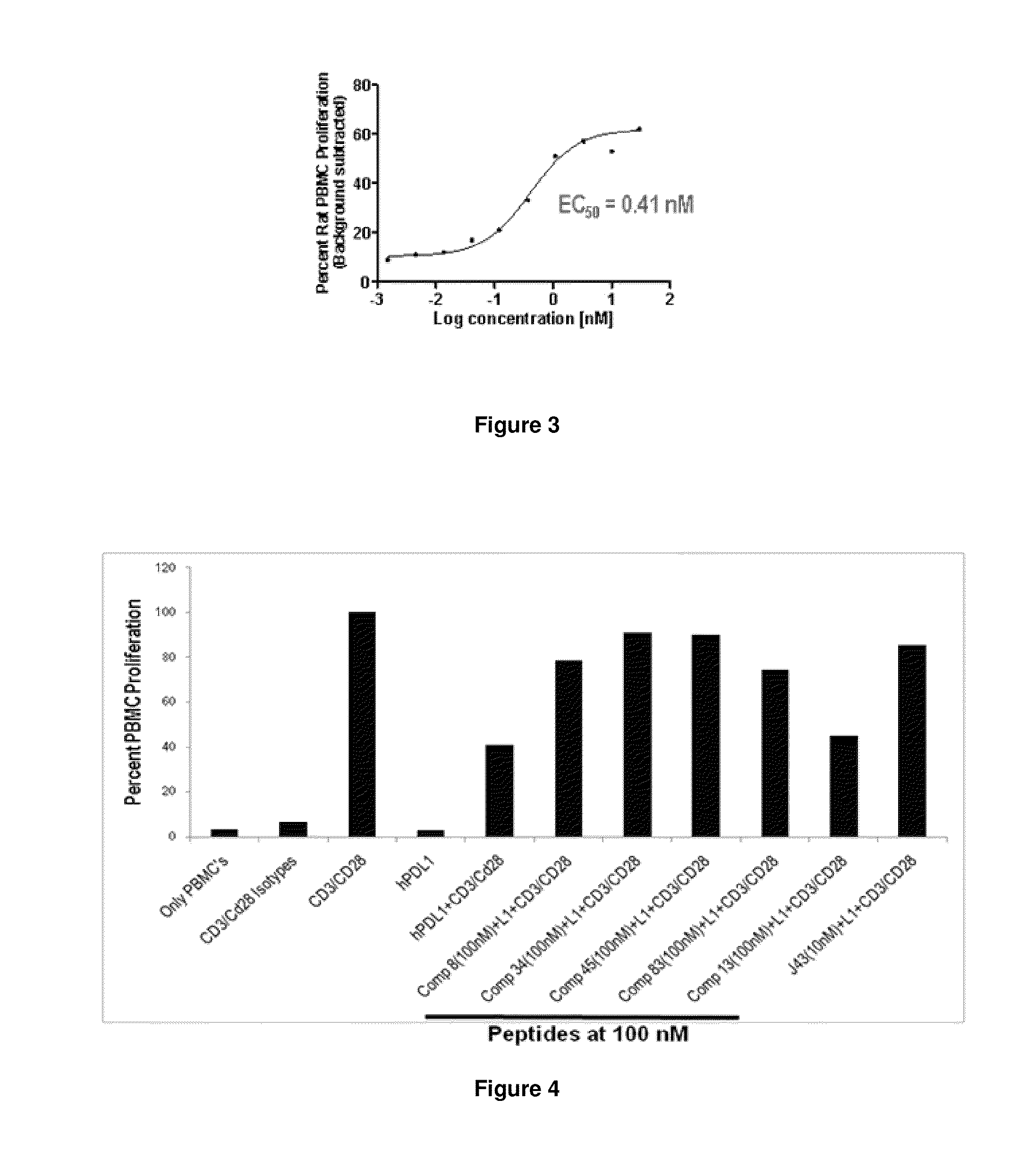
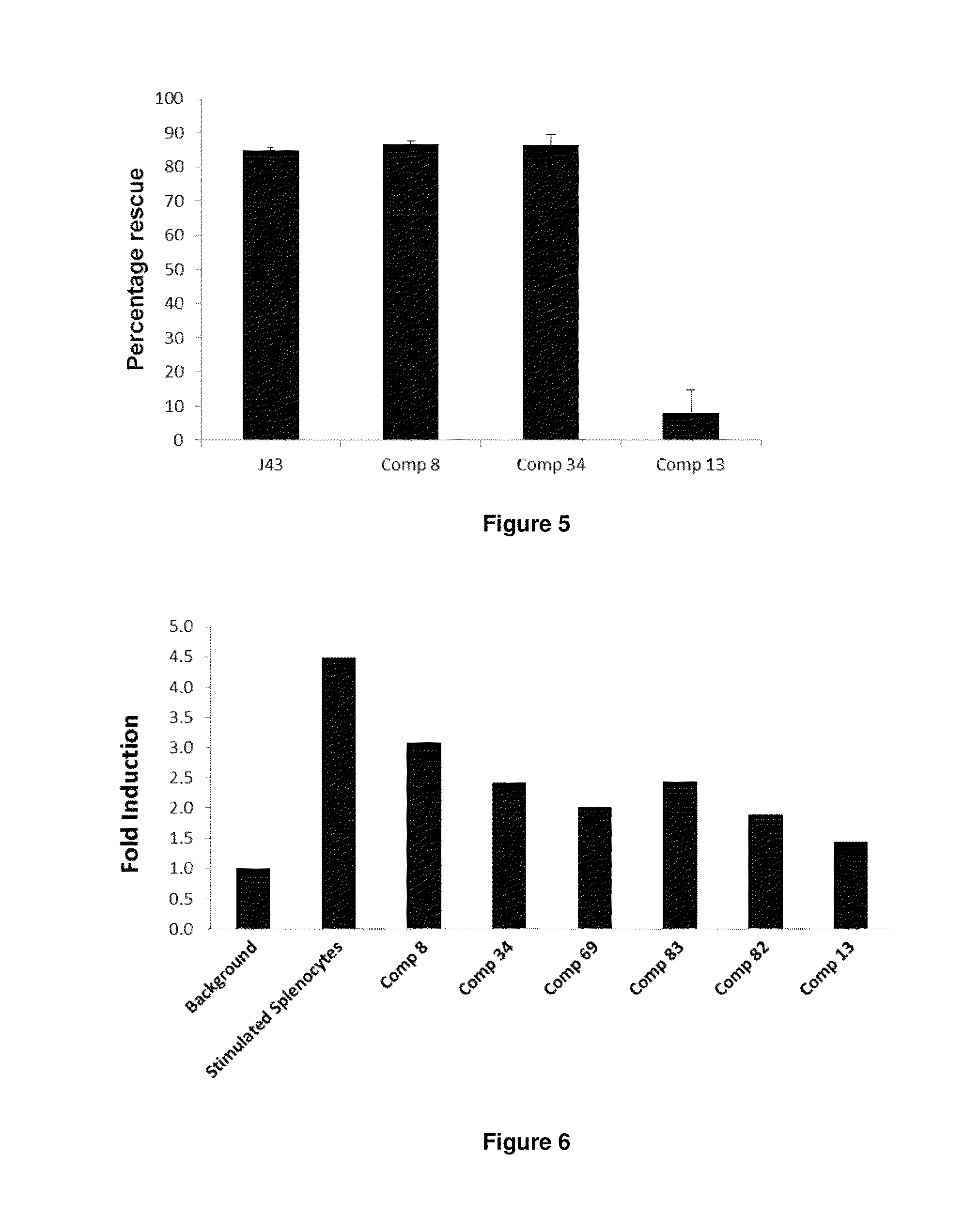
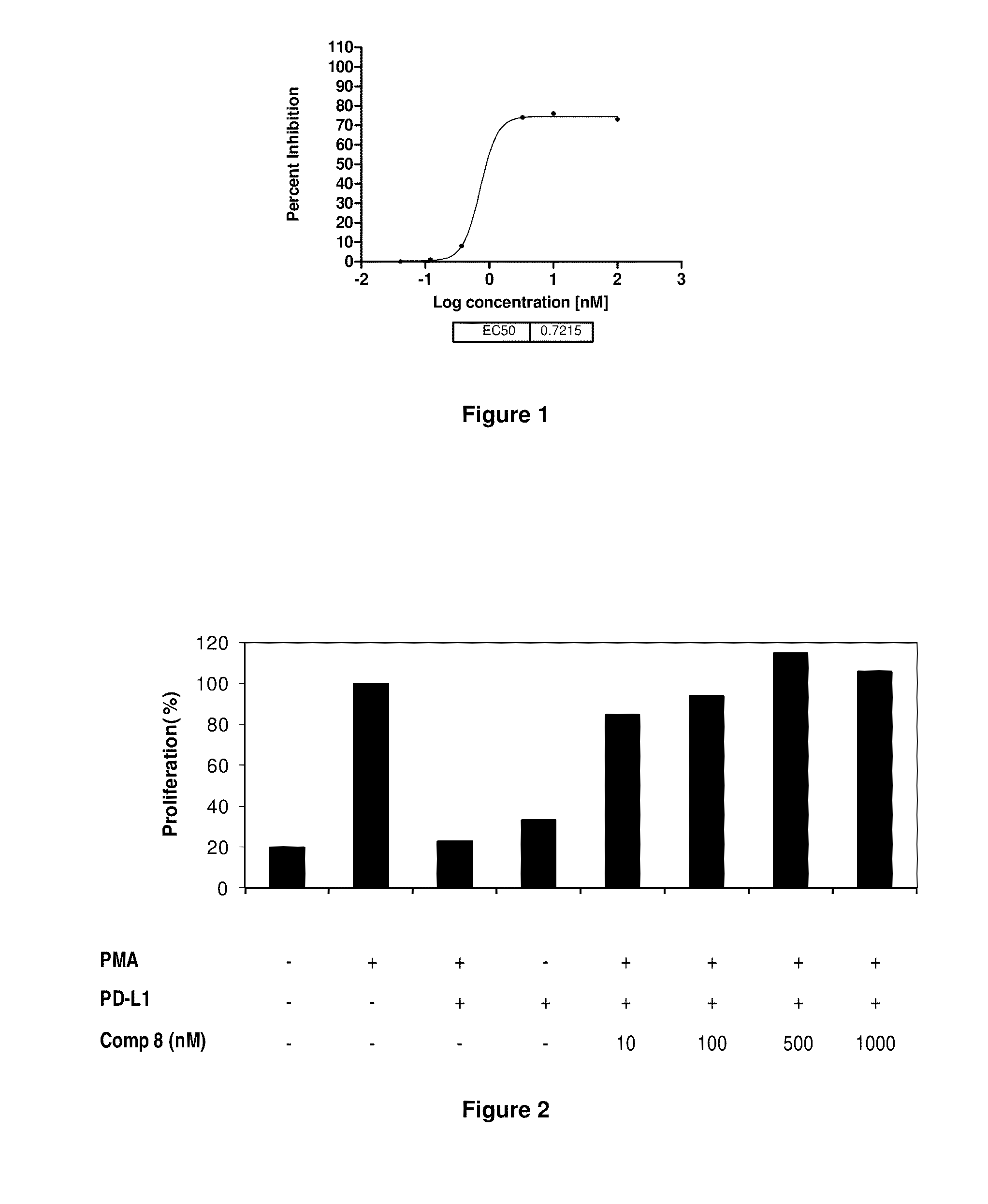
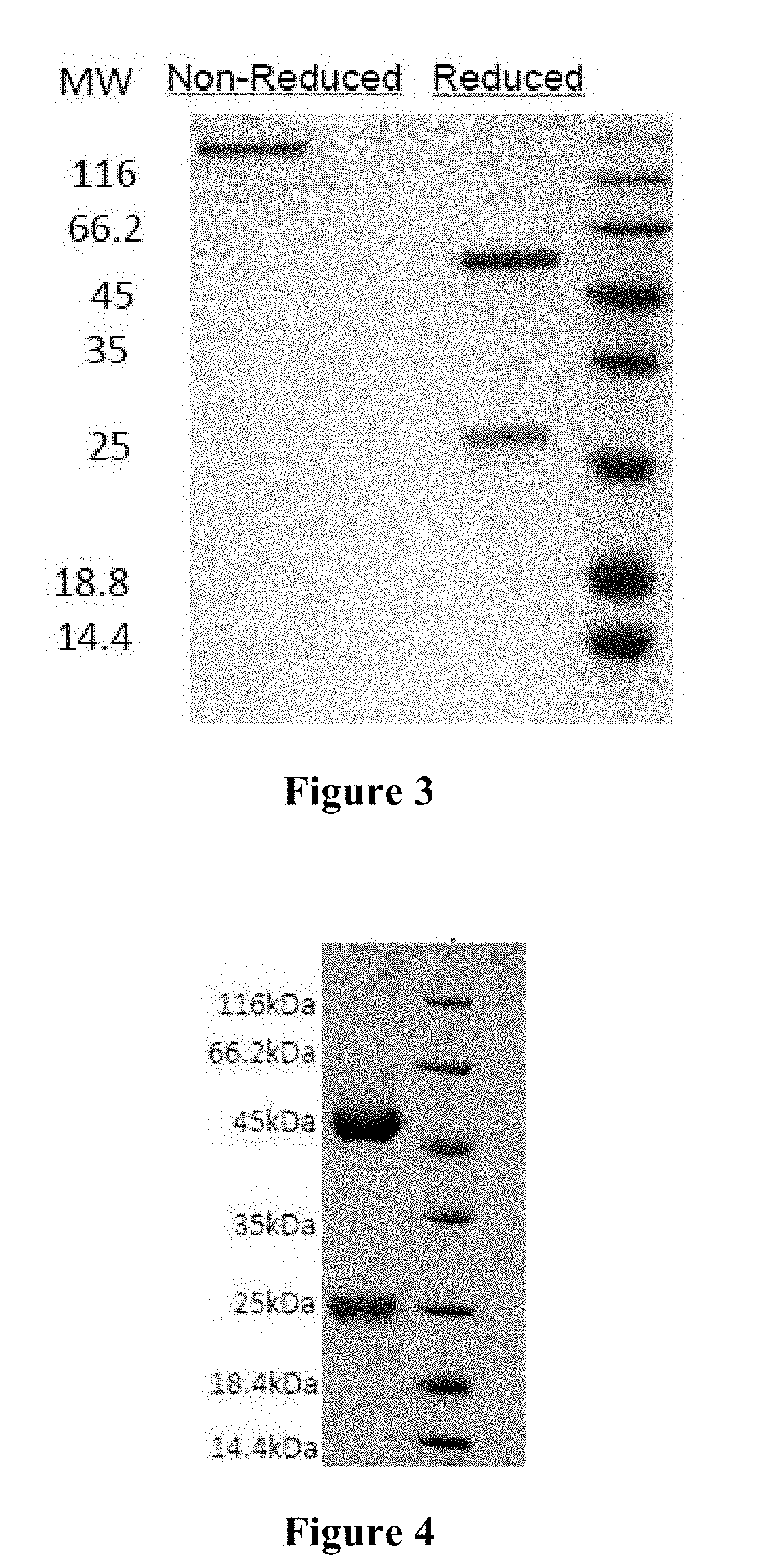
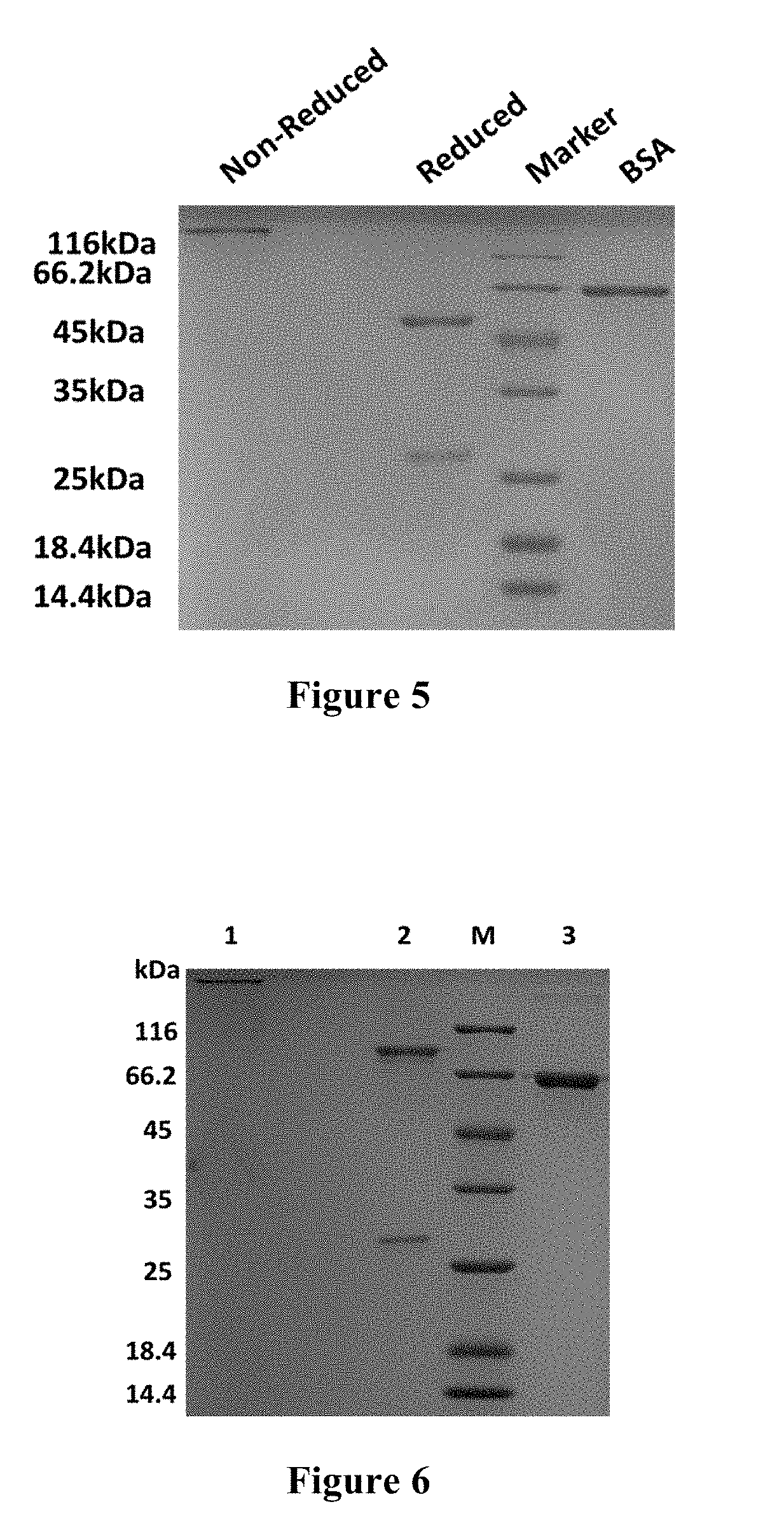
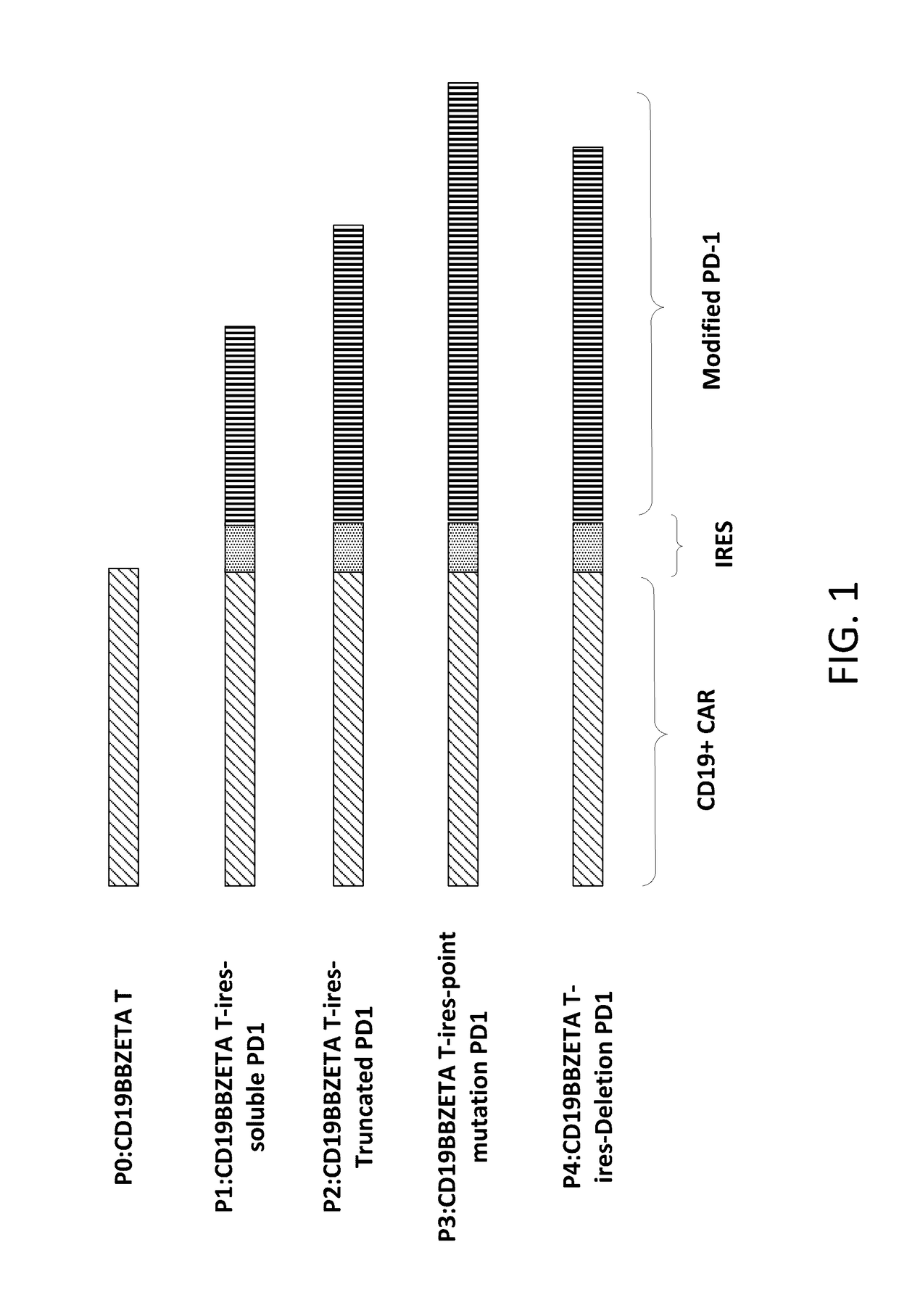
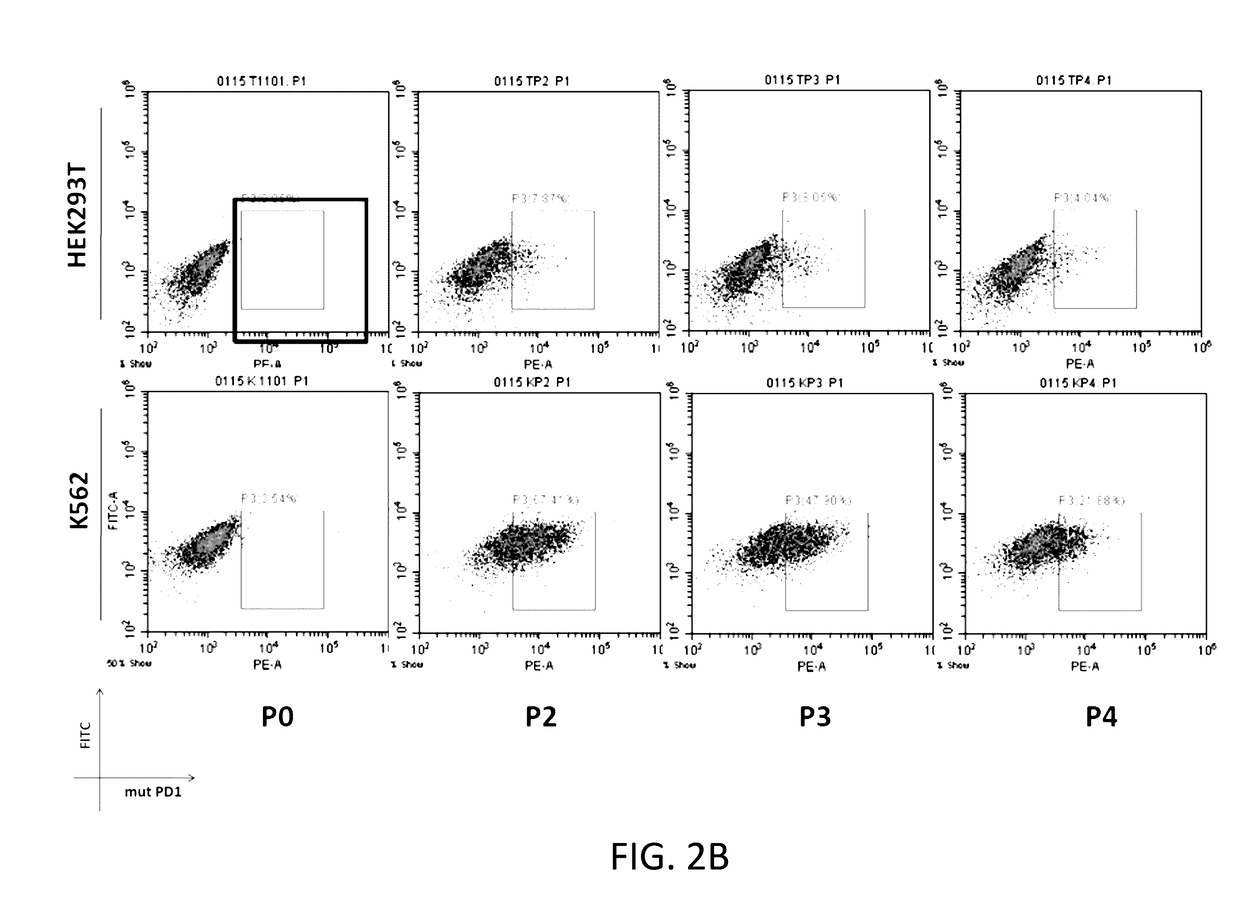
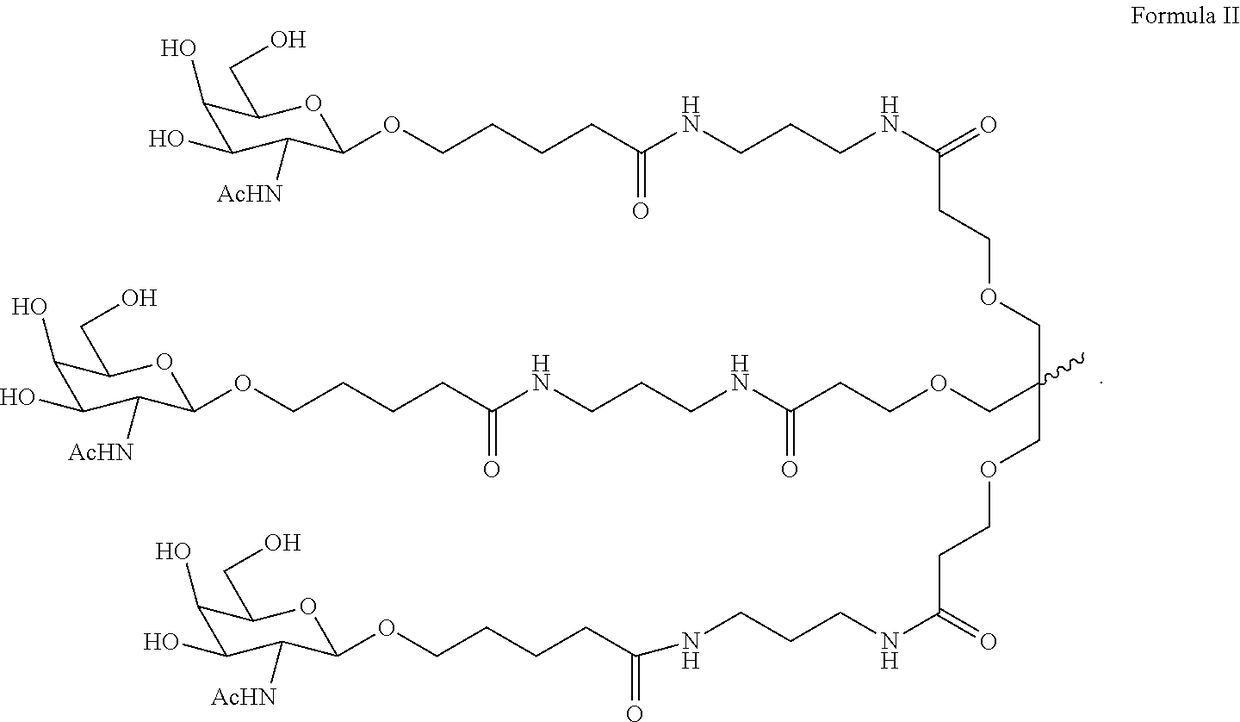
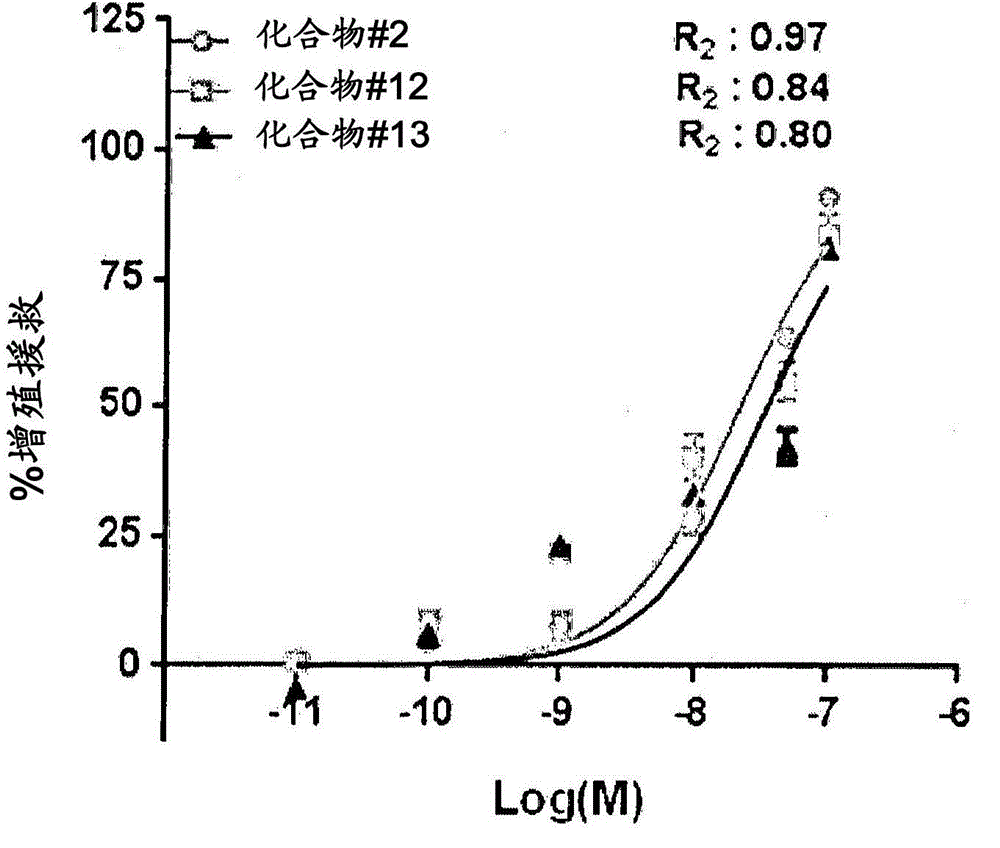
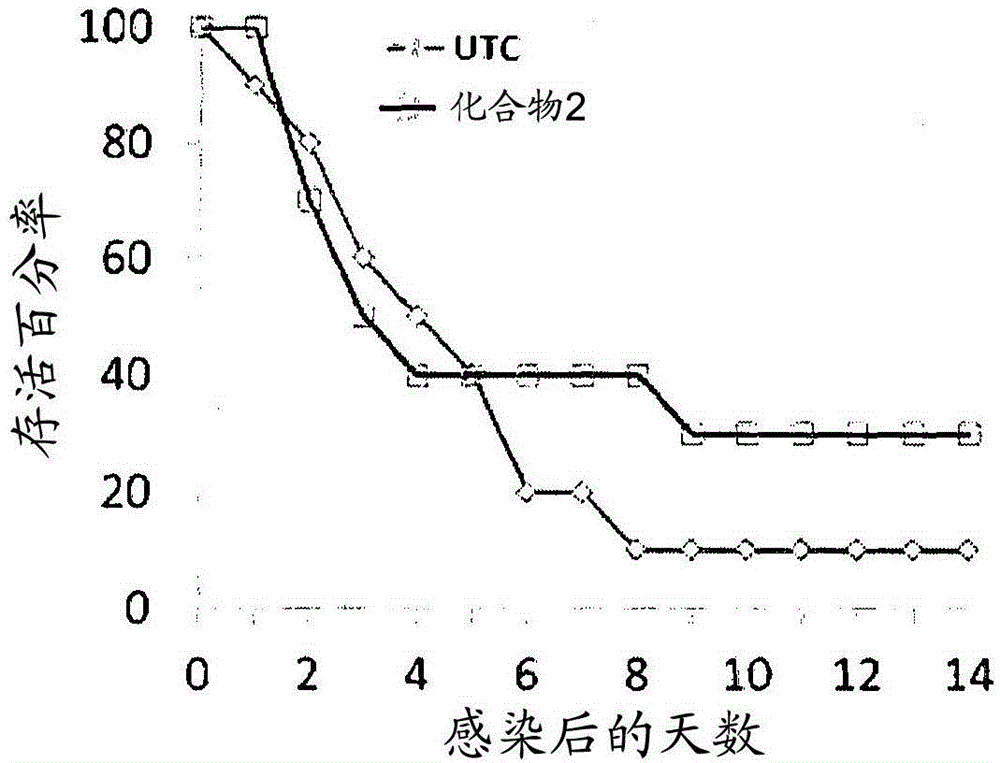
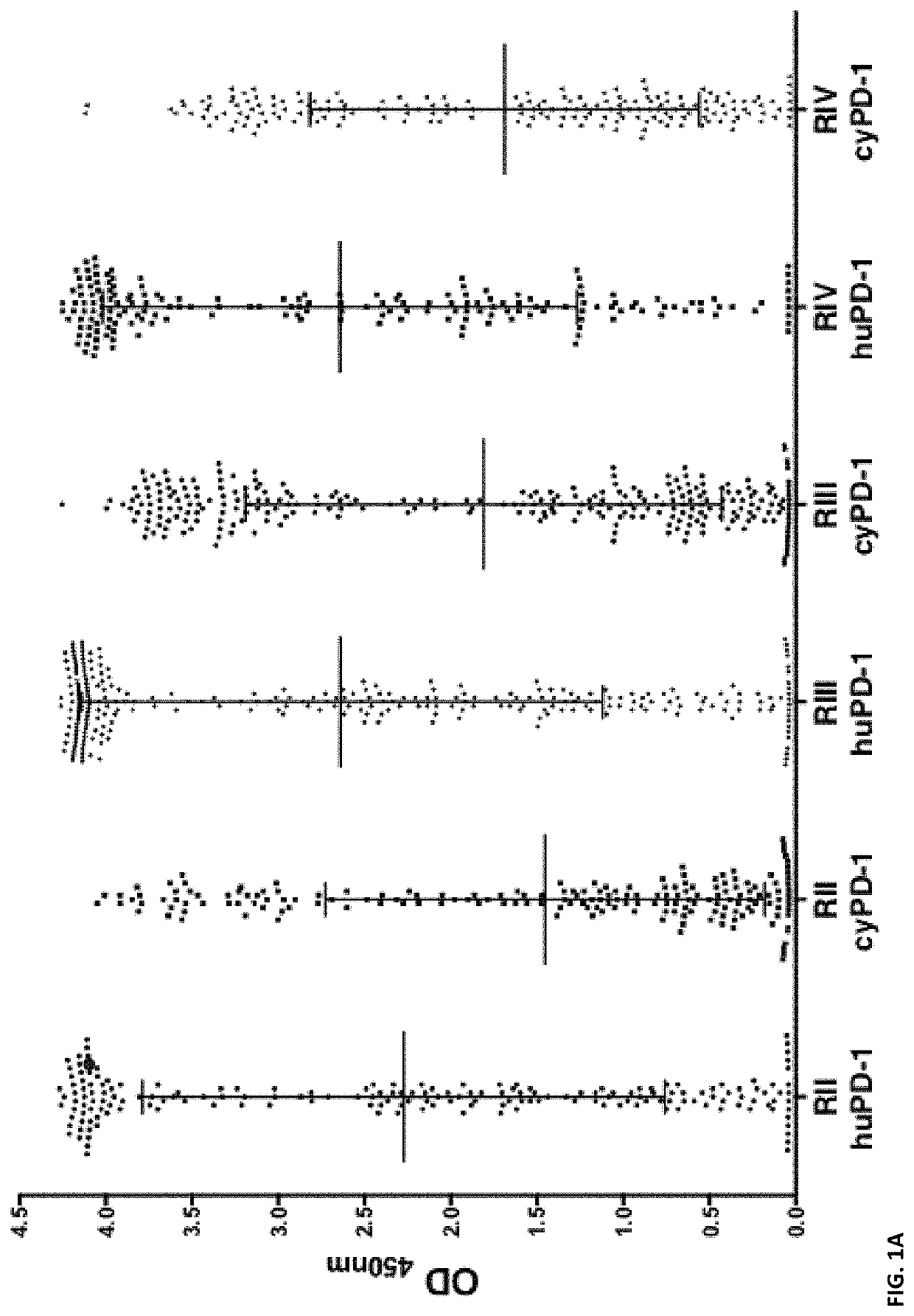
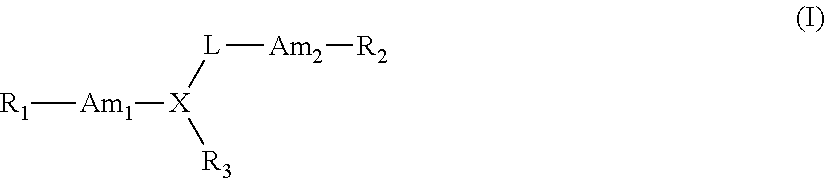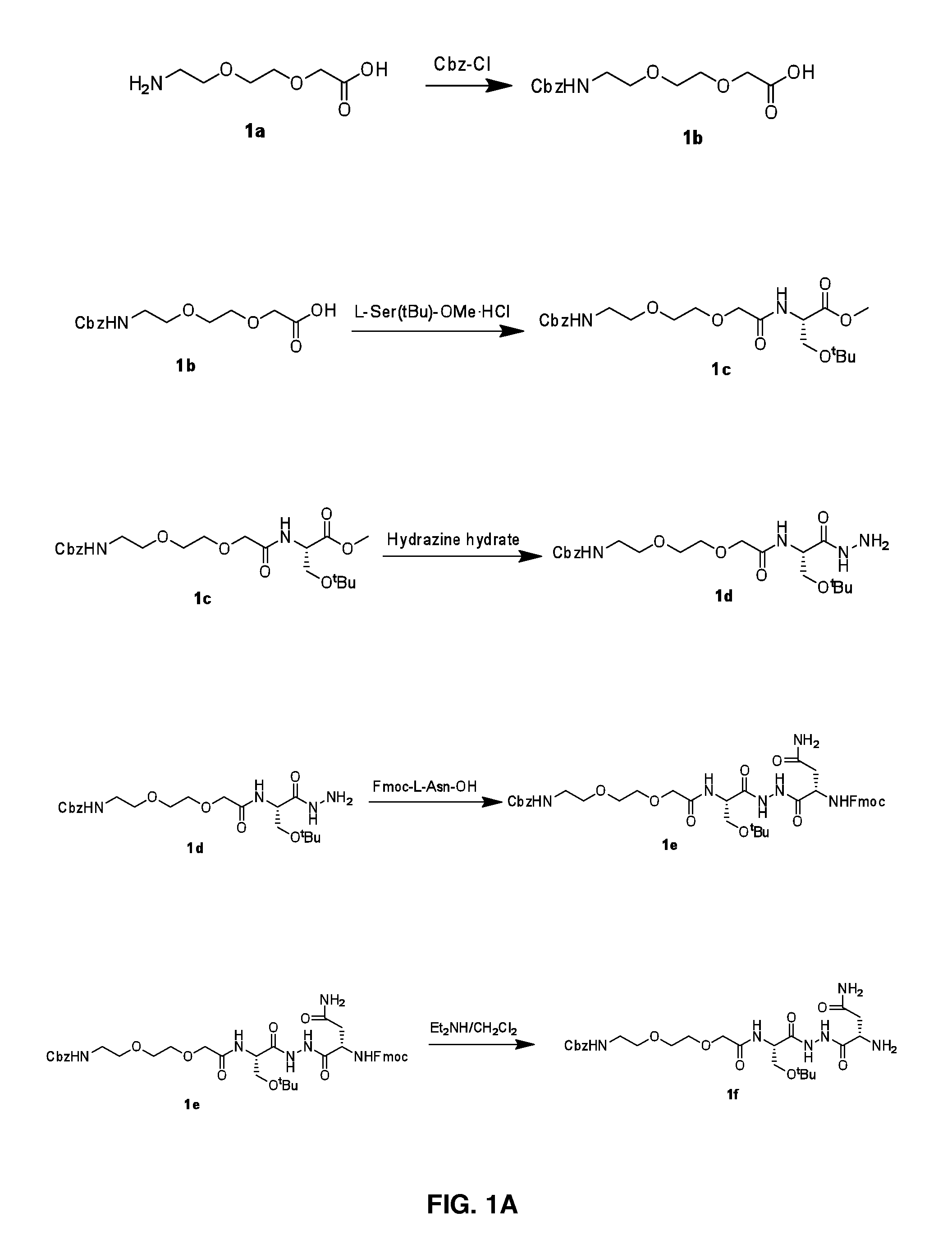Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
103 results about "Programmed cell death 1" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
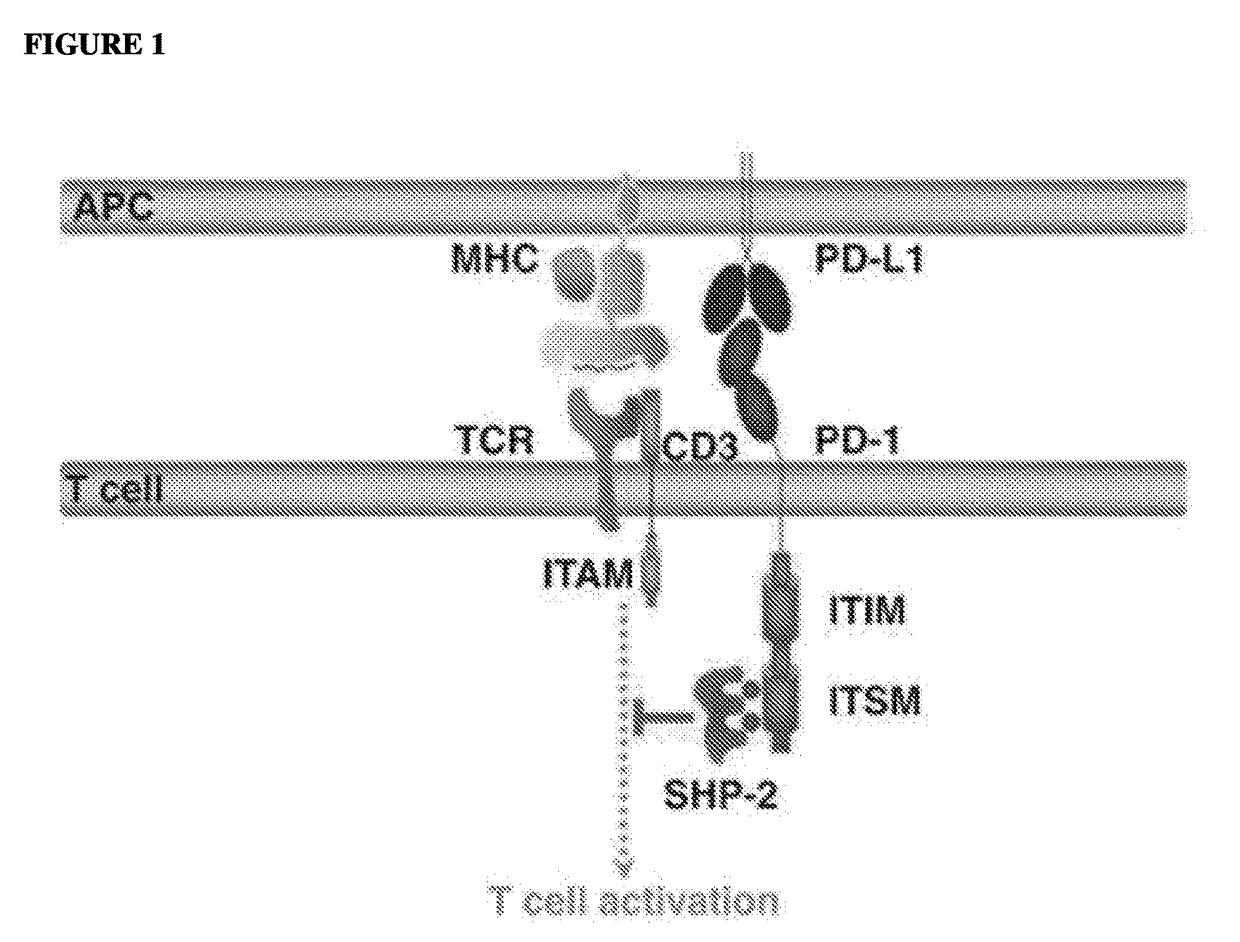
Programmed cell death protein 1, also known as PD-1 and CD279 (cluster of differentiation 279), is a protein on the surface of cells that has a role in regulating the immune system's response to the cells of the human body by down-regulating the immune system and promoting self-tolerance by suppressing T cell inflammatory activity. This prevents autoimmune diseases, but it can also prevent the immune system from killing cancer cells.
Immunosuppression modulating compounds
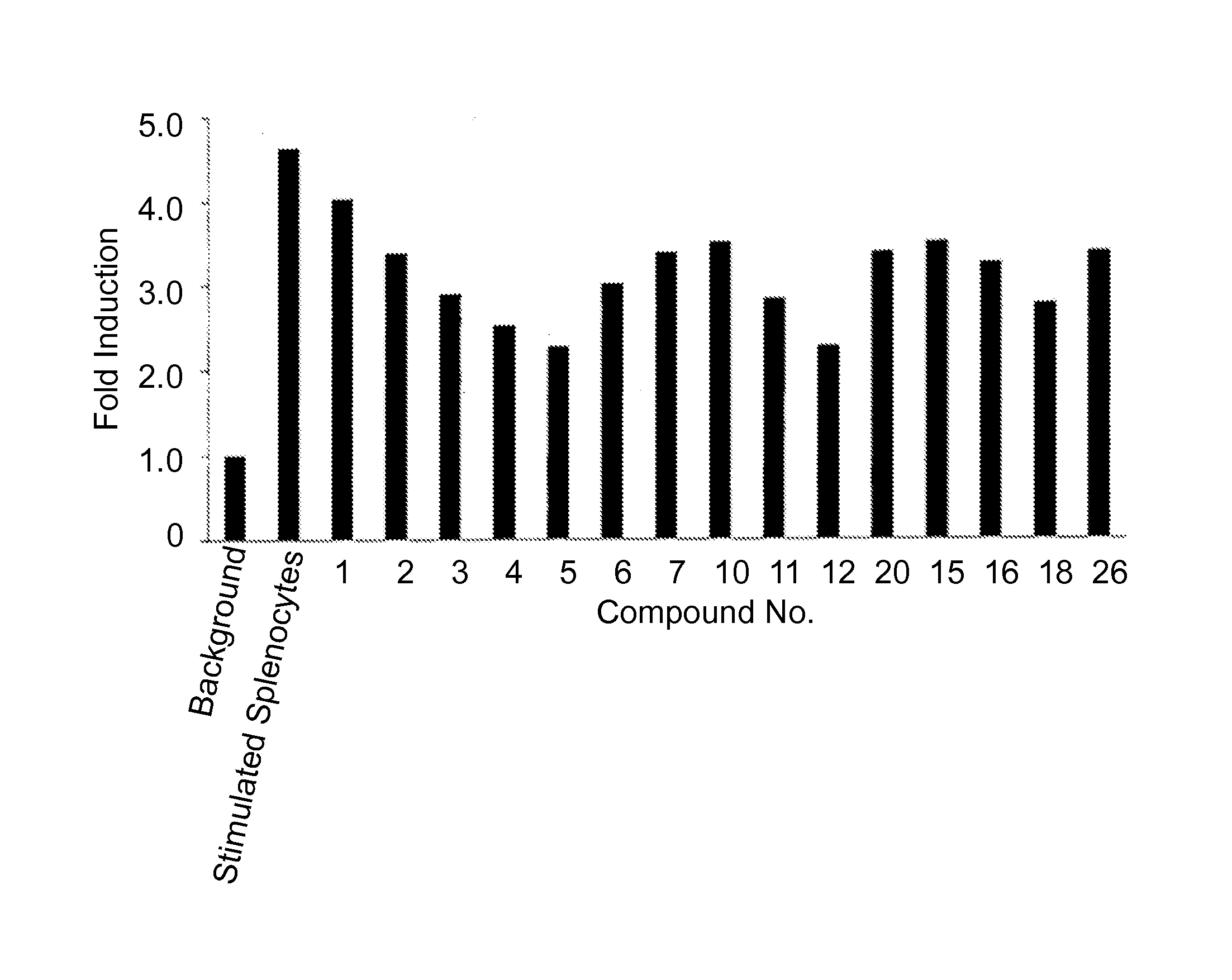
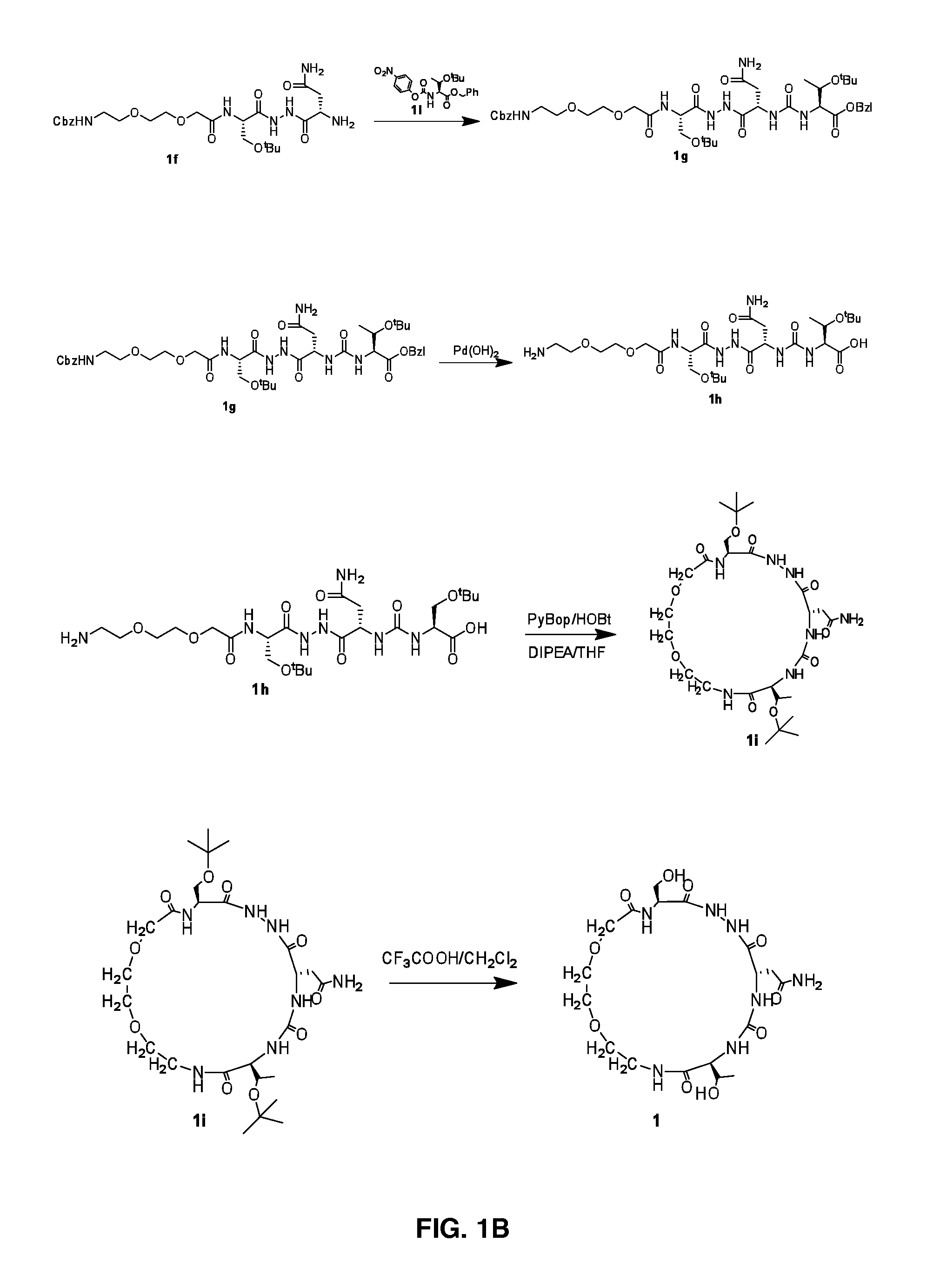
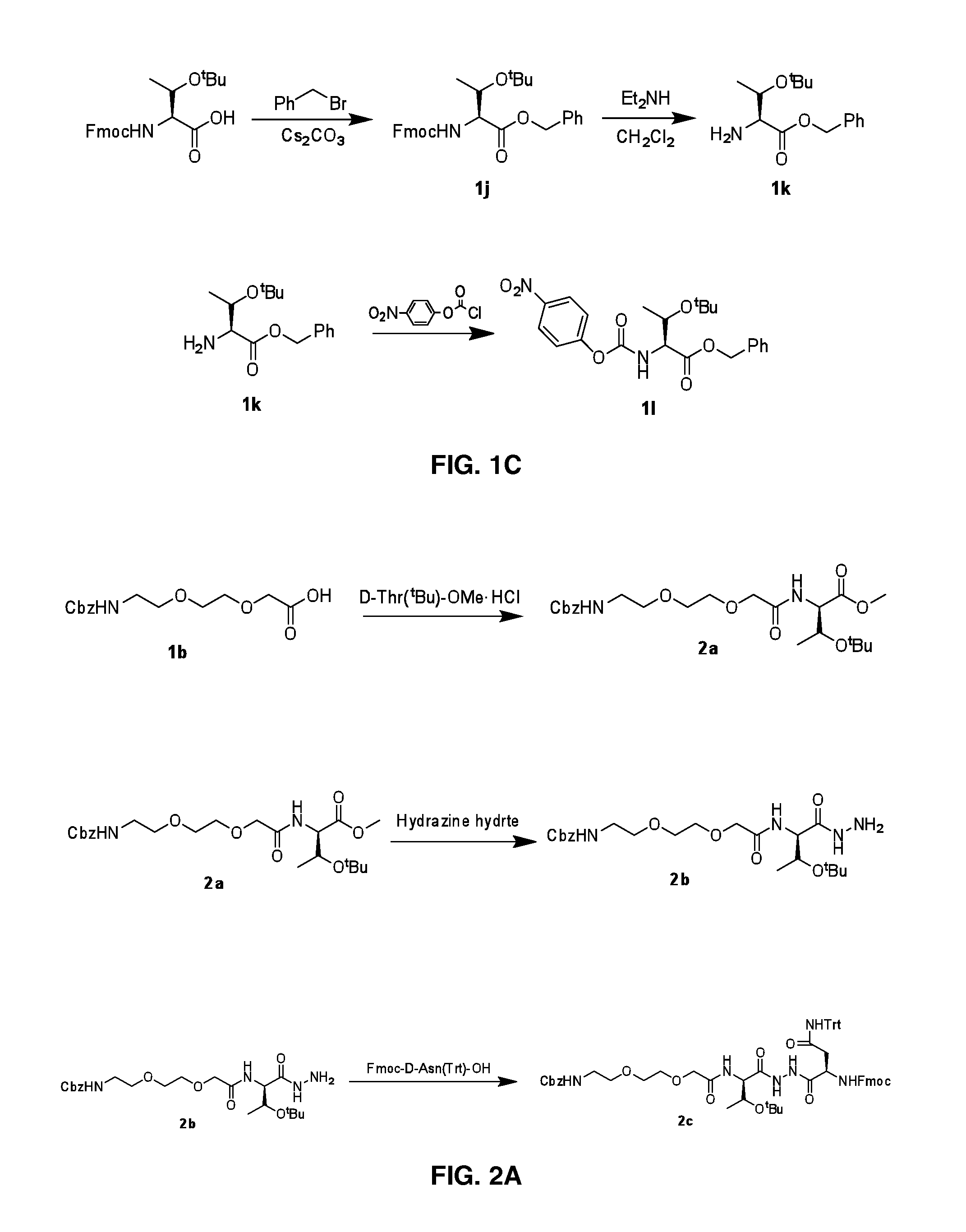
The present invention provides immunosuppression compounds capable of inhibiting the programmed cell death 1 (PD1) signalling pathway. The present invention further provides peptide based compositions for treatment of cancer or treatment of infections via immunopotentiation caused by inhibition of immunosuppressive signaling induced by PD-1, PD-L1, or PD-L2 and therapies using them, immunopotentiative substrates included as the active ingredient. Further, the invention provides an application of the compositions containing the peptide moieties for preventive and / or therapeutic agents for cancer, cancer metastasis, immunodeficiency, an infectious disease or the like and an application of peptide moieties as a testing or diagnostic agent or a research agent for such a disease.
Owner:AURIGENE DISCOVERY TECH
Immunosuppression modulating compounds
InactiveUS20110318373A1Reduce the binding forceAntibacterial agentsBiocideDiseaseSignalling pathways
The present invention provides immunosuppression compounds capable of inhibiting the programmed cell death 1 (PD1) signalling pathway. The present invention further provides peptide based compositions for treatment of cancer or treatment of infections via immunopotentiation caused by inhibition of immunosuppressive signaling induced by PD-1, PD-L1, or PD-L2 and therapies using them, immunopotentiative substrates included as the active ingredient. Further, the invention provides an application of the compositions containing the peptide moieties for preventive and / or therapeutic agents for cancer, cancer metastasis, immunodeficiency, an infectious disease or the like and an application of peptide moieties as a testing or diagnostic agent or a research agent for such a disease.
Owner:AURIGENE DISCOVERY TECH
Immunological reagents
ActiveUS9982053B2Immunoglobulins against virusesAntiviralsAutoimmune responsesHuman immunodeficiency
Owner:MABQUEST
Therapeutic compounds for immunomodulation
The present invention provides Immunosuppressive compounds capable of inhibiting the programmed cell death 1 (PD1) signalling pathway. The present invention further provides peptide based compositions for treatment of cancer or treatment of infections via immunopotentiation caused by inhibition of immunosuppressive signalling induced by PD-1, PD-L1, or PD-L2 and therapies using them, immunopotentiative substrates included as the active ingredient. Further, the invention provides pharmaceutical compositions comprising the Immunosuppressive peptide compounds or modified peptide moieties for preventive and / or therapeutic agents for cancer, cancer metastasis, immunodeficiency, an infectious disease or the like and an application of PD-1 or PD-L1 as a testing or diagnostic agent or a research agent for such a disease.
Owner:AURIGENE DISCOVERY TECH
Cyclic Peptidomimetic Compounds as Immunomodulators
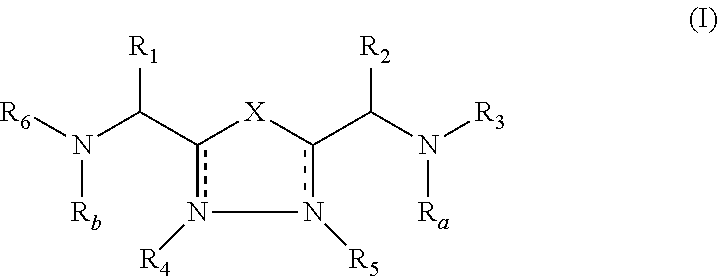
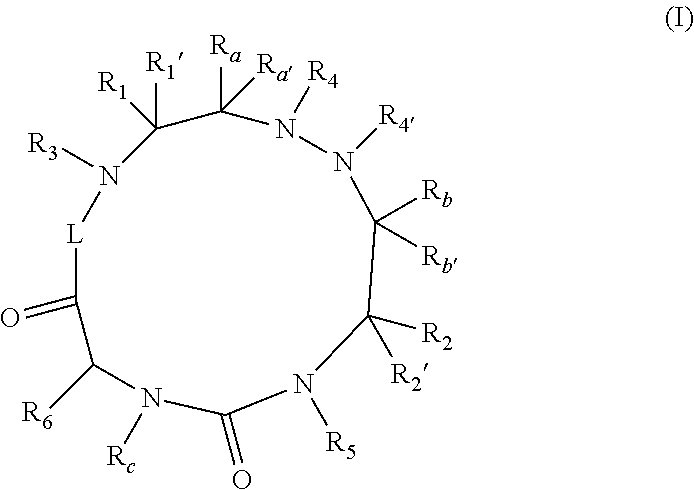
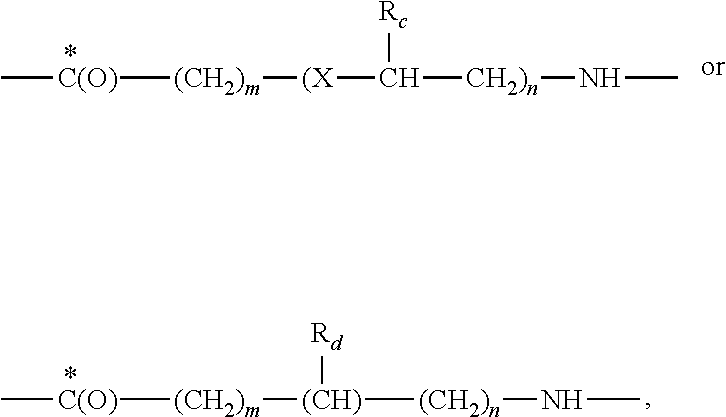
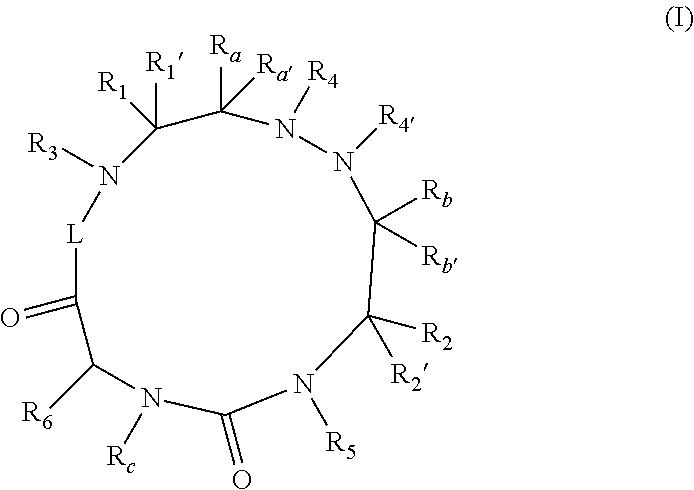
The present invention relates to cyclic peptidomimetic compounds as therapeutic agents capable of inhibiting the programmed cell death 1 (PD1) signalling pathway. The invention also relates to derivatives of the therapeutic agents. The invention also encompasses the use of the said therapeutic agents and derivatives for treatment of disorders via immunopotentiation comprising inhibition of immunosuppressive signal induced due to PD-1, PD-L1, or PD-L2 and therapies using them.
Owner:AURIGENE DISCOVERY TECH
1,2,4-oxadiazole and thiadiazole compounds as immunomodulators
ActiveUS20180044303A1Suppress and inhibit programmed cell death (PD1) signaling pathwaySuppress and/or inhibit the programmed cell death 1 (PD1) signaling pathwayAntibacterial agentsOrganic active ingredientsPD-L1Thiadiazoles
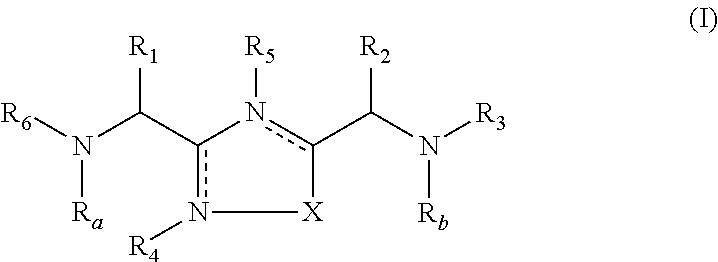
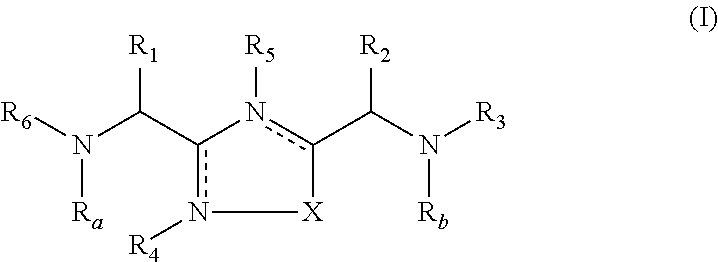
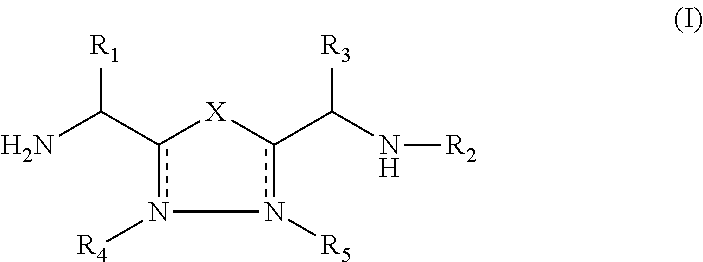
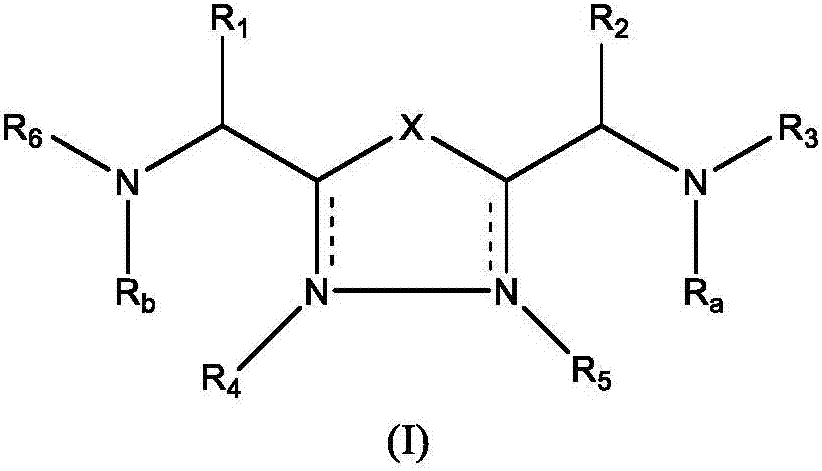
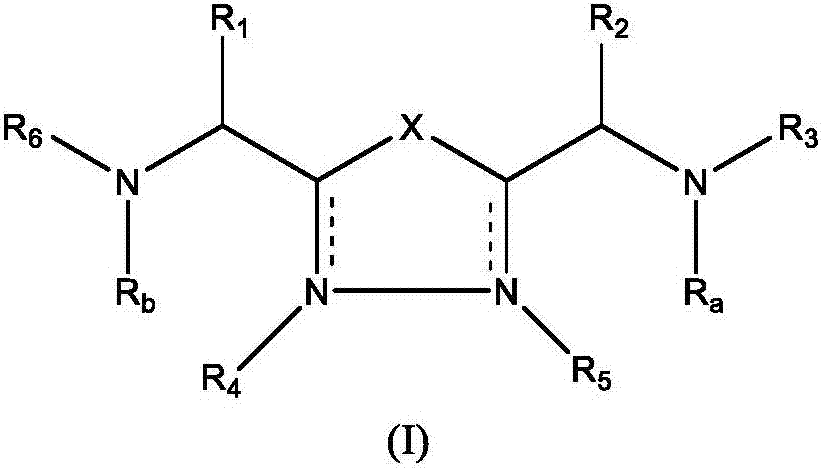
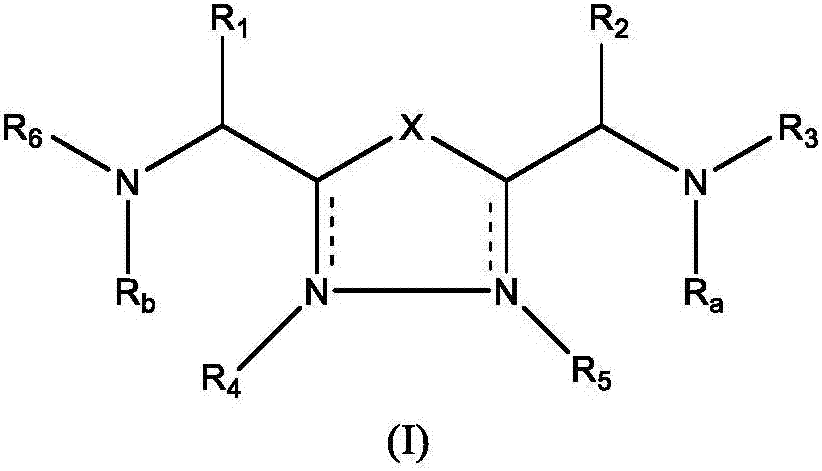
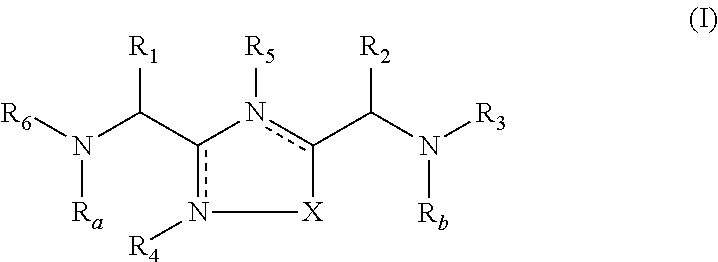
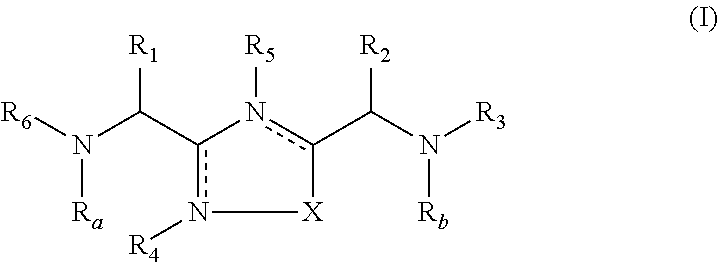
The present invention relates to 1,2,4-oxadiazole compounds of formula (I) and their use to inhibit the programmed cell death 1 (PD-1) signaling pathway and / or for treatment of disorders by inhibiting an immunosuppressive signal induced by PD-1, PD-L1 or PD-L2.
Owner:AURIGENE ONCOLOGY LTD
Anti-PD-1 (programmed cell death-1) humanized antibody
ActiveCN104479020AHigh expressionHigh affinityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsHeavy chainHumanized antibody
The invention relates to the technical field of biology, and in particular relates to an anti-PD-1 humanized antibody. The anti-PD-1 humanized antibody is characterized in that the amino acid sequence of a variable region of a heavy chain of the antibody is shown as SEQ ID NO: 2 or is a conserved variation sequence; the amino acid sequence of a variable region of a light chain of the antibody is shown as SEQ ID NO: 4 or is a conserved variation sequence. The anti-PD-1 humanized antibody shows relatively high expression quantity in mammalian cells, and is obvious in affinity with PD-1 and able to inhibit the combination of a ligand and a receptor.
Owner:SHANGHAI HENLIUS BIOTECH INC
1,3,4-oxadiazole and thiadiazole compounds as immunomodulators
InactiveUS20180044304A1Suppress and inhibit programmed cell death (PD-1) signaling pathwaySuppress and/or inhibit the programmed cell death 1 (PD-1) signaling pathwayOrganic active ingredientsOrganic chemistryPD-L1Thiadiazoles
The present invention relates to 1,3,4-oxadiazole and thiadiazole compounds of formula (I) and their use to inhibit the programmed cell death 1 (PD-1) signaling pathway and / or for treatment of disorders by inhibiting an immunosuppressive signal induced by PD-1, PD-L1 or PD-L2.
Owner:AURIGENE DISCOVERY TECH
Therapeutic cyclic compounds as immunomodulators
InactiveUS20180044350A1Suppress and inhibit programmed cell death (PD-1) signaling pathwaySuppress and/or inhibit the programmed cell death 1 (PD-1) signaling pathwayAntibacterial agentsAntimycoticsSignalling pathwaysPD-L1
The present invention relates to cyclic compounds of formula (I) and their use to inhibit the programmed cell death 1 (PD-1) signaling pathway and / or for treatment of disorders by inhibiting an immunosuppressive signal induced by PD-1, PD-L1 or PD-L2.
Owner:AURIGENE DISCOVERY TECH
Anti-PD-1 (programmed cell death 1) monoclonal antibody as well as pharmaceutical composition and application thereof
InactiveCN106632674ABinding blockRelief of immunosuppressionRadioactive preparation carriersImmunoglobulins against cell receptors/antigens/surface-determinantsMolecular ImmunologyHeavy chain
The invention belongs to the fields of oncotherapy and molecular immunology, and relates to an anti-PD-1 (programmed cell death 1) monoclonal antibody as well as pharmaceutical composition and an application thereof, in particular to a monoclonal antibody or an antigen binding fragment thereof. The heavy chain variable region of the monoclonal antibody contains CDR with amino acid sequences represented as SEQ ID NO: 13-15; and / or the light chain variable region of the monoclonal antibody contains CDR with amino acid sequences represented as SEQ ID NO: 16-18. The monoclonal antibody can be specifically bound with PD-1 very well, specifically remove immunosuppression of PD-1 on an organism and activate T lymphocytes.
Owner:ZEDA BIOPHARMACEUTICALS INC
Tumor immunization method combining with chimeric antigen T cells targeting at PD-1 (programmed cell death protein 1) and EGFR (epidermal growth factor receptor)
InactiveCN107034235AIncrease lethalityImprove homingGenetically modified cellsMammal material medical ingredientsTumor targetEGFR Antibody
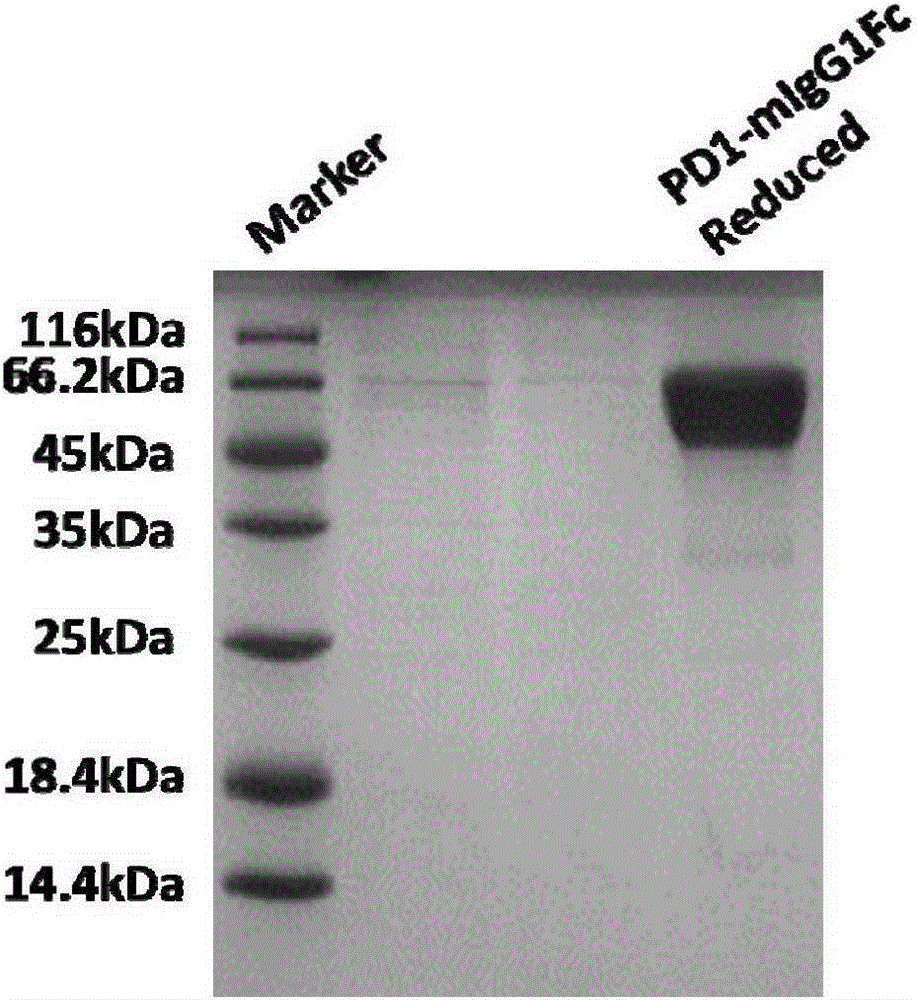
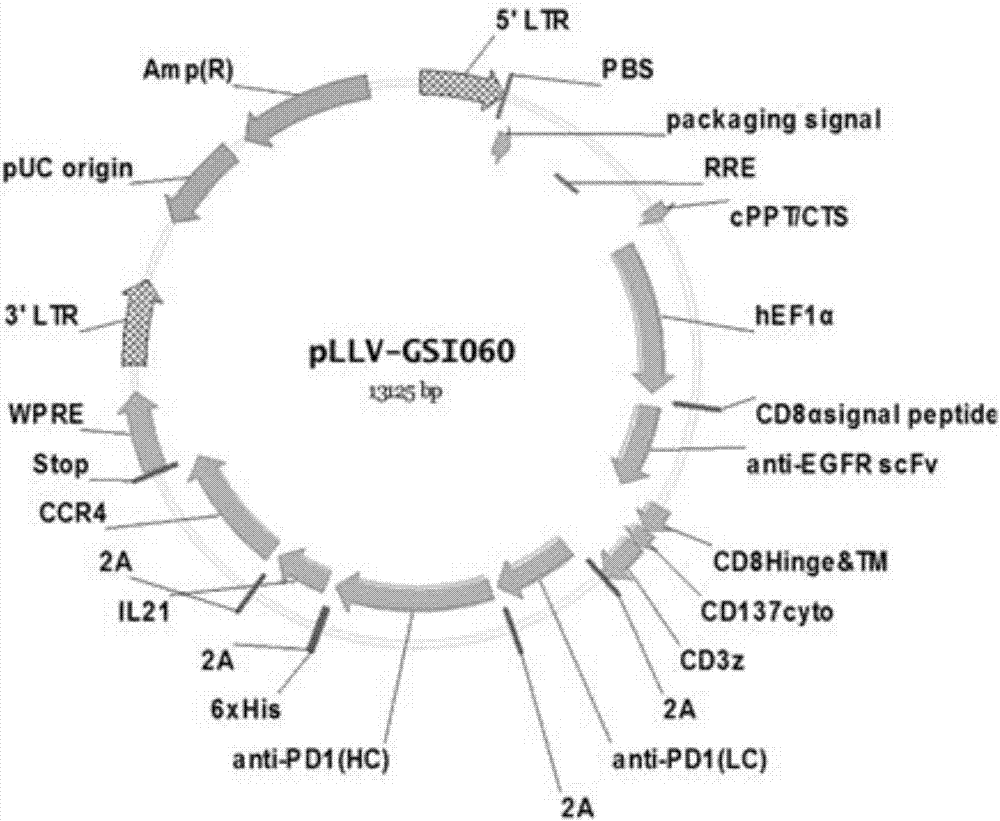
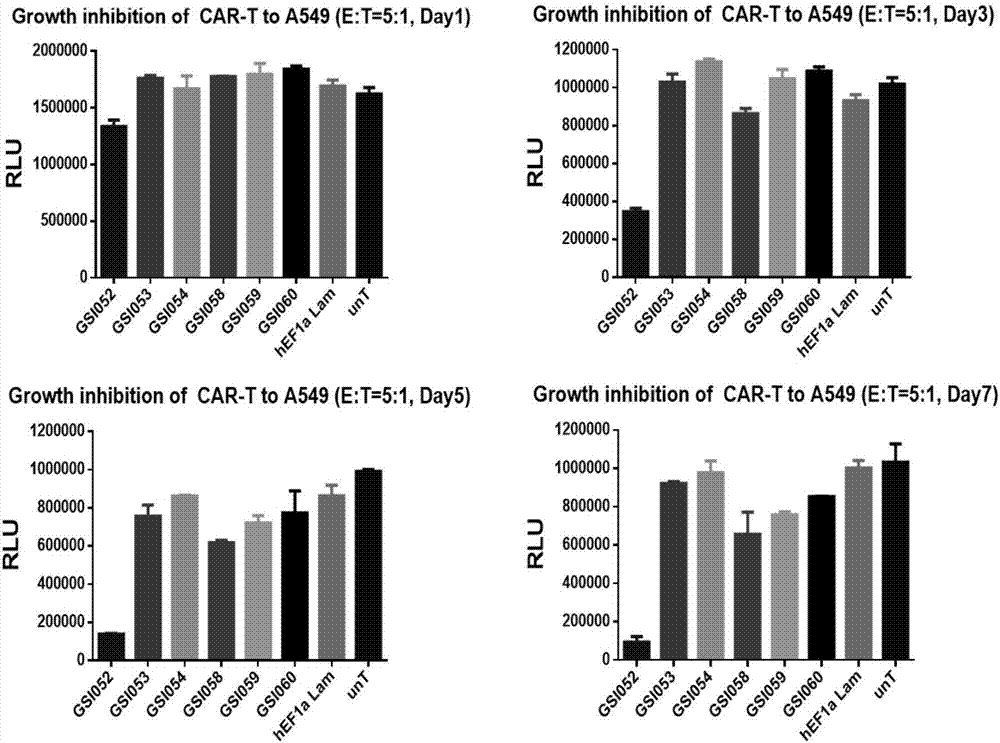
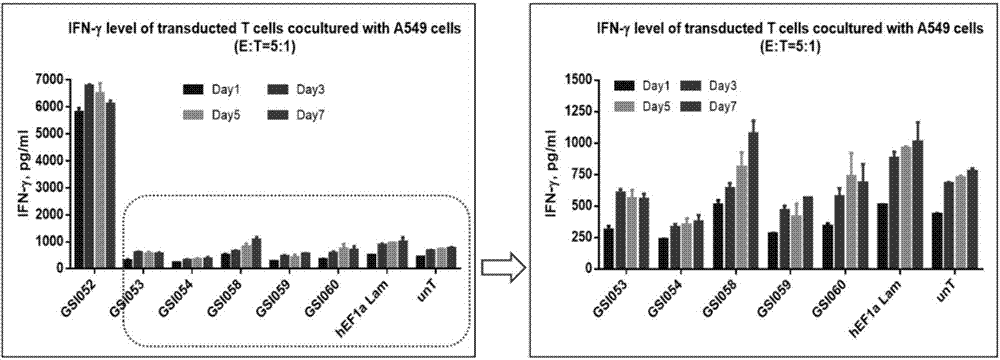
The invention discloses a tumor immunization method combining with chimeric antigen T cells targeting at PD-1 (programmed cell death protein 1) and EGFR (epidermal growth factor receptor) and also discloses a plasmid vector for implementing the method. In combination with fourth-generation CAR-T (chimeric antigen receptor T) cells targeting at PD-1 and EGFR, a lentiviral vector is used as a CAR-T vector basic structure, and truncated EGFR antibody is selected as a CAR core to give tumor targeted enrichment to play, tumor-killing effect is given to play in conformity with overexpressed immune checkpoint inhibitor PD-1 monoclonal antibody; by constructing the fourth-generation CAR-T vector for co-expressing various regulatory factors such as IL21, CCR4 and Bcl2, the killing, homing and persistent proliferating abilities of CAR-T cells are improved. EP-CAR T (esophageal papilloma chimeric antigen receptor T) cells are treated by transducing patient's autologous T-lymphocytes in vitro, amplifying suitably and transducing back to the patient's body via autologous transfusion, and no reports on similar designs of CAR-T cells are provided at present.
Owner:尹荣
Methods and compositions for the treatment of persistent infections and cancer by inhibiting the programmed cell death 1 (pd-1) pathway
ActiveUS20140178370A1Reduced activityReduce expressionAntibacterial agentsOrganic active ingredientsProgrammed cell death 1Chronic infection
The present invention provides methods and compositions for the treatment, prevention, or reduction of persistent infections, such as chronic infections, latent infections, and slow infections and cancer. The methods and compositions of the invention are also useful for the alleviation of one or more symptoms associated with such infections and cancer.
Owner:EMORY UNIVERSITY +3
Immunosuppression modulating compounds
InactiveCN103096915AReduce the binding forceAntibacterial agentsSenses disorderCancer metastasisImmunodeficiency
The present invention provides immunosuppression compounds capable of inhibiting the programmed cell death 1 (PDl) signalling pathway. The present invention further provides peptide based compositions for treatment of cancer or treatment of infections via immunopotentiation caused by inhibition of immunosuppressive signaling induced by PD-1, PD-L1, or PD-L2 and therapies using them, immunopotentiative substrates included as the active ingredient. Further, the invention provides an application of the compositions containing the peptide moieties for preventive and / or therapeutic agents for cancer, cancer metastasis, immunodeficiency, an infectious disease or the like and an application of peptide moieties as a testing or diagnostic agent or a research agent for such a disease.
Owner:AURIGENE DISCOVERY TECH
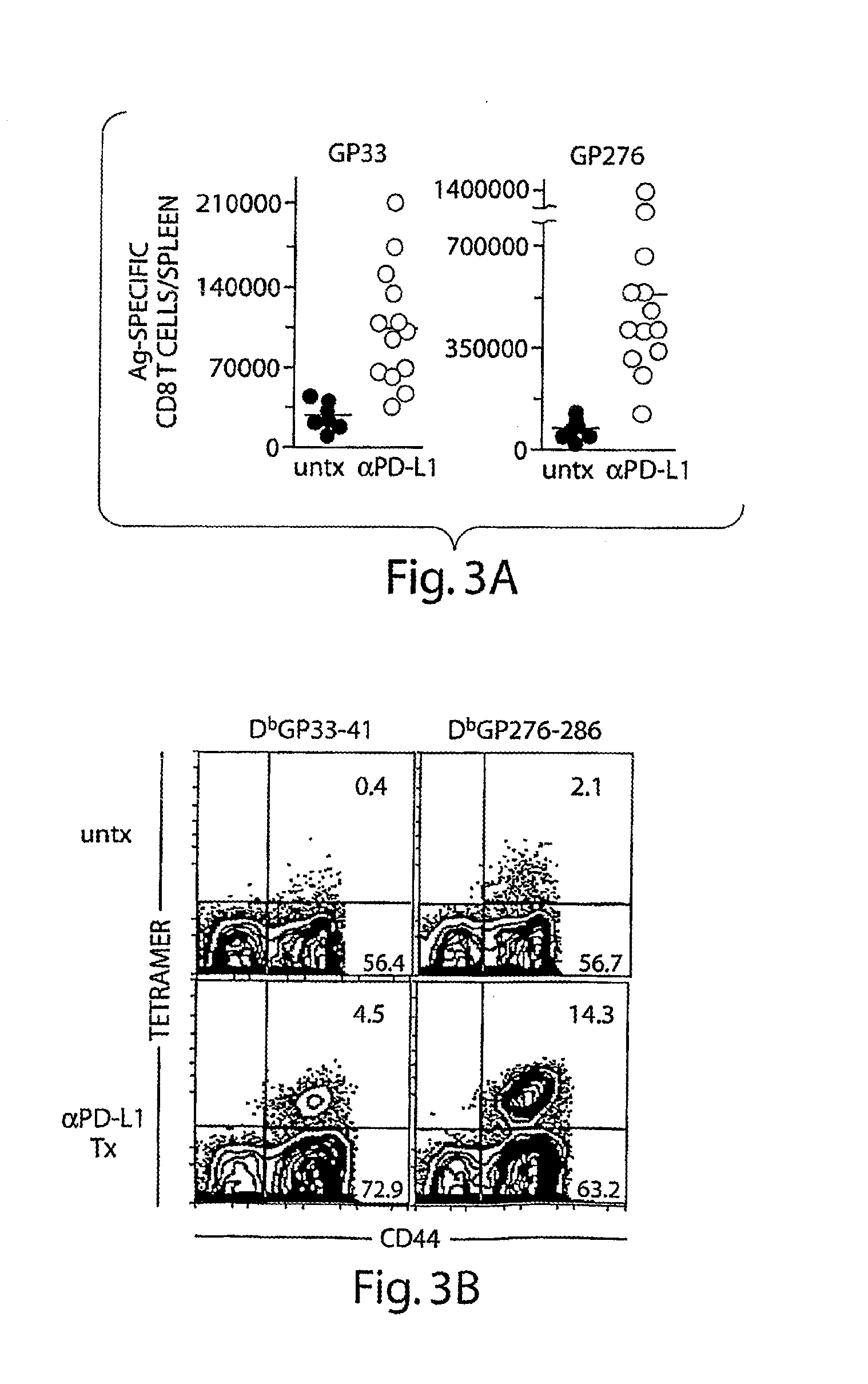
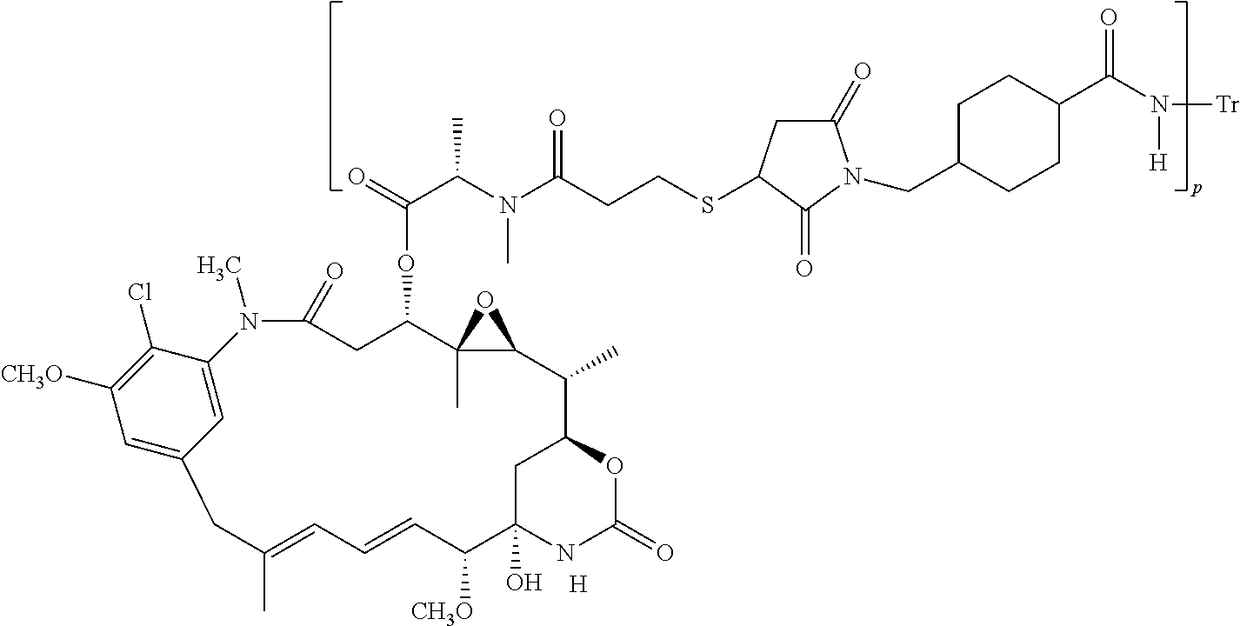
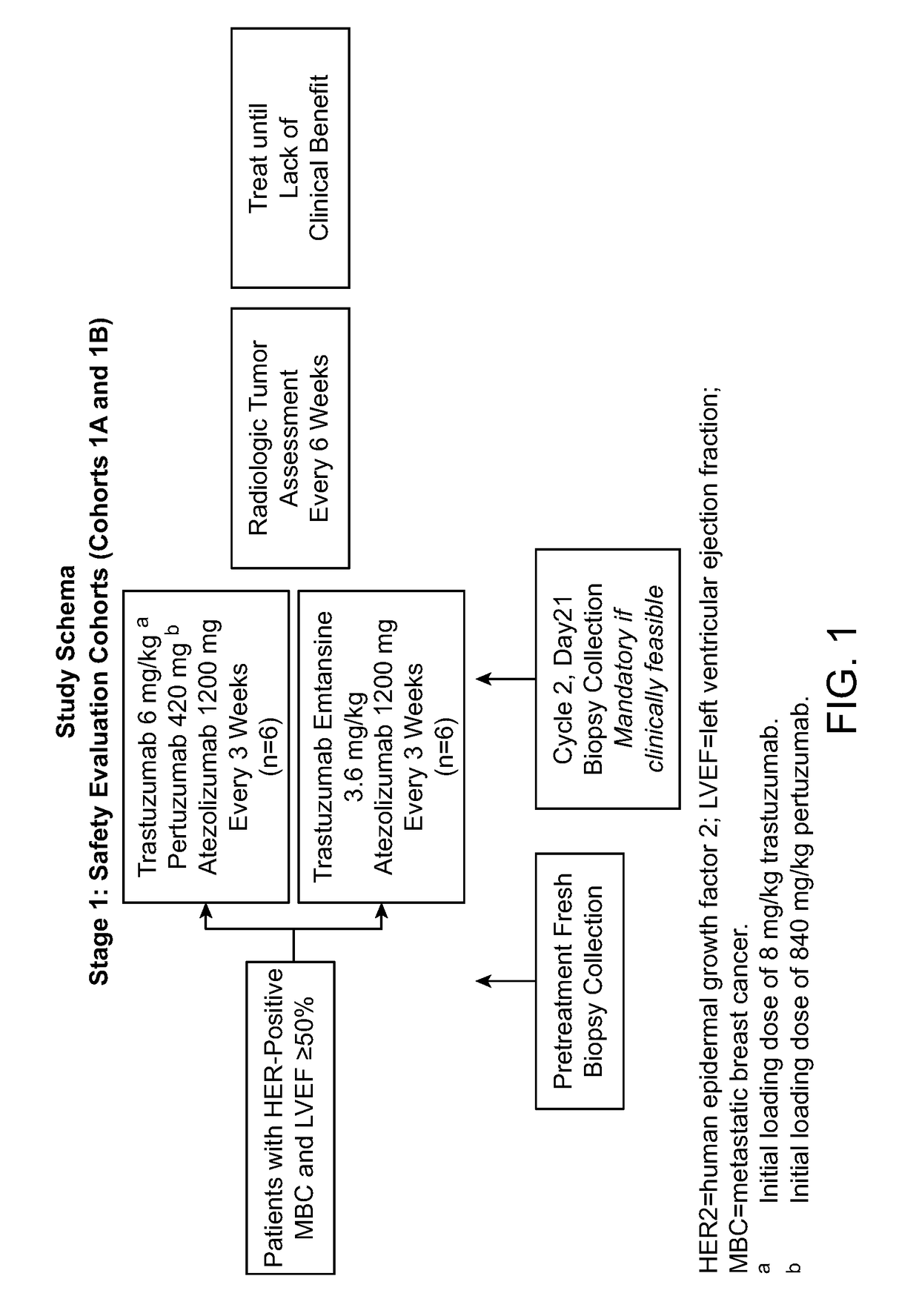
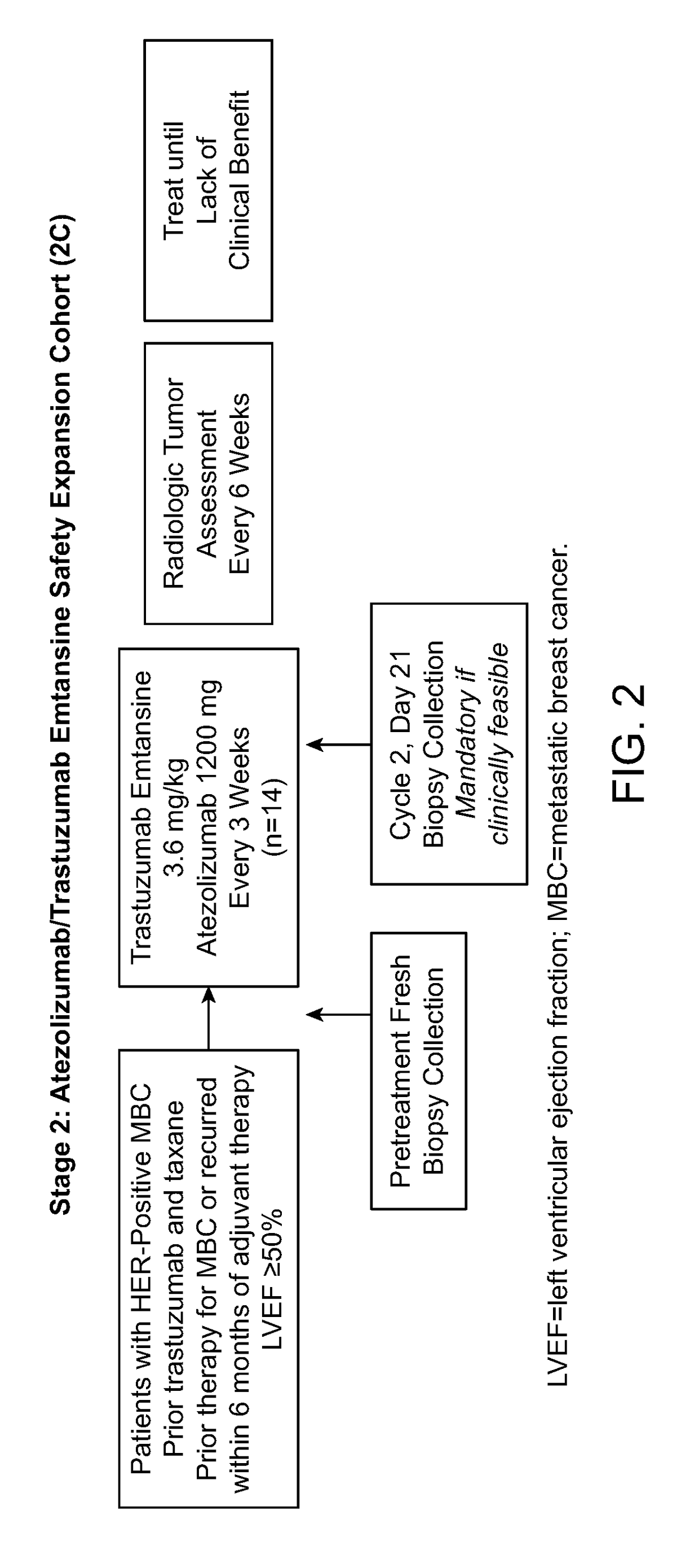
Methods of treating her2-positive cancer
InactiveUS20180251557A1The dosage is easy to controlEnable patient complianceOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsHER2 Positive Breast CancerClinical settings
Methods of treating patients having HER2-positive cancer are provided. Certain methods involve treatment of HER2 positive breast cancer using a programmed cell death protein 1 (PD-1) binding antagonist or a programmed death ligand 1 (PD-L1) binding antagonist in combination with trastuzumab and pertuzumab or with trastuzumab emtansine. The treatment regimen may be used in various clinical settings, for example, for treatment in the neoadjuvant or metastatic setting.
Owner:GENENTECH INC
1,3,4-Oxadiazole and 1,3,4-Thiadiazole Derivatives as Immunomodulators
InactiveUS20160194295A1Suppressing and inhibiting programmed cell death (PD1) signaling pathwaySuppressing and/or inhibiting the programmed cell death 1 (PD1) signaling pathwayBiocideOrganic chemistryImmunomodulating AgentPD-L1
The present invention relates to 1,3,4-oxadiazole and 1,3,4-thiadiazole compounds as therapeutic agents capable of inhibiting the programmed cell death 1 (PD1) signalling pathway. The invention also refers to derivatives of the therapeutic agents. The invention also encompasses the use of the said therapeutic agents and derivatives for treatment of disorders via immunopotentiation comprising inhibition of immunosuppressive signal induced due to PD-1, PD-L1, or PD-L2 and therapies using them.
Owner:AURIGENE DISCOVERY TECH
Anti-PD1 antibodies, activatable anti-PD1 antibodies, and methods of use thereof
ActiveUS10513558B2Inhibit progressReduce capacityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsProgrammed cell death 1Virology
The invention relates generally to antibodies that specifically bind programmed cell death protein 1 (PD-1), activatable antibodies that specifically bind to PD-1 and methods of making and using these anti-PD-1 antibodies and anti-PD-1 activatable antibodies in a variety of therapeutic, diagnostic and prophylactic indications.
Owner:CYTOMX THERAPEUTICS
Immunological Reagents
ActiveUS20170166642A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAutoimmune responsesFunctional activity
This disclosure relates to binding agents with specificity for programmed cell death 1 (PD-1) and to methods for using the same to treat, prevent and / or ameliorate an infectious disease (e.g., human immunodeficiency virus (HIV)), cancer and / or autoimmunity. In addition, this disclosure identifies a novel binding patch (“P2”) on PD-1 that is linked with a previously unidentified functional activity of PD-1 that is distinct from the interaction site involved with either the PD-L1 or PD-L2 ligands. Furthermore, we demonstrate that antibodies that interact with this region of PD-1 are able to act as antagonists of PD-1 and that this antagonism is further enhanced with the addition of antibodies that act through the blockade of the PD-1 / PD-L1 / L2 interaction.
Owner:MABQUEST
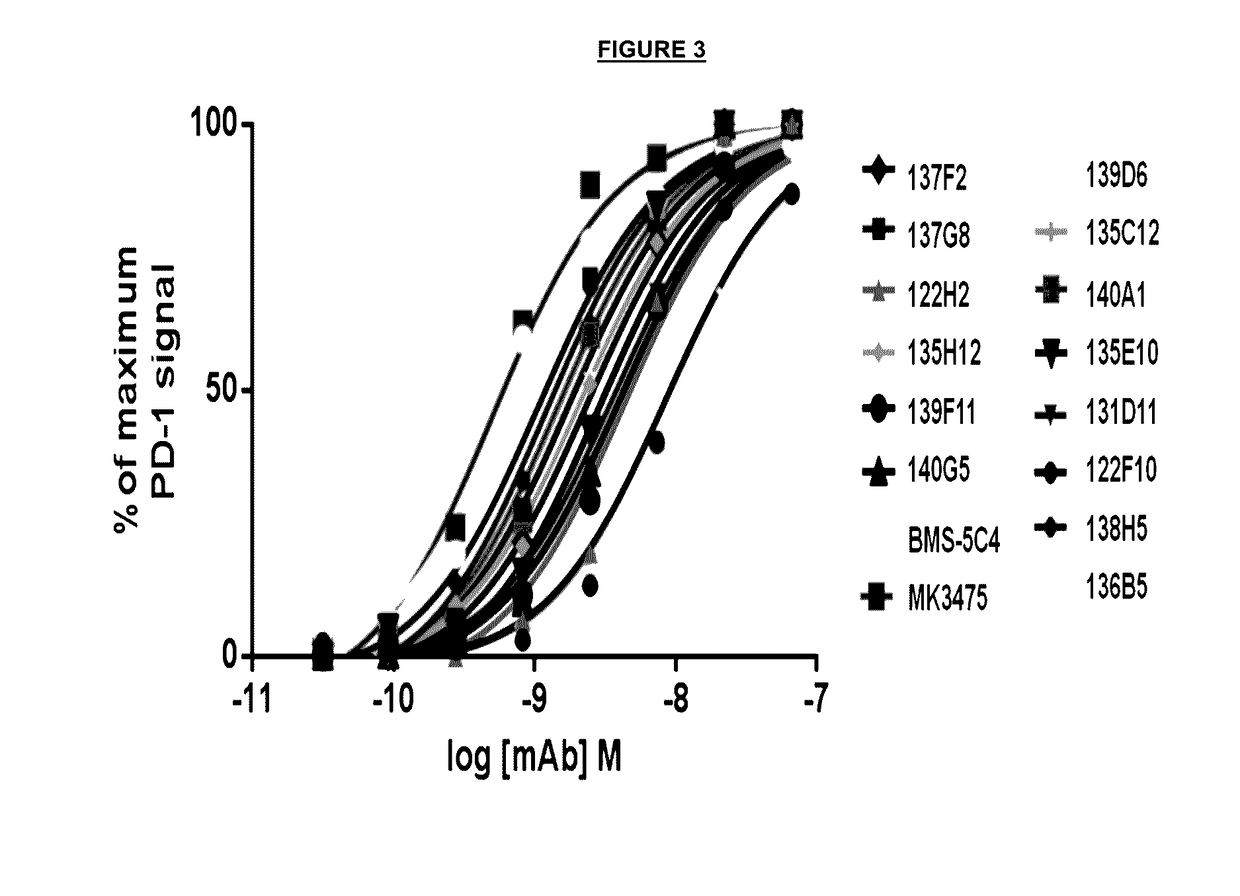
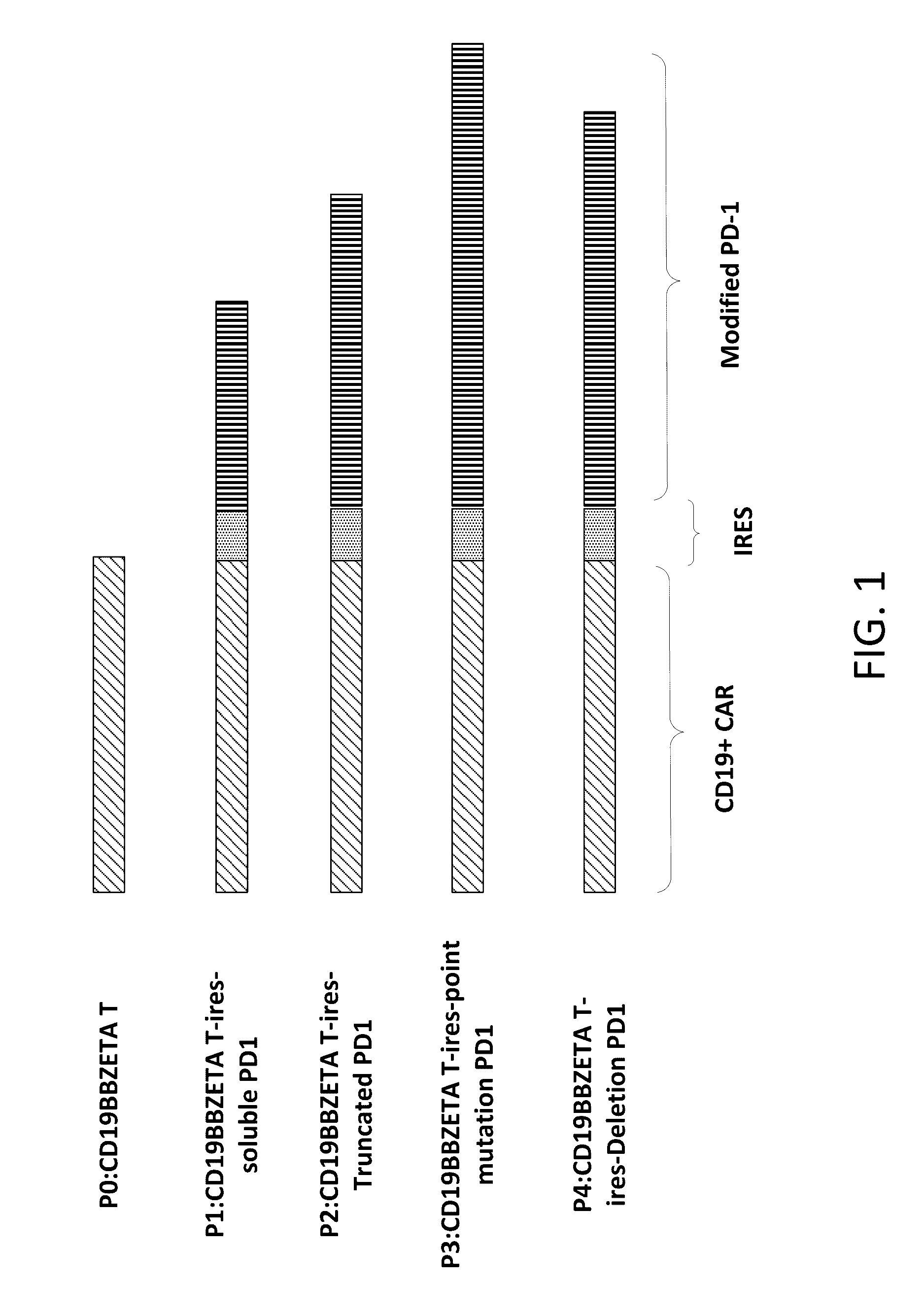
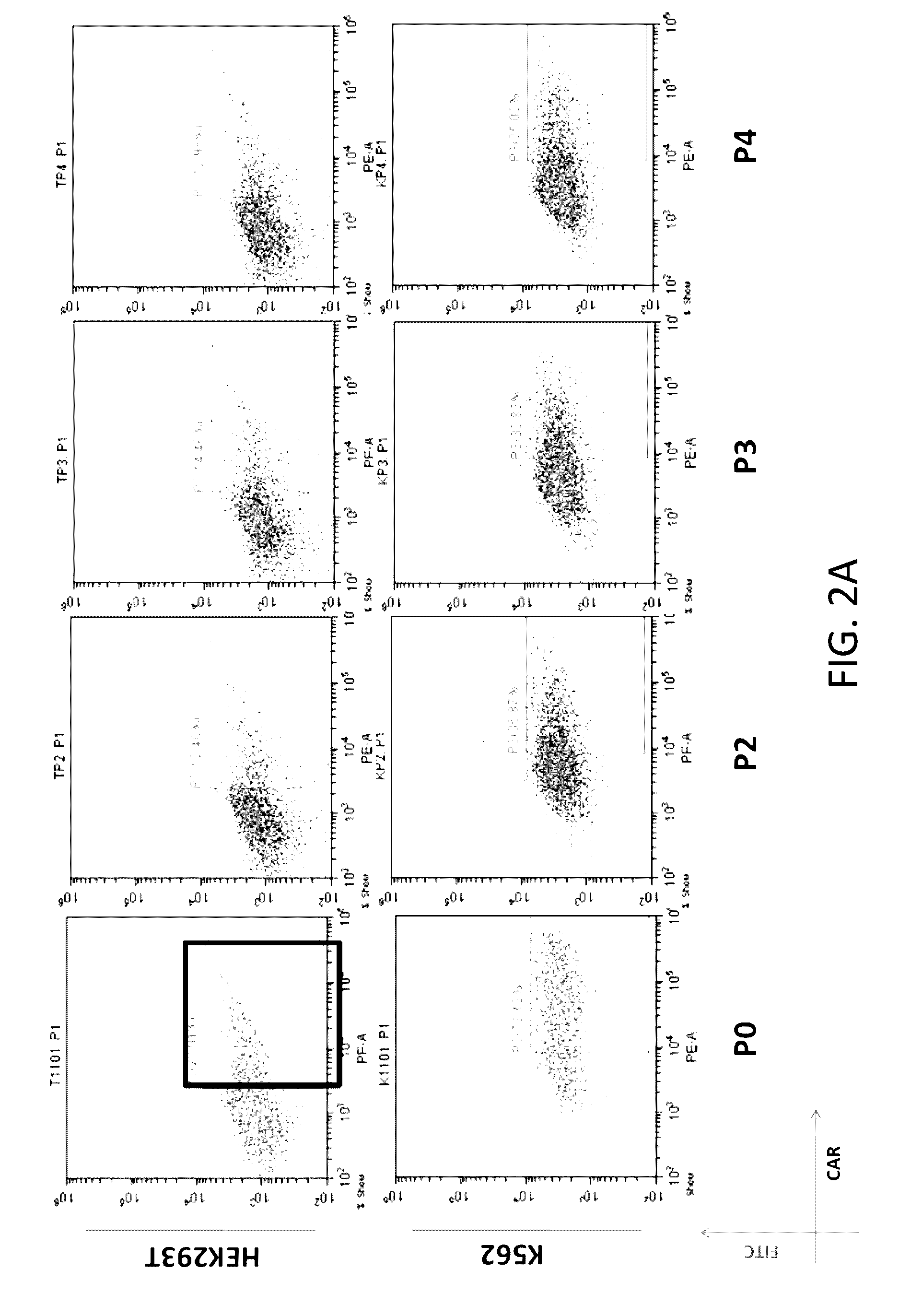
Reducing immune tolerance induced by PD-L1
ActiveUS9572837B2Less effectAntibody mimetics/scaffoldsGenetic material ingredientsCAR T-cell therapyAntigen receptors
The present disclosure relates to compositions and methods for reducing immune tolerance associated with CAR T cell therapy. Embodiments of the present disclosure include isolated nucleic acid sequence comprising a nucleic acid sequence that encodes modified programmed cell death protein 1 (PD-1) and a nucleic acid sequence that encodes chimeric antigen receptor (CAR).
Owner:INNOVATIVE CELLULAR THERAPEUTICS HLDG LTD
Anti-ctla4 and Anti-pd-1 bifunctional antibody, pharmaceutical composition thereof and use thereof
ActiveUS20190185569A1Avoid interactionEliminate immunosuppressionHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsT lymphocyteAnti ctla4
An anti-CTLA4 (cytotoxic T lymphocyte associated antigen 4) and anti-PD-1 (programmed cell death 1) bifunctional antibody. a pharmaceutical composition thereof and use thereof. Particularly, the anti-CLTA4 and anti-PD-1 bifunctional antibody comprises a first protein functional domain that targets PD-1 and a second protein functional domain that targets CTLA-4. The bifunctional antibody can bind to CTLA-4 and PD-1 specifically, relieve immunosuppression of CTLA4 and PD-1 on an organism specifically, activate T lymphocytes, and thus has good application prospects.
Owner:AKESO PHARMA INC
Reducing Immune Tolerance Induced by PD-L1
ActiveUS20170096638A1Antibody mimetics/scaffoldsGenetic material ingredientsCAR T-cell therapyNucleic acid sequencing
The present disclosure relates to compositions and methods for reducing immune tolerance associated with CAR T cell therapy. Embodiments of the present disclosure include isolated nucleic acid sequence comprising a nucleic acid sequence that encodes modified programmed cell death protein 1 (PD-1) and a nucleic acid sequence that encodes chimeric antigen receptor (CAR).
Owner:INNOVATIVE CELLULAR THERAPEUTICS HLDG LTD
Immunological reagents
ActiveUS10294299B2Promote infectionAntiviralsImmunoglobulins against cell receptors/antigens/surface-determinantsAutoimmune responsesFunctional activity
This disclosure relates to binding agents with specificity for programmed cell death 1 (PD-1) and to methods for using the same to treat, prevent and / or ameliorate an infectious disease (e.g., human immunodeficiency virus (HIV)), cancer and / or autoimmunity. In addition, this disclosure identifies a novel binding patch (“P2”) on PD-1 that is linked with a previously unidentified functional activity of PD-1 that is distinct from the interaction site involved with either the PD-L1 or PD-L2 ligands. Furthermore, we demonstrate that antibodies that interact with this region of PD-1 are able to act as antagonists of PD-1 and that this antagonism is further enhanced with the addition of antibodies that act through the blockade of the PD-1 / PD-L1 / L2 interaction.
Owner:MABQUEST
Polynucleotide agents targeting programmed cell death 1 ligand 1 (pd-l1) and methods of use thereof
ActiveUS20180371465A1Inhibit and reduce expressionGuaranteed functionAntibacterial agentsOrganic active ingredientsPD-L1Apoptotic programmed cell death
The invention relates to polynucleotide agents targeting programmed cell death 1 ligand 1 (PD-L1) gene, and methods of using such polynucleotide agents to inhibit expression of PD-L1 and to treat subjects having a PD-L1-associated disorder.
Owner:ALNYLAM PHARM INC
Peptidomimetic compounds as immunomodulators
InactiveCN104159911ASuppresses and/or inhibits the death 1 (PD1) signaling pathwayAmide active ingredientsAntineoplastic agentsPD-L1Signal pathway
The present invention relates to novel peptidomimetic compounds as therapeutic agents capable of inhibiting the programmed cell death 1 (PD1) signalling pathway. The invention also relates to derivatives of the therapeutic agents. The invention also encompasses the use of the said therapeutic agents and derivatives for treatment of disorders via immunopotentiation comprising inhibition of immunosuppressive signal induced due to PD-1, PD-L1, or PD-L2 and therapies using them.
Owner:AURIGENE DISCOVERY TECH
PD1 binding agents
ActiveUS10858435B2Improve the preparation effectImprove humanityImmunoglobulins against cell receptors/antigens/surface-determinantsAntineoplastic agentsProgrammed cell death 1Cell biology
Provided herein are antibody molecules that bind specifically to Programmed cell death 1 (PD1) and related nucleic acid molecules, vectors and host cells. Also provided herein are medical uses of such antibody molecules.
Owner:ULTRAHUMAN EIGHT LTD
ANTI-PD-1 antibodies, activatable ANTI-PD-1 antibodies, and methods of use thereof
ActiveCN108368170AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntiendomysial antibodiesAnti antibody
The invention relates generally to antibodies that specifically bind programmed cell death protein 1 (PD-1), activatable antibodies that specifically bind to PD-1 and methods of making and using theseanti-PD-1 antibodies and anti-PD-1 activatable antibodies in a variety of therapeutic, diagnostic and prophylactic indications.
Owner:CYTOMX THERAPEUTICS
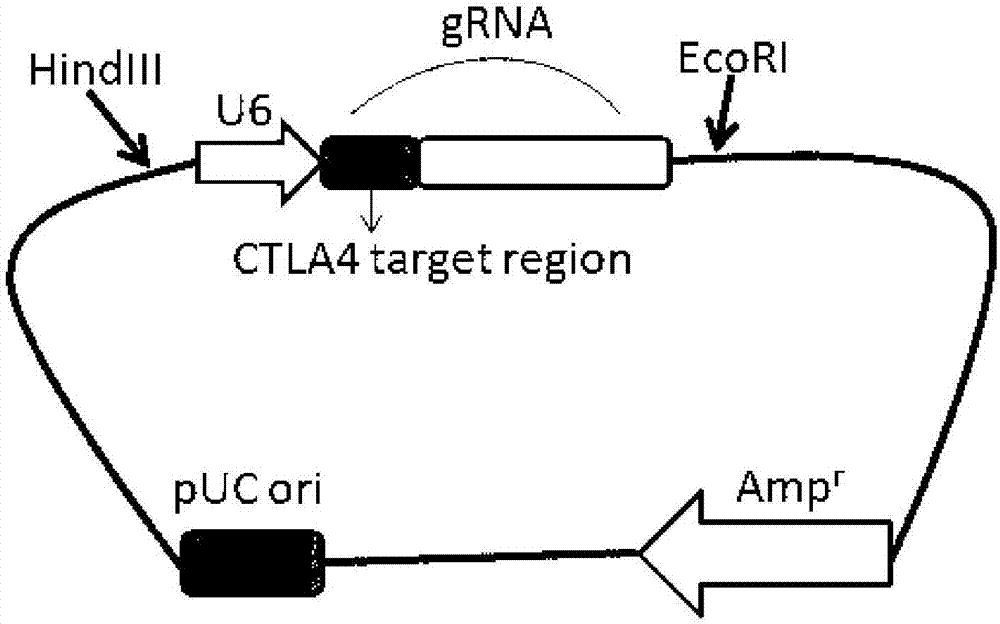
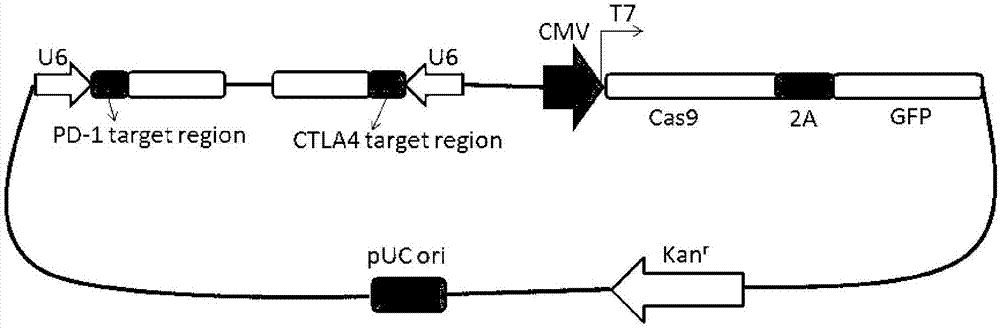
Preparation method of PD-1 (Programmed cell death protein 1) and CTLA4 (Cytotoxic T-Lymphocyte Antigen 4) double-gene defect type T lymphocyte preparation
InactiveCN107384959AReduce mortalityEfficiently obtainedGenetically modified cellsBlood/immune system cellsEnzyme digestionT lymphocyte
The invention belongs to the technical field of molecular biology and in particular relates to a preparation method of a PD-1 (Programmed cell death protein 1) and CTLA4 (Cytotoxic T-Lymphocyte Antigen 4) double-gene defect type T lymphocyte preparation. Construction of a PD-1 and CTLA4 double-gene knockout vector, T lymphocyte separation and activation, electric transfection of T lymphocytes and T7E1 enzyme digestion identification, and flow cytometer screening and sequencing analysis are carried out. According to the production of a gene defect type immune cell preparation, on one hand, a production procedure of cells is simplified; on the other hand, the capability of killing tumor cells by the T lymphocytes is improved and the long-period tumor immunity can be enhanced.
Owner:山东百福基因科技有限公司 +1
Immunological reagents
ActiveUS9982052B2Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAutoimmune responsesFunctional activity
This disclosure relates to binding agents with specificity for programmed cell death 1 (PD-1) and to methods for using the same to treat, prevent and / or ameliorate an infectious disease (e.g., human immunodeficiency virus (HIV)), cancer and / or autoimmunity. In addition, this disclosure identifies a novel binding patch (“P2”) on PD-1 that is linked with a previously unidentified functional activity of PD-1 that is distinct from the interaction site involved with either the PD-L1 or PD-L2 ligands. Furthermore, we demonstrate that antibodies that interact with this region of PD-1 are able to act as antagonists of PD-1 and that this antagonism is further enhanced with the addition of antibodies that act through the blockade of the PD-1 / PD-L1 / L2 interaction.
Owner:MABQUEST
Anti-pd-l1 immunotoxin for use in therapy
InactiveUS20180064825A1Effective targetingFast penetrationRadioactive preparation carriersImmunoglobulins against cell receptors/antigens/surface-determinantsIndiumPD-L1
The present disclosure relates to a combination of an anti-PD-L1 (Programmed Cell Death 1 Ligand 1) with an ‘immunotoxin’ for use in a method for treating a tumor in a patient. Experimental results are provided for a combination of an anti POL 1 antibody with the radioisotope Indium 111.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +3
1,3,4-oxadiazole and thiadiazole compounds as immunomodulators
The present invention relates to 1,3,4-oxadiazole and thiadiazole compounds of formula (I) and their use to inhibit the programmed cell death 1 (PD-1) signaling pathway and / or for treatment of disorders by inhibiting an immunosuppressive signal induced by PD-1, PD-L1 or PD-L2.
Owner:AURIGENE DISCOVERY TECH
1,2,4-Oxadiazole and thiadiazole compounds as immunomodulators
ActiveUS10781189B2Suppress and/or inhibit the programmed cell death 1 (PD1) signaling pathwayAntibacterial agentsOrganic active ingredientsDitazoleDisease
The present invention relates to 1,2,4-oxadiazole compounds of formula (I) and their use to inhibit the programmed cell death 1 (PD-1) signaling pathway and / or for treatment of disorders by inhibiting an immunosuppressive signal induced by PD-1, PD-L1 or PD-L2.
Owner:AURIGENE ONCOLOGY LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com