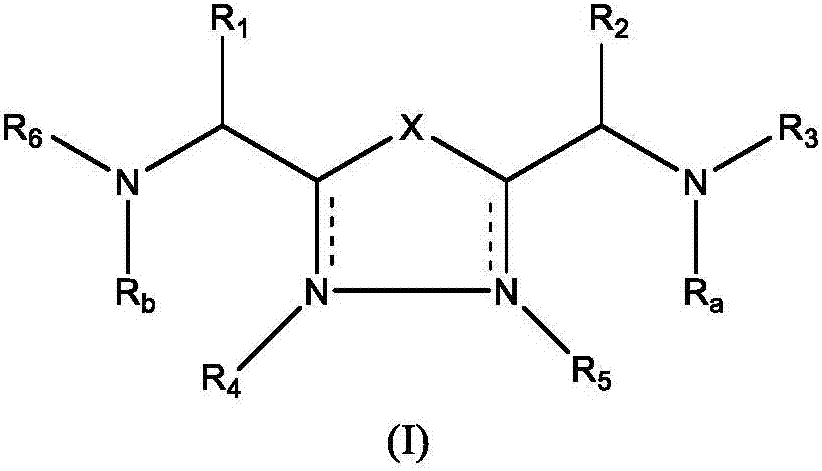
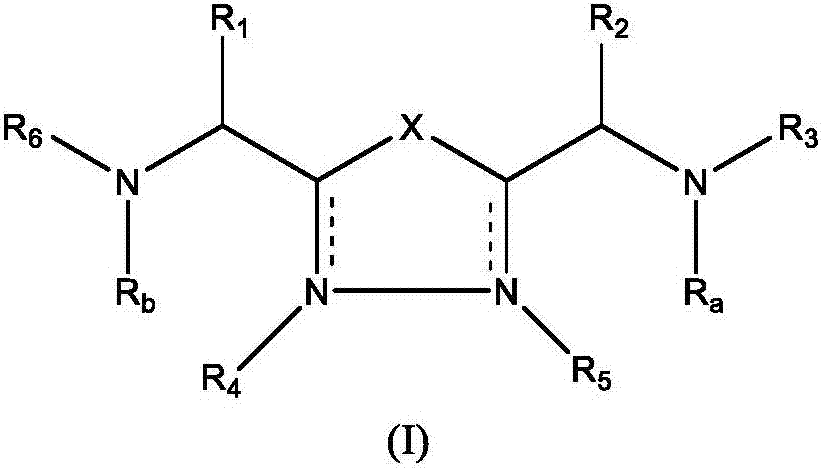
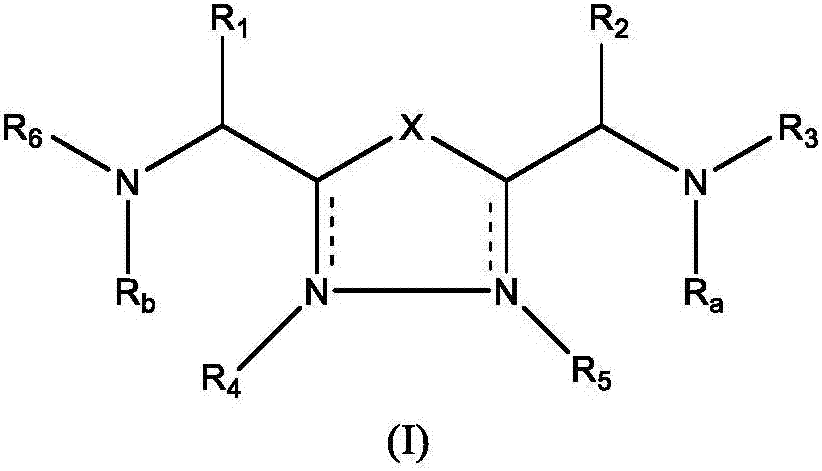
1,3,4-oxadiazole and thiadiazole compounds as immunomodulators
A compound, C1-C6 technology, applied in the direction of organic chemistry, drug combination, medical preparations containing active ingredients, etc., can solve problems such as the breakdown of immune tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0367] Embodiment 1: the synthesis of compound 1
[0368] Step 1a:
[0369]
[0370] Potassium carbonate (1.8 g, 13.0 mmol) and methyl iodide (0.65 mL, 10.4 mmol) were added to a solution of compound 1a (3.0 g, 8.7 mmol) in DMF (25 mL) and stirred at room temperature for 2 hours. Reaction completion was confirmed by TLC analysis. The reaction mixture was partitioned between water and ethyl acetate. The organic layer was washed with water and brine, washed with Na 2 SO 4 Drying and evaporation under reduced pressure gave 3.0 g of compound 1b. LCMS: 361.1(M+H) + .
[0371] Step 1b:
[0372]
[0373] Hydrazine hydrate (3.3 mL, 8.3 mmol) was added to a solution of compound 1b (3.0 g, 8.3 mmol) in methanol (20 mL) and stirred at room temperature for 5 hours. Reaction completion was confirmed by TLC analysis. The volatiles were evaporated under reduced pressure and the residue obtained was partitioned between water and ethyl acetate. The organic layer was washed with...
Embodiment 2
[0397] Embodiment 2: the synthesis of compound 32
[0398] Step 32a:
[0399]
[0400] 4-Nitrophenol (1.3 g, 9.99 mmol) and pyridine (0.8 mL, 9.99 mmol) were dissolved in Et at -78 °C under argon atmosphere 2 A solution in O (20 mL) was added dropwise to SO 2 Cl 2 (0.8mL, 9.99mmol) in Et 2 solution in O (20 mL). The reaction mixture was allowed to reach room temperature and stirred for 4 hours. Reaction completion was confirmed by TLC analysis. The reaction mixture was evaporated under reduced pressure to obtain crude compound. The crude compound was purified by silica gel column chromatography (eluent: 0%-3% ethyl acetate in hexanes) to afford 1.2 g of compound 32a. 1 H NMR (400MHz: CDCl 3 ) 8.39-8.36 (m 2H), 7.61-7.57 (m 2H).
[0401] Step 32b:
[0402]
[0403] Compound 32b (0.6g, 2.59mmol), molecular sieves (1.0g), 4-nitrophenol (0.72g, 5.18mmol) and Et 3 N (1.1 mL, 7.77 mmol) in anhydrous CH 2 Cl 2 (25.0 mL) was added dropwise to compound 32a (1.2 g, 5....
Embodiment 3
[0416] Example 3: Rescue of mouse splenocyte proliferation in the presence of recombinant PD-L1
[0417] Recombinant mouse PD-L1 (rm-PDL-1, catalog number: 1019-B7-100; R&D Systems) was used as a source of PD-L1.
[0418] Require:
[0419] Mouse splenocytes were harvested from 6-8 week old C57BL6 mice; RPMI 1640 (GIBCO, Cat# 11875); DMEM with high glucose (GIBCO, Cat# D6429); fetal bovine serum [Hyclone, Cat# SH30071.03] ; penicillin (10,000 units / mL)-streptomycin (10,000 μg / mL) liquid (GIBCO, cat. no. 15140-122); MEM sodium pyruvate solution 100 mM (100x), liquid (GIBCO, cat. no. 11360); not essential Amino Acid (GIBCO, Cat. No. 11140); L-Glutamine (GIBCO, Cat. No. 25030); Anti-CD3 Antibody (eBiosciences–16-0032); Anti-CD28 Antibody (eBiosciences–16-0281); ACK Lysis Buffer (1 mL ) (GIBCO, catalog number - A10492); Histopaque (density - 1.083 gm / mL) (SIGMA 10831); Trypan blue solution (SIGMA-T8154); 2 mL Norm Ject Luer Lock syringe (Sigma 2014-12); 40 μm nylon cells Filter ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com