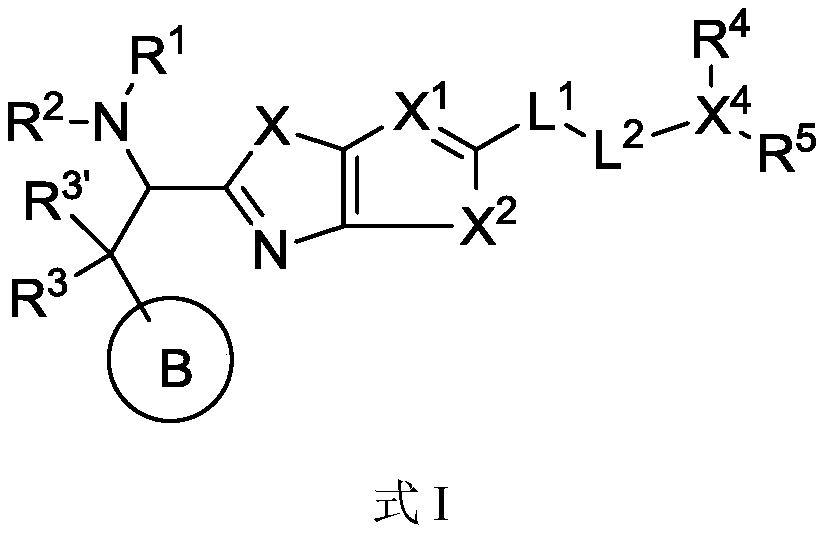
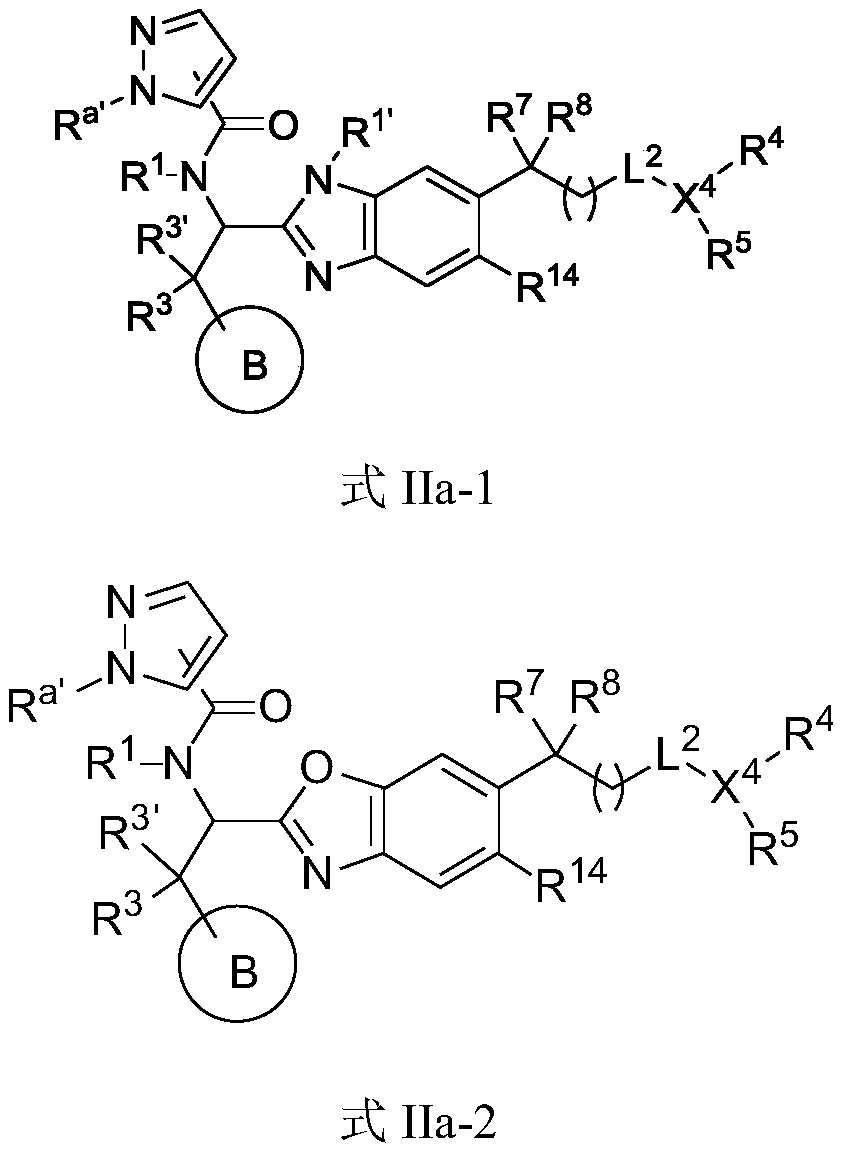
Immunomodulator
A heterocycloalkyl group, selected from the technology, applied in the field of immunomodulators and the preparation of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0173] The preparation of embodiment 1 intermediate 1
[0174]
[0175] Step 1 Preparation of intermediate 1-1
[0176]
[0177] Ethyl nitroacetate (28.5g, 130mmol) and 5-bromo-2-chlorobenzaldehyde (17.3g, 130mmol) were dissolved in anhydrous tetrahydrofuran (400mL), and titanium tetrachloride was slowly added dropwise at 0°C under nitrogen protection (28.5mL, 260.0mmol), after the dropwise addition, continue to stir the reaction at 0°C for 1 hour. N-methylmorpholine (57.8 mL, 520.0 mmol) was slowly added dropwise to the reaction solution, and after the dropwise addition, the temperature was slowly raised to room temperature, and the reaction was continued to stir for 2 hours. Add distilled water to quench the reaction, extract with ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, filter, evaporate the solvent under reduced pressure, and purify by column chromatography (eluent: petroleum ether: ethyl acetate = 20:1 ) to obtain intermediate ...
Embodiment 2
[0190] The preparation of embodiment 2 intermediate 2
[0191]
[0192] Referring to the method for preparing intermediate 1 in Example 1, using o-chlorobenzaldehyde as the starting material, condensation, Grignard reaction, nitro reduction, Boc protected amino group, hydrolysis, and finally separated by SFC chiral separation column to prepare Four single chiral isomers 2-a, 2-b, 2-c, 2-d of intermediate 2 were obtained. MS m / z:242[M-99] + , 286 [M-55] + .
Embodiment 3
[0193] The preparation of embodiment 3 intermediate 3
[0194]
[0195] Referring to the method for preparing intermediate 1 in Example 1, using m-chlorobenzaldehyde as a starting material, condensation, Grignard reaction, nitro reduction, Boc protection of amino groups, hydrolysis, and finally separated by SFC chiral separation column can be prepared respectively Four single chiral isomers 3-a, 3-b, 3-c, 3-d of intermediate 3 were obtained. MS m / z:242[M-99] + , 286 [M-55] + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com