Novel method for preparing tenofovir
A compound and selected technology, applied in chemical instruments and methods, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve the problems of low optical purity and unstable chiral enrichment treatment yield
Inactive Publication Date: 2010-12-08
INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
View PDF0 Cites 17 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
This route is comparatively brief, but this document (US5935946A1, 1999-08-10) points out simultaneously: the optical purity of the target object obtained is not high (R-type content is about 90~94%), can improve the purity of the product through chiral enrichment treatment. optical purity
However, the literature data show that the yield of chiral enrichment treatment is very unstable
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
specific Embodiment approach
Embodiment 1
Embodiment 2
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
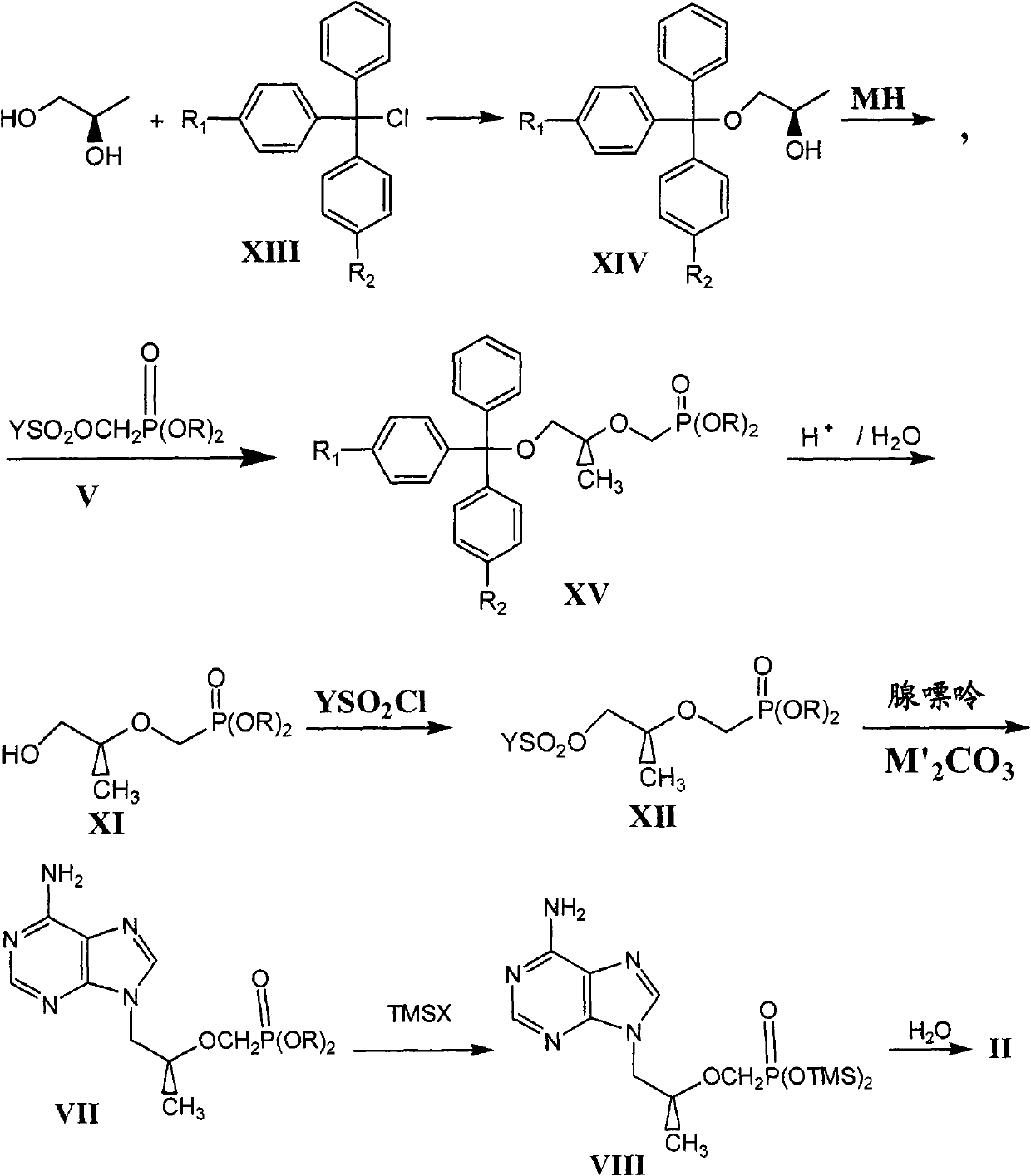
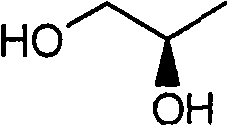
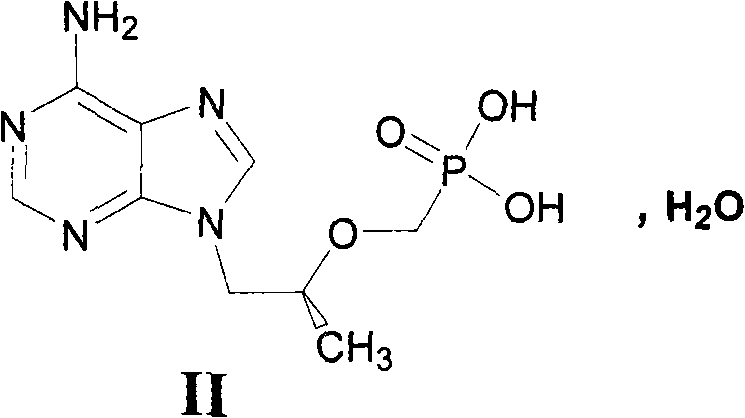
The invention relates to a novel method for preparing tenofovir, in particular to a method for preparing a tenofovir compound, in particular the monohydrate of the tenofovir for resisting hepatitis B viruses and human immunodeficiency viruses. The method comprises the following steps of: using (substituted) triphenylchloromethane XIII to protect hydroxy in the end position of (R)-1,2-propanediol,condensing the obtained product with dialkyl sulfonyloxy methyl phosphonate V to obtain XV, hydrolyzing the XV in the aqueous solution of an organic acid to remove the protective group of the hydroxyl at the end position of XV to obtain an intermediate XI, condensing sulfonate XII of the intermediate XI with adenine to obtain an intermediate VII, transforming the intermediate VII into the corresponding trimethylsilyl phosphonate VIII by trimethylsilyl bromide (TMSBr), and hydrolyzing the VIII to obtain the target product. The method has the advantages of short process route, convenient operation, low cost and easy access of raw materials and favorability for large scale production.
Description
Technical field: The invention relates to a preparation method of tenofovir (Tenofovir, PMPA), an anti-hepatitis B virus and AIDS virus compound. Background technique: The chemical name of tenofovir (I) is (R)-{[2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl}phosphonic acid or (R) -9-(2-phosphonomethoxypropyl) adenine, its structural formula is: Tenofovir belongs to the class of acyclic nucleoside phosphonates. Since its preparation generally goes through a hydrolysis step, the product is provided in the form of tenofovir monohydrate (II): There are three synthetic routes of II reported in the literature, which are as follows. Synthetic route 1 In this route, the hydroxyl group in D-(+)-isobutyl lactate is protected with dihydropyran (DHP), and then the ester is reduced to alcohol III with bis(methoxyethoxy)aluminum hydride. After condensation of p-toluenesulfonate and adenine (Adenine), deprotection can give intermediate IV. Protect the hydroxyl group of IV w...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07F9/6561A61K31/675A61P31/18A61P31/20
CPCY02P20/55
Inventor 仲伯华朱红元何新华刘河陈兰福
Owner INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Features
- Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com