Novel method for preparing tenofovir
A compound and reaction technology, applied in the new field of preparation of tenofovir, can solve problems such as unstable yield of chiral enrichment treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0078] The present invention will be further illustrated by specific intermediates and examples below. However, it should be understood that these intermediates and examples are only used for more detailed and specific explanation, and should not be understood as limiting the present invention in any form. invention.
[0079] The present invention provides a general and / or specific description of the materials and test methods used in the test. Although many materials and operating methods used to achieve the purpose of the present invention are well known in the art, the present invention is still described here in as much detail as possible. It is clear to those skilled in the art that, in the following, unless otherwise specified, the materials and operating methods used in the present invention are well known in the art.
Embodiment 1
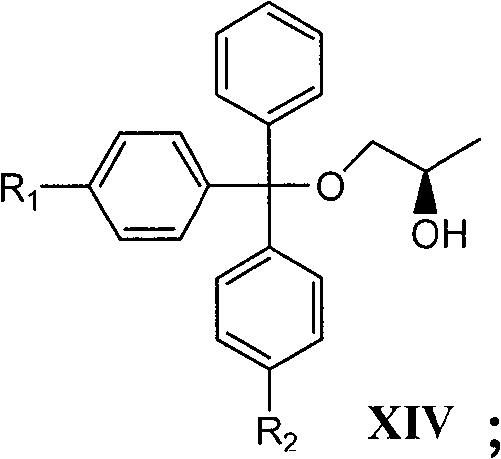
[0080] Example 1: (R)-1-Trityloxy-2-propanol (Formula XIV, R 1 =R 2 =H) Preparation
[0081] Add (R)-1,2-propanediol (24.0g, 0.315moL), N,N-dimethylaminopyridine (0.25g, 2.00mmoL), dichloromethane (350mL) and TEA (46.0g, 0.45 moL), cooled in an ice water bath, magnetically stirred. Triphenylchloromethane (88.7g, 0.315moL) was added in batches, and the addition was completed in about 1 hour. After 1.5 hours of reaction, the ice-water bath was removed, and then the temperature was raised to room temperature, and then reacted at room temperature for about 15 hours (TLC detection, until triphenylchloromethane disappeared). Add distilled water (100mL) to dissolve the triethylamine hydrochloride generated by the reaction, transfer to a separatory funnel for layering, and wash the oil phase with 5% sodium bicarbonate solution (2×100mL) and distilled water (100mL) in turn, and then use anhydrous After drying over sodium sulfate and filtering, the filtrate was evaporated to remove...
Embodiment 2
[0082] Example 2: (R)-[(2-Trityloxy-1-methylethoxy)methyl]phosphonic acid diisopropyl ester (Formula XV, R 1 =R 2 =H) Preparation
[0083] The crude product obtained in Example 1 (96.6 g, about 0.30 moL) and anhydrous THF (500 mL) were added to the flask, stirred magnetically, and cooled in an ice-water bath. Sodium hydride (70% oil powder, 11.3g, 0.33mol) was added in batches, and the addition was completed in about 40 minutes. After 0.5 hours, remove the ice-water bath, then let the temperature rise to room temperature and react for about 2 hours, and then heat and reflux for about 3 hours until the reaction liquid surface is basically free of gas foam. The temperature is lowered to 5℃, and the compound of formula Va (diisopropyl p-toluenesulfonyloxymethylphosphonate, where Y is p-tolyl and R is isopropyl, 106.2g, 0.30moL) is added dropwise through a constant pressure funnel. Anhydrous THF (200 mL) solution was added in about 0.5 hours, the ice-water bath was removed af...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com