Lyophilized inactivated Japanese encephalitis vaccine
A Japanese encephalitis and inactivation technology is applied in the field of new freeze-dried and inactivated Japanese encephalitis whole virus vaccines, which can solve the problems of low miscellaneous protein content and instability of purified vaccines, and achieve clear components, easy quantitative and qualitative, and stable long term effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Comparison of freeze-dried state of different formulation excipients
[0039] a) Preparation of Japanese encephalitis virus inactivation stock solution
[0040] Use a 175cm2 culture flask (Corning Company) to resuscitate a 136-generation working cell bank Vero cell (ATCC, CCL81), culture it in a carbon dioxide incubator at 37 degrees for 3-5 days, discard the culture medium, and add 0.25% trypsin (GIBCO) Digest for 3-5 minutes, add fresh growth medium (serum-free medium PV2, Tianjin Bairuoke Company) to disperse the cells by blowing, 1:6 split into six 175cm2 culture flasks, and culture under the same conditions. In this way, 24 10-layer 1750 flasks (Corning Company) were amplified, which were digested and inoculated into a bioreactor after forming a dense monolayer.
[0041] Adopt the 7.5L cell culture reactor produced by APPLIKAN; cytodex1 microcarrier (GE Company), the concentration is 20g / l. Serum-free medium PV2 was used, the culture temperature was 37 degrees, t...
Embodiment 2
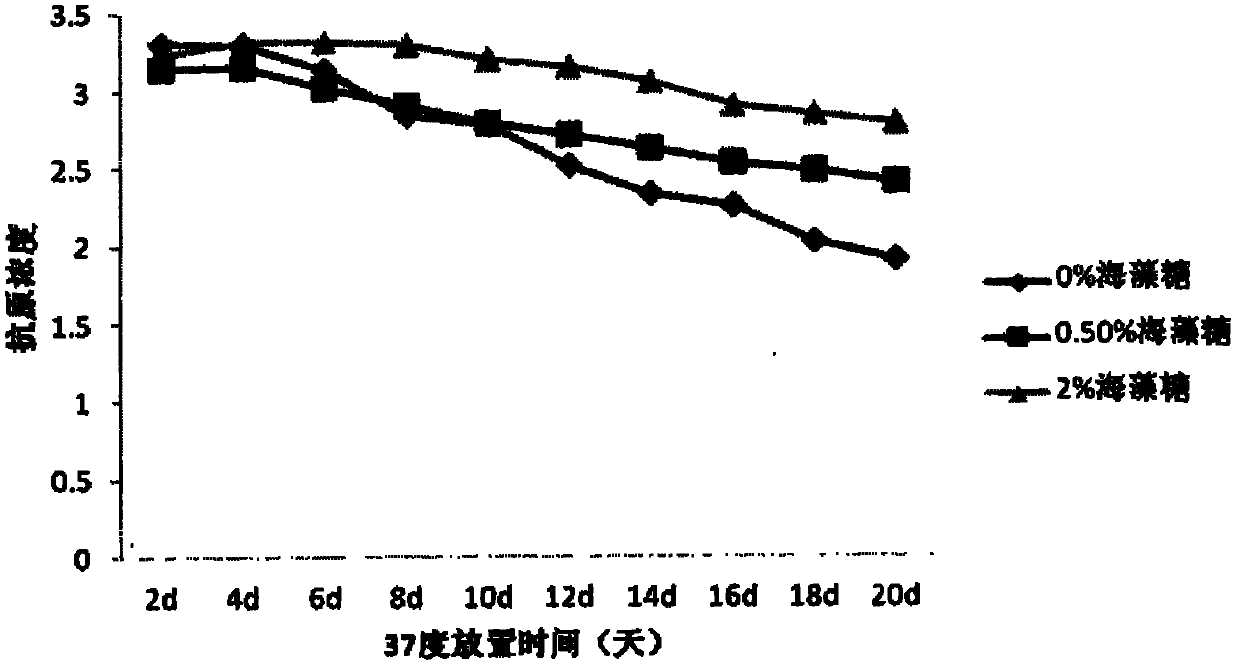
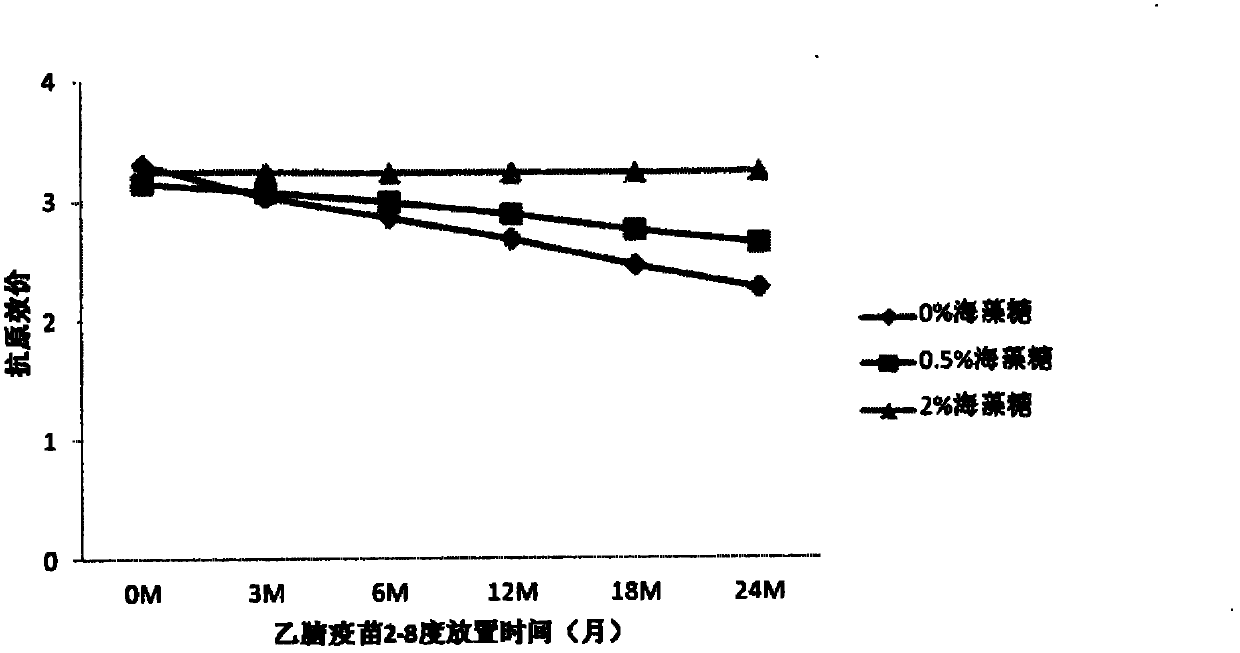
[0074] Comparison of Stability of JE Vaccines with Different Concentrations of Trehalose
[0075] a) Preparation of JE vaccine inactivated stock solution
[0076] The method was the same as that in Experiment 1, and the antigen concentration of the inactivated stock solution was measured to be 30 EU / ml by the double-antibody sandwich method.
[0077] b) On the basis of Experiment 1 formula B, add different concentrations of trehalose (2%, 0.5%, 0%), then place the vaccine at 4 degrees and 37 degrees respectively, take samples regularly, and use the double antibody sandwich method to determine the The antigenic content of the vaccine, and the vaccine potency were determined using a mouse immune neutralizing antibody assay.
[0078] c) Antigen results at 37 degrees are as follows: figure 1 , the antigen was measured every two days. With the prolongation of the storage time of the vaccine at 37 degrees, the antigen content decreased, but the decline rate of the vaccine containi...
Embodiment 3
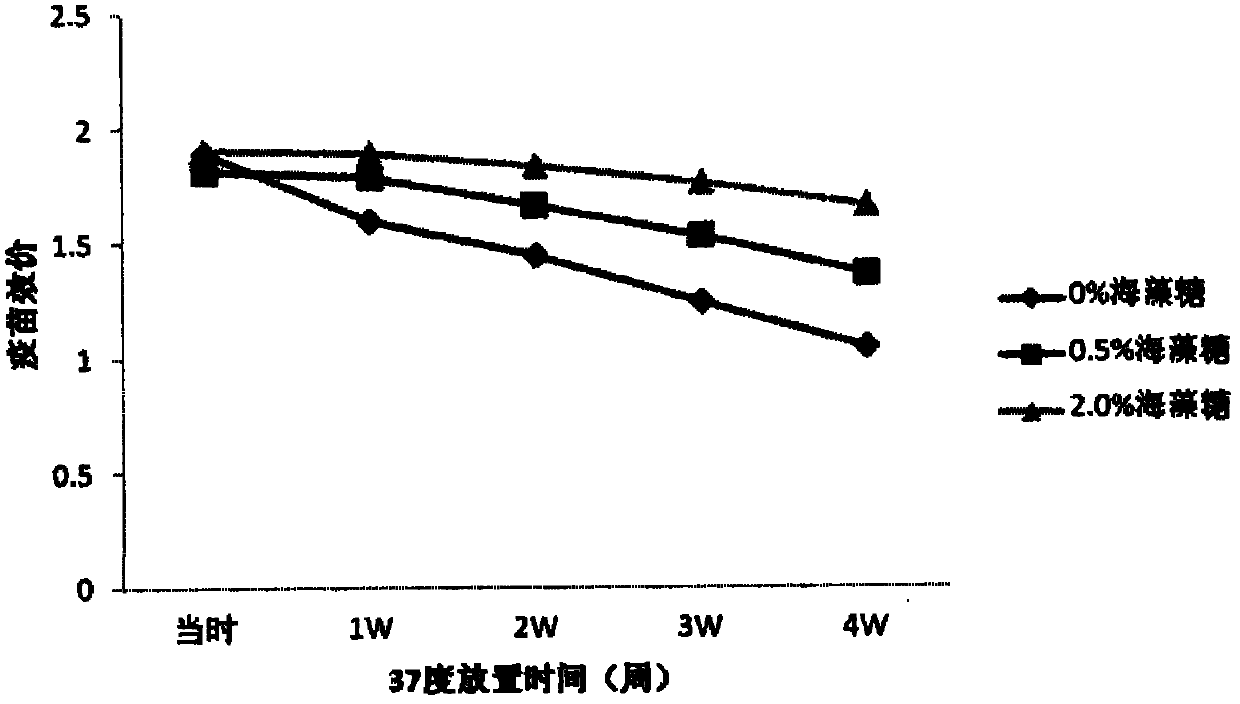
[0082] Stability test results of freeze-dried vaccine
[0083] a) Vaccine preparation
[0084] Vero cells, passage 139, recovered and expanded to a 5L reactor; cytodex1 microcarrier, 10g / L;
[0085] Japanese encephalitis virus: the Vero cell passage virus species P3V5 of the P3 strain. MOI, 0.02
[0086] Cell culture temperature: 36.5 degrees, stirring speed: 80RPM, 80% dissolved oxygen, pH7.2;
[0087] Virus culture temperature: 34.5 degrees, stirring speed: 80rpm, 80% dissolved oxygen, pH7.8
[0088]The virus harvested solution was filtered at 1.0+0.22 microns, concentrated 100 times with a 100KD ultrafiltration membrane, centrifuged through a sucrose density gradient, desugared by ultrafiltration, inactivated with formaldehyde, and the antigen concentration of the inactivated stock solution was determined.
[0089] b) Preparation of semi-finished products:
[0090] Prepare the semi-finished product with 0.01M phosphate buffer containing maltose, lactose, arginine and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com