Method for detecting IFA neutralizing antibody of PCV2
An antibody detection and cell technology, which is applied in the biological field, can solve the problem that the research on PCVAD prevention and control of the diagnostic standard virus is not very clear, and achieve the effects of accuracy and representativeness, complete antigen structure and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
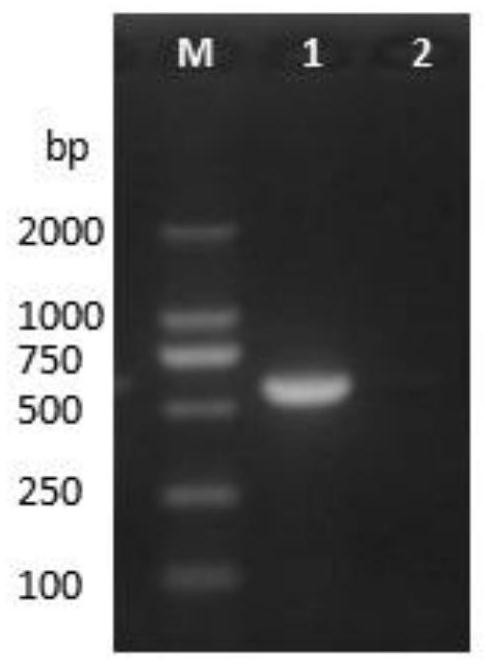
[0022] Example 1 PCV2 virus isolation and identification
[0023] According to the PCV2 gene sequence in GenBank (sequence number: FJ667592.1), design primers, the amplification length is 600bp, and the specific primer sequences are as follows:
[0024] PCV2-F: 5'-gatctcaaggacaacggagt-3'; SEQ ID NO.1;
[0025] PCV2-R: 5'-catatggaaattcagggcatgg-3'; SEQ ID NO.2.
[0026] Grind clinically collected diseased tissues such as lymph nodes, add 1mL PBS to dissolve 1mg tissue samples, freeze and thaw 3 times, take supernatant, then centrifuge at 12000rpm for 5min, and filter with 0.22μm filter to obtain PCV2 virus liquid. Take 1mL virus liquid to inoculate PK15 cells. After the virus proliferates for 48 hours, freeze and thaw 3 times, collect the virus liquid, centrifuge at 12000rpm for 10min, take the supernatant, and extract PCV2 DNA according to Baobio DNA extraction kit.
[0027] Carry out PCR amplification, the system is 25 μL: DNA template 1 μL, PCV2-F 0.5 μL, PCV2-R 0.5 μL, Ex...
Embodiment 2
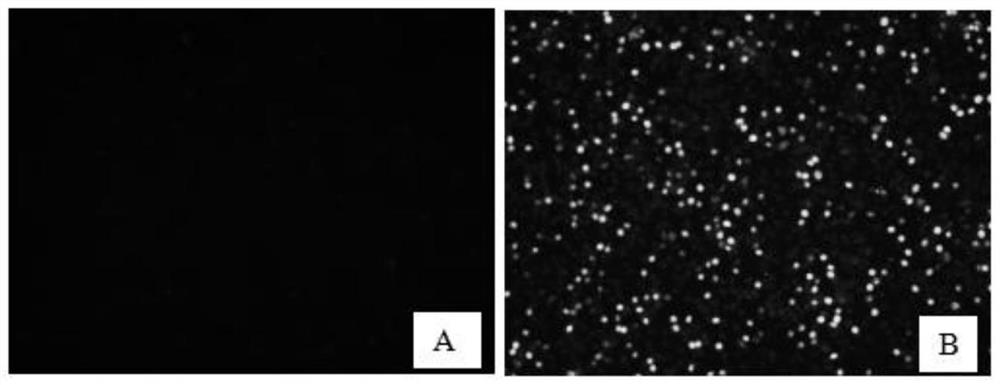
[0029] Example 2 Establishment of Clinical Neutralizing Antibody Detection Method
[0030] PK15 cells were digested and centrifuged to prepare 1.0×10 5 cells / mL cell suspension, spread 100 μL per well on a 96-well plate, and store at 37°C in 5% CO 2 Cultivate in the incubator for 12 hours. After the cells are completely adhered to the wall, incubate an equal volume of PCV2 virus solution (50 μL) and clinical serum at 37°C for 1 hour, then inoculate them on PK15 cells in a 96-well plate, and culture them for 48 hours. Take out after the cells are full. 96-well plate; at the same time, set up a control well for normal inoculated cells; fix with pre-cooled absolute ethanol, add pre-cooled absolute ethanol, 50 μL per well, and place at -20°C for 30 min. Then take it out, discard absolute ethanol, wash 5 times with PBST, pat dry, add 5% skimmed milk powder for blocking, 200 μL per well, and place in a 37°C incubator for 2h. Wash 5 times with PBST, pat dry, add PCV2 positive antib...
Embodiment 3I
[0032] Embodiment 3IFA reaction conditions
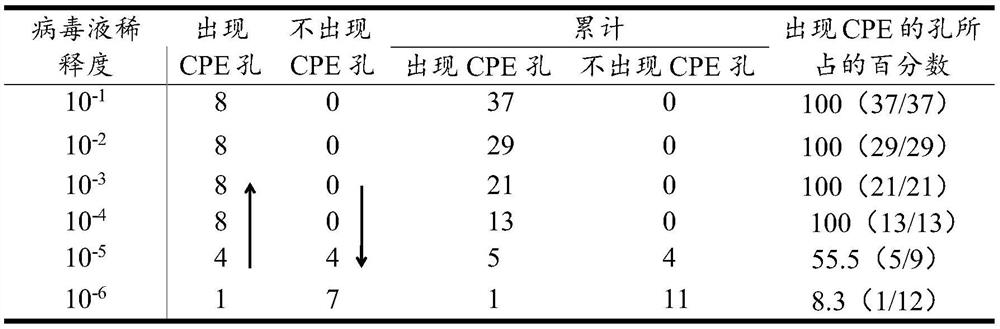
[0033] 1) PCV2 TCID 50 determination
[0034] The PK15 cells were expanded and cultured, and then inoculated with PCV2 virus liquid at an appropriate dosage. After 48 hours, the virus was harvested, frozen and thawed three times, and centrifuged at 12,000rpm for 10 minutes to obtain a large amount of PCV2 virus liquid. Digest the PK15 cells grown to a single layer with 0.25% trypsin, add DMEM containing 10% fetal bovine serum and blow repeatedly, count the cells, and dilute to 1.0×10 5 cells / mL, 100 μL per well was added to a 96-well cell culture plate. After the cells adhered to the wall, the PCV2 virus liquid was diluted with 10-fold gradient with DMEM containing 2% fetal bovine serum, and then 10 -1 、10 -2 、10 -3 、10 -4 、10 -5 、10 -6 、10 -7 、10 -8 Each gradient, each gradient was inoculated into a 96-well cell culture plate, 8 wells per gradient, 100 μL per well, and a negative control was set up at the same time. Put ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com