Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
91 results about "Acute viral encephalitis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The majority of viral cases of encephalitis have an unknown cause, however the most common identifiable cause of viral encephalitis is from herpes simplex infection. Other causes of acute viral encephalitis are rabies virus, poliovirus, and measles virus.
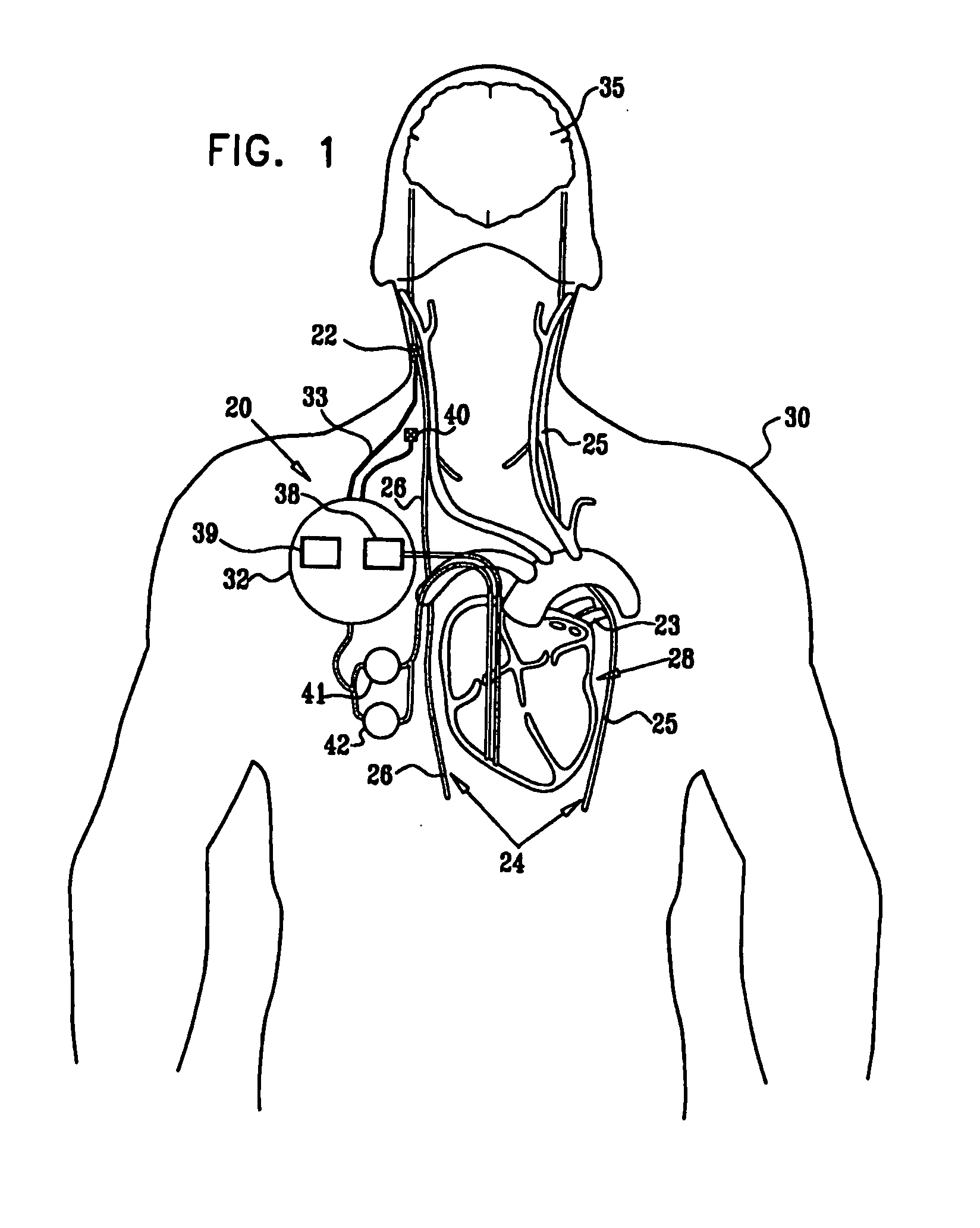
Parasympathetic stimulation for heart conditions
InactiveUS20080125819A1Enhancing and sustaining efficacyImprove efficiencyHeart defibrillatorsHeart stimulatorsMyelitisNervous system
A method is provided for treating a subject, including applying a current to a site of the subject selected from the list consisting of: a vagus nerve of the subject, an epicardial fat pad of the subject, a pulmonary vein of the subject, a carotid artery of the subject, a carotid sinus of the subject, a vena cava vein of the subject, and an internal jugular vein of the subject. The method also includes configuring the current so as to treat a condition of the subject selected from the list consisting of: an autoimmune disease, an autoimmune inflammatory disease, multiple sclerosis, encephalitis, myelitis, immune-mediated neuropathy, myositis, dermatomyositis, polymyositis, inclusion body myositis, inflammatory demyelinating polyradiculoneuropathy, Guillain Barre syndrome, myasthenia gravis, inflammation of the nervous system, inflammatory bowel disease, Crohn's disease, ulcerative colitis, SLE (systemic lupus erythematosus), rheumatoid arthritis, vasculitis, polyarteritis nodosa, Sjogren syndrome, mixed connective tissue disease, glomerulonephritis, thyroid autoimmune disease, sepsis, meningitis, a bacterial infection, a viral infection, a fungal infection, sarcoidosis, hepatitis, and portal vein hypertension.
Owner:MEDTRONIC INC
Minimal-heart-rate reduction parasympathetic stimulation
ActiveUS20080275514A1Enhancing and sustaining efficacyImprove efficiencyHeart defibrillatorsInternal electrodesMyelitisNervous system
A method is provided for treating a subject, including applying a current to a site of the subject selected from the list consisting of: a vagus nerve of the subject, an epicardial fat pad of the subject, a pulmonary vein of the subject, a carotid artery of the subject, a carotid sinus of the subject, a vena cava vein of the subject, and an internal jugular vein of the subject. The method also includes configuring the current so as to treat a condition of the subject selected from the list consisting of: an autoimmune disease, an autoimmune inflammatory disease, multiple sclerosis, encephalitis, myelitis, immune-mediated neuropathy, myositis, dermatomyositis, polymyositis, inclusion body myositis, inflammatory demyelinating polyradiculoneuropathy, Guillain Barre syndrome, myasthenia gravis, inflammation of the nervous system, inflammatory bowel disease, Crohn's disease, ulcerative colitis, SLE (systemic lupus erythematosus), rheumatoid arthritis, vasculitis, polyarteritis nodosa, Sjogren syndrome, mixed connective tissue disease, glomerulonephritis, thyroid autoimmune disease, sepsis, meningitis, a bacterial infection, a viral infection, a fungal infection, sarcoidosis, hepatitis, and portal vein hypertension.
Owner:MEDTRONIC INC
DNA-based vaccine against the encephalitis alphaviruses
This invention relates to the development of a mammalian expression vector, under which expression of the structural genes of western equine encephalitis virus have been placed under the control of an eucaryotic promoter. When the recombinant vector is administered to mammalian cell culture or using a cell-free transcription / translation system, in vitro, authentic structural proteins of western equine encephalitis virus are produced as verified by reactivity with monoclonal antibodies developed to western equine encephalitis virus. When the recombinant DNA molecule is administered in vivo, a protective immune response is induced, thereby enhancing protection of the individual against subsequent infection by western equine encephalitis virus. In a similar manner, DNA vaccines to related alphaviruses (Venezuelan and eastern equine encephalitis viruses) could also be developed.
Owner:HER MAJESTY THE QUEEN AS REPRESENTED BY THE MINIST OF NAT DEFENCE OF HER MAJESTYS CANADIAN GOVERNMENT
Interferon antagonists useful for the treatment of interferon related diseases
InactiveUS20030138404A1Extended half-lifeLow immunogenicityBiocideNervous disorderDiseaseAutoimmune responses
The present invention relates to a process for ameliorating or preventing diseases that are caused, in part, by an increased level of, and / or an abnormal responsivity to, interferon. Alzheimer's disease, HIV infection, Down syndrome, transplant rejection, autoimmune disease, and infant encephalitis are examples of such diseases. Specifically, the invention provides a method for treating subjects suffering from, or at risk for, such diseases by the administration of a pharmacological preparation of interferon binding proteins of mammalian and / or viral origin that antagonize interferon's action. This invention comprises compositions of interferon binding proteins that can inhibit the activity of interferon gamma plus interferon alpha such compositions along with their method of production and modification being described herein.
Owner:MEIOGEN BIOTECH CORP
West nile vaccine
InactiveUS7153513B2Safe and effectiveSuitable for useAntibacterial agentsSsRNA viruses negative-senseEquidaeDisease
The present invention provides a safe and effective vaccine composition against West Nile virus disease. An immunogenically active component of West Nile virus or plasmid DNA, an adjuvant such as a metabolizable oil, and a pharmacologically acceptable carrier are formulated into an immunizing vaccine. The invention also provides a method for the prevention or amelioration of West Nile disease, such as encephalitis, in equidae by administering the vaccine composition herein set forth.
Owner:ZOETIS SERVICE LLC
Methods and compositions for treatment and diagnosis of encephalitis or epilepsy
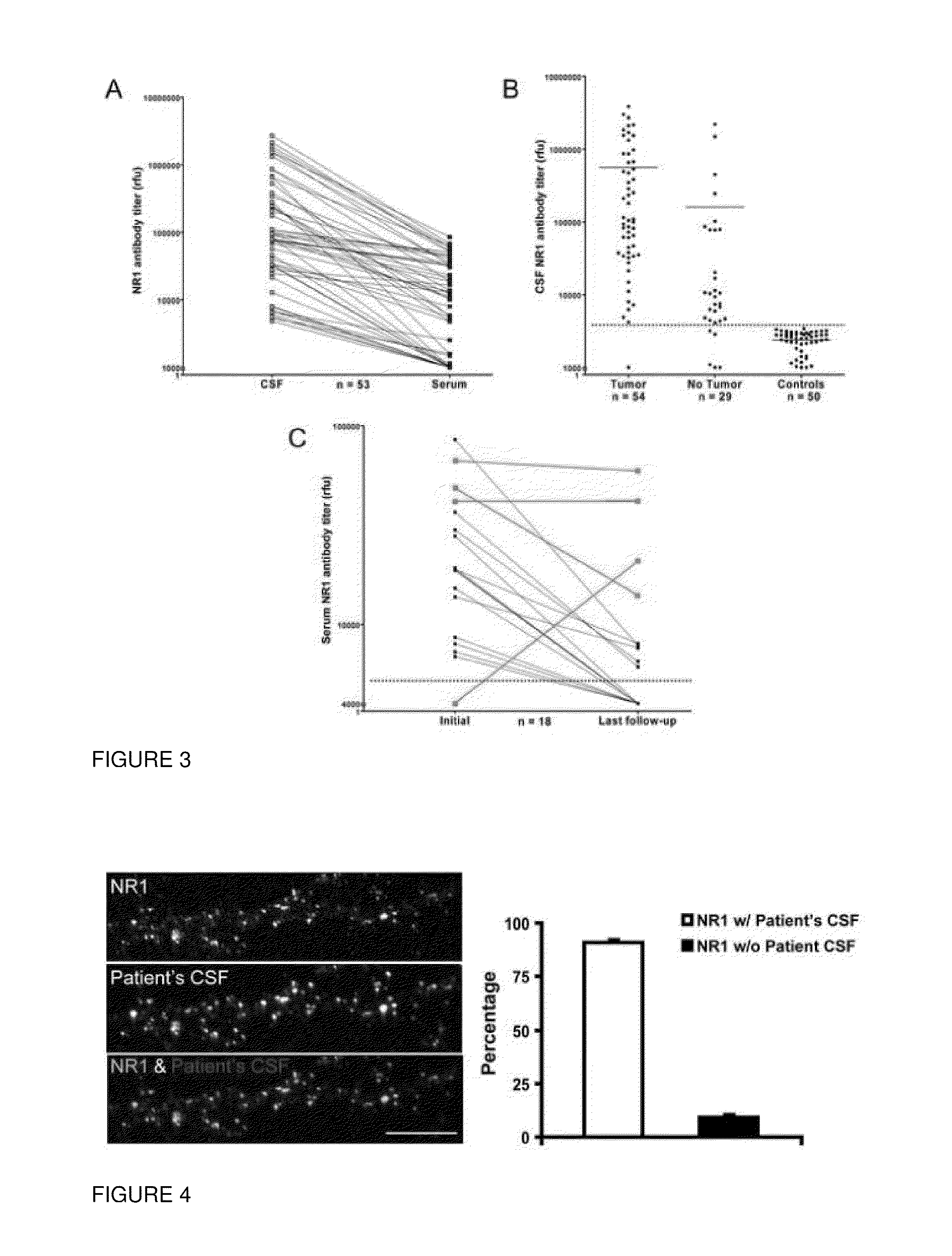
This invention provides methods of diagnosing or determining a cause of an autoimmune encephalitis or an epilepsy in a subject and of diagnosing a tumor in a subject, comprising the step of testing a biological sample of the subject for an antibody to an NR1 subunit of the NMDA receptor. This invention further provides methods of treating an autoimmune encephalitis or an epilepsy, comprising the steps of detecting an antibody to an NR1 subunit of the NMDA receptor and treating a tumor associated with the disease.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
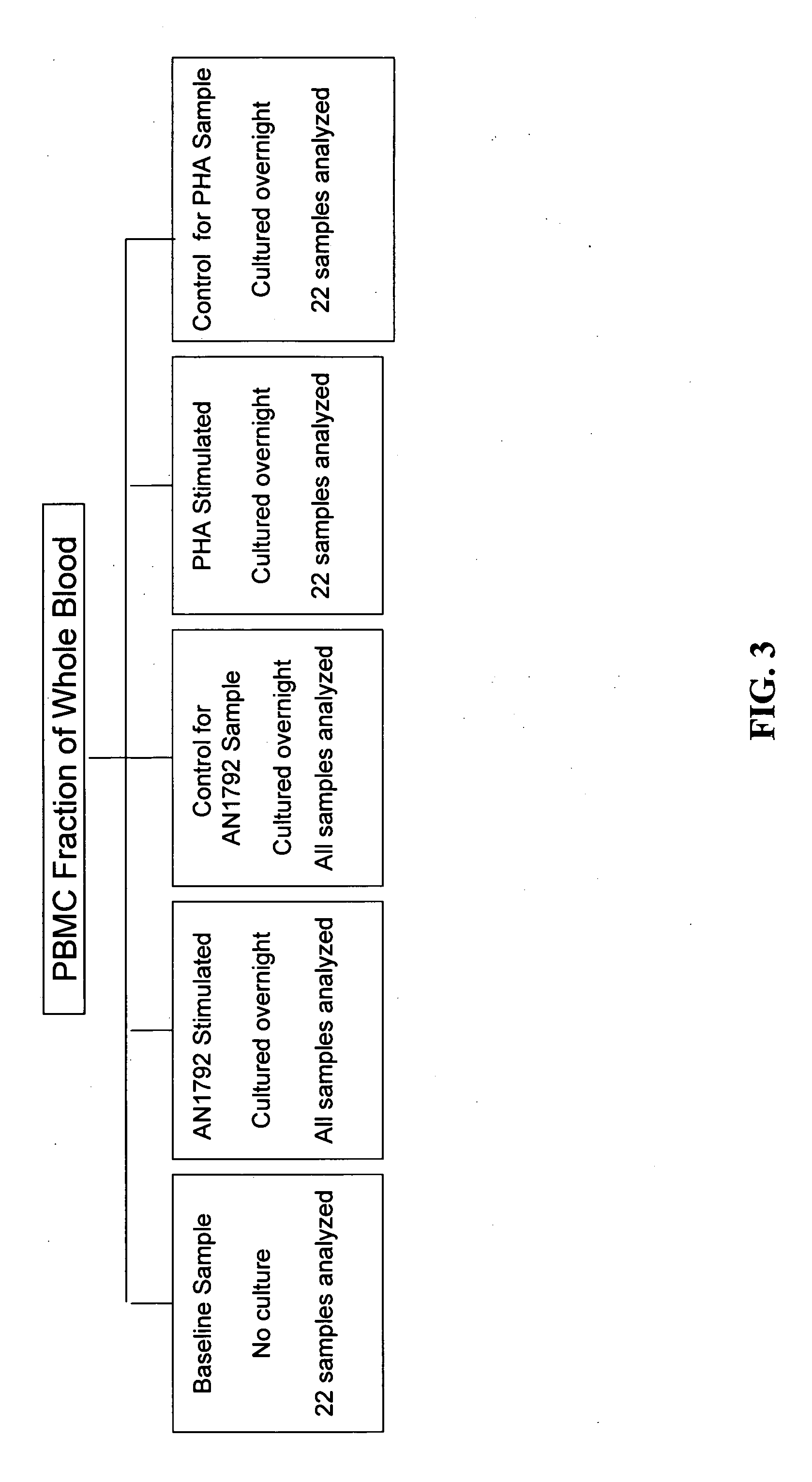
Methods of identifying patients at risk of developing encephalitis following immunotherapy for Alzheimer's disease
InactiveUS20060073496A1Improve accuracyImprove responseData processing applicationsHealth-index calculationSafety profileΒ amyloid
The present invention generally relates to a method for an improved treatment for Alzheimer's disease (AD) using immunotherapy, e.g., immunotherapy targeting β amyloid (Aβ), e.g., immunotherapy based on AN1792. In one embodiment, the method allows for predicting an adverse clinical response, and therefore allows for an improved safety profile of AN1792. In another embodiment, the method allows for predicting a favorable clinical response, and therefore allows for an improved efficacy profile of AN1792. The methods of the present invention may be combined to predict a favorable clinical response and the lack of an adverse clinical response.
Owner:WYETH LLC +1
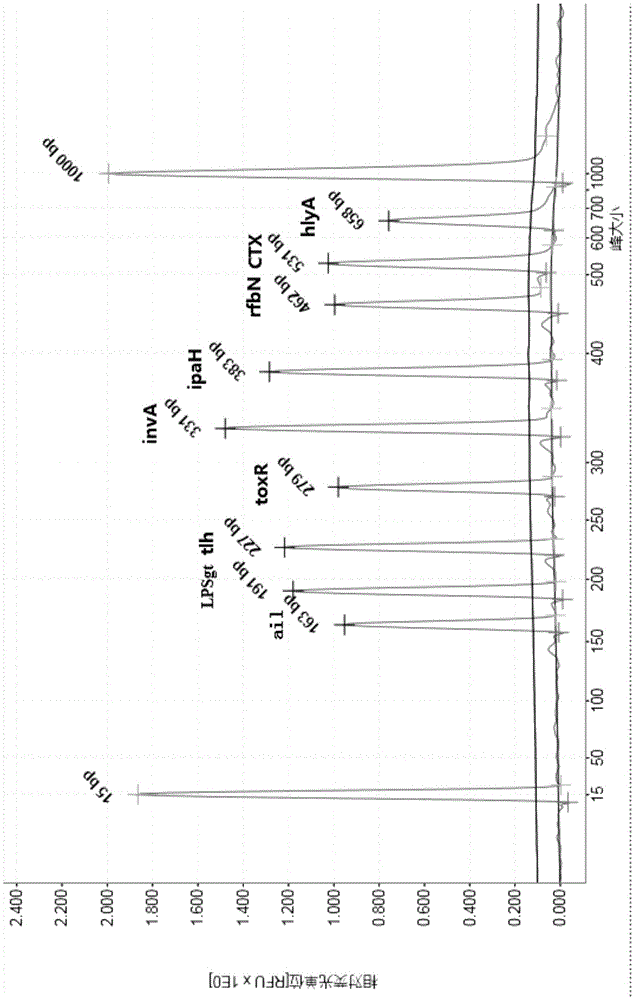
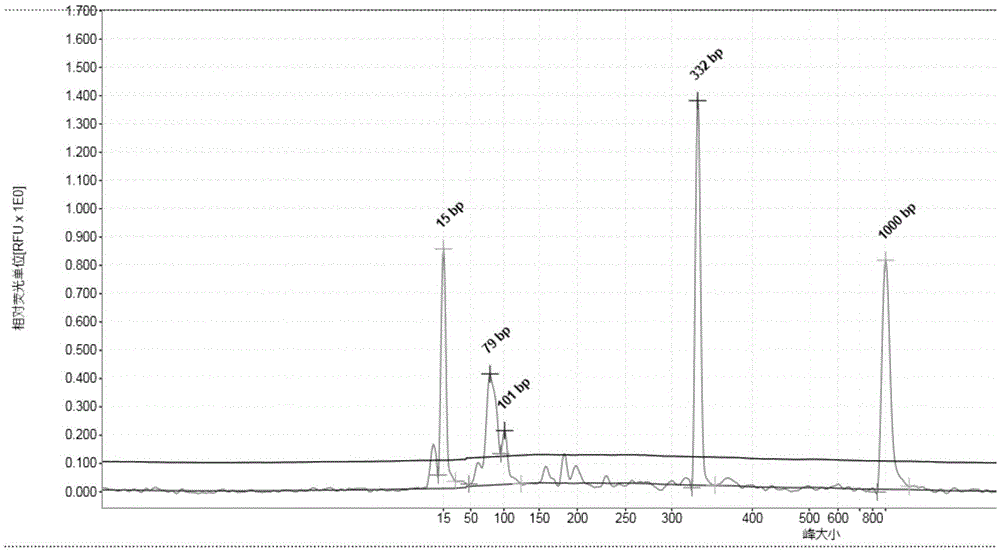
Multiple PCR detection kit for 11 intestinal pathogen nucleic acid and application of detection kit
ActiveCN105525031ASuitable for emergency testingReduce testing costsMicrobiological testing/measurementMicroorganism based processesEscherichia coliVibrio parahaemolyticus
The invention discloses a multiple PCR detection kit for 11 intestinal pathogen nucleic acid and application of the detection kit. The detection kit comprises primers for amplifying 11 intestinal pathogens which include vibrio cholerae serotype O1, vibrio cholerae serotype O139, salmonella, Shigella, vibrio parahaemolyticus, Yersinia enterocolitica, enterophathogenic escherichia coli (EPEC), enteroinvasive escherichia coli (EIEC), enteroaggregative escherichia coli (EAEC), enterotoxigenic escherichia coli (ETEC) and escherichia coli O157:H7. The multiple PCR detection kit has the advantages that the kit is capable of performing multiple detection, high in sensitivity and fast and convenient to use; specific primer sequences are used to guarantee detecting result reliability; the detection method is simple to operate, time saving, labor saving, high in detection throughput, low in reagent consumable cost, capable of directly detecting the nucleic acid extracted from encephalitis pathogens, low in detection platform and staff technical level requirements and capable of being widely popularized in conventional detection.
Owner:NANJING MOKOBIO BIOTECH +2
A group of antigen epitope polypeptide and uses thereof
InactiveCN101492495AViral antigen ingredientsMicroorganism based processesCarrier proteinB-Cell Epitopes
The invention discloses a set of epitope polypeptides, particularly relates to a neutralizing B cell epitope sequence of encephalitis B virus E protein, and further discloses application of controlling and diagnosing encephalitis B virus by these epitopes. The amino acid sequences of the epitope polypeptides in the invention are respectively any amino acid sequence of SEQ ID NO: 64, SEQ ID NO: 79, SEQ ID NO: 95, SEQ ID NO: 108, SEQ ID NO: 124 and SEQ ID NO: 139. After the epitope polypeptides in the invention are coupled or fused with carrier proteins to be expressed as immunogenic or vaccine immuno animal organism, neutralizing antibodies aiming at JEV can be generated, and the JEV can be neutralized in vivo or in vitro so as to prevent virus from infecting animal organism. The epitope polypeptides in the invention or the junctional complex thereof can be used as reagents for detecting encephalitis B virus antibodies or encephalitis B virus polypeptide antibodies.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Hsv-1 and hsv-2 vaccines and methods of use thereof
ActiveUS20110177125A1Reduce morbidityReducing pathogenesisSenses disorderNervous disorderVaccinationGenital ulcer
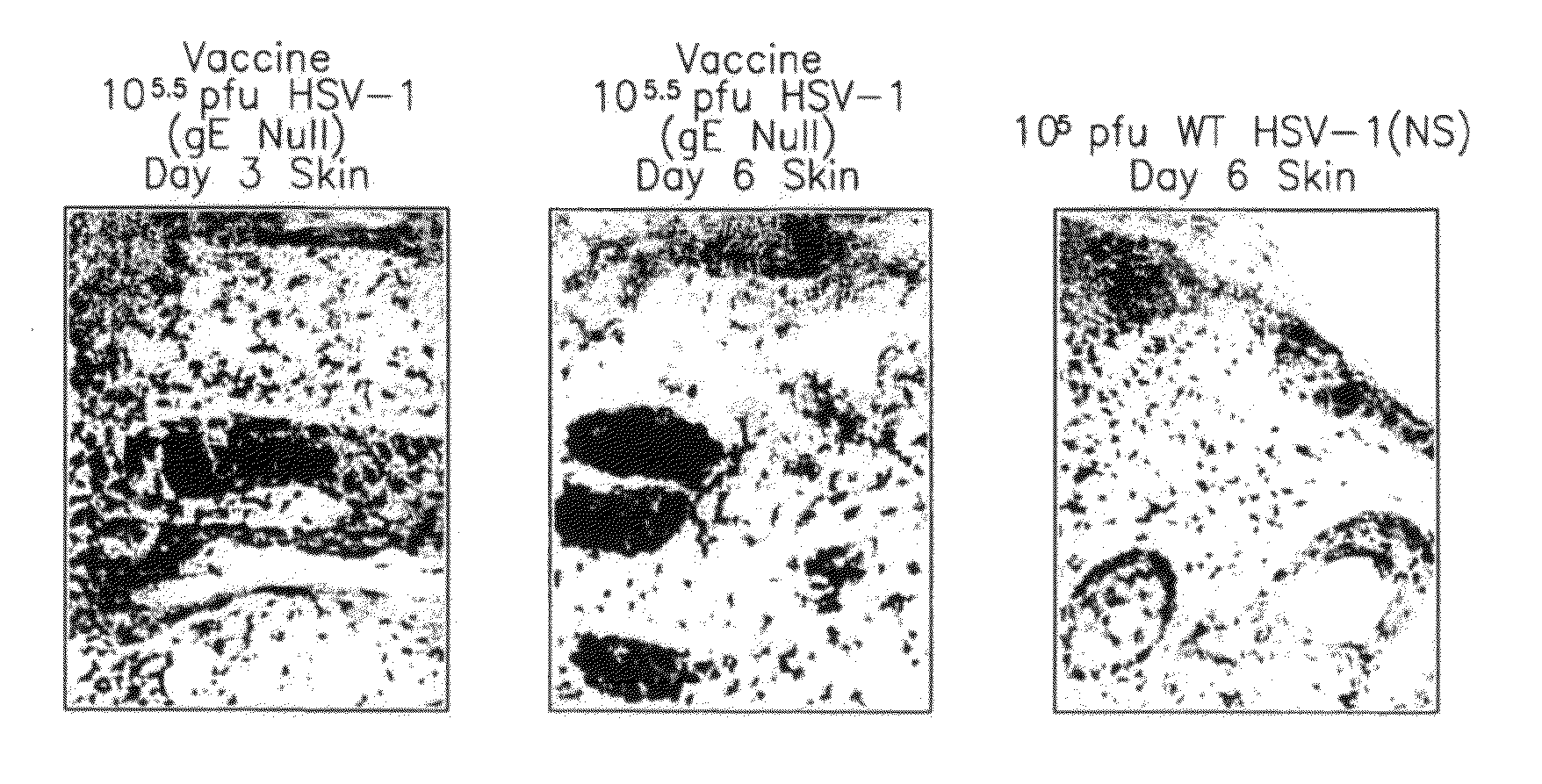
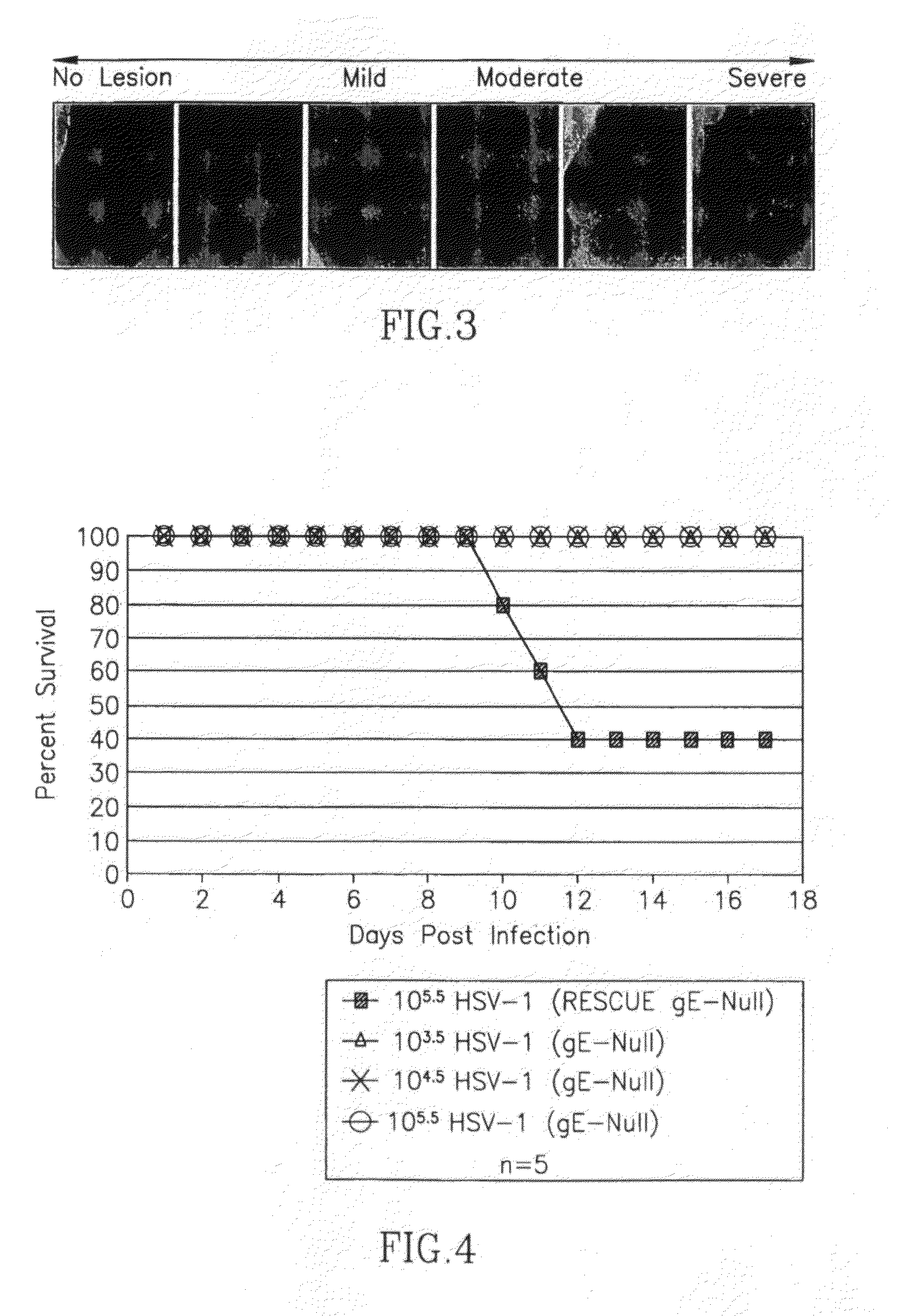
This invention provides methods of vaccinating a subject against a Herpes Simplex Virus (HSV) infection and disorders and symptoms associated with same, and impeding, inhibiting, reducing the incidence of, and suppressing HSV infection, neuronal viral spread, formation of zosteriform lesions, herpetic ocular disease, herpes-mediated encephalitis, and genital ulcer disease in a subject, comprising the step of contacting the subject with a mutant strain of the HSV, containing an inactivating mutation in a gene encoding a gE, gl, Us9, or other proteins.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Specific B cell epitope polypeptide of NS1 protein of encephalitis B virus and use thereof
ActiveCN102206249AStrong specificityReduce false positive ratePeptidesGenetic engineeringMolecular ImmunologyScreening method
The invention discloses the sequence of a specific B cell epitope polypeptide of an NS1 protein of encephalitis B virus and the use of the epitope polypeptide in the diagnosis of encephalitis B virus and a screening method of specific B cell epitope polypeptide of NS1 protein of encephalitis B virus, and belongs to the field of molecular immunology. The amino sequence of the epitope polypeptide is represented by SEQ ID No.1 or SEQ ID No.2. The specific B cell epitope synthetic polypeptide of the JEV NS1 protein, the coupling antigen of the synthetic epitope polypeptide and the epitope fusion expression protein can be used for specifically detecting a JEV NS1 protein antibody generated in body which is immunized and infected. The epitope polypeptide can be used for diagnosis of JEV infection, evaluation on immunity effect and identification and diagnosis of immunity of inactivated vaccine and natural infection.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
'Xingnaojing' dripping pills for treating cephalitis and hepatic coma and preparation process thereof
InactiveCN1634145AQuality improvementDefinite curative effectMammal material medical ingredientsDrug compositionsJapanese encephalitisDisease
The invention discloses a pharmaceutical composition for treating diseases including epidemic type B encephalitis and hepatic coma, which has the advantages of wherein the bolus has the advantages of high biological availability, quick-speed medicine release, quick-speed effect, less toxic and side effects, higher medicinal content, smaller amount of administration, accurate administration dosage, easy administration, low price, and facilitated carrying. The preparation is prepared through the conventional injection extracting processes.
Owner:SHANDONG WOHUA PHARMACEUTICALS CO LTD
Anti-herpes simplex virus I-form medicament composition and uses thereof
InactiveCN101322714AOrganic active ingredientsDigestive systemStructural formulaHerpes simplex disease
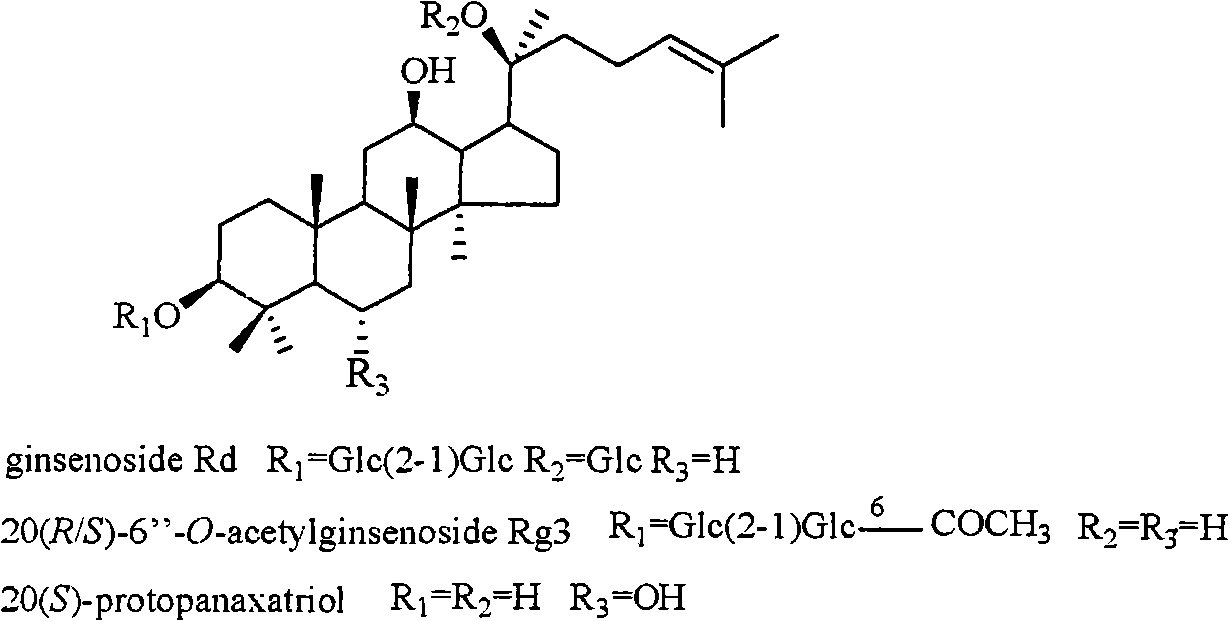
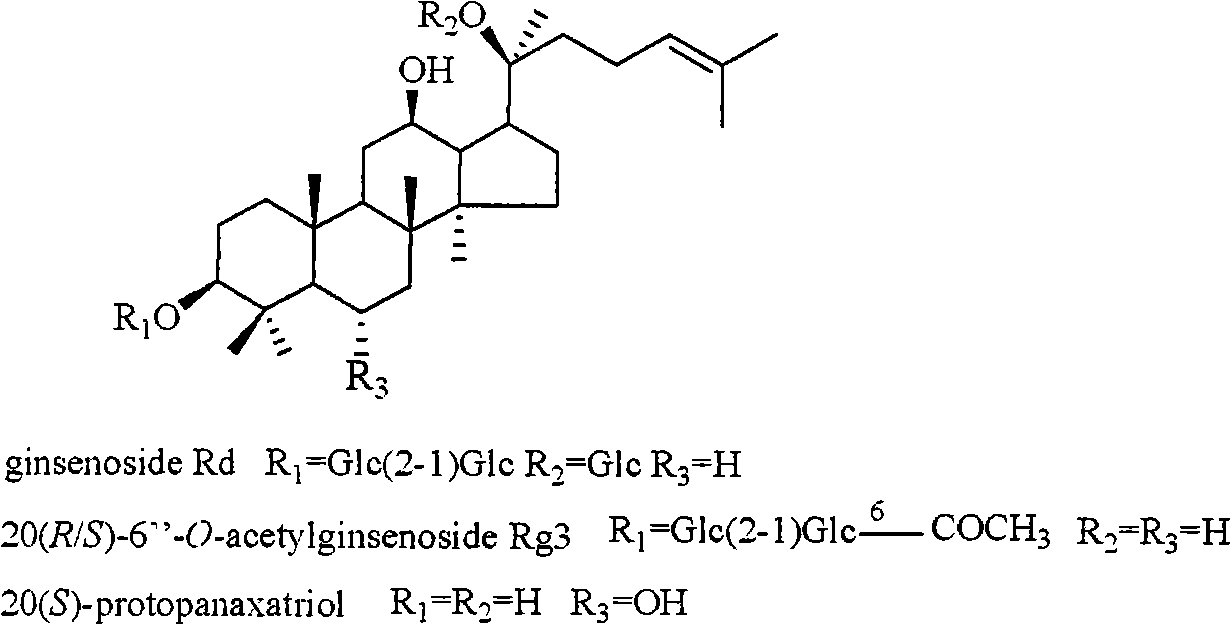
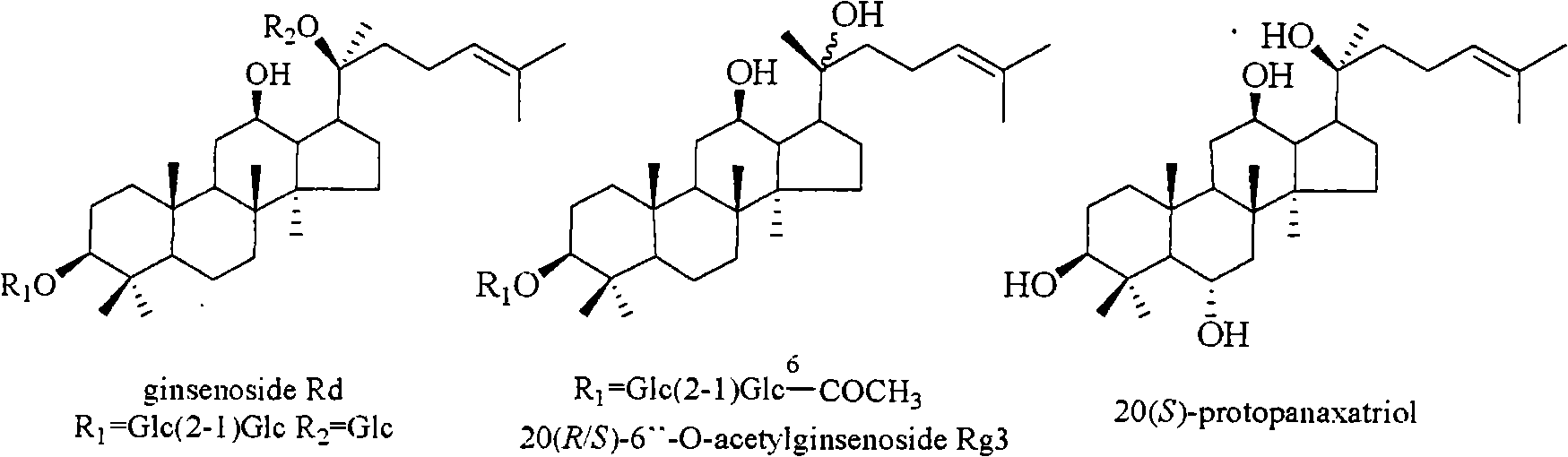
The invention relates to a pharmaceutical composition with dammarane-type tetracyclic triterpene compound as an active component and an application thereof to the pharmaceutical field. Panax notoginseng is separated to obtain three dammarane-type tetracyclic triterpene compounds as shown in the following structural formula, in vitro pharmacological experiments show that the pharmaceutical composition has good in vitro inhibitory activity for herpes simplex virus type I and can be applied to the preparation of medicines for resisting herpes simplex virus type I and is used for treating herpetic keratitis, encephalitis, pneumonia, ulcerative stomatitis, blister and the like caused by herpes simplex virus type I.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI
Preparation method of room air virus pathogenic-bacteria jinx
ActiveCN101491243AResolve inhibitionSolving the Killing ProblemBiocideGaseous substancesBacterial virusStaphylococcus aureus
The invention discloses a method for preparing an indoor air virus pathogen invincible opponent. The indoor air virus pathogen invincible opponent prepared by menthol, thymol, weeping forsythia oil and radix bupleuri oil has the killing rate of between 76 and 99.9 percent to idemic encephalitis, influenza viruses, hand-foot-and-mouth disease, avian influenza, legionnella, pneumoniae, hepatitis C viruses, hepatitis A viruses, Staphylococcus albus, Staphylococcus aureus, Klebsiella pneumoniae, Candida albicans, typhoid viruses, Escherichia coli, cholera viruses, infectious germs and bacteria, and harmful germs and bacteria. The indoor air virus pathogen invincible opponent is mainly applied to rooms, schools, hospitals, hotels, air conditioners, automobiles, public places, and the like, and has obvious effect on the control of diseases such as influenza, upper respiratory tract infection. The indoor air virus pathogen invincible opponent has outstanding substantive characteristics and broad application prospect.
Owner:上海钮爱环保科技有限公司
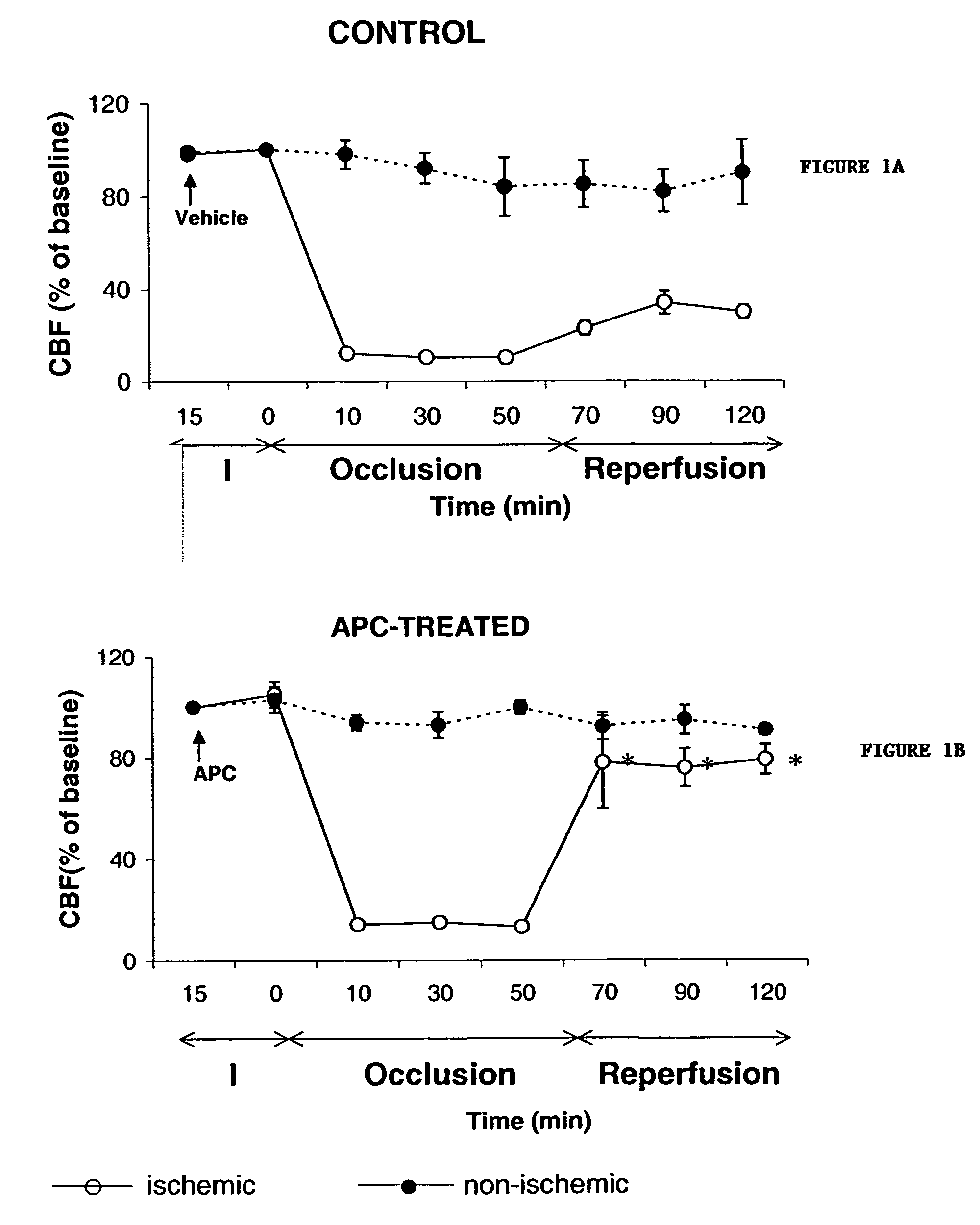
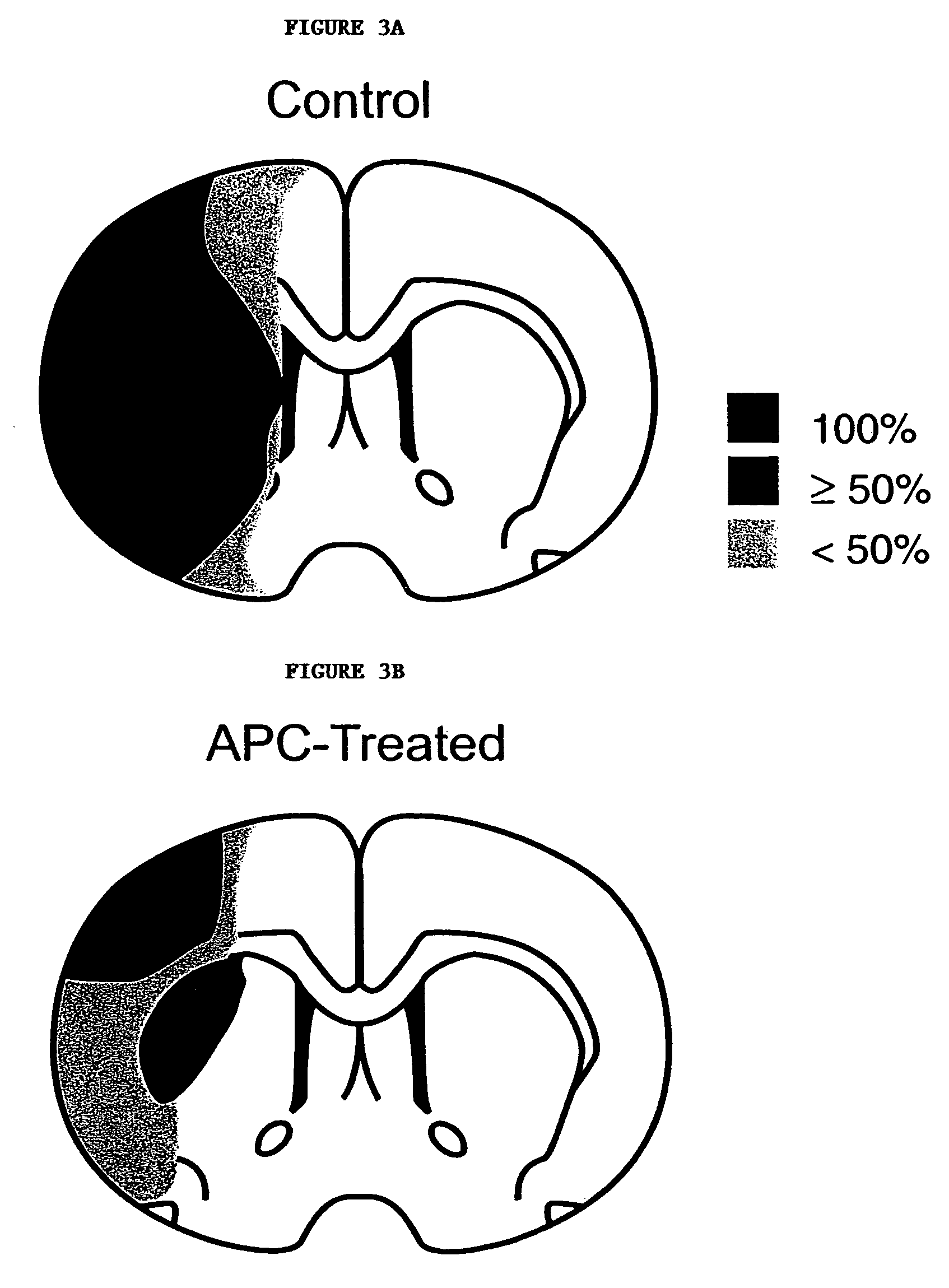
Neuroprotective, antithrombotic and anti-inflammatory uses of activated protein C (APC)
InactiveUS7074402B2Reduce inflammationReduce neuroinflammationBiocideNervous disorderRisk strokeDisease cause
The present invention provides methods for treating subjects having or at risk of having a neuropathological disorder or brain inflammatory diseases with and without vascular involvement, and systemic inflammatory vascular disease by administering a therapeutically effective amount of Activated Protein C (APC) to the subject. Brain disorders and brain inflammatory vascular diseases that can be treated by the invention method include all neurodegenerative diseases with different types of neuronal dysfunction, including stroke, Alzheimer's disease, Parkinson's disease, Huntington disease, neuroimmunological disorders such as multiple scelrosis and Gullian-Barre, encephalitis, meningitis, as well as other peripheral vascular diseases, such as diabetes, hypertension, artheriosclerosis. Also included are methods of treatment using APC in combination with a co-factor, such as Protein S.
Owner:UNIV OF SOUTHERN CALIFORNIA +1
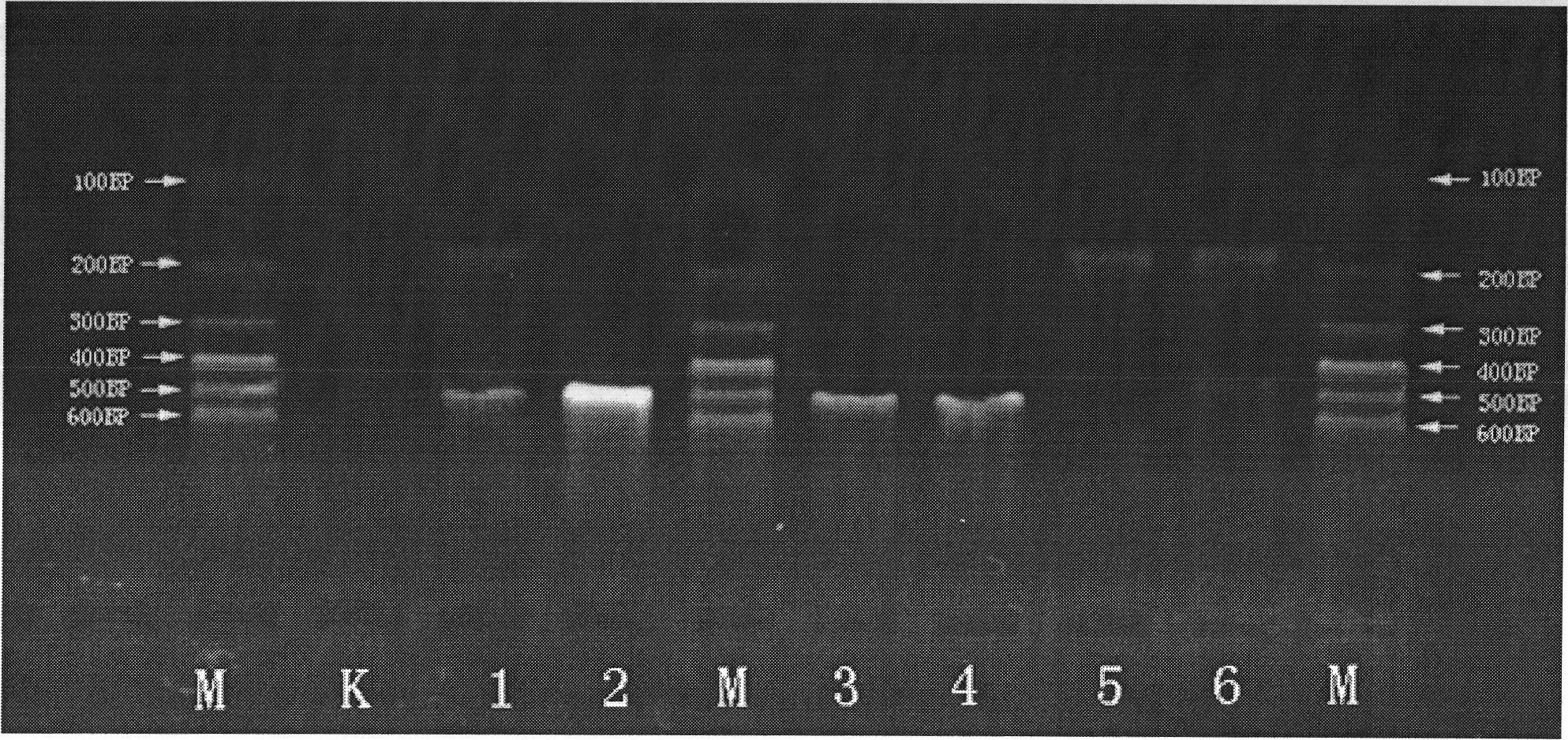
Multiple polymerase chain reaction (PCR) kit and method for detecting mosquito-borne pathogens
InactiveCN101979665AReliable informationEffective early warning dataMicrobiological testing/measurementAgainst vector-borne diseasesTissue fluidMultiplex pcrs
The invention provides a multiple polymerase chain reaction (PCR) kit for detecting mosquito-borne pathogens. The kit comprises six pairs of specific primers. The invention also provides a method for detecting the mosquito-borne pathogens. Electrophoresis is performed on a PCR-amplified product. Whether pathogens, such as encephalitis B virus, dengue fever virus, yellow fever virus, plasmodium falciparum, plasmodium vivax, plasmodium knowlesi, plasmodium ovale, plasmodium malariae, wuchereria malayi, wuchereria bancrofti and the like, exists or not is detected and identified according to the length of a PCR-amplified fragment. By the method, various reported mosquito-borne pathogens, and the yellow fever virus and west nile virus which come from other countries can be detected quickly, accurately and sensitively at the same time, and can be applied to the detection of various samples, such as mosquitoes, blood of patients, tissue fluid and the like. The invention provides a low-cost and high-efficient method for early monitoring and finding mosquito-borne disease prevalence for prevention and control work of mosquito-borne diseases in China.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Fluorescent quantitative RT-PCR detection kit and detection method for enterovirus
InactiveCN101407846ANo cross reactionQuick checkMicrobiological testing/measurementFluorescence/phosphorescenceCerebrospinal fluidHand-foot-and-mouth disease
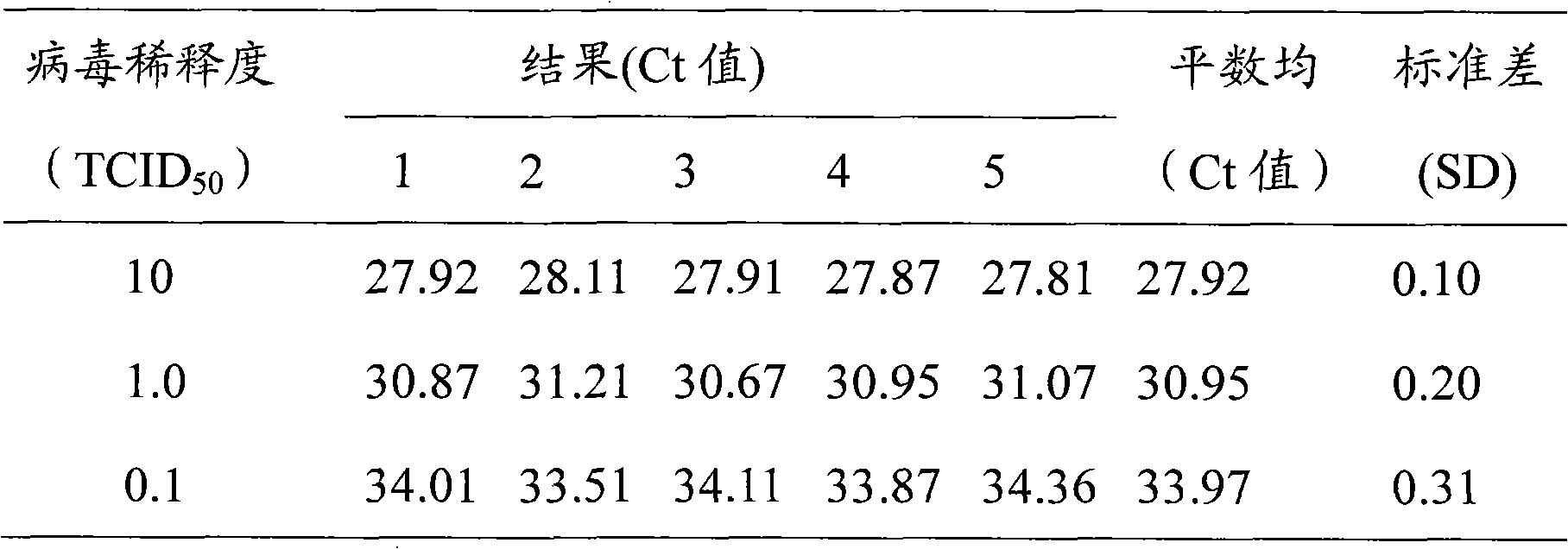
The invention provides a fluorescence quantitative RT-PCR detecting kit for an enterovirus and a detecting method thereof; and the sequences of an upstream primer and a downstream primer and a specific probe of the fluorescence quantitative RT-PCR detecting kit are as follows: the upstream primer EV(YG)F is 5'-GGCTGCGYTGGCGGCC-3', the downstream primer EV(YG)R is 5'-CCAAAGTAGT CGGTTCCGC-3' and the specific probe EV(YG)PB is 5'-CTCCGGCCCCTGAATGCGG-3'. The method has high specificity on detecting the enterovirus and does not have cross reactions with other enteroviruses such as hepatitis A, hives, rubella, parotitis, encephalitis, dengue fever, adenovirus, and the like. The detection sensitivity of the method achieves 0.1TCID50; the method can directly detect the nucleic acid of the enterovirus from the samples of ncurolymph, herpes liquid, dejecta and the like of suspected patients; only about 3h is needed from extracting the nucleic acid of the enterovirus to finishing the detection; and the detecting kit and the detecting method are suitable for the lab early diagnosis of sudden epidemic caused by the infection of the enteroviruses such as the hand-foot-and-mouth disease and the like.
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION
Epidemic encephalitis B/forest encephalitis hybrid virus and application of virus
InactiveCN103352029AImprove securityStable attenuation characteristicsViral antigen ingredientsMicrobiological testing/measurementLethal doseEncephalitis Viruses
The invention discloses an epidemic encephalitis B / forest encephalitis hybrid virus and an application of the virus. The epidemic encephalitis B / forest encephalitis hybrid virus takes an epidemic encephalitis virus attenuated strain as a gene skeleton, and is a recombinant virus obtained by replacing a deoxyribonucleotide fragment of an encoded epidemic encephalitis virus prM-E delta 3 protein in epidemic encephalitis virus genome RNA (Ribonucleic Acid) with a deoxyribonucleotide fragment of an encoded forest encephalitis virus prM-E delta 3 protein in forest encephalitis virus genome RNA. The epidemic encephalitis B / forest encephalitis hybrid virus has the advantages that the virus is high in safety, and strong in stability, and can be induced to generate a good immune response to a forest encephalitis virus envelope protein, and protect an animal from being attacked by the exogenous forest encephalitis virus of lethal dose. The epidemic encephalitis B / forest encephalitis hybrid virus has a good application prospect in preventing and / or treating forest encephalitis virus infection as a vaccine.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
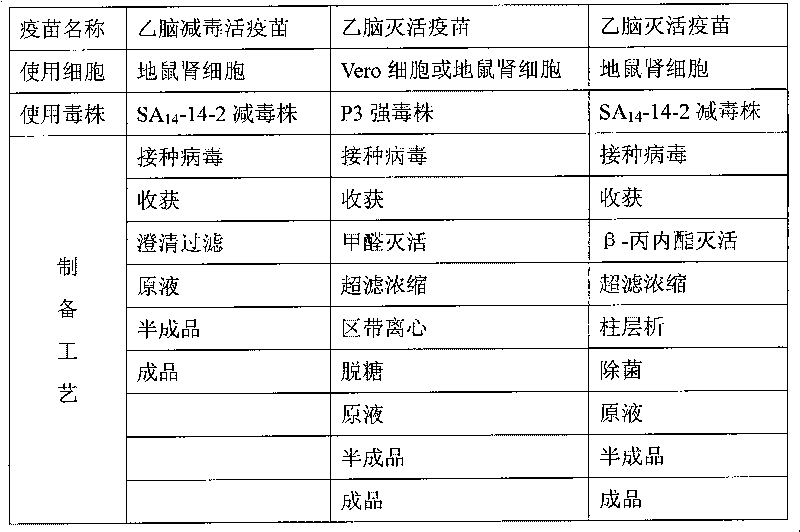
Diploid somatic cell encephalitis B vaccine and method for preparing purified encephalitis B vaccine
ActiveCN101352569ATotal protein content decreasedImprove immunityViral antigen ingredientsAntiviralsJapanese encephalitis vaccineSerum ige
The invention relates to a preparation method for diploid cell Japanese encephalitis vaccine and purified encephalitis vaccine. The method uses a serum-free medium to culture human diploid cell Japanese encephalitis purified vaccine, thus greatly reducing anaphylactic reaction and being beneficial to purification.
Owner:崔栋
Freeze dried isatis root powder for injection and its prepn
InactiveCN1453033AImprove stabilityQuick effectAntibacterial agentsPowder deliveryDiseaseTreatment effect
The present invention discloses one kind of freeze dried isatis root powder for injection, which is prepared with isatis root as main material and through extraction, refining, blending with proper amount of medicinal supplementary material and freeze drying. The present invention has high stability, long effective period, obvious antiseptic, antiviral, heat-clearing away and immunity-strengthening functions, good safety and no negative effect.it has excellent treating effect on influenza, parotitis, hepatitis, encephalitis B, upper respiratory tract infection and other diseases.
Owner:吴梅春
Vaccine for virus of encephalitis B and preparation method
ActiveCN1695736AImproving immunogenicityGood protective effectViral antigen ingredientsAntiviralsAcute viral encephalitisDiploid cells
A vaccine of the encephalitis B virus for preventing viral encephalitis B is prepared through inoculating said virus into human diploid cells, culturing, naturalizing, adapting, amplifying, concentrating and purifying.
Owner:复星安特金(成都)生物制药有限公司
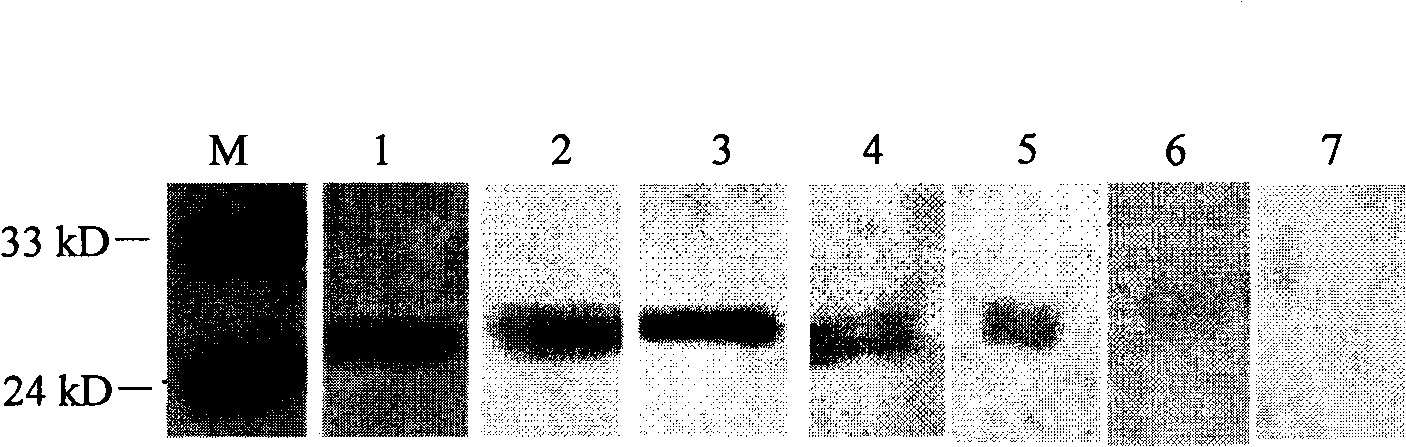
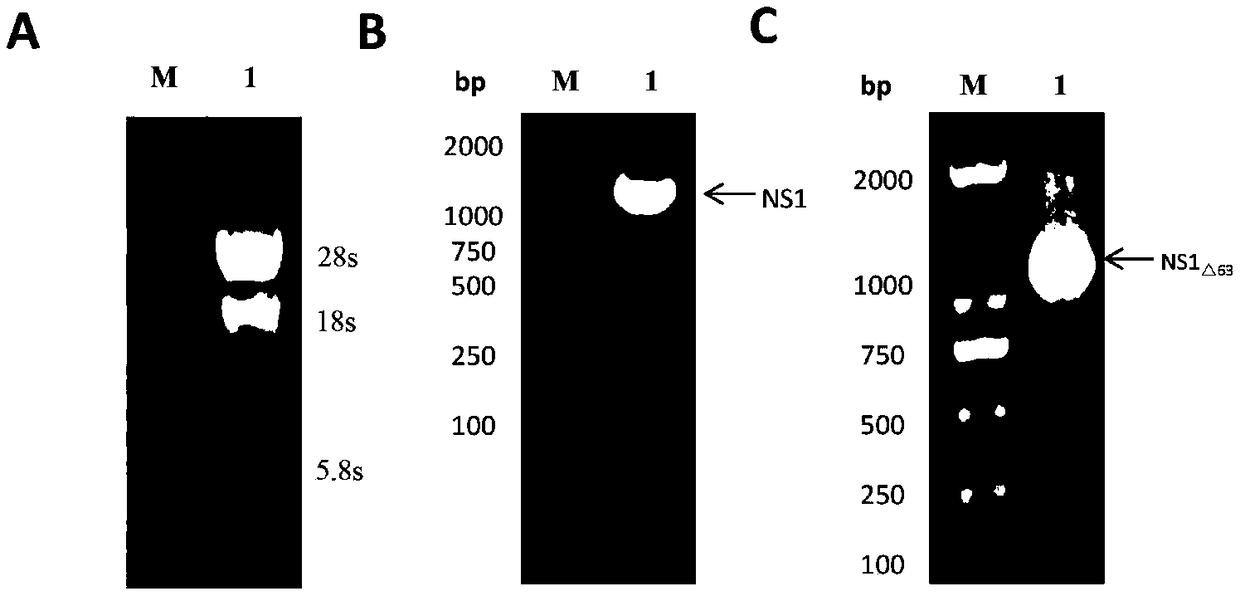
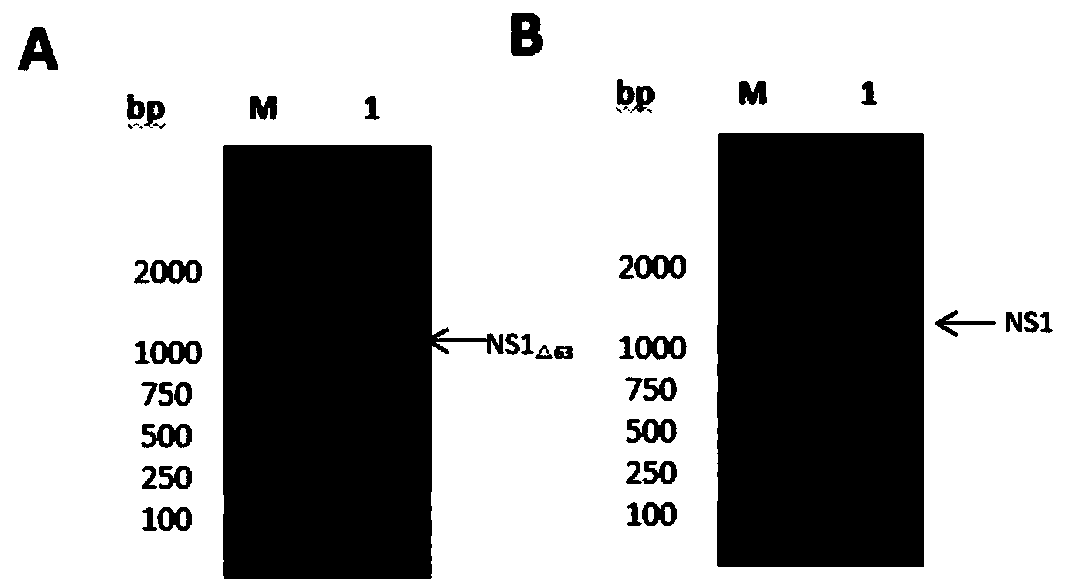
Encephalitis b virus non-structural protein NS1 truncated mutant as well as coding gene and application thereof
ActiveCN108707191AEfficient and stable expressionSsRNA viruses positive-senseViral antigen ingredientsEscherichia coliAgricultural science
The invention relates to an encephalitis b virus non-structural protein NS1 truncated mutant as well as a coding gene and application thereof, which belongs to the technical field of viruses. According to the encephalitis b virus non-structural protein NS1 truncated mutant, on the basis of original NS1, two strong hydrophobic zones on an end C are deleted, a truncated mutant NS1 delta63 gene with63 amino acids on the end C being deleted, then the truncated mutant NS1 delta63 gene is cloned to a prokaryotic expression vector pET-28a(+), a recombinant expression vector pET28a-NS1delta63 is obtained, escherichia coli is converted, and by virtue of the induction of IPTG, the NS1 delta63 can be efficiently and stably expressed in the escherichia coli. The NS1 delta63 protein expressed in the Escherichia coli purifies and immunizes a mouse, and the NS1 anti-serum titer of the immunized mouse reaches 1:12150 by virtue of ELISA detection. The protection capability test of the immunized mouseproves that the NS1 delta63 has the protection activity. The NS1 delta63 can lay a foundation for later research of the virus vaccine and diagnosis kit.
Owner:JIANGXI AGRICULTURAL UNIVERSITY
Amide derivative
There is provided a pharmaceutical drug, particularly a novel compound useful for prophylaxis or a therapeutic treatment of various diseases involving infections with viruses of the herpesvirus family, specifically various herpesvirus infections such as varicella (chicken pox) via varicella zoster virus, varicella zoster via recurrent infection with latent varicella zoster virus, herpes labialis and herpes encephalitis via HSV-1 and genital herpes via HSV-2 infection. An N-{2-[(4-substituted phenyl)amino]-2-oxoethyl}tetrahydro-2H-thiopyran-4-carboxamide derivative, of which phenyl group is substituted at position 4 with a specific 5- or 6-membered heteroaryl group, and a salt thereof have an effective anti-virus activity, and the oral administration thereof at a low dose enabled the therapeutic treatment of the above diseases.
Owner:ASTELLAS PHARMA INC
Detection material resisting NMDAR autoantibodies in human body fluid, preparation method and application
InactiveCN110606887AIncrease concentrationIncreased sensitivityPeptide preparation methodsNucleic acid vectorHuman bodyAntigen
The invention discloses a detection material resisting NMDAR autoantibodies in human body fluid and a preparation method and application thereof. According to the invention, NMDAR antigens are arranged on a carrier membrane through coating to prepare an anti-NMDAR-receptor detection material, specific NMDAR antibodies in human serum and cerebrospinal fluid can be combined with the antigens, alkaline phosphatase substrate-ligand reaction is utilized for color development, the sealing material is added in the color development reaction, and whether a detected sample contains the NMDAR antibodiesor not can be directly judged through naked eye observation. The method has the advantages of high sensitivity, easy and convenient operation and rapid detection, and is conducive to recognition anddiagnosis of anti-NMDAR-receptor encephalitis.
Owner:SHAANXI MYBIOTECH CO LTD
Zika virus and encephalitis B virus combined inactivated vaccine
InactiveCN108187036AStable physical and chemical propertiesReduce the number of vaccinationsSsRNA viruses positive-senseViral antigen ingredientsZika virusDisease
The invention provides a zika virus and encephalitis B virus combined inactivated vaccine, and belongs to the technical field of preparation of biological products. The combined vaccine consists of 0.5-10ug / ml of encephalitis B virus and 0.5-10ug / ml of zika virus. The invention also provides a preparation method of the zika virus and encephalitis B virus combined inactivated vaccine; the preparation method comprises steps of inoculating, purifying and inactivating the encephalitis B virus and the zika virus, wherein inoculated MOI of the encephalitis B virus is 0.001-0.1CCID50 / ml; and inoculated MOI of the zika virus is 0.001-0.1CCID50 / ml. The vaccine provided by the invention can be used for simultaneously immunizing the zika virus and the encephalitis B virus, so that immunizing times are reduced and infection and allergic reactions caused by attenuated vaccines are avoided; and the combined inactivated vaccine has a good capacity of preventing and controlling diseases caused by thezika virus and the encephalitis B virus.
Owner:SINOVAC RES & DEV
Incense for eliminating plague
The invention discloses incense for eliminating plague, which is prepared from Chinese atractylodes rhizome, dried duckweed, dahurian angelica root, argyi leaf, realgar, nard, kaempferia galamga, nutgrass galingale rhizome and sweet wormwood. The incense can dispense fragrance so as to sterilize air, purify filth, clear away heat and toxic material such as influenza virus, encephalitis B virus and the like, kill bacteria such as tubercule bacillus, Klebs-Loeffler bacillus, streptococcus hemolyticus, and the like. After the incense is fired, the generated smoke has no harm and toxic and side effects to persons and livestock, the smelt is pure and mild, no peculiar smell is emitted, and the incense can be used for prevention of infectious diseases, evil influence, epidemic virus, pestilence, arashiyama and influenza virus and sterilization in stations, schools, hospitals, hotels and large cultivation farms.
Owner:南通市海门江海建设投资有限公司
Neutral B cell antigen epitope polypeptide of encephalitis b virus E protein and uses thereof
InactiveCN101333246AAvoid infectionViral antigen ingredientsPeptidesMolecular ImmunologyJapanese encephalitis virus Antibody
The invention discloses a polypeptides epitope in Japanese encephalitis virus E protein neutralizing B cell, also discloses the application of the polypeptides epitope in the prevention, treatment and diagnosis of Japanese encephalitis, belonging to the field of molecular immunology. The amino acid sequence of the polypeptides epitope disclosed in the invention is shown in SEQ ID NO: 1 or SEQ ID NO: 2. After being used as immunogen or vaccine immune animal organisms, the JEV E protein neutralizing B cell polypeptides epitope of the invention can produce neutralizing antibody against Japanese encephalitis virus, and can neutralize the Japanese encephalitis virus in vivo or in vitro so as to prevent the virus infecting animal organisms. The polypeptides epitope of the invention can immunise animals if being self-connected or inter-connected with carriers; the anti-peptide antibodies or the anti-Japanese encephalitis virus antibodies produced after the immunising of animals can neutralize the Japanese encephalitis virus in vitro and in vivo and generate immune protection.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Preparation method of vaccine for epidemic encephalitis B
InactiveCN101732708AImprove securityAvoid damageViral antigen ingredientsInactivation/attenuationSodium bicarbonateViral culture
The invention relates to a preparation method of a vaccine for epidemic encephalitis B, which comprises the processes of cell culture, virus propagation and vaccine preparation. After cells overgrow with a single layer, a seed culture of viruses and a culture solution are inoculated, and the use quantity of the seed culture of the viruses is that every 100cm<2> of cells is inoculated with 1ml of seed culture of the viruses. The culture solution of the viruses mainly comprises 90-95% of MEM culture solution, 3-5% of calf serum and 7.5% of sodium bicarbonate solution the pH of which is regulated to 7.2-7.6. The vaccine is prepared by adding human serum albumin with the final concentration of 0.3% after being clarified and filtered. The invention adopts an SA14-14-2 attenuated strain to produce an encephalitis purified vaccine. Compared with a vaccine produced by a P3 strain, the attenuated strain is safer and more effective. The invention has the advantages that: (1) the attenuated strain is used for preparing the purified vaccine, which has good safety; (2) the inactivation effect of inactivating the viruses by using beta-propiolactone is good, and an inactivator is naturally degraded and has no residue; and (3) the column chromatography purification property is mild, and the injury to the viruses is small.
Owner:ZHEJIANG TIANYUAN BIO PHARM CO LTD
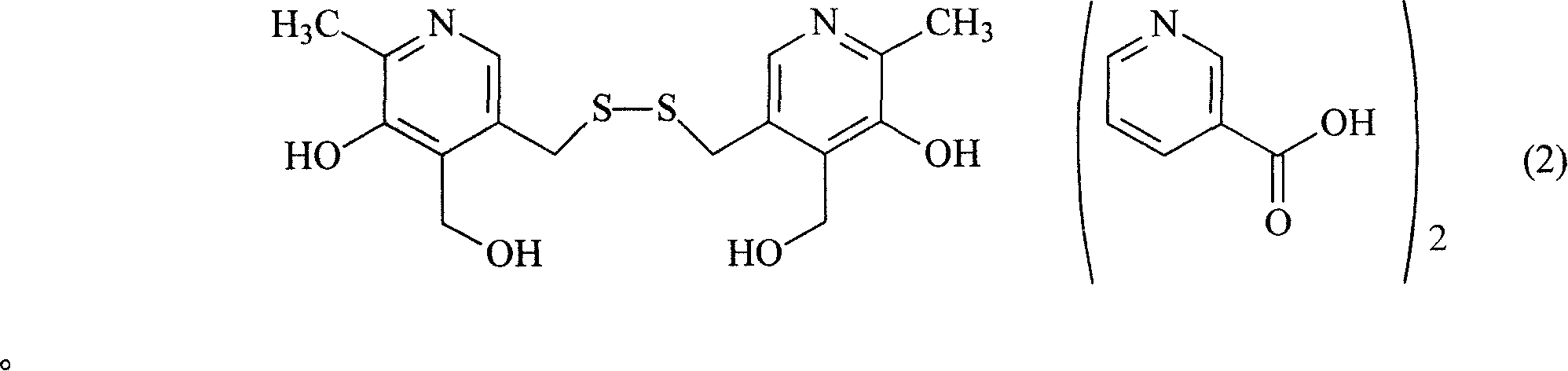
Organic salt of pyritinol and its prepn process
InactiveCN101066266ASolve solubilityResolve irritationOrganic active ingredientsNervous disorderHydroxybutyric acidSenile dementia
The present invention relates to organic salt of pyritinol as shown for treating cerebral concussion, brain trauma, encephalitis, meningitis sequelae and senile dementia. The organic salt exists in hydrate and is named as 3, 3-(dithio methylene) bis(5-hydroxy-6-methyl-pyridyl methane) organic salt chemically. The organic salt is salt of nicotinic acid, lactobionic acid, malic acid, etc. Experiments show that the organic acid and pyritinol have synergistic effect of protecting cardiac muscle, and the nicotinate of pyritinol is especially hopeful in developing pyritinol medicine.
Owner:GUANGDONG ZHONGKE DRUG R&D
Vero cell fick-borne encephalitis inactivated vaccine
InactiveCN1513553AHave effectSafeViral antigen ingredientsAntiviralsCulture fluidEncephalitis Viruses
An deactivated vero cell vaccine for forest encephalitis virus is composed of the deactivated forest encephalitis virus and vaccine adjuvant. Its preparing process includes inoculating the forest encephalitis virus to vero cells, culturing to obtain the culturing liquid, purifying, and preparing said vaccine. Its advantages are high purity, high immunizing effect and high safety.
Owner:长春生物制品研究所有限责任公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com