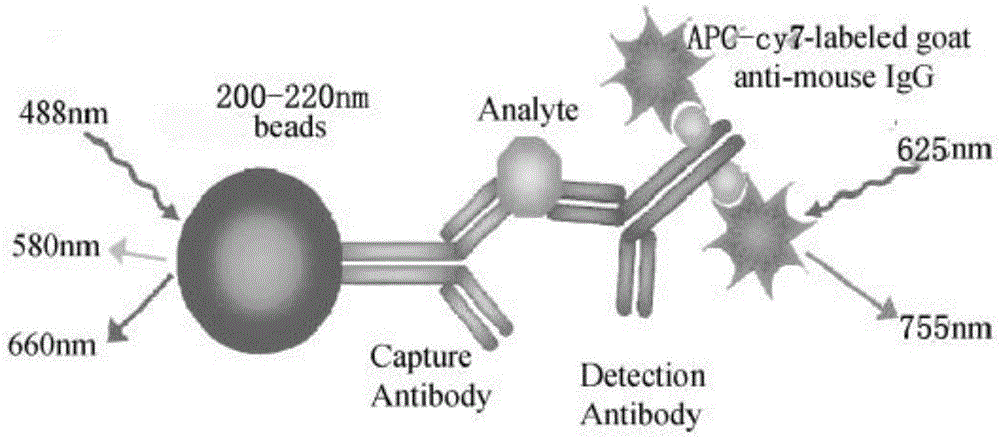
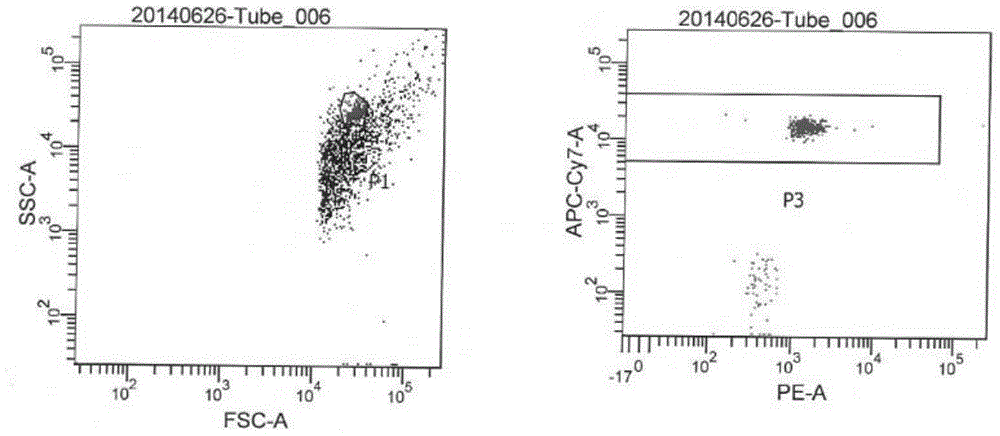
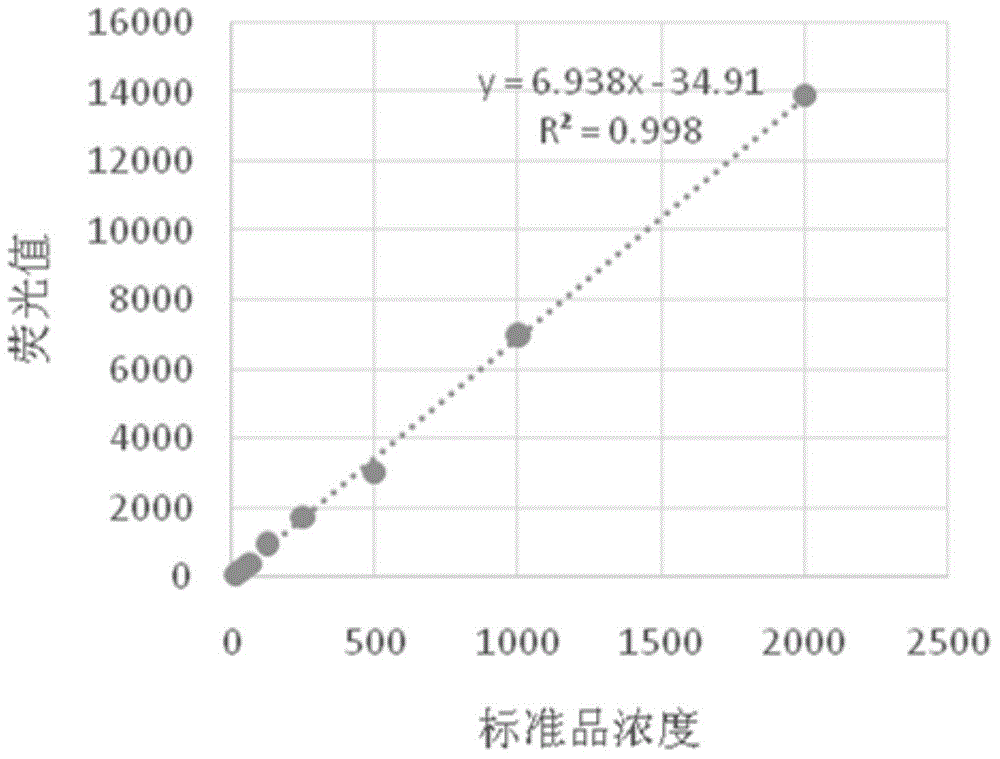
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
97results about How to "Reduced risk of cross-contamination" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
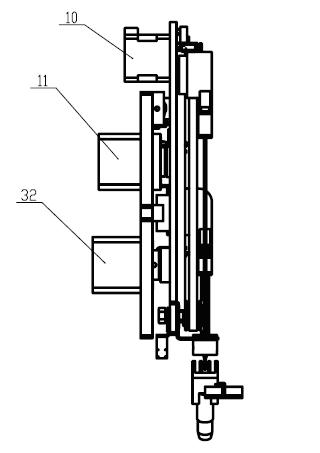
Automatic micro-droplet array screening system using method with pico-liter-scale precision
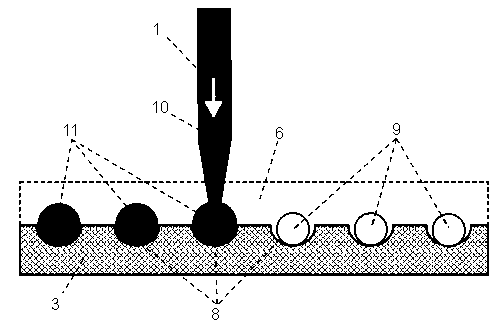
ActiveCN103008037AReduce adsorptionReduced activityLaboratory glasswaresMaterial analysisPhysical chemistryCapillary Tubing
The invention relates to the field of high-throughput screening and particularly relates to an automatic micro-droplet array screening system using method with pico-liter-scale precision. The using method provided by the invention comprises the following steps of: firstly, using liquid with a low thermal expansion coefficient and filled in a liquid driving system and a capillary tube as carrier liquid, and entirely emptying bubbles inside the capillary tube; secondly, soaking a sampling end of the capillary tube in an oil phase sample immiscible with a water phase sample, extracting a section of oil phase sample into the capillary tube, so as to isolate the water phase sample and the carrier liquid; thirdly, soaking the sampling end of the capillary tube into a sample / reagent storage tube and extracting a certain volume of the water phase sample solution into the capillary tube; and finally, moving the sampling end of the capillary tube into the oil phase sample above micro-pores of a micro-porous array chip, and pushing the sample solution in the capillary tube into the micro-pores, so as to form sample droplets. With the adoption of the using method provided by the invention, the liquid is quantitatively measured, and the generated droplets have the pico-liter-scale volume precision, so that the consumption of the samples / reagents in the high-throughput screening can be effectively reduced, and the experiment cost can be saved.
Owner:ZHEJIANG UNIV
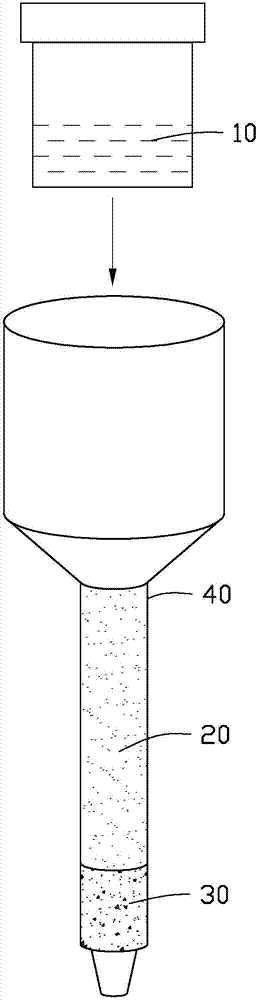
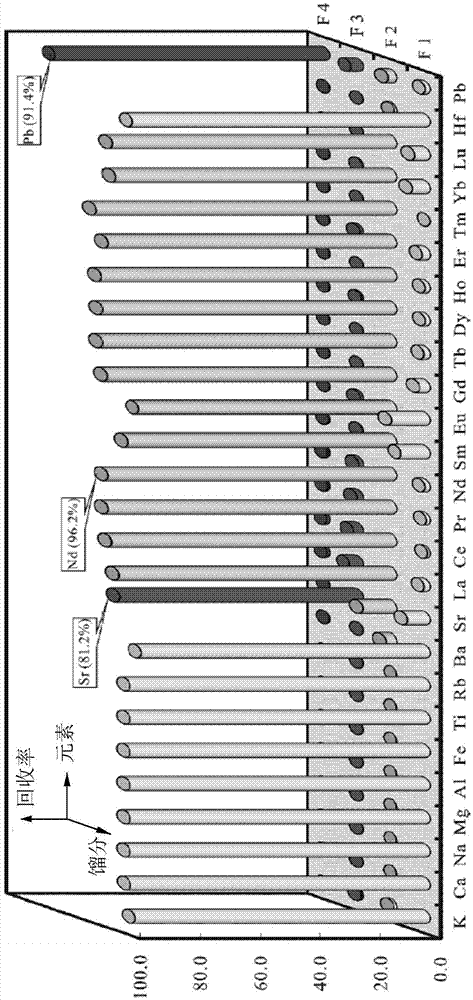
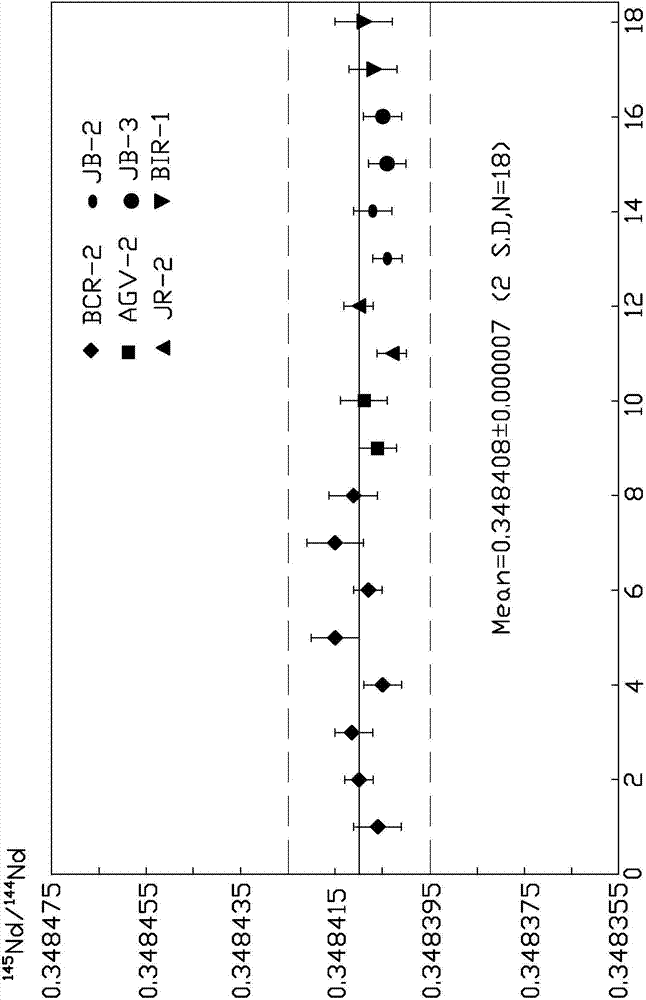
Method for one-step column separation of Sr, Nd and Pb in geologic sample
ActiveCN104713757ASimple and fast operationImprove efficiencyComponent separationPreparing sample for investigationOrganic solventStrong acids
The invention provides a method for one-step column separation of Sr, Nd and Pb in a geologic sample. The method comprises the following steps: dissolving the geologic sample to obtain a sample solution; and chemically separating, allowing the sample solution to go through an exchange column supported with A cation resin and Sr specific resin to respectively separate Sr, Nd and Pb, wherein the A cation resin is positioned at the upper layer of the exchange layer, the Sr specific resin is positioned at the lower layer of the exchange column, the A cation resin is a strong acid cation exchange column containing a sulfonic acid exchange group and adopting a styrene-divinyl benzene copolymer as a skeleton, the cross-linking degree of the A cation resin is 12%, and the Sr specific resin is an inert resin support supported with a solution formed by dissolving dicyclohexyl crown ether in a liquid organic solvent.
Owner:INST OF GEOLOGY & GEOPHYSICS CHINESE ACAD OF SCI
Method, primer group and kit for capturing novel coronavirus whole genome
ActiveCN111118226AEasy to operateShorten the timeMicrobiological testing/measurementAgainst vector-borne diseasesMolecular biologyRNA
The invention relates to a super-sensitivity method, primer group and kit for capturing whole genome of novel coronavirus. The method can conveniently and quickly amplify the whole genome of the novelcoronavirus by using a small amount of RNA, and can directly dock with a second-generation sequencing library-building reagent and a third-generation sequencing platform so as to obtain a whole genome sequence of the novel coronavirus.
Owner:北京微未来科技有限公司
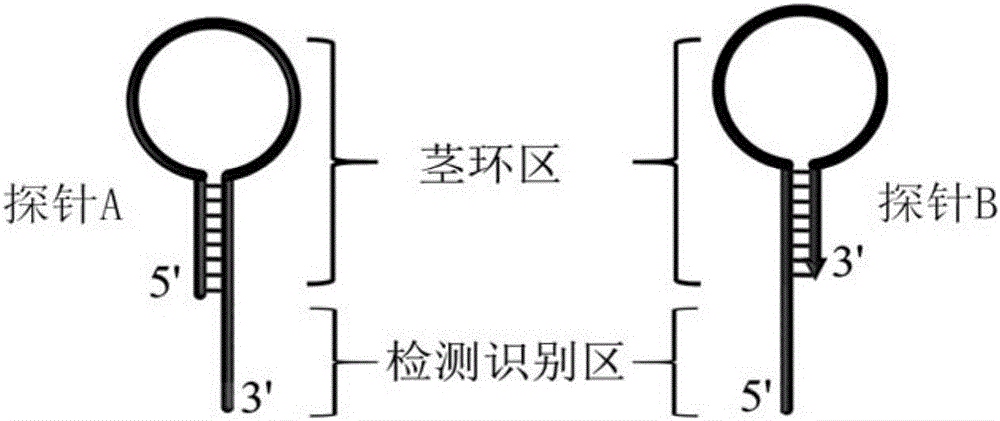
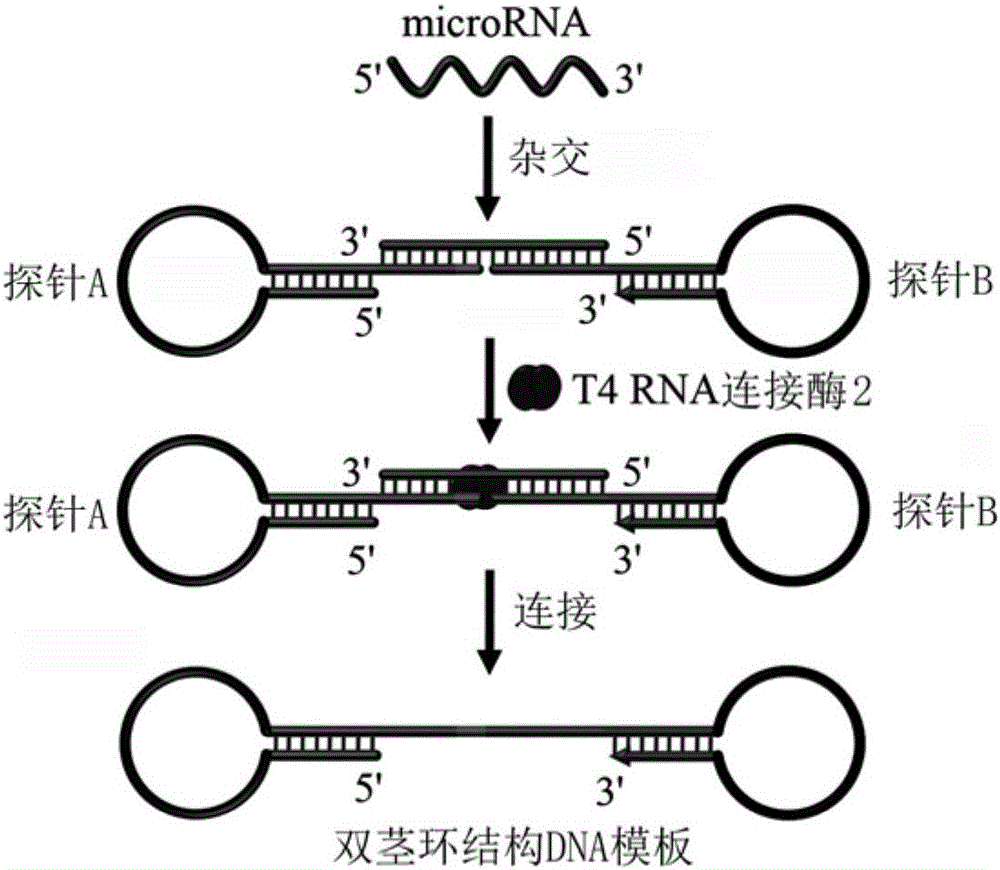
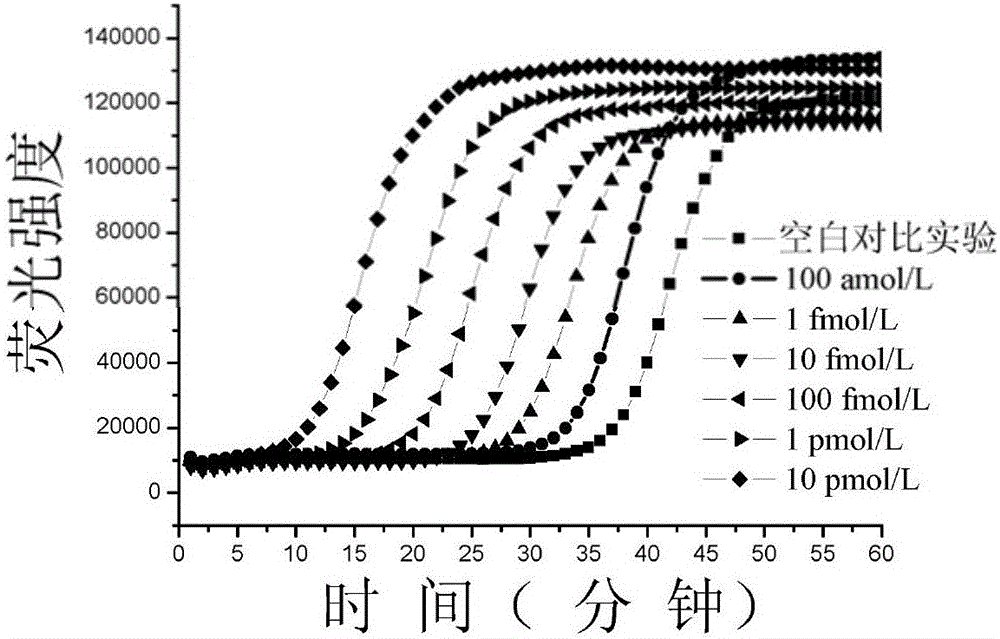
Method for constructing double-stem-loop structure DNA template to detect nucleic acid based on ligation reaction
InactiveCN105821138AAvoid Radiation HazardsLow costMicrobiological testing/measurementNucleic acid detectionFluorescence
The invention discloses a method for constructing a double stem loop structure DNA template to detect nucleic acid based on a ligation reaction. The method includes the steps that ligase is used for connecting a probe containing a stem-loop structure and complemented with nucleic acid to be detected to form a double-stem-loop structure DNA template, the template can guide fast and efficient loop-mediated isothermal amplification reaction to achieve high-sensitivity nucleic acid detection, and meanwhile the method can specifically distinguish nucleic acid with single-base difference. It is provided for the first time that double stem loop structure DNA is constructed through the high-specificity ligation reaction, the specificity template is provided for the loop-mediated isothermal amplification reaction in the next step, fluorescence labeling is not needed in the method, cost is low, the precise heat cycle process in the PCR process is avoided, and fast amplification of the nucleic acid to be detected can be realized at constant temperature. The method can be used for quantitative analysis, methylation detection, SNP detection and the like on RNA or DNA and provides a new strategy for high-sensitivity nucleic acid analysis, early cancer diagnosis and other researches.
Owner:SHAANXI NORMAL UNIV
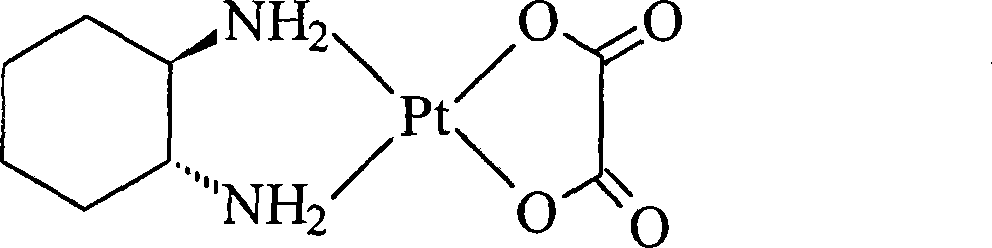
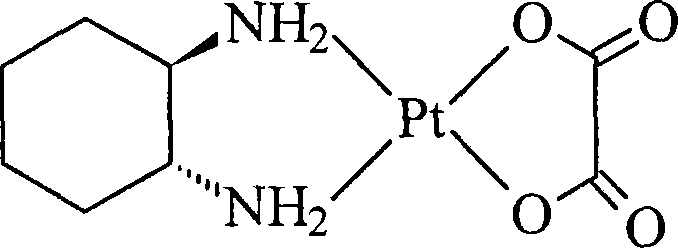
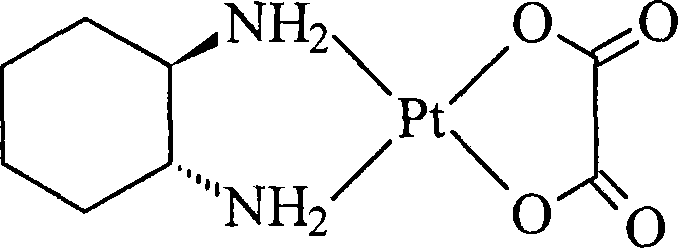
Oxaliplatin lyophilized powder injection and preparing method thereof
ActiveCN101199506AGood reproducibilityLess impuritiesPowder deliveryPharmaceutical non-active ingredientsCITRATE ESTERMANNITOL/SORBITOL
The invention relates to oxaliplatin freeze-dried injection, which is characterized in that the invention is prepared by the method that aqueous solution is freeze-dried. The aqueous solution contains oxaliplatin, mannitol and citrate, wherein, the concentration of the oxaliplatin in the aqueous solution is 2.5 to 6.25 mg / ml; the concentration of the mannitol in the aqueous solution is 25 to 200 mg / ml; and the concentration of the citrate in the aqueous solution is 2 to 20 mg / ml. And sodium citrate can also be added into the aqueous solution so as to adjust the pH of the aqueous solution. The preparation processes are as following: the oxaliplatin is placed inside the container, 80 percent amount of water for injection is added, and then the water for injection is mixed so that the oxaliplatin can be dissolved and mixed evenly in the water for injection; after that, the mannitol and the citrate are added into the water for injection, and then the water for injection is mixed so that the mannitol and the citrate can be dissolved and mixed evenly in the water for injection; the content of the intermediate is measured; if the content of the intermediate is qualified, the volume of the water for injection is fixed to full amount; in the aseptic condition, the water for injection is filtered until to be clear by a microporous membrane of 0.22 microns; the filtered solution is filled into an aseptic silin bottle; part of the aseptic silin bottle is plugged with a butyl rubber closure; and then the aseptic silin bottle is filled in the tray to be sent into the freeze dryer to be freeze-dried; the mouth of the aseptic silin bottle is rolled; the quality of the filtered solution is inspected; and the aseptic silin bottle is packaged.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Automatic sample injection system based on micro-fluidic chip
ActiveCN106984370AIncrease the level of automationImproved sampling methodLaboratory glasswaresInjection portPressure controlled ventilation
The invention discloses an automatic sample injection system based on a micro-fluidic chip. The automatic sample injection system comprises a micro-fluidic chip and a sample injection device for inputting samples into the micro-fluidic chip, and further comprises a negative pressure control device for generating negative pressure, wherein the sample injection device comprises a porous plate for accommodating samples; a suction needle is connected to the sample injection port of the micro-fluidic chip in a sealed manner; the negative pressure control device generates air pressure from the porous plate to the micro-fluidic chip, so that the samples in the porous plate are sucked into the micro-fluidic chip through the suction needle. According to the automatic sample injection system, the samples are directly led into the micro-fluidic chip through the suction needle by virtue of the negative pressure, manual sample adding is avoided, the detection automation degree is improved, and the cross contamination risk is reduced.
Owner:北京旌准医疗科技有限公司
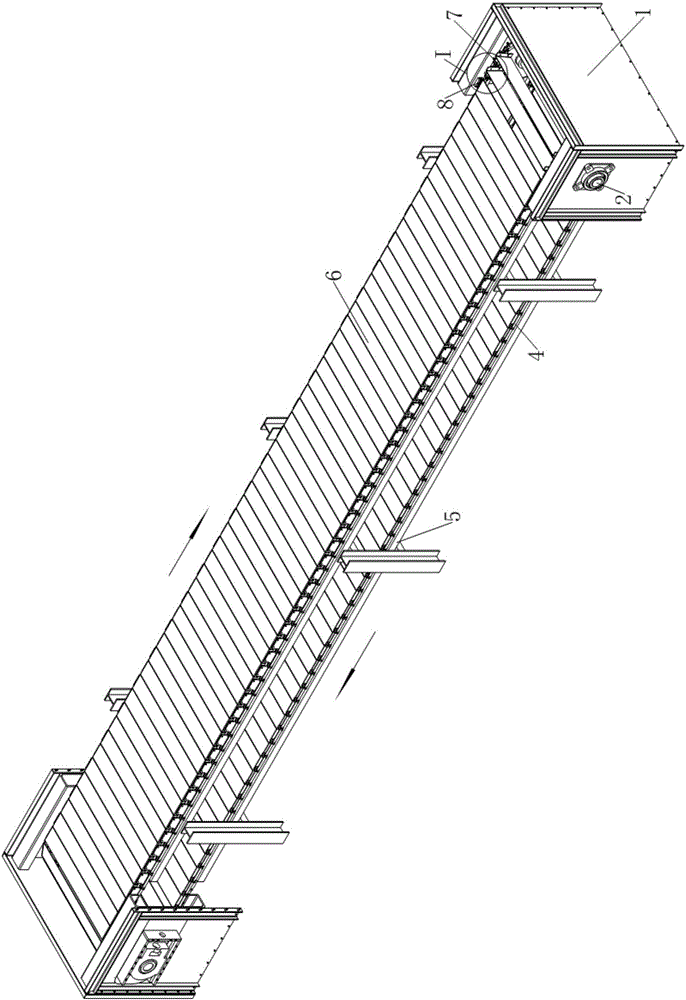
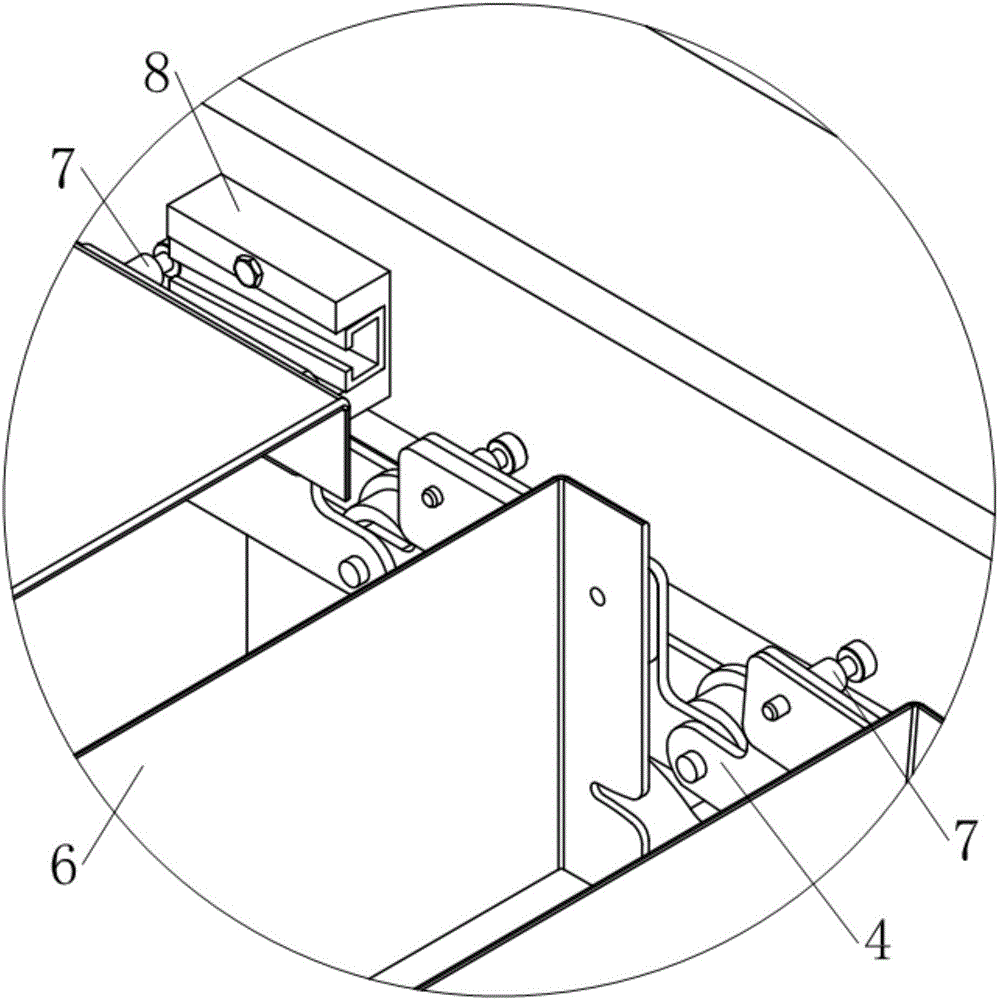
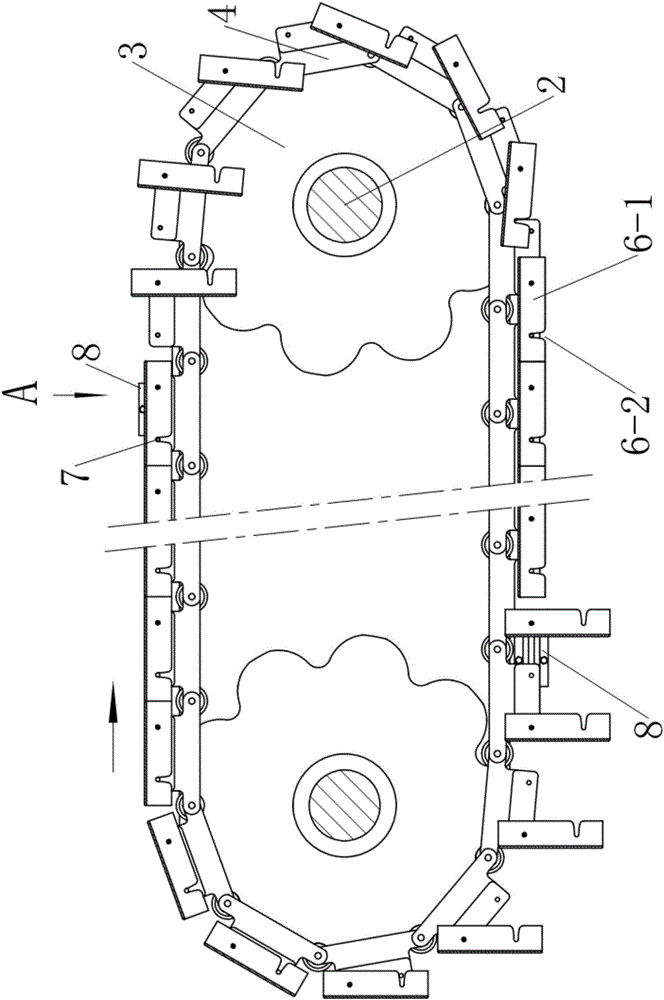
Plate chain type conveyor
The invention discloses a plate chain type conveyor. The plate chain type conveyor comprises racks, two pairs of chain wheels supported at the two ends of the racks, and conveyor belts which are wound on the two pairs of chain wheels, wherein each conveyor belt is composed of two conveying chains and one group of material plate. The plate chain type conveyor is characterized in that a support lug is arranged at one edge, close to a chain plate on one side of the material plate, of each of the two conveying chains in a manner of extending outwards; a connection hole is formed in one front end of each support lug along the movement direction of the conveying chains, and a spring pin is arranged at the other end of each support lug; the spring pins are fixedly arranged on the outer sides of the support lugs and nail rods of the pins penetrate through the support lugs to enter the inner sides of the support lugs; when the conveying chains are at a stretched state, the distances from each connection hole to the adjacent two spring pins are equal; when the conveyor belts are located on the upper sides of the chain wheels, one end of each material plate is hinged with the connection holes in the support lugs of one pair of chain sections, and the other end of each material plate is supported on one rear spring pin adjacent to the connection holes. Unloading control devices are arranged on the rack close to the rail ends of the upper and lower layers of conveyor belts respectively.
Owner:GUANGZHOU UNIVERSITY
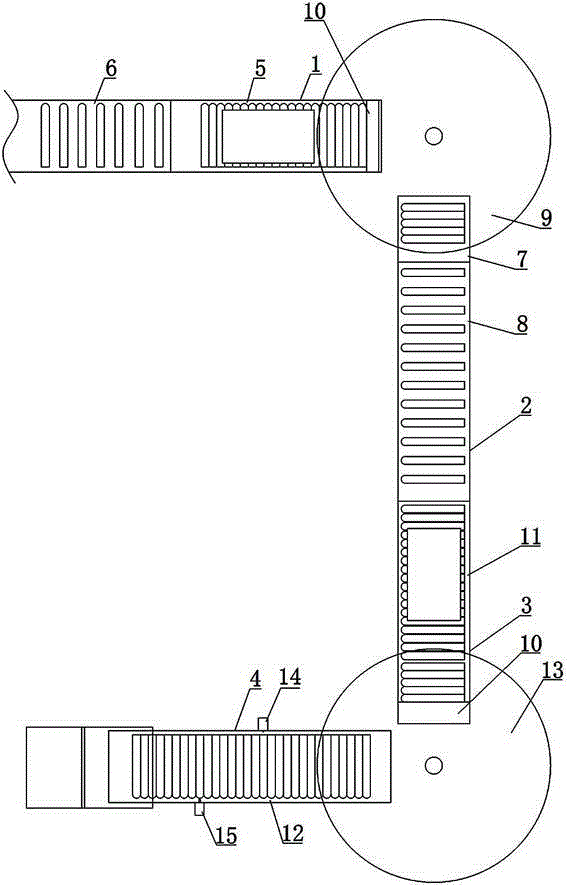
Blood collection tube labeling and assembling automatic connecting device
ActiveCN104150206ARealize automatic and orderly transmissionReduce direct contactConveyor partsBlood Collection TubeUltimate tensile strength
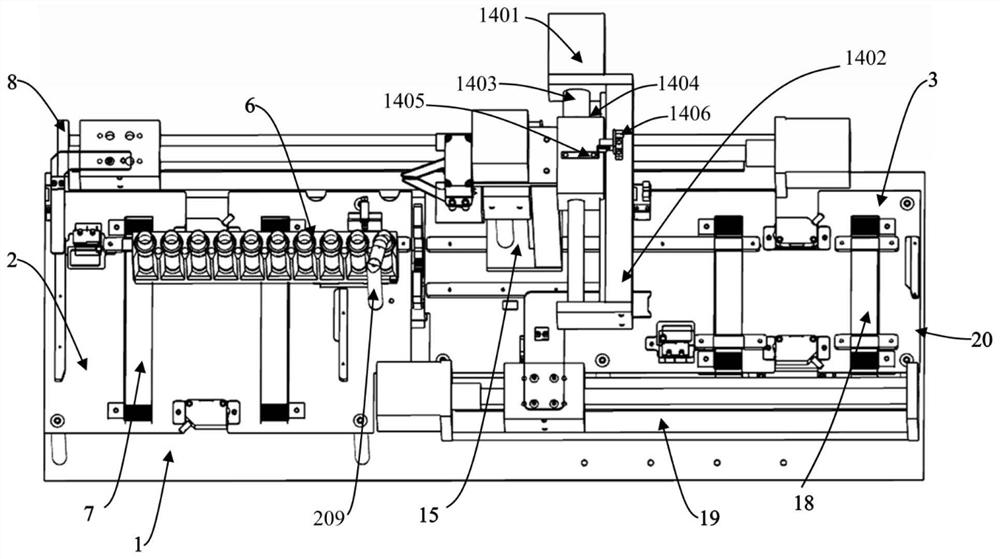
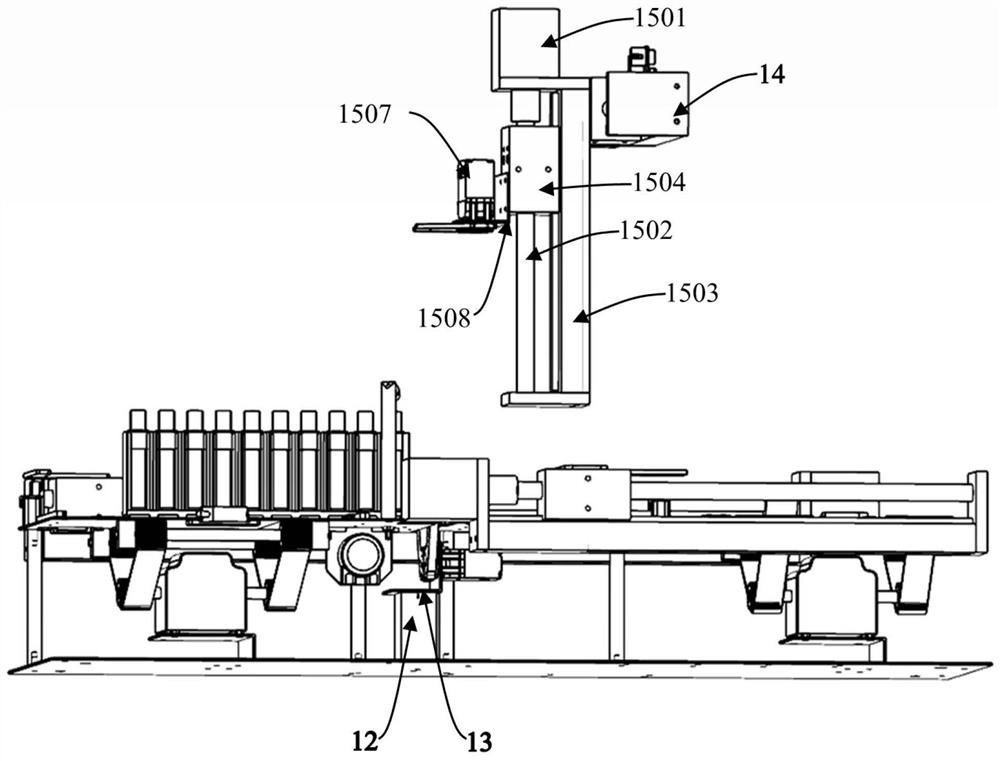
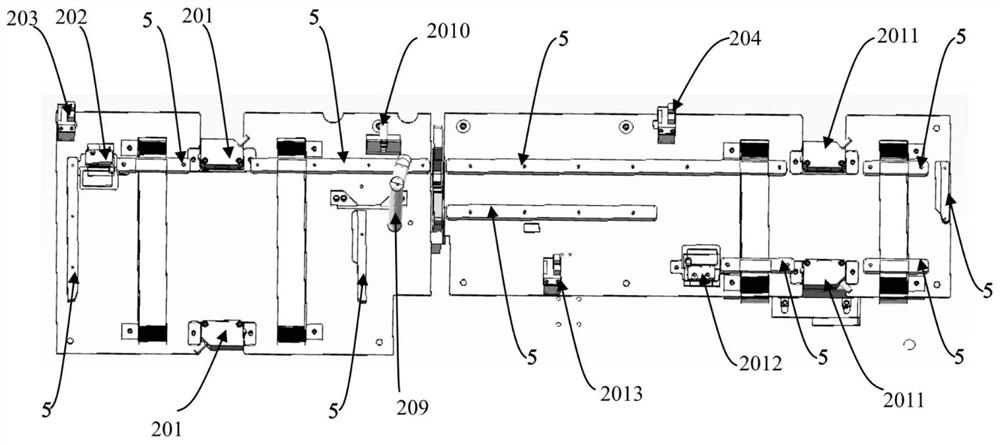
The invention discloses a blood collection tube labeling and assembling automatic connecting device which comprises a transfer device A (1), a lifting section (2), a transfer device B (3) and a reverse removing section (4). Each transfer device comprises a carrying platform and a rotating disc, wherein vacuum material suction devices are evenly distributed on the lower surface of the rotating disc circumferentially, and the rear end of the carrying platform is located at the lower portion of the rotating disc. The transfer device A (1) conveys materials outputted by a labeling machine (6) to a discharge bin A (7). The discharge port of the discharge bin A (7) is located at the upper portion of a lifting conveying belt (8). The transfer device B (3) conveys materials outputted by the lifting conveying belt (8) to the reverse removing section (4) and a horizontal conveying belt (12). A detecting device (14) and a removing device (15) are arranged on one side of the horizontal conveying belt (12). The blood collection tube labeling and assembling automatic connecting device has the advantages that full automation is achieved in the production process from labeling to assembling, personnel for arranging, transferring and placing products are omitted, work efficiency is improved, labor intensity is lowered, and production cost is saved.
Owner:江西科伦医疗器械制造有限公司
Automatic reagent dropping system of urine analysis meter
ActiveCN102221627AAccurate sampling positionImprove cleanlinessMaterial analysisEvery HourEngineering
The invention relates to an automatic reagent dropping system of a urine analysis meter, belonging to the field of the urine analysis meter. The automatic reagent dropping system provided by the invention is of a double-probe structure and comprises a sampling mechanism, a dropping mechanism, a sampling cleaning mechanism and a dropping cleaning mechanism. The automatic reagent dropping system provided by the invention effectively enhances the speed of dropping and realizes the testing result of 480 parts every hour. In the automatic reagent dropping system provided by the invention, the sampling and dropping system drives a synchronous belt to make a motion by adopting a motor in a transmission mode, and the extraction and sampling of samples are carried out by utilizing an injection pump, an electromagnetic valve and a pipeline. In the automatic reagent dropping system provided by the invention, the accuracy of the sampling and the dropping is improved to the great extent; and the double probes are alternately operated in parallel, thereby greatly improving the work efficiency. The two paths of cleaning mechanisms avoids the cross contamination among the samples to the great extent and improves the accuracy of the test result.
Owner:DIRUI MEDICAL TECH CO LTD
Fast joint inspection kit for human immunodeficiency viruses, hepatitis B viruses and hepatitis C viruses and preparation and application thereof
ActiveCN105695631AReduced risk of cross-contaminationHigh detection sensitivityMicrobiological testing/measurementMicroorganism based processesMultiplexImmunodeficiency virus
The invention belongs to the field of biotechnology detection, and particularly relates to a fast joint inspection kit for human immunodeficiency viruses (HIV), hepatitis B viruses (HBV) and hepatitis C viruses (HCV) and preparation and application thereof. The kit comprises an HIV detecting primer and probe, an HBV detecting primer and probe and an HCV detecting primer and probe. A multiplex fluorescent PCR technology is adopted, three kinds of viral nucleic acid of the HIV, the HBV and the HCV are detected in a single PCR reaction tube at the same time, detecting sensitivity is high, good specificity is achieved, the human error rate is low, and time consumed in experiments is short. Fluorescence signals are detected in real time in the amplified reaction process, the whole process is conducted in a sealed mode, the risk of cross infection among samples is reduced, and the kit is suitable for being applied to large-scale blood screening and clinical examinations.
Owner:INSPECTION & QUARANTINE TECH CENT OF GUANGDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU +1
Fluorescent label amplification kit for simultaneously amplifying human 27 STR loci and application of fluorescent label amplification kit
InactiveCN110607374AHigh sensitivityCompatibleMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceAmelogenin
The invention provides a fluorescently label amplification kit for simultaneously amplifying human 27 STR loci. The fluorescently label amplification kit comprises 24 autosomal STR loci, one Y-chromosome STR locus, and two specific amplified primer pairs for gender-identified STR loci. The loci are D3S1358, TH01, D21S11, D18S51, Penta E, D5S818, D13S317, D7S820, D16S539, CSF1PO, D2S1338, D6S1043,D22S1045, D19S433, D1S1656, D12S391, D10S1248, D2S441, vWA, D8S1179, TPOX , FGA, SE33, Penta D, DYS391, Y-INDEL and Amelogenin. The loci contain 20 core loci and four preferred loci as prescribed by the Ministry of Public Security, all sites of mainstream kits currently on the market are covered, the risk of incorrect gender identification due to the deletion of the Y-chromosome can be effectivelyprevented, and the advantages of high discrimination power and high probability of paternity exclusion are achieved.
Owner:百特元生物科技(北京)有限公司
Processing for electromechanical systems and equipment for same
InactiveCN104040708ALarge amount of processingReduce the risk of contaminationSemiconductor/solid-state device manufacturingSelf-assembled monolayerEngineering
This disclosure provides systems, methods and apparatus for processing multiple substrates in a batch cluster tool. A batch cluster tool can include a transfer chamber, an etch process chamber, and one or both of an ALD process chamber and an SAM process chamber. Each of the batch process chambers can be a common chamber where the substrates are open to one another, or can include multiple process subchambers that are isolated from one another in operation. Multiple substrates are transferred into an etch chamber. The substrates are exposed to a vapor phase etchant. The substrates can then be transferred to an atomic layer deposition (ALD) chamber and exposed to vapor phase reactants to form a thin film. The substrates can be transferred either from the etch process chamber or the ALD chamber to a third chamber and exposed to vapor phase reactants to form a self-assembled monolayer (SAM).
Owner:IDC LLC
Triple fluorescent PCR detection kit for infectious spleen and kidney necrosis virus, largemouth bass ranavirus and siniperca chuatsi rhabdovirus
ActiveCN111733283AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationNecrovirusReverse transcriptase
The invention belongs to the technical field of virus detection, and specifically relates to a triple fluorescent PCR detection kit for the infectious spleen and kidney necrosis virus, largemouth bassranavirus and siniperca chuatsi rhabdovirus. The kit of the invention comprises virus-specific amplification primers and probes, a positive standard, a negative standard, Taq enzyme, reverse transcriptase, a RNA enzyme inhibitor, a reaction buffer, dNTP, nuclease-free water and a freeze-drying protective agent. The kit of the invention can perform multiple channel detection at the same time by using triple fluorescent PCR, uses the different fluorescently labeled probes, can detect the three viruses simultaneously in one reaction system, reduces the detection difficulty, shortens the detection time, and can help raisers accurately get detection results in time. The kit of the invention has high detection sensitivity, high stability and strong specificity, has no cross-reactions with otheraquatic viruses, and can be used for early monitoring and prevention and control of epidemic diseases.
Owner:GUANGDONG HAID ANIMAL HUSBANDRY & VETERINARY RES INST
Method for suspending or re-suspending particles in a solution and apparatus adapted thereto
ActiveCN101772379AReduced risk of cross-contaminationMagnetic separationSuspended particlesEngineering
A method for suspending or re-suspending magnetically attractable particles is provided. Thereby at least a mixing vessel (10) is provided filled at least partially with a mixture (30) containing magnetically attractable particles (40) at least partially precipitated at the bottom (11) of the mixing vessel (10). An effective magnetic field acting at least in the front end area (3) of the mixing bar (1) is switched on by the magnetic field generating apparatus (4) while the mixing bar (1) is immersed in the mixture (30). Subsequently, the magnetic field is moved away from the bottom (11) of the mixing vessel (10) along with the mixing bar, whereby the movement of the magnetic field along with the mixing bar is carried out such that at least a part of the magnetically attractable particles (40) is raised from the bottom (11) of the mixing vessel (10) and the portion of the particles sticking to the bar is minimized. The magnetic field is switched off in a predefined distance from the bottom which is greater than the distance from the bottom at the time when the magnetic field is switched on. Thereafter, repeated mixing movements of the mixing bar (1) are carried out until the magnetically attractable particles present in the mixture (30) are sufficiently suspended or re-suspended whereby a magnetic field which is switched on does not exist at the front end (3) of the mixing bar (1).
Owner:QIAGEN GMBH
System and method for collinearly preparing multi-batch biological products
InactiveCN108611264AReduced risk of cross-contaminationIncrease productivityBioreactor/fermenter combinationsBiological substance pretreatmentsEngineeringAtmospheric pressure
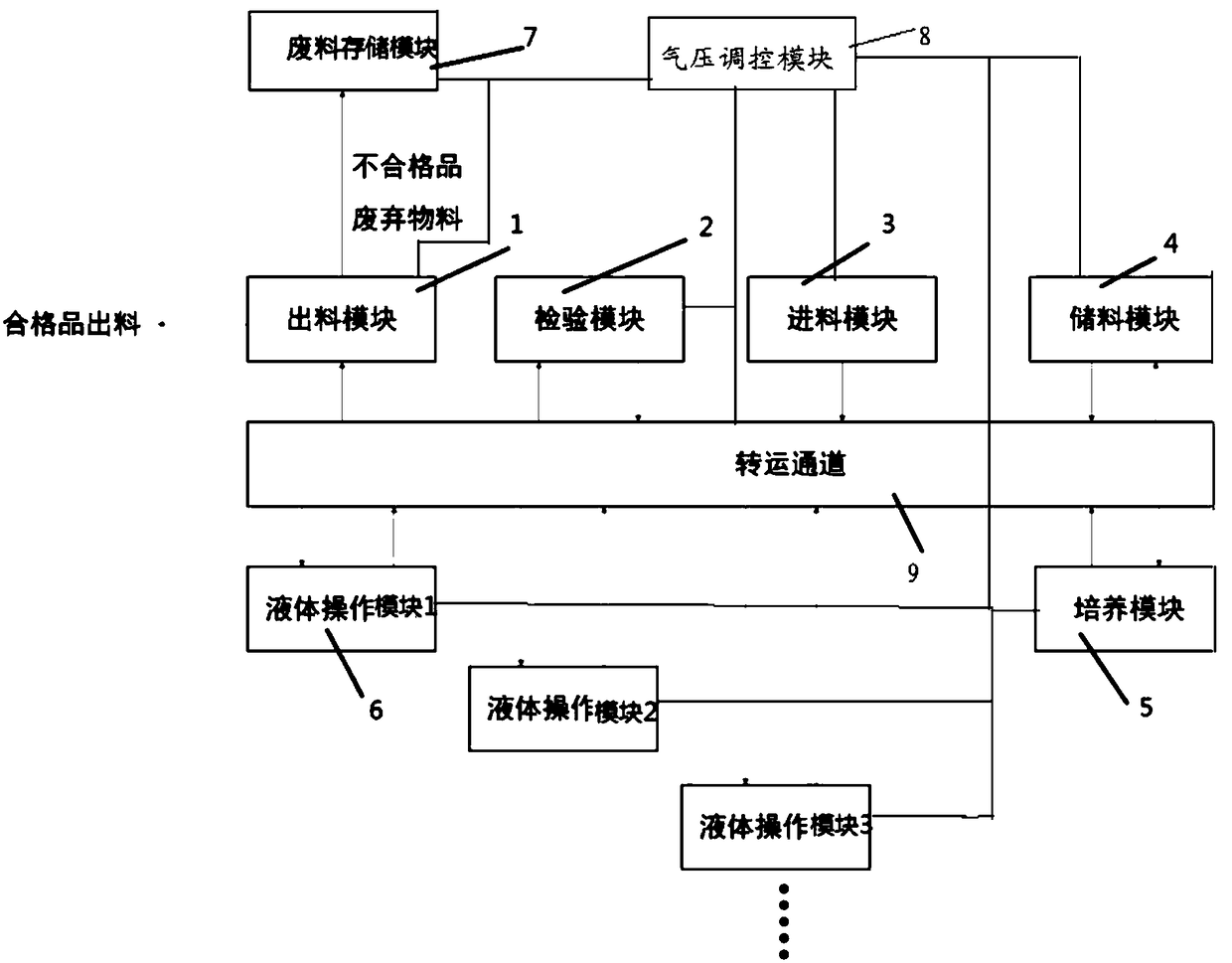
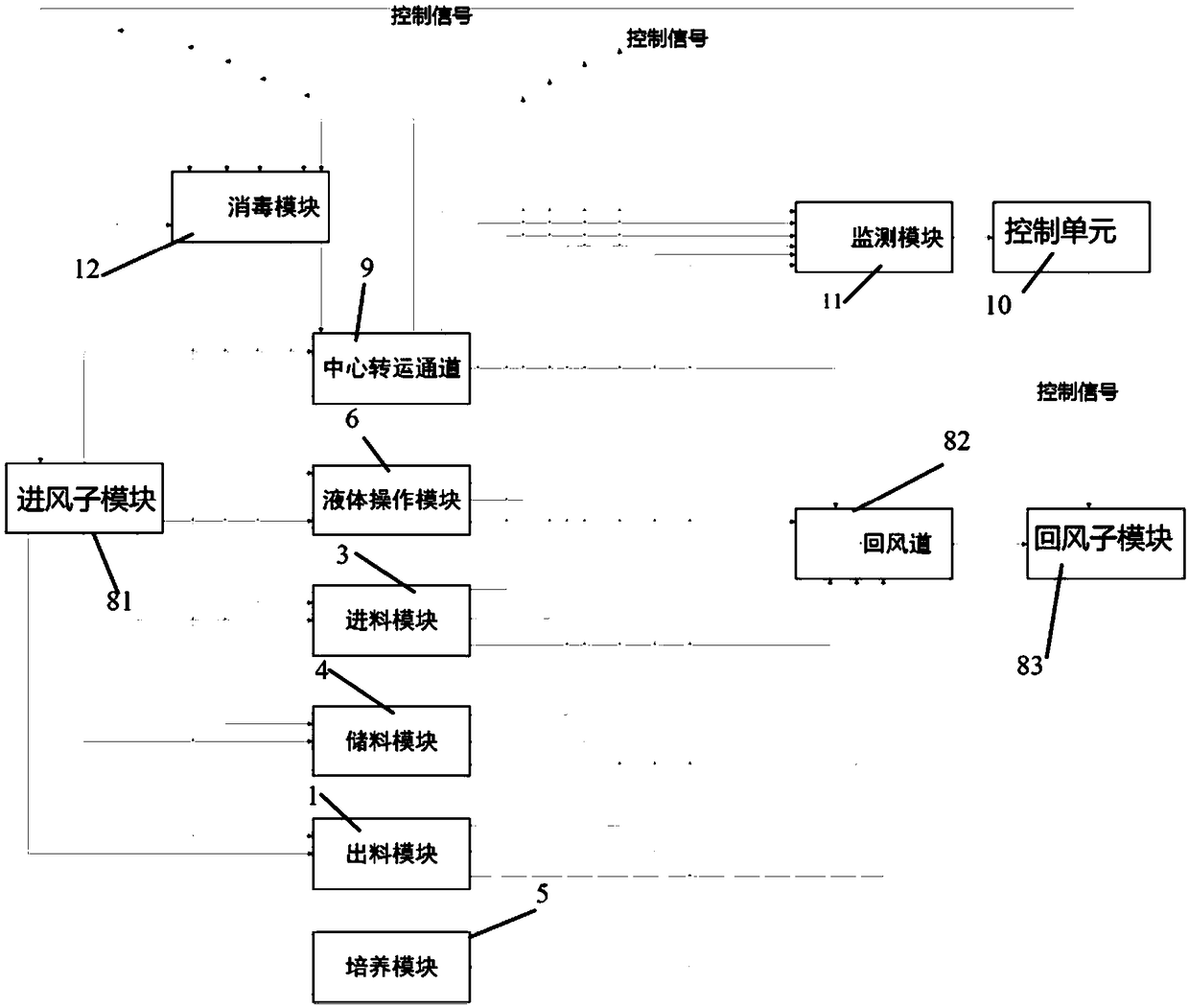
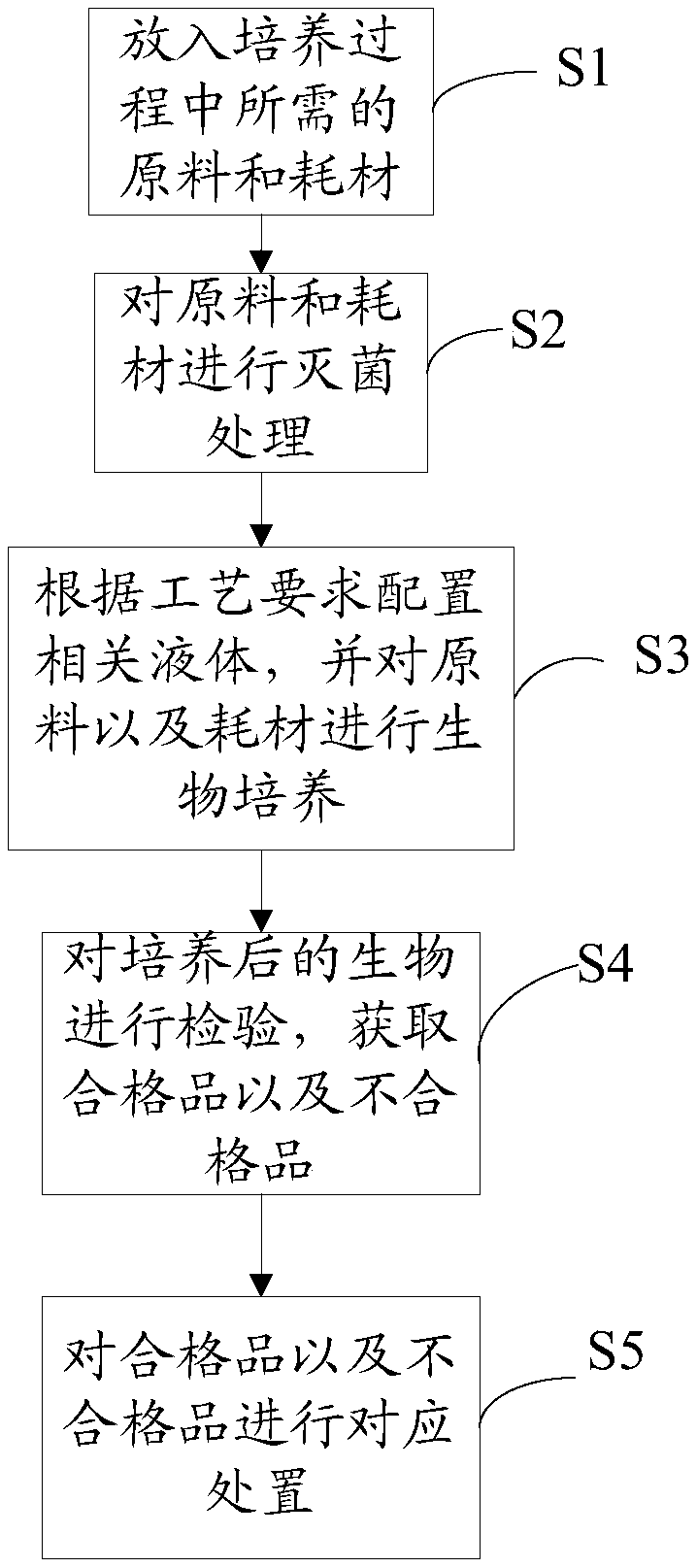
The invention relates to a system and a method for collinearly preparing multi-batch biological products. The system comprises a feed module, a discharge module, an inspection module, a transfer passage, a culture module and a plurality of liquid operating modules, wherein the feed module feeds raw materials and consumables required during preparation; the liquid operating modules separate and sort the raw materials and purify the raw materials before production; the culture module prepares cells; the inspection module detects and checks the prepared cells; the discharge module discharges thecells; the transfer passage transfers the cells among the feed module, the discharge module, the inspection module, the liquid operating modules and the culture module; air pressure control modules are arranged inside the feed module, the discharge module, the inspection module, the transfer passage, the culture module and the plurality of liquid operating modules respectively; the air pressure ofthe transfer passage is higher than those of the liquid operating modules. Through the system and the method, a cross-contamination risk during collinear production of non-finally sterilized biological preparations between different batches is lowered and the production efficiency is improved.
Owner:朱丹
Respiratory tract pathogen detecting kit
InactiveCN105018644AReduced risk of cross-contaminationLow costMicrobiological testing/measurementMicroorganism based processesAvian influenza virusLegionella pneumophila
The invention discloses a respiratory tract pathogen detecting kit. The kit is used on the basis of multiple PCR combining nucleic acid intrusion reaction and the nano-gold color developing principle. The kit comprises one or more of 6 primer probe combinations and can be used for detecting influenza A / avian influenza viruses (FluA), influenza B viruses (FluB), SARS coronaviruses, legionella pneumophila (LP), neisseria meningitidis (N. men) and adenoviruses (ADV). The kit has the advantages that the kit is high in specificity and sensitivity during respiratory tract pathogen detecting, expensive equipment is not needed, detecting results can be observed through naked eyes, and the kit can be applied at primary level.
Owner:JIANGSU PROVINCIAL CENT FOR DISEASE PREVENTION & CONTROL
Quantitative detection method for gamma-interferon and kit
The invention relates to the technical field of in-vitro diagnosis, and in particular relates to a quantitative detection method for gamma-interferon and a kit. The quantitative detection method for the gamma-interferon disclosed by the invention comprises the following steps: (1) preparing a fluorescent trapping microsphere by adopting a carbodiimide method; (2) releasing the gamma-interferon in vitro; (3) combining the fluorescent trapping microsphere with the gamma-interferon; (4) preparing an antigen-antibody complex; and (5) quantitatively detecting. The quantitative detection kit for gamma-interferon disclosed by the invention comprises the fluorescent microsphere, a coupling buffer solution, carbodiimide, an NHS activating agent and a fluorescein labelled anti-human gamma-interferon antibody. The quantitative detection method for the gamma-interferon and the kit, disclosed by the invention, are simpler to operate; and simultaneously, quantitative detection of the gamma-interferon is realized effectively.
Owner:HEBEI CHEST HOSPITAL
Internal reference detection system and kit for isothermal nucleic acid amplification reaction
ActiveCN102618627AFully enclosed detectionStrong specificityMicrobiological testing/measurementFalse Negative ReactionsBiology
The invention designs an internal reference detection system, and concretely relates to an internal reference detection system for the isothermal nucleic acid amplification reaction, a kit containing the internal reference detection system and an application of the system. The internal reference detection system is used for detecting the false negative reaction in the isothermal the nucleic acid amplification reaction, and comprises template DNA with a hairpin structure, a specific primer 1 and a specific primer 2, wherein the specific primer 1 is complementary with the sequence of a ring in the hairpin structure, and the specific primer 2 is complementary with the 3'-terminal sequence at the stem in the hairpin structure. The invention also relates to the kit of the internal reference detection system for the isothermal nucleic acid amplification reaction, and the kit comprises a strand displacement-function DNA polymerase-mediated isothermal nucleic acid amplification system and an internal reference detection system. The internal reference detection system of the invention has the advantages of exquisite design, sensitive reaction and suitableness for the large-scale popularization and use.
Owner:USTAR BIOTECHNOLOGIES (HANGZHOU) CO LTD
A kind of respiratory pathogen detection kit
InactiveCN105018644BReduced risk of cross-contaminationLow costMicrobiological testing/measurementMicroorganism based processesAvian influenza virusLegionella pneumophila
Owner:JIANGSU PROVINCIAL CENT FOR DISEASE PREVENTION & CONTROL
Direct use type high-fidelity PCR amplification reagent mixture
The invention provides a direct use type high-fidelity PCR amplification reagent mixture which is high in fidelity, stability and using convenience. The reagent mixture comprises DNA polymerase, KCl, Tris-HCl, MgSO4, (NH4)2SO4, dNTPs and glycerinum, and ddH2O serves as the solvent. In certain optimized embodiments, the reagent mixture further comprises an optimizer which is one or more of DMSO, tetramethylene sulfoxide, hydroxyproline, trehalose, ethanediol and BSA. In use, PCR can be conducted simply by adding a template and a primer to an amplification system, operation processes are simplified greatly, operation time is shortened, and pollution is reduced; meanwhile, due to the fact that the system contains the optimizer, PCR amplification fidelity and stability can be improved remarkably, and the fidelity of the reagent mixture reaches 2.3*10<-7> to 3.9*10<-7>.
Owner:赛特斯(海南)生物医学有限公司
Preparation method for single-phase crosslinked sodium hyaluronate gel
ActiveCN108395552AReduce the risk of useAvoid situations where it is difficult to mix evenlyPharmaceutical non-active ingredientsCross-linkButanediol
The invention discloses a preparation method for a single-phase crosslinked sodium hyaluronate gel. The method specifically comprises the following steps: sodium hyaluronate alkali treatment-a 1,4-butanediol diglycidyl ether cross-linking reaction-dialysate dialysis-preliminary granulation-and colloid mill grinding granulation. The method provided by the invention controls a crosslinking degree ofa crosslinking agent and HA through designing reaction conditions, so that a gel with suitable viscoelastic properties is obtained, a gel particle diameter suitable for secondary granulation of a colloid mill is obtained by subsequent preliminary crushing, and finally the crosslinked sodium hyaluronate gel with a target diameter is obtained by selection of appropriate colloid mill granulation parameters; and since the final product has suitable rheological properties, small particle sizes and uniform distribution, artificial operation in the production process can be reduced, the risk of cross contamination is reduced, the production filling process can be met without adding lubricants, the production cycle is shortened, the situation that two-phase cross-linked products are difficult tomix uniformly is avoided, the risk of clinical use is reduced, and product quality is improved.
Owner:ZHEJIANG JINGJIA MEDICAL TECH CO LTD
Set of multicomponent cartridges
ActiveCN103748020AUniversal availabilityReduce the numberDispensing apparatusBottlesElectrical and Electronics engineeringEngineering
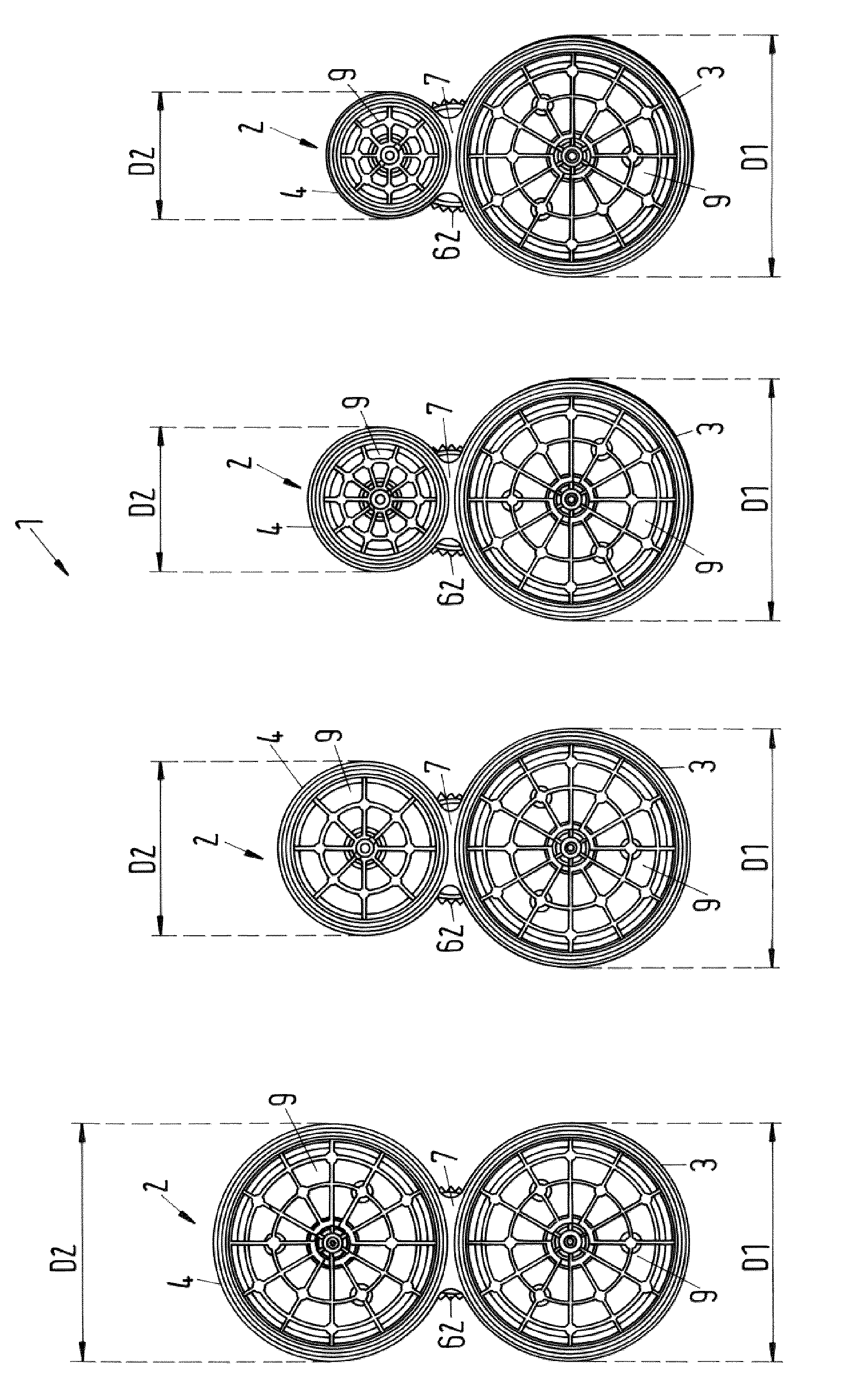
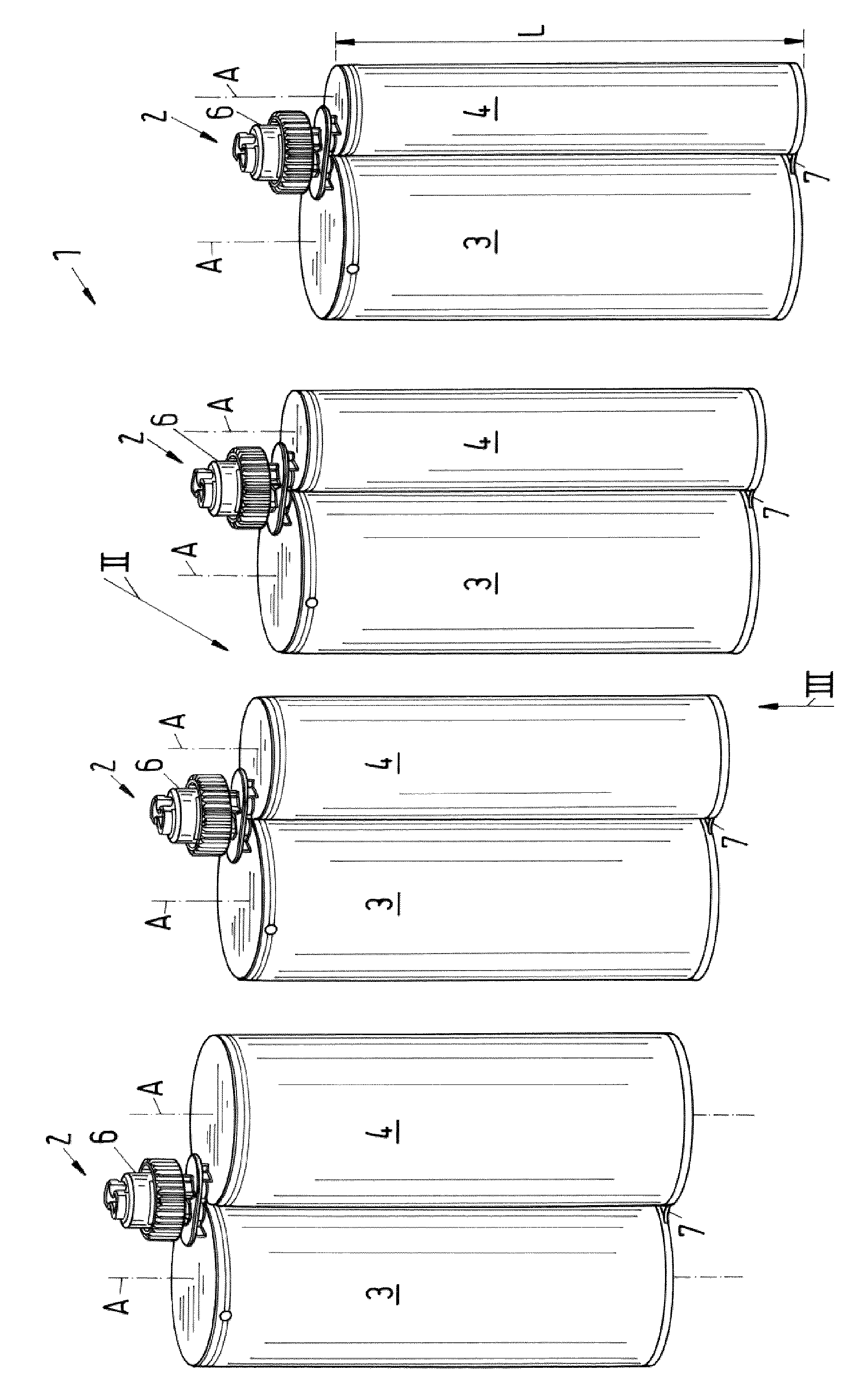
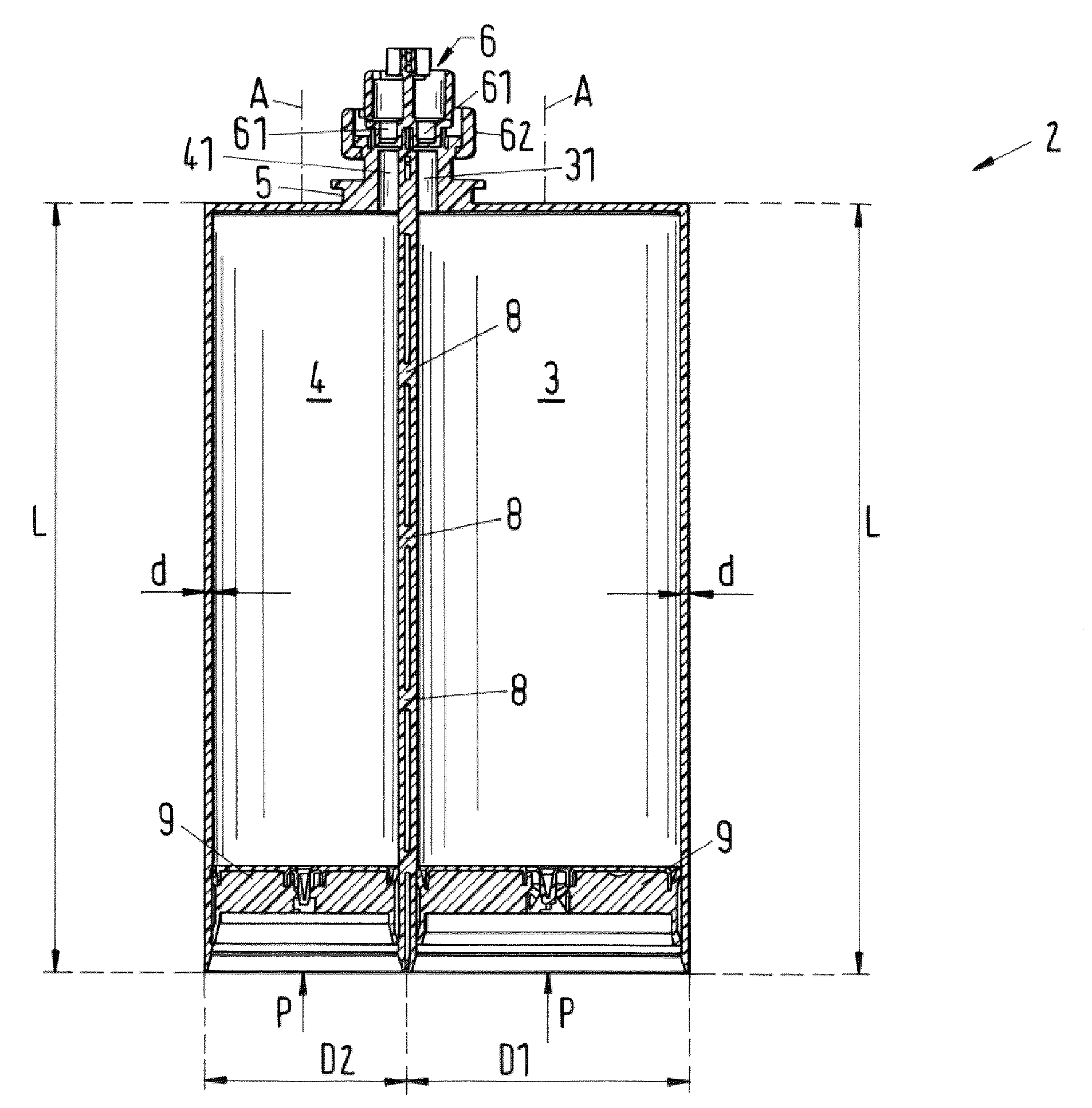
The invention relates to a set of multicomponent cartridges having at least two multicomponent cartridges (2), wherein each multicomponent cartridge (2) comprises at least a first and a second receiving chamber (3, 4) for components to be discharged, wherein each receiving chamber (3, 4) is configured substantially cylindrically and extends in a longitudinal direction (A), wherein the receiving chambers (3, 4) are arranged parallel to one another and have the same extent (L) in the longitudinal direction (A), wherein each multicomponent cartridge (2) is produced in one piece, so that the receiving chambers (3, 4) thereof are non-detachably connected to one another, and wherein the first receiving chamber (3) of each multicomponent cartridge (2) of the set (1) has the same external diameter (D1).
Owner:MEDMIX SWITZERLAND AG
Oxaliplatin lyophilized powder injection and preparing method thereof
ActiveCN101199506BGood reproducibilityLess impuritiesPowder deliveryPharmaceutical non-active ingredientsCITRATE ESTERMANNITOL/SORBITOL
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
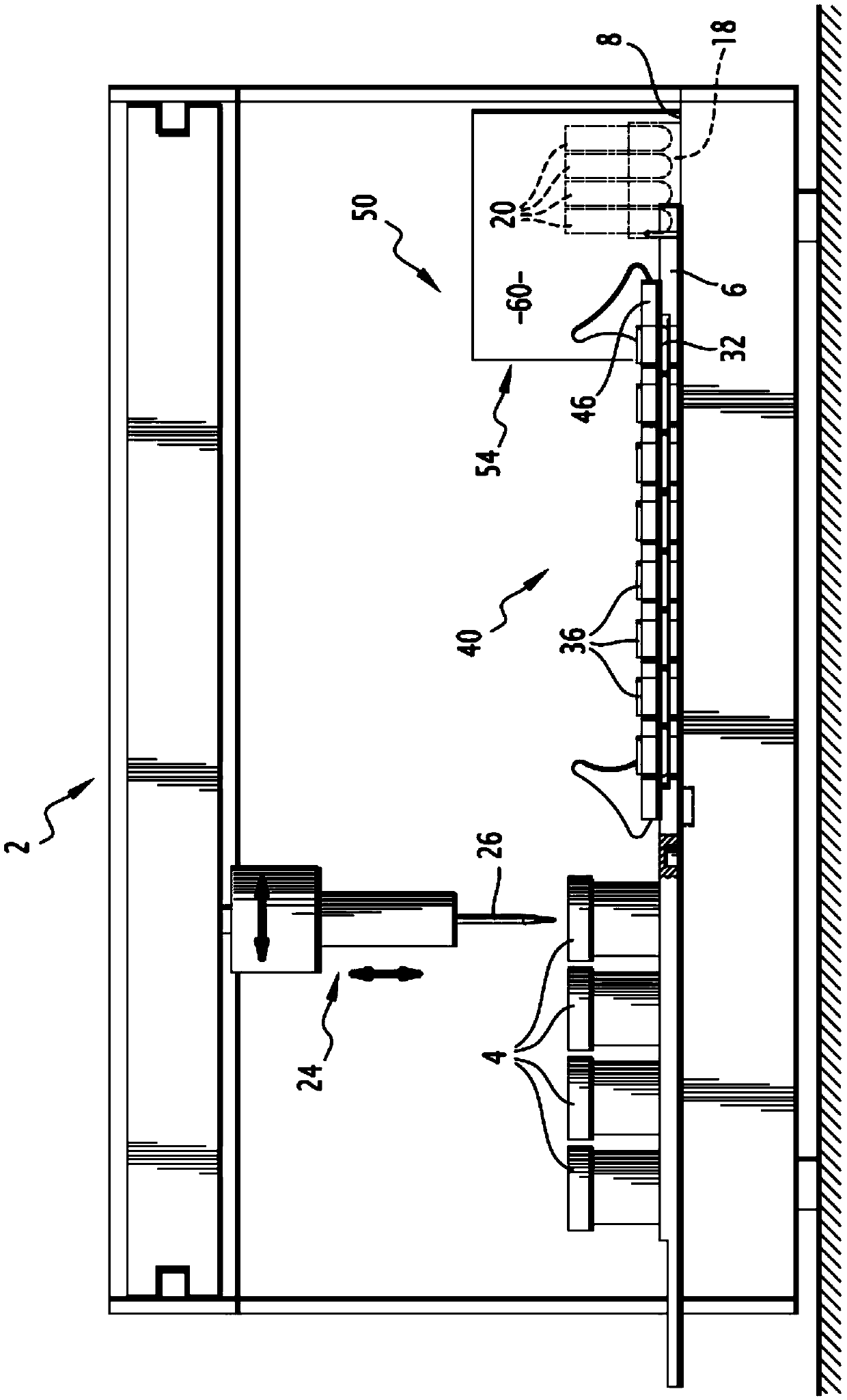
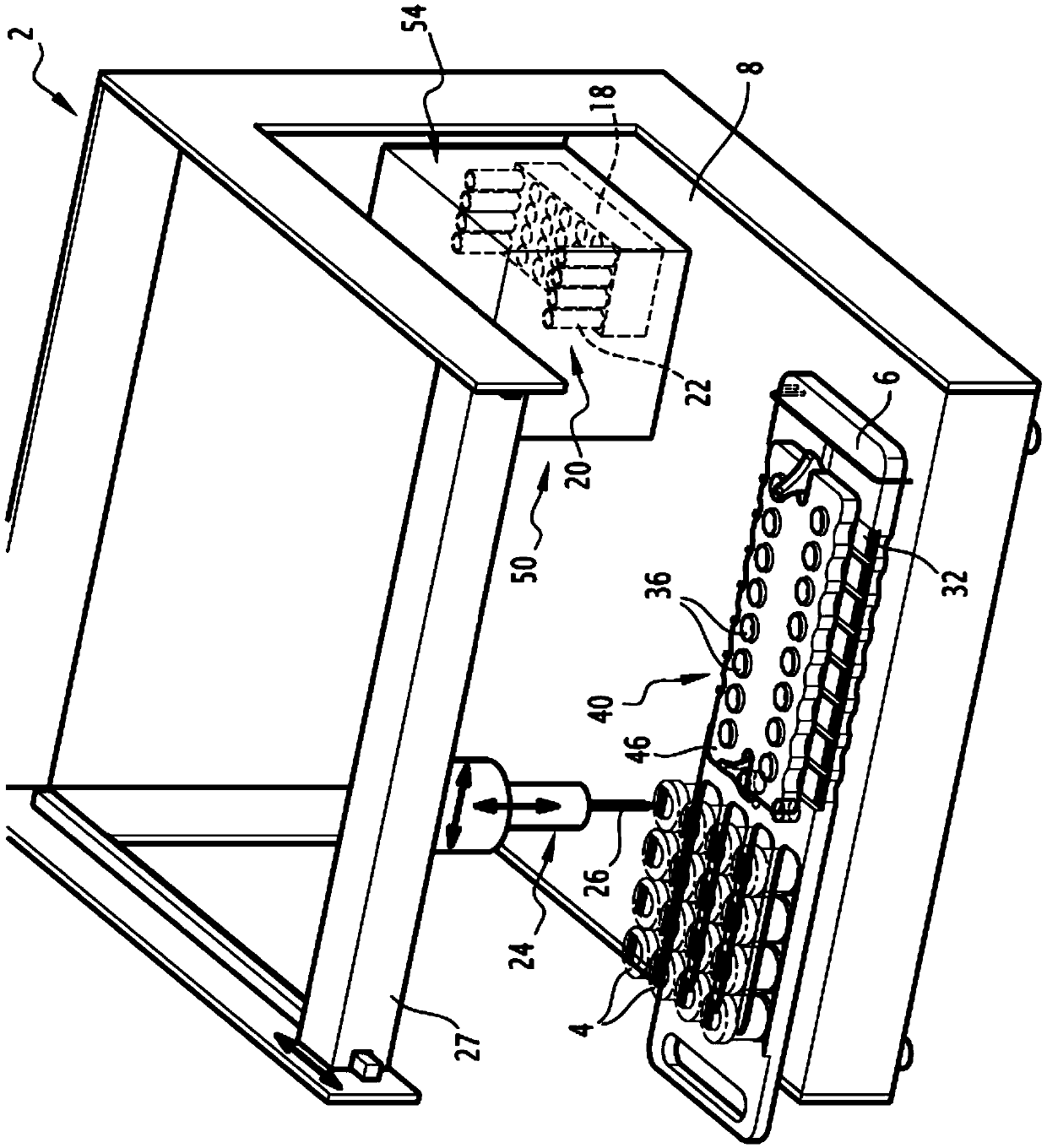
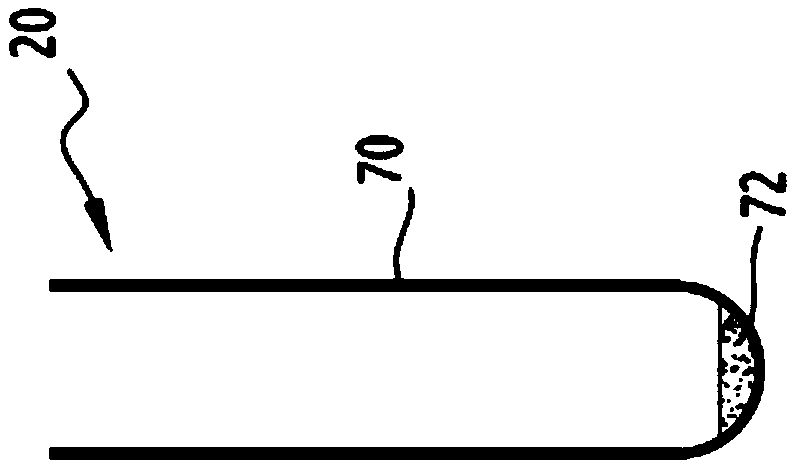
Automatic method and automated device for processing a plurality of cell suspensions
InactiveCN105518463AReduced risk of cross-contaminationPreparing sample for investigationCell processingEngineering
This method for processing a plurality of cell suspensions comprises at least the following steps: (a) loading a plurality of phials (4) onto a receiving plate (6), each phial (4) comprising a cell suspension to be analysed; (b) loading a plurality of intermediate containers (20) into a receptacle (18); (c) extracting, by virtue of pipetting means (24), a sample of a cell suspension from a phial (4) and depositing this sample in an intermediate container (20); and (e) taking a sample of a cell suspension from an intermediate container (20) and depositing this sample in an analysis container (32, 36); steps (c) and (e) being repeated for each phial (4) to be analysed. The method also comprises, between the extracting step (c) and the sample-taking step (e), a step (d) of cell treatment of the sample extracted, by virtue of cell treatment means, step (d) being repeated for each phial (4) to be treated. The invention also relates to an automated device implementing this method.
Owner:NOVACYT
Low-lactose low-lactoprotein depth hydrolysis infant formula food and preparation method thereof
The invention provides a low-lactose low-lactoprotein depth hydrolysis infant formula food. The food comprises, by mass, 740-800 parts of vegetable fat powder, 160-200 parts of depth hydrolysis lactoprotein powder, 26-32 parts of mineral substances, 8-12 parts of fructo-oligosaccharide, 8-12 parts of vitamins and 0.3-0.5 part of nucleotide. The low-lactose low-lactoprotein depth hydrolysis infantformula food is good in stability in a goods shelf period, the risk of cross pollution is small in the preparation process, and the residual oxygen content is low.
Owner:贝因美(杭州)食品研究院有限公司
Specific amplification primer group for simultaneously amplifying 25 human STR (short tandem repeat) gene loci, fluorescence labeling amplification kit, application and method
ActiveCN113416791ARapid expansionHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationNucleotideY chromosome deletions
The invention relates to a specific amplification primer group for simultaneously amplifying 25 human STR (short tandem repeat) gene loci, a fluorescence labeling amplification kit, application and a method, and belongs to the technical field of molecular genetics. The specific amplification primer group disclosed by the invention comprises primers with nucleotide sequences as shown in SEQ ID NO: 1-50, and 25 corresponding human STR gene loci comprise 23 autosomal STR gene loci and 2 sex identification STR gene loci. The loci corresponding to the specific amplification primer group comprise all loci required by mainstream cases in the market at present, and also comprise two gender identification loci, so that the risk of gender identification errors caused by Y chromosome deletion can be effectively prevented. and the 25 gene loci are combined, so that the method has the characteristics of high individual recognition capability and high non-father exclusion rate.
Owner:百特元生物科技(北京)有限公司
Vaginal secretion sample detection pretreatment system
PendingCN111830267AEfficient processing capacityEasy to handlePreparing sample for investigationApparatus instrumentsVaginal secretion sample
The embodiment of the invention discloses a vaginal secretion sample detection pretreatment system, and relates to the technical field of medical instruments. The system comprises a sample sending module, a processing module and a control module, wherein the processing module comprises a diluent adding module, a soft test tube extrusion module and a cotton swab clamping and discarding module, thesample feeding module, the diluent adding module, the soft test tube extruding module and the cotton swab clamping and discarding module are all connected with the control module and are controlled bythe control module, the sample feeding module is used for pushing a tube bank frame to sequentially pass through the diluent adding module, the soft test tube extrusion module and the cotton swab clamping and discarding module, the tube rack is used for placing soft test tubes containing cotton swabs, the diluent adding module is used for adding a diluent into the soft test tube, the soft test tube extrusion module is used for uniformly mixing a leucorrhea sample on the cotton swab with a diluent, and the cotton swab clamping and discarding module is used for discarding the cotton swab. The system can improve sample pretreatment efficiency.
Owner:SHENZHEN HALDEX STC BIOLOGICAL ENG CO LTD
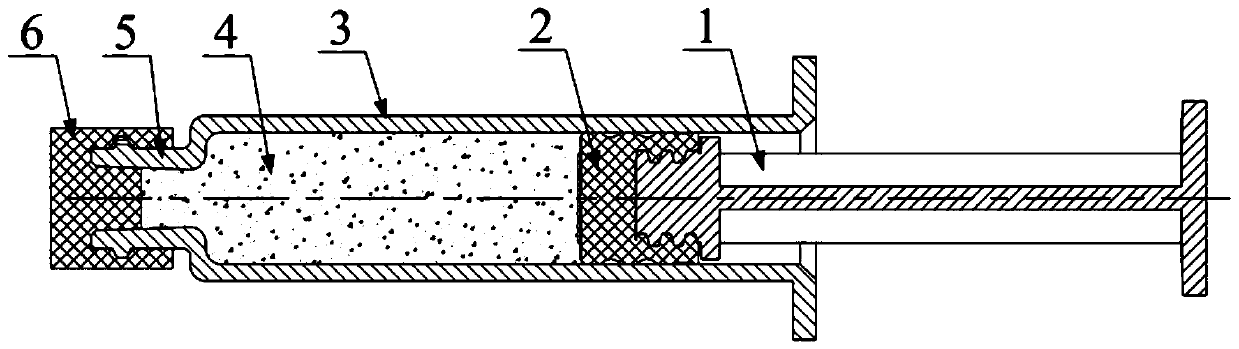
Prefilled injector for medicine dissolution and dispensing device
PendingCN110496274ALess consumablesReduce workloadInfusion syringesPharmaceutical containersDissolutionWorkload
The invention discloses a prefilled injector for medicine dissolution and a dispensing device. The prefilled injector comprises a push rod, a piston, a needle tube and a protective cap, wherein the needle tube is provided with a medicine dissolution joint, the medicine dissolution joint is in matched connection with an external cone joint of a prefilled injector for injection, and the protective cap is in sealed connection to an opening of the medicine dissolution joint. During medicine dispensing, the protective cap of the prefilled injector for the medicine dissolution and a protective cap of the prefilled injector for injection are taken off, then, the medicine dissolution joint is directly in matched connection with the external cone joint of the prefilled injector for injection, pushrods of the two injectors are pushed repeatedly for medicine dispensing, after medicines are sufficiently dissolved, the prefilled injector for medicine dissolution is directly taken down, finally, the needle is mounted at the external cone joint of the prefilled injector for injection for injection. The scheme simplifies the dispensing process, the workload of a medical worker is reduced, and puncture scrap falling and cross pollution can be avoided. The invention also discloses the prefilled injector for injection and the dispensing device for the prefilled injector for medicine dissolution.
Owner:SHANDONG WEGO PREFILLS PHARM PACKAGING CO LTD
Automatic transmission cabinet
InactiveCN105775672APrevent mutual flowAchieve mutual isolationControl devices for conveyorsPower-operated mechanismProduction lineAutomatic transmission
The invention relates to an automatic transmission cabinet. The automatic transmission cabinet comprises a cabinet body respectively provided with a front cabinet door and a back cabinet door at the front and back two ends; the front cabinet door and the back cabinet door have the same structure; the front cabinet door includes a front door frame, an automatic transmission line I and a front automatic door sheet; the upper end of the front automatic door sheet is hinged with the front door frame; the automatic transmission line I is arranged above a lower wall plate of the front door frame; a front electric pushing mechanism is fixedly mounted on an upper wall plate of the front cabinet door, and is connected with the upper end of the front automatic door sheet; and cabinet doors on two sides of the front electric pushing mechanism are respectively provided with a photoelectric induction device I and a photoelectric induction device II. The automatic transmission cabinet has the following beneficial effects: when materials are automatically transmitted, mutual isolation of two areas is realized, so that mutual air flowing between the areas is stopped, the risk of cross contamination is reduced, and the saving of energy is facilitated; the labor intensity is relieved; the pollution of external materials to indoor spaces is effectively prevented; and the cabinet doors can be singly used, and can be conveniently matched with other automatic production lines for use.
Owner:贺红宇
Reagent set, kit and detection method for detecting fungal infection
ActiveCN109295174AAvoid Nucleic Acid LossReduced risk of cross-contaminationMicrobiological testing/measurementDNA/RNA fragmentationChemistryLysozyme
The invention discloses a reagent set, a kit and a detection method for detecting fungal infection, and relates to the field of biotechnology. The reagent set comprises one of the following reagents or a combination of two of the following reagents: a PCR buffer and a complex enzyme preparation. The PCR buffer contains the following components: potassium acetate, MgCl2, glycerol, DMSO, Tween-20, and PEG-200. The complex enzyme preparation contains the following components: snail enzyme, lysozyme, papain and laccase. The reagent set can directly detect samples such as blood without the steps such as nucleic acid extraction and purification, and has the advantages of high sensitivity, good specificity and short detection time.
Owner:SUREXAM BIO TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com