Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
138 results about "Drug usage" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Drug use, use of drugs for psychotropic rather than medical purposes. Among the most common psychotropic drugs are opiates (opium, morphine, and heroin), hallucinogens (LSD, mescaline, and psilocybin), barbiturates, cocaine, amphetamines, tranquilizers, and cannabis. Alcohol and tobacco are also sometimes classified as drugs.
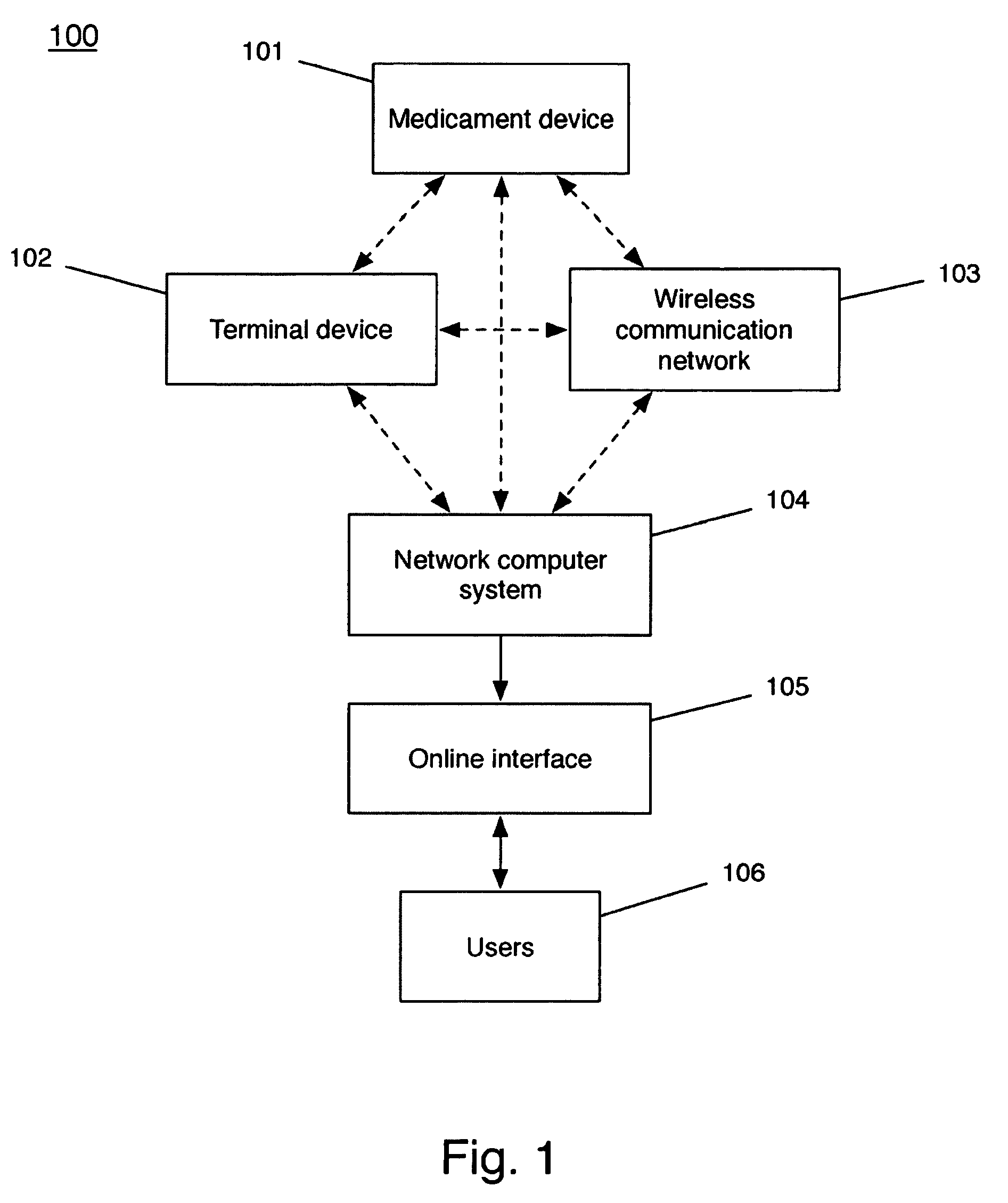
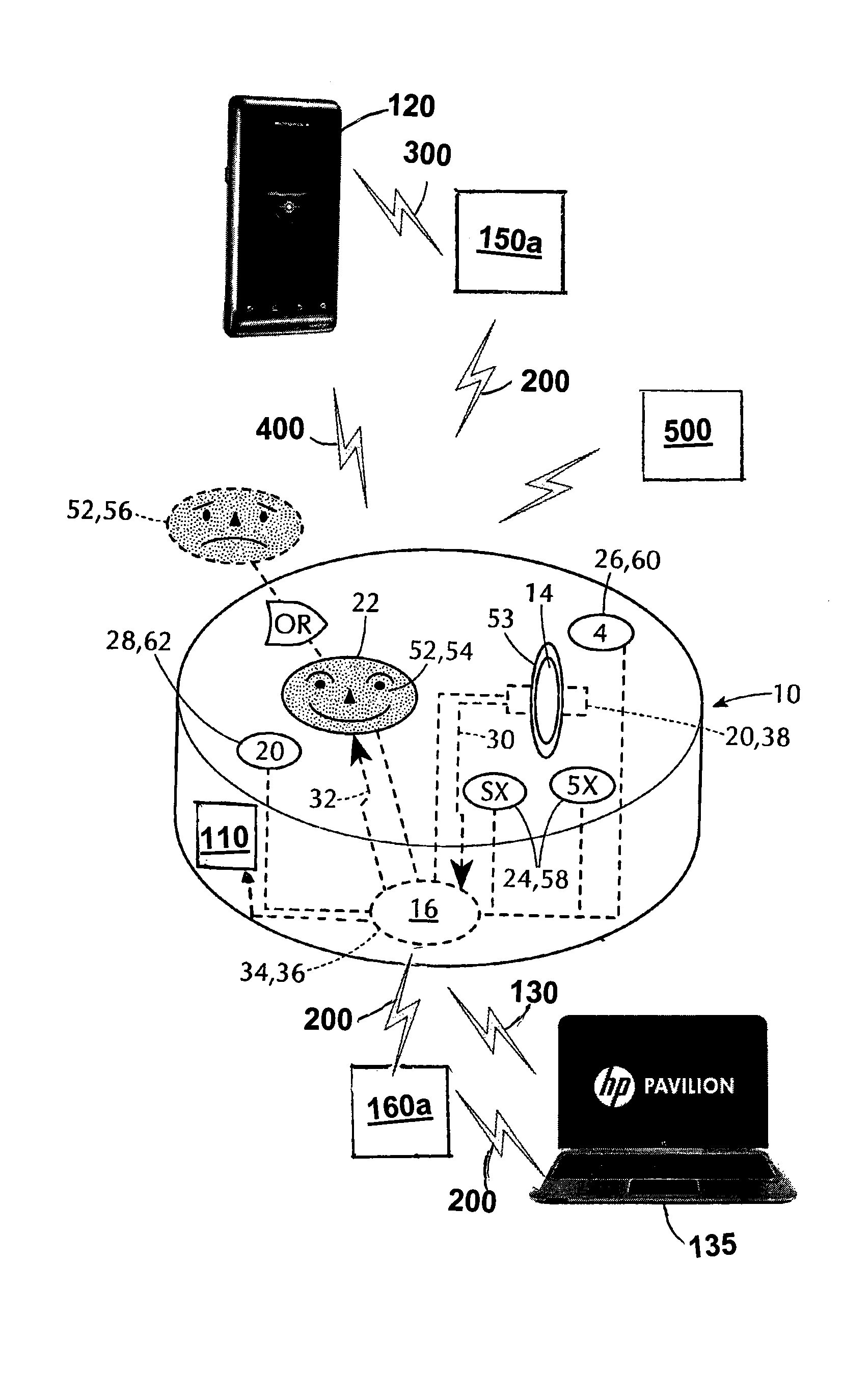
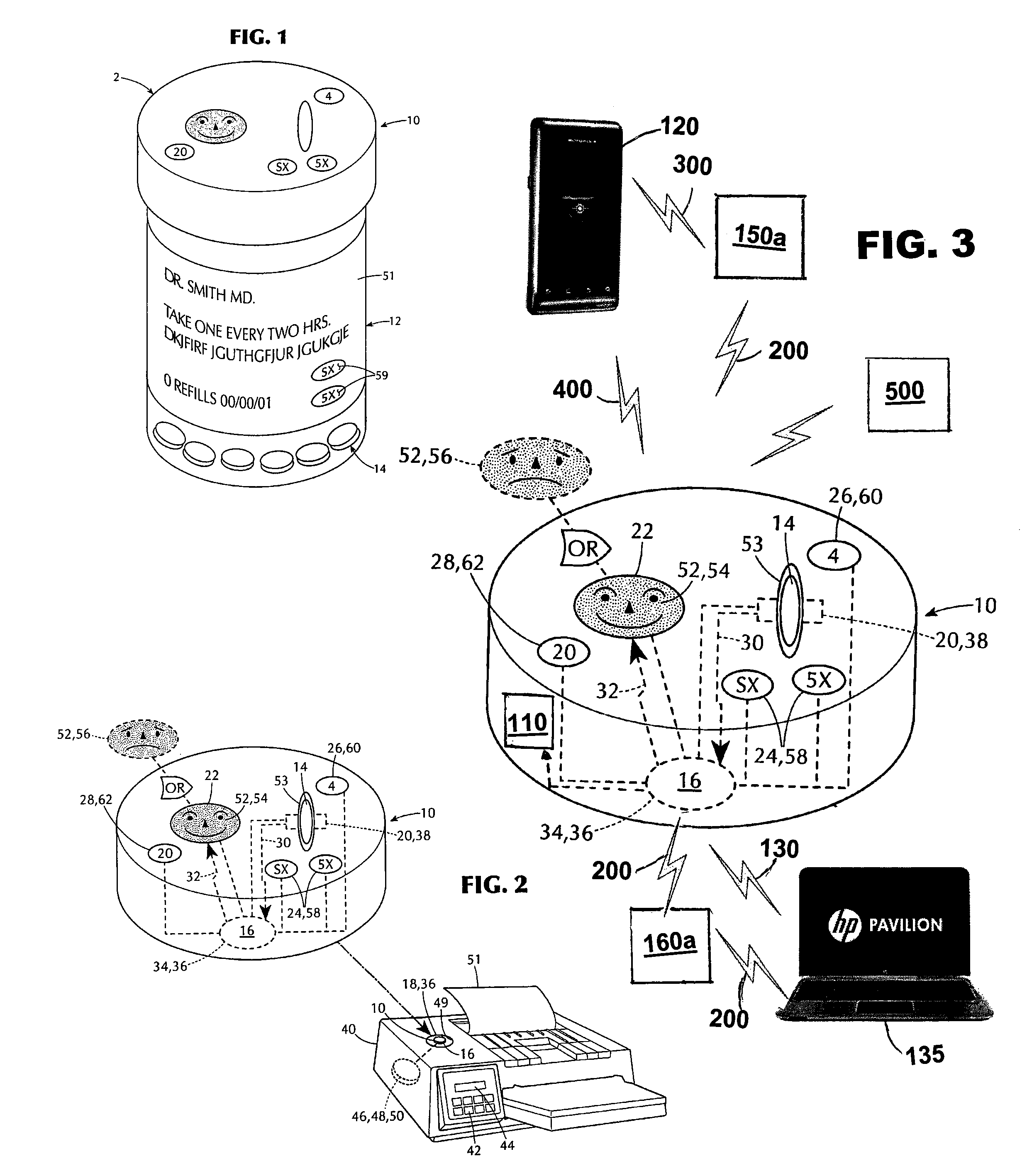
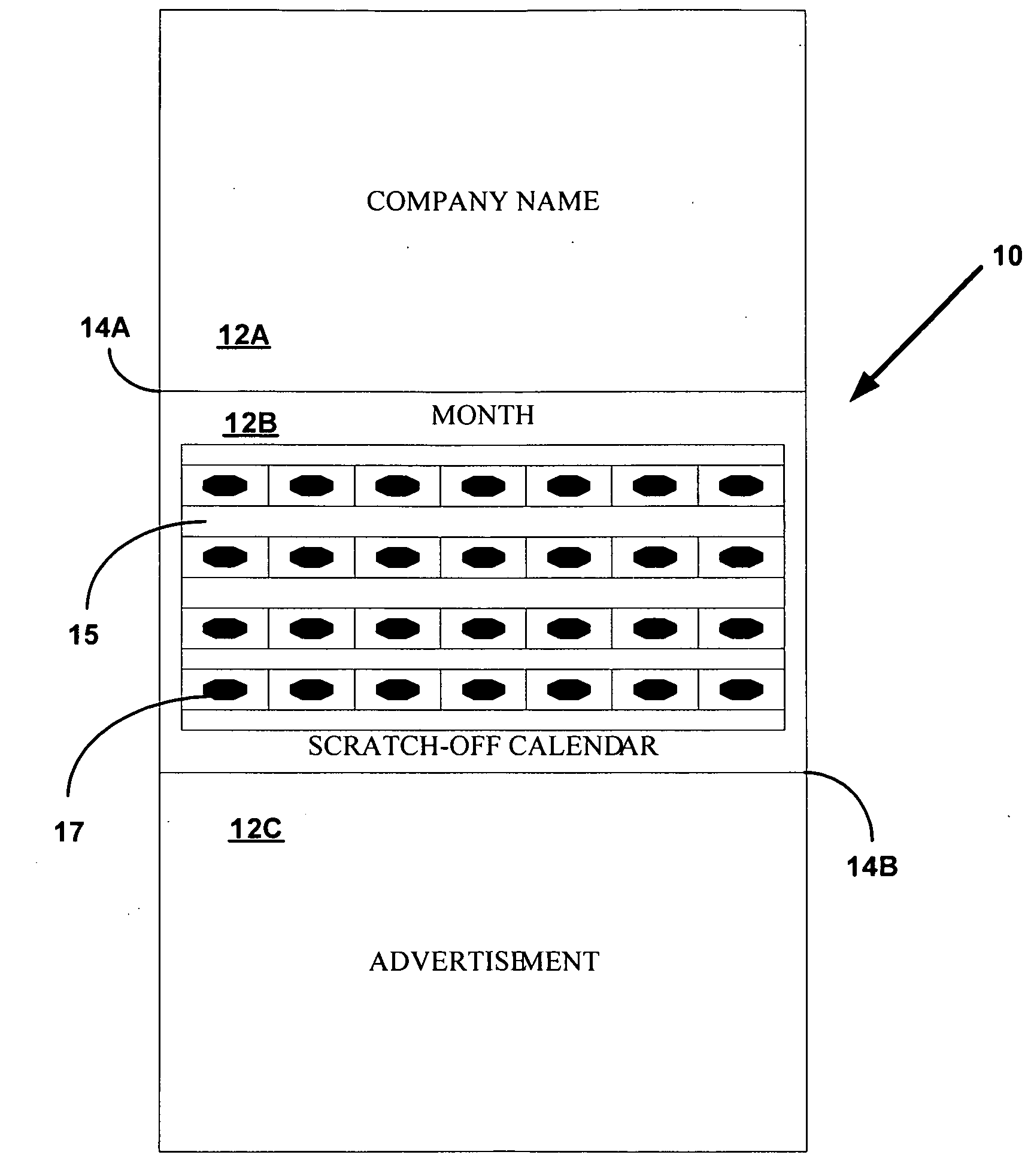
Device and method to monitor, track, map, and analyze usage of metered-dose inhalers in real-time
ActiveUS20090194104A1Easy to manageEasy to identifyRespiratorsDrug and medicationsClinical careComputer science
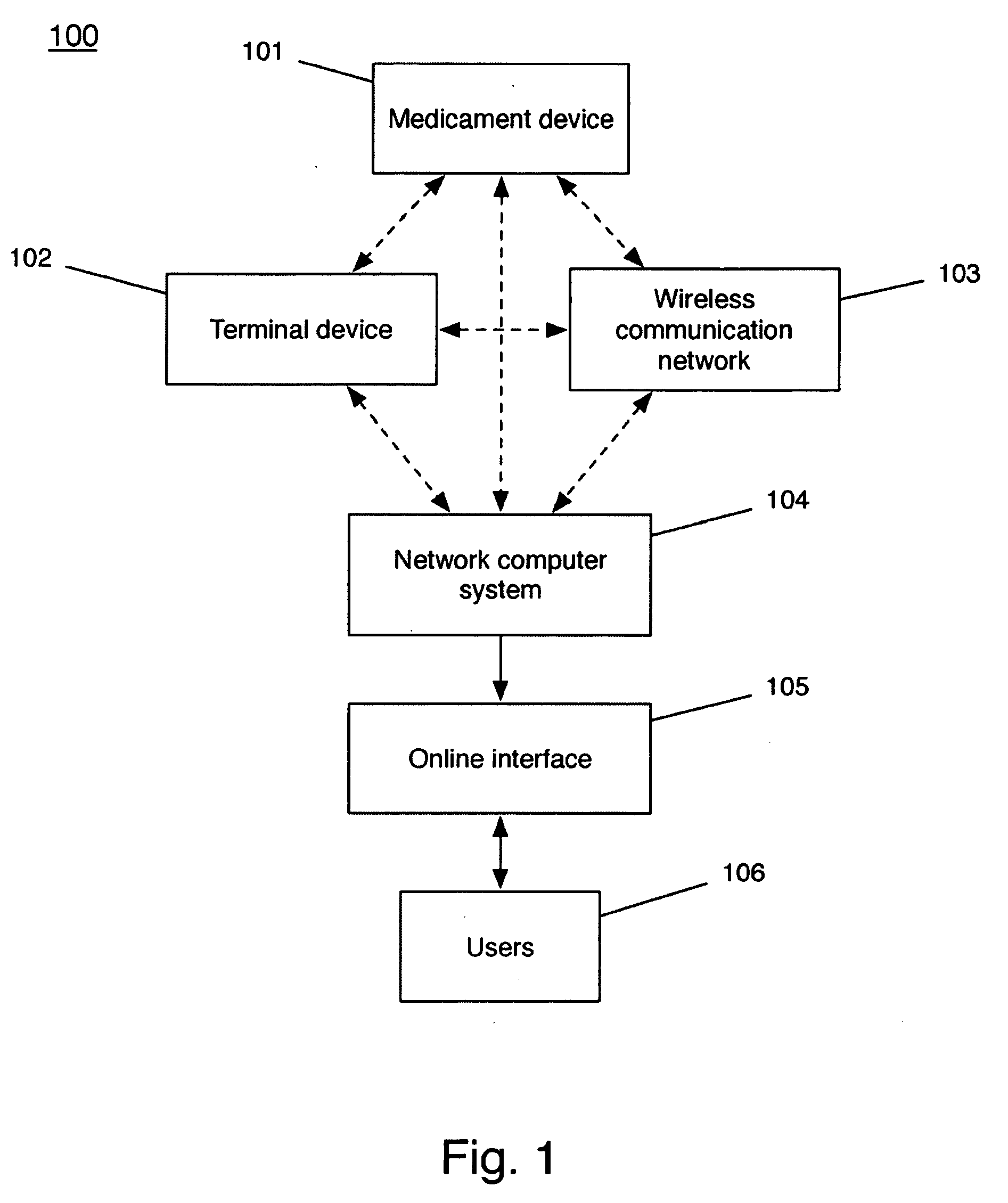
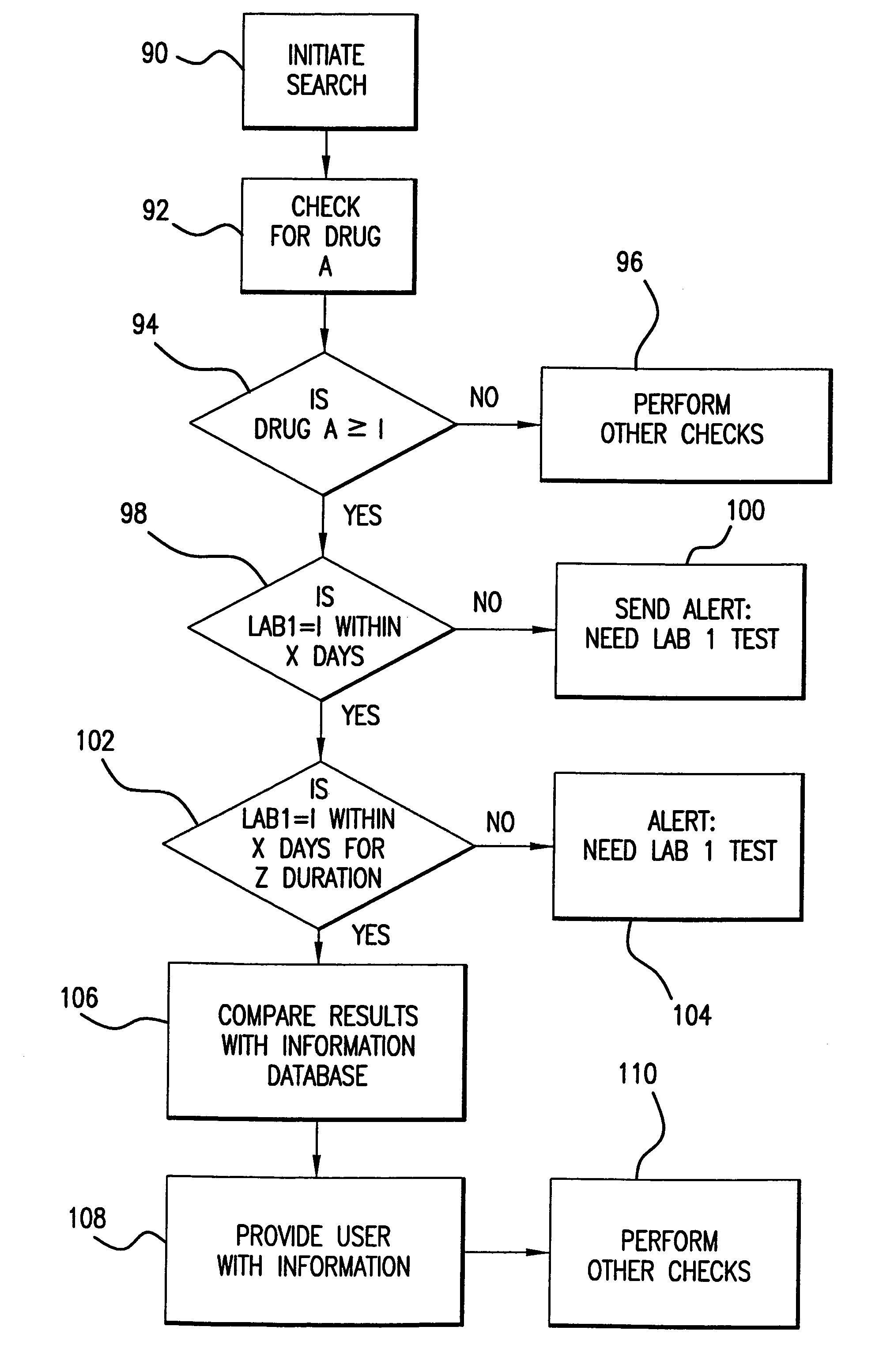
A system and method for accurately and reliably determining and recording the time, date and location where a medication is used, and a system and method for transmitting, collecting, and using that data to improve clinical care, disease management, and public health surveillance. The device allows information concerning drug usage, including the time, date and location of use, to be transmitted to a remote network computer system so that the data can be evaluated to determine current impairment and future risk, and to identify changes in the frequency, timing, or location of medication usage indicative of change in disease control or management, and to examine spatial, temporal or demographic patterns of medication use or absence of use among individuals and groups. In addition, the device may further be configured to transmit signals indicative of its status, condition or other results to the remote network computer system.
Owner:RECIPROCAL LABS CORP D B A PROPELLER HEALTH
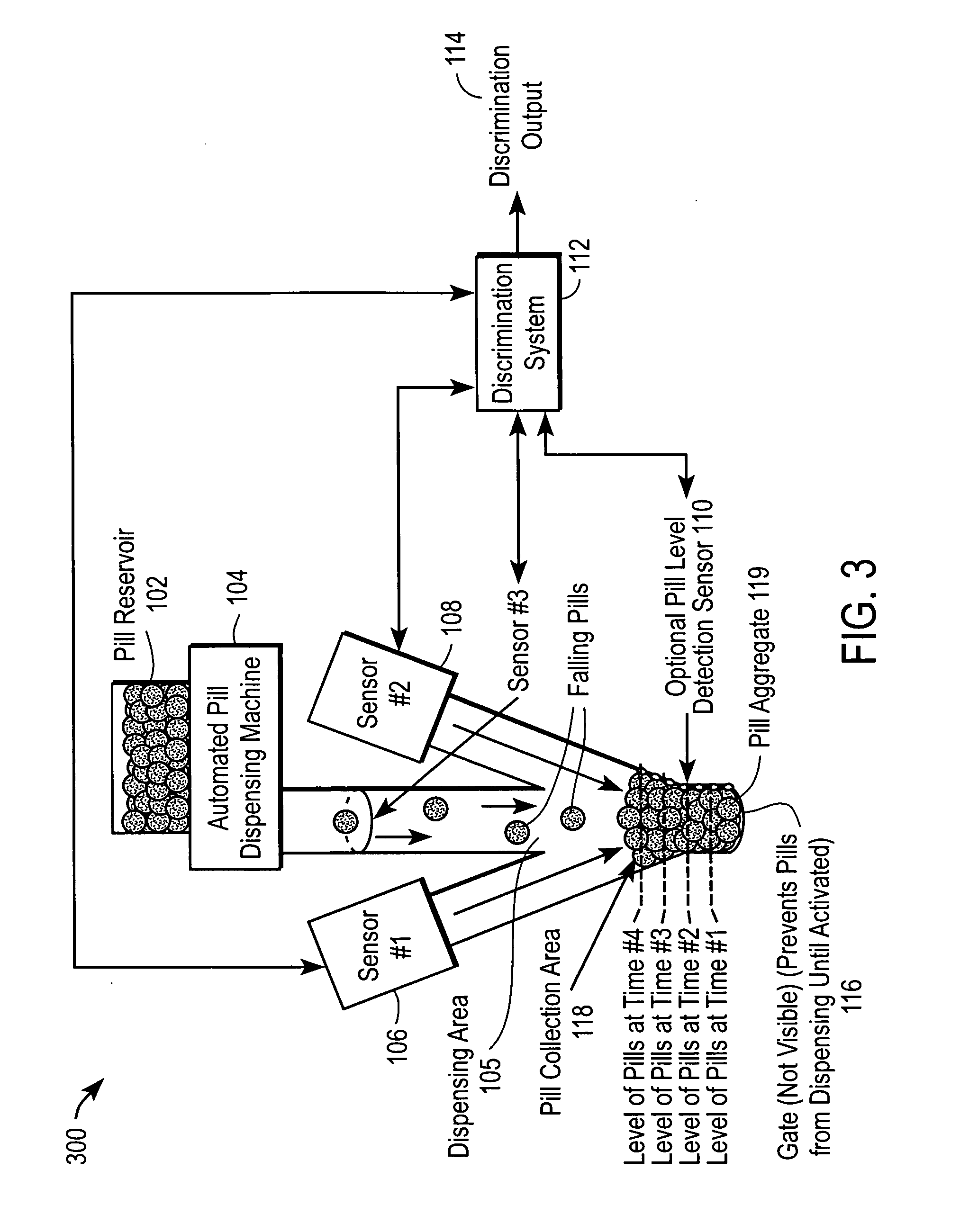
Automated drug discrimination during dispensing
ActiveUS20060124656A1Coin-freed apparatus detailsApparatus for meter-controlled dispensingBiomedical engineeringPharmacist
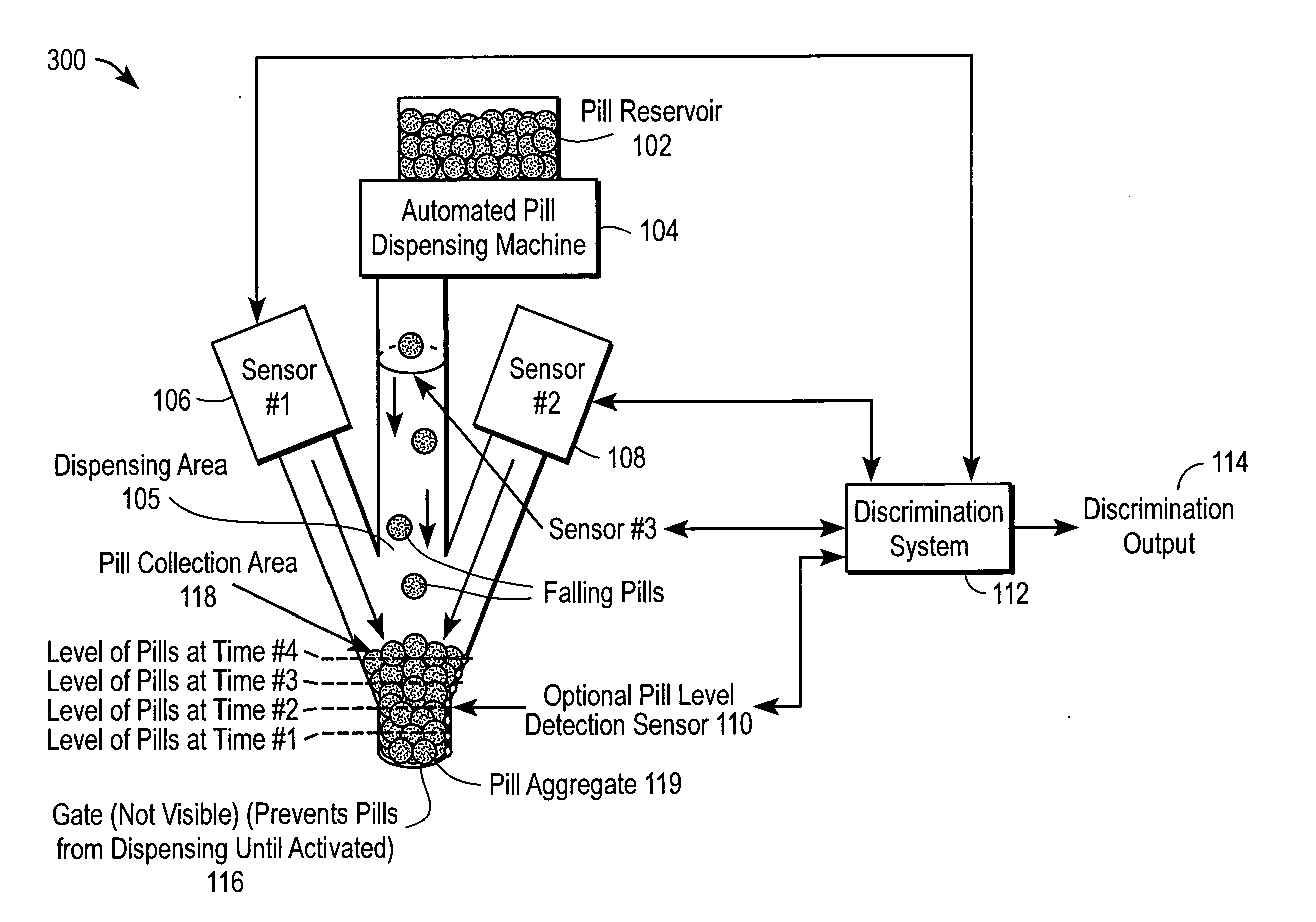
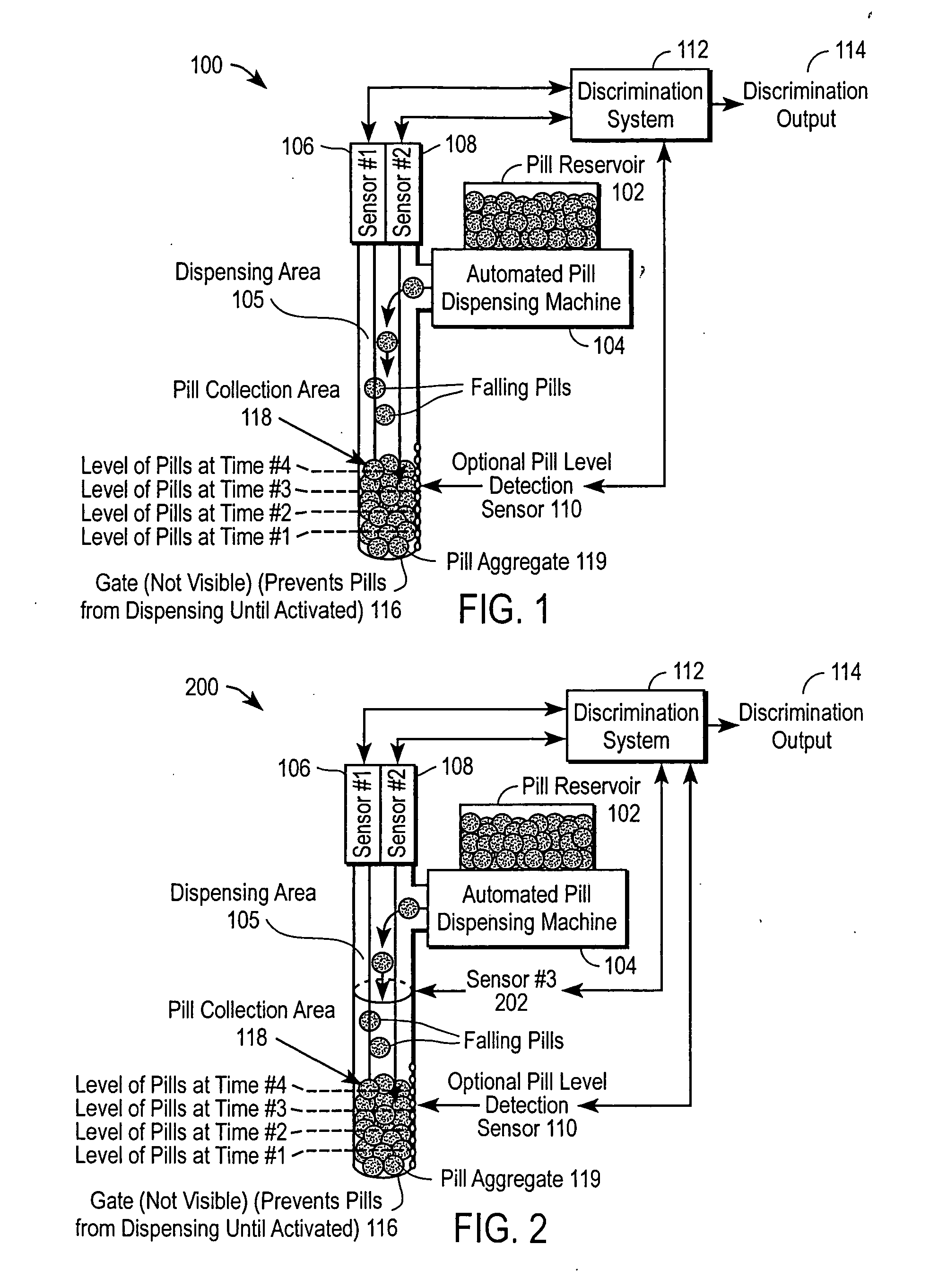
The automated drug discrimination system inspects the drug being dispensed during the dispensing process so that the pharmacist can be certain the correct formulation, dosage and quality of pharmaceuticals were dispensed so the pharmacist does not need to spend as much time examining the dispensed drug. The pills are dispensed through a dispensing area using a dispensing apparatus and are collected in a collection area. At least two sensors take a plurality of measurements of an aggregate of the pills during the dispensing process or of each pill as it moves through the dispensing area. A discrimination system compares the measurements taken to verify that the pills dispensed are the type of pharmaceuticals intended to be dispensed as identified in the individual prescription for at least one of formulation and dosage of the pill.
Owner:PARATA SYST
System and Method for Intelligent Administration and Documentation of Drug Usage
A system and method are provided for automated and precise administration and documentation of medical drugs. An identification mechanism such as barcodes or RFID technology is incorporated into a syringe that can be loaded into a syringe gun having an appropriate reader. The syringe gun includes or communicates with a control system that can store or communicate with a database comprising drug, patient and inventory information. The information can be used by the control system to control the administration of the drug, provide suitable safeguards based on the nature of the drug and the patient, and to update inventory and initiate billing as required.
Owner:HASSAN SAMEER +4
Inhalation device and system for the remote monitoring of drug administration
The present invention is directed to a device for monitoring the usage of inhaled drugs by a patient. The device includes an inhaler, a use sensor, a microprocessor, a wireless transmitter and a battery compartment. These components allow information concerning drug usage to be transmitted to health care personnel that can evaluate the data to determine whether there are changes in drug usage characteristics that are indicative of an impending acute attack. The invention includes not only the device, but also the systems and methods in which the device is employed.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
System for monitoring regulation of pharmaceuticals from data structure of medical and labortory records
InactiveUS7124031B1Strong specificityHigh strengthPhysical therapies and activitiesDigital data processing detailsDiseaseLaboratory Test Result
A system is provided that integrates of records of clinical laboratory services into the assessment and optimization of patient health care and, in particular, regulation of the use of pharmaceuticals. Laboratory test result records are used in conjunction with other health care benefits records to monitor regulation of use of pharmaceuticals by patients. The incorporation of laboratory tests and results into such a utilization system allows improvement in the management of a patient's therapy based on a more precise picture of the patient's level of illness as revealed by the laboratory test results. The system of the present invention also allows optimization of the selection of laboratory tests to be performed, and also provides an outcome assessment of the risk of hospitalization due to pharmaceutical treatments resulting in physician intervention, leading to a change in physician prescribing behavior and, accordingly, a decrease in drug induced hospitalizations and improved quality of patient care and savings of health care costs.
Owner:PROVANTAGE INC +1
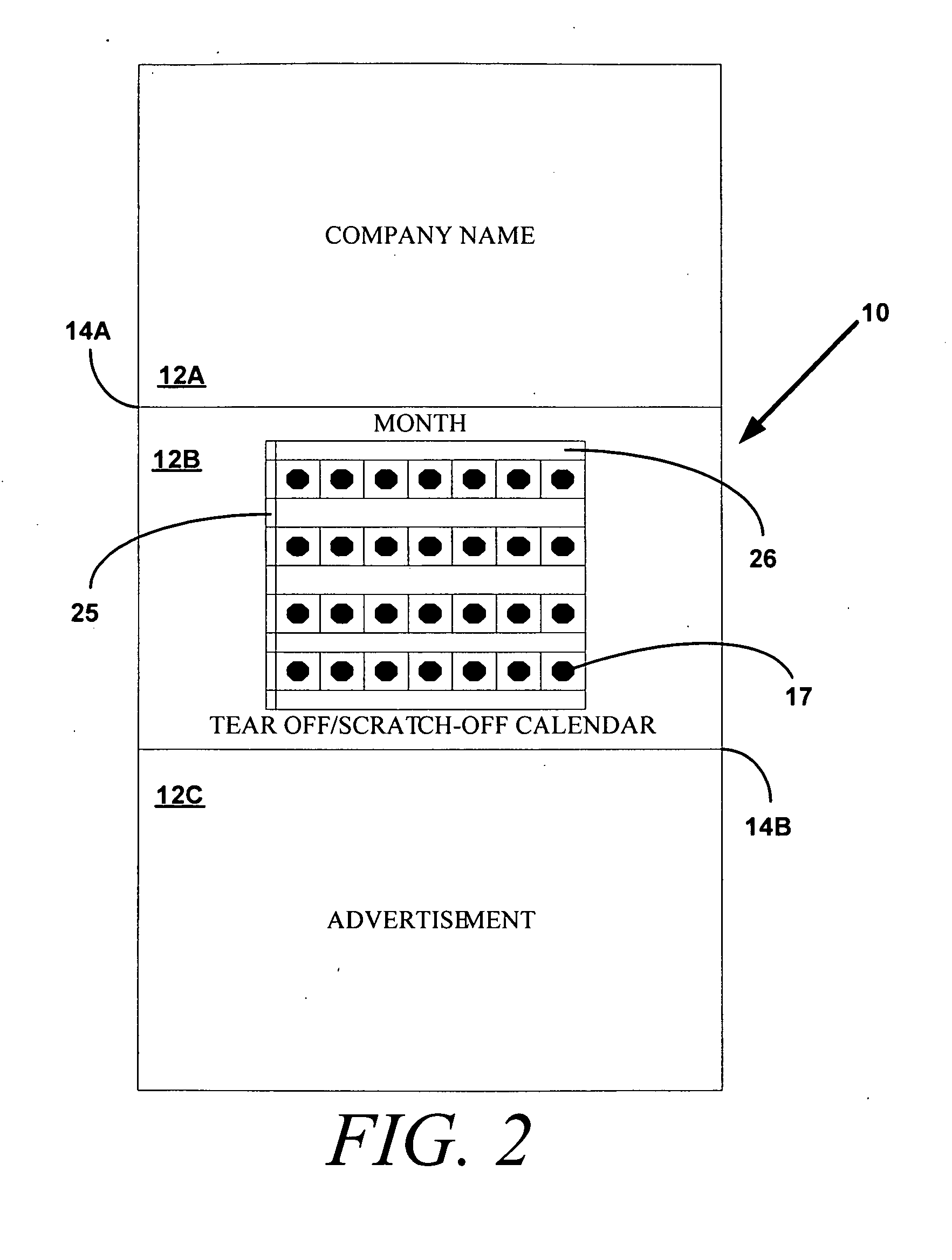
Device and method to monitor, track, map, and analyze usage of metered-dose inhalers in real-time
ActiveUS9550031B2Easy to manageEasy to identifyRespiratorsDrug and medicationsClinical careComputer science
A system and method for accurately and reliably determining and recording the time, date and location where a medication is used, and a system and method for transmitting, collecting, and using that data to improve clinical care, disease management, and public health surveillance. The device allows information concerning drug usage, including the time, date and location of use, to be transmitted to a remote network computer system so that the data can be evaluated to determine current impairment and future risk, and to identify changes in the frequency, timing, or location of medication usage indicative of change in disease control or management, and to examine spatial, temporal or demographic patterns of medication use or absence of use among individuals and groups. In addition, the device may further be configured to transmit signals indicative of its status, condition or other results to the remote network computer system.
Owner:RECIPROCAL LABS CORP D B A PROPELLER HEALTH
Smart cap with communication function
ActiveUS8319613B2Prevent improper dispensingSave effortData processing applicationsDrug and medicationsPharmacyMedicine
A smart cap for a medical container for the containment of solid medications having unique indicia. The cap is provided with an optical scanner configured with at least one locally contained or external data base having general medication identification data and optionally patient-specific information to scan and identify the medication (and optionally the dosage, specific formulations, manufacturing source, etc.) and to record and correlate information regarding patient medication usage (scanning of a medication is generally considered indicative of actual patient taking of the medication). The cap further comprises communication elements configured to transmit / receive “usage” through scanning of a unit dosage of the medication, to an external data base such as the patient's cell phone and or computer (such as with blue tooth or RF communication) or via a telephone call or internet transmission to a data base of a pharmacy or physician or other health care provider.
Owner:LAZAR STEVEN
Compositions for control of drug abuse
InactiveUS20130295170A1Challenge can be overcomePrevent degradationBiocidePowder deliveryBenzodiazepinePersulfate
Opiates, amphetamines, barbiturates and other drugs such as benzodiazepines are extensively abused or misused and are frequently the cause of death by overdosing. These drugs are also prone to oxidation and the final degradation products depend on the reactants and the reaction conditions. This invention describes the use of inactivating agents such as permanganates, peroxides, persulfates, bismuthates, periodates or other oxidants in a dosage form as an approach to minimize abuse and overdose. The product is designed such that the inactivating agent is released if there is an attempt to extract the drug from the formulation or in cases of overdose. Once released, the inactivating agent quickly degrades the drug and converts it into inactive compounds. Since the reactants (drug and inactivating agent) are incompatible in situations of normal drug usage, they are kept separated within the vehicle of the invention, but released for interaction in case of misuse. A catalyst may be included in the formulation to facilitate the reaction.
Owner:KYDES PHARMA
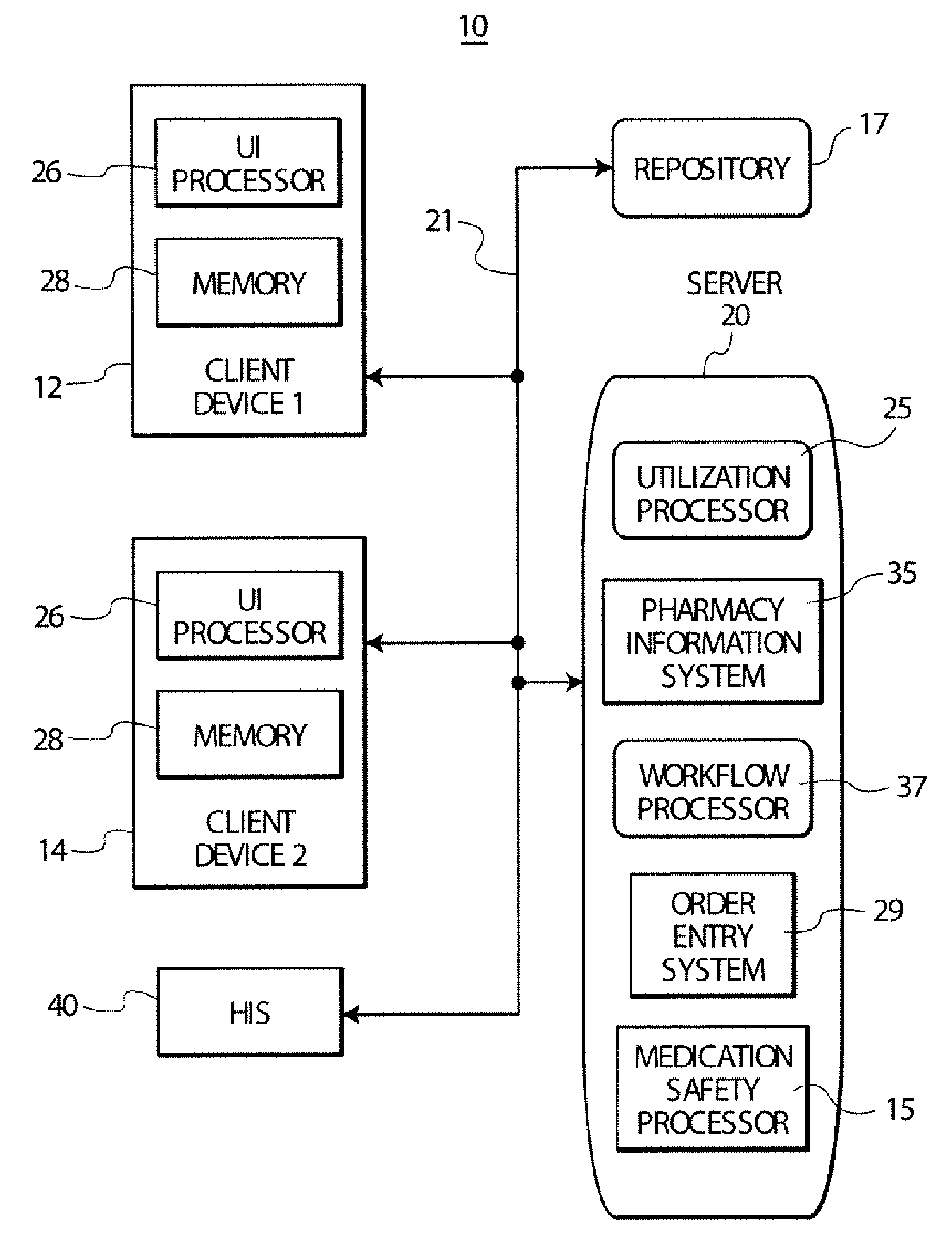
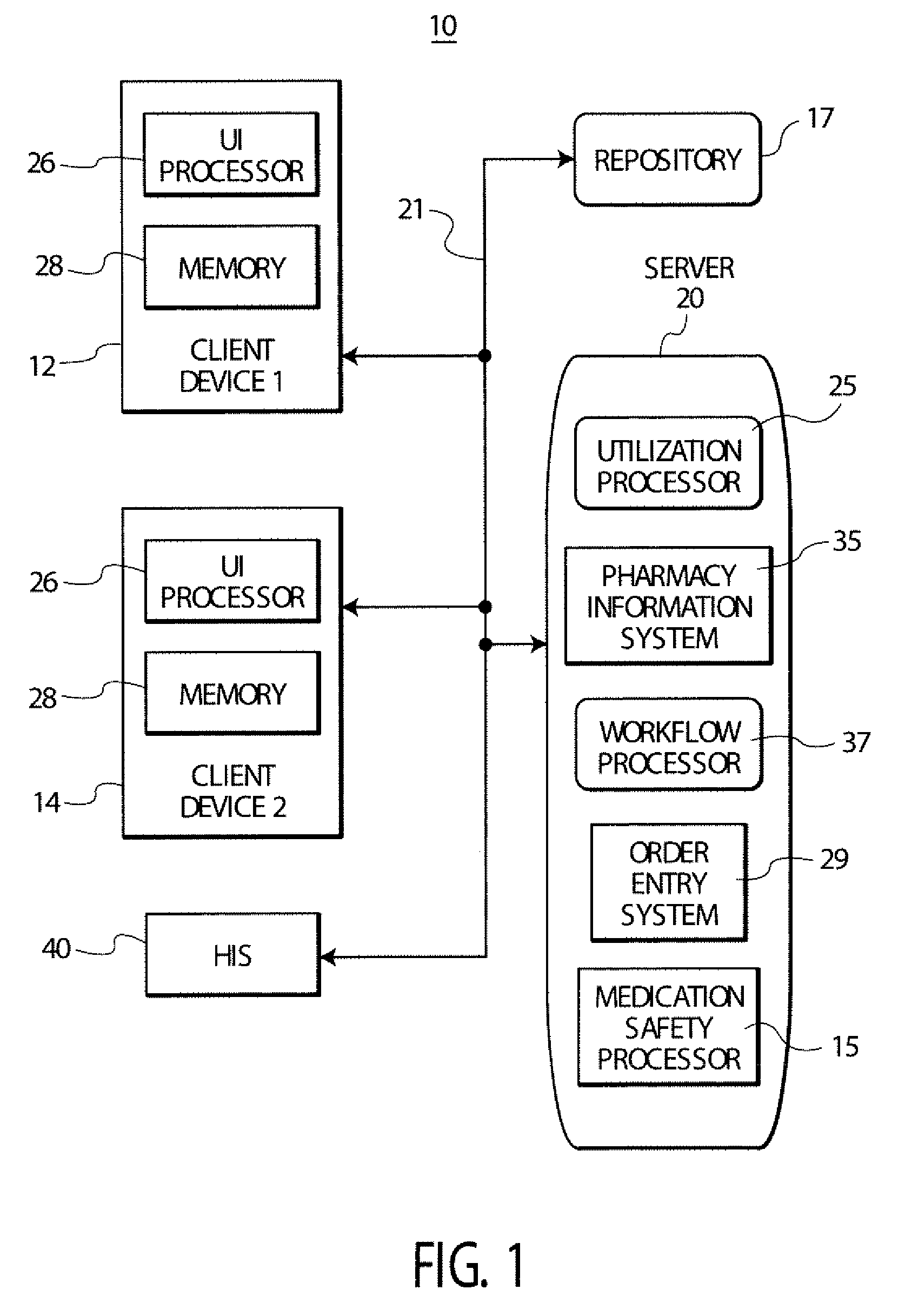
Treatment order processing system suitable for pharmacy and other use
A system links a standardized coded diagnosis associated with a medication order with information in a pharmacy information system to supplement medication utilization data. A pharmacy user interface system includes a user interface processor for providing data representing at least one display image supporting user entry of a coded diagnosis identifier for association with an order identifier identifying a medication order record indicating an order for a particular medication to be administered to a particular patient during an inpatient stay in a medical facility. A repository stores information associating multiple order identifiers and associated multiple coded diagnosis identifiers A utilization processor uses the stored repository information and processing data associating multiple order identifiers and associated multiple coded diagnosis identifiers to determine utilization information indicating usage characteristics of a particular medication for treatment of a condition indicated by a particular coded diagnosis.
Owner:CERNER INNOVATION
Apparatus and method for advertising
InactiveUS20090039640A1Encourages interactionPromote extended and repeated viewingOther printing matterArticle advertisingMedicineInternet privacy
Owner:NIJJER JAY +2
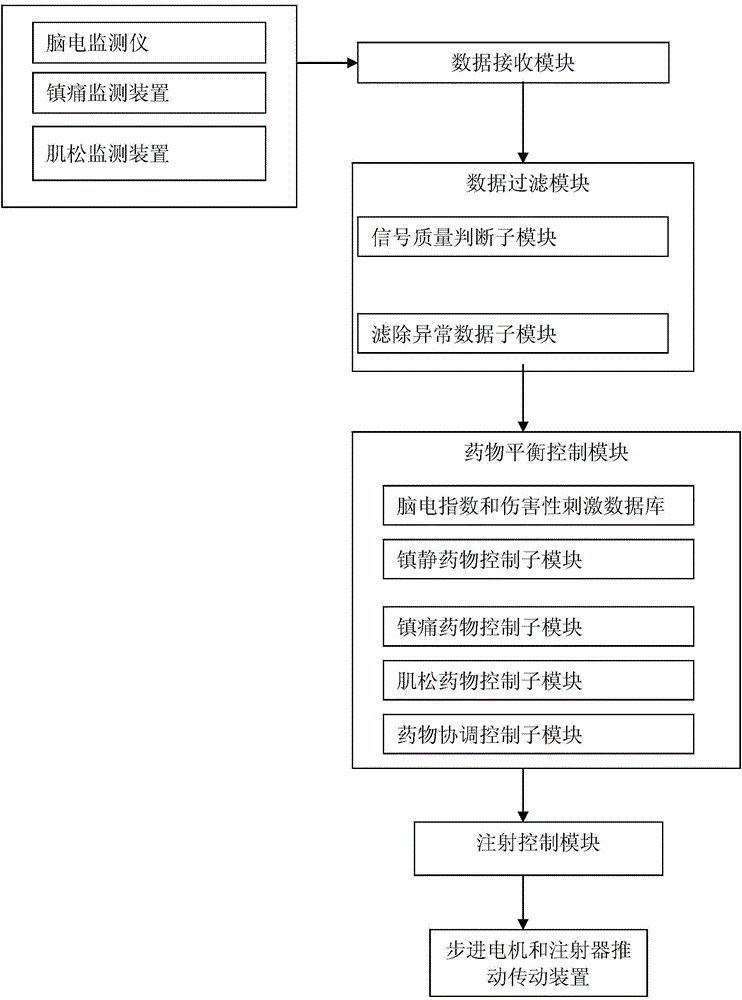
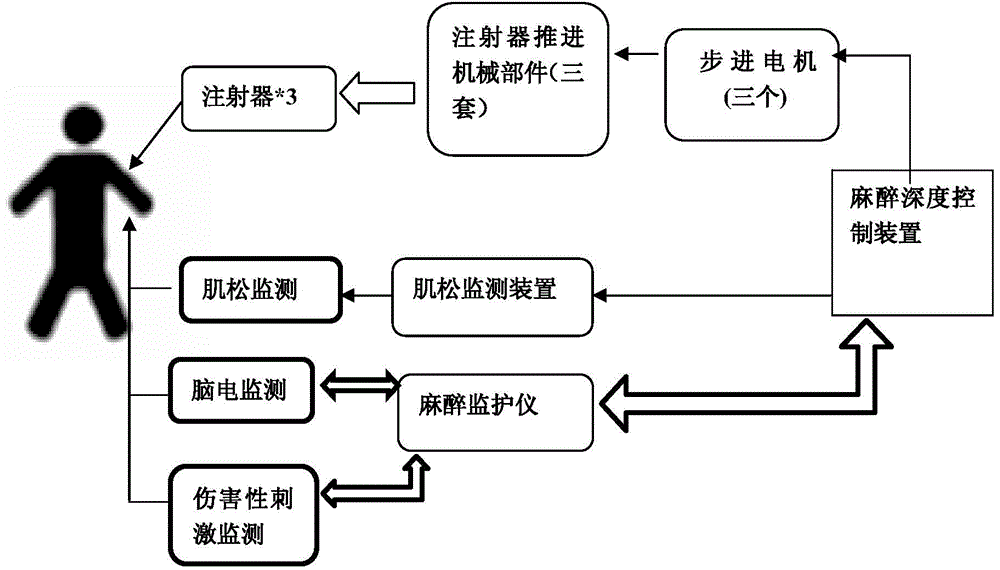
Anaesthetic balance control device and control method
ActiveCN103908249AAddressing Individual DifferencesEasy to controlAutomatic syringesDiagnostic recording/measuringBispectral Index MonitorNociceptive Stimulus
The invention aims at providing an anaesthetic balance control device. The device comprises a data filter module and a drug balance control module. Pre-stored electrocerebral indexes, noxious stimulation data drug usage data are divided into electrocerebral index-sedative data and noxious stimulation data-sedative data. Corresponding drug target control indexes are selected according to electrocerebral index and noxious stimulation data changes in certain period, threshold values are preset, and injection of muscle relaxants are increased or stopped based on the set threshold values. Meanwhile, bispectral index, noxious stimulation data, neuromuscular retarding depth data change tendency slope and the like can be analyzed dynamically, and transverse balance adjusting control is performed on three signals. The invention further provides a work method of the device. According to the device and the method, the problem the imbalanced usage of three drugs is solved, effects of individual differences are prevented, the safety during anesthesia is guaranteed to the largest extent, and good social values and application prospects are provided.
Owner:GUANGXI VERYARK TECH
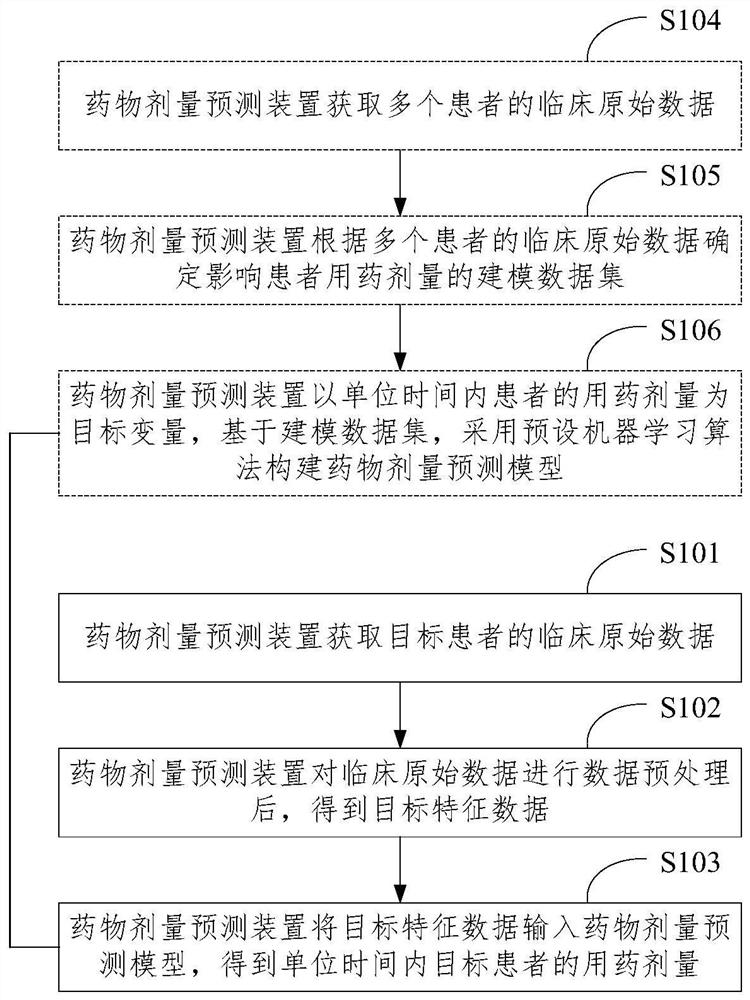
Drug dose prediction method and device, electronic equipment and storage medium
PendingCN113270203APrecise dosageAccurately determineMedical data miningDrug referencesOriginal dataAdjuvant therapy
The invention provides a drug dose prediction method and device, electronic equipment and a storage medium, is applied to the technical field of data processing, and can determine more accurate drug use doses for different patients. The method comprises the following steps: acquiring clinical original data of a target patient, wherein the clinical original data comprises demographic information, therapeutic drug use information, drug combination information, adjuvant therapy means, gene polymorphism and inspection information; performing data preprocessing on the clinical original data to obtain target feature data, the data preprocessing including at least one of data cleaning, data normalized coding and data screening; and inputting the target feature data into a drug dose prediction model to obtain the drug dose of the target patient in unit time.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV +1
Treatment Order Processing System Suitable for Pharmacy and Other Use
A system links a standardized coded diagnosis associated with a medication order with information in a pharmacy information system to supplement medication utilization data. A pharmacy user interface system includes a user interface processor for providing data representing at least one display image supporting user entry of a coded diagnosis identifier for association with an order identifier identifying a medication order record indicating an order for a particular medication to be administered to a particular patient during an inpatient stay in a medical facility. A repository stores information associating multiple order identifiers and associated multiple coded diagnosis identifiers A utilization processor uses the stored repository information and processing data associating multiple order identifiers and associated multiple coded diagnosis identifiers to determine utilization information indicating usage characteristics of a particular medication for treatment of a condition indicated by a particular coded diagnosis.
Owner:CERNER INNOVATION
Compound sodium sulfadimidine injection liquid for pig and preparation method thereof
InactiveCN101606945ADelay drug resistanceIncreased sensitivityAntibacterial agentsOrganic active ingredientsDoxofyllineTrimethoprim
The invention discloses a compound sodium sulfadimidine injection liquid for a pig and a preparation method thereof, aiming at providing a compound kanamycin sulfate injection liquid which has fast effect for treating toxoplasmosis of the pig, addresses both the symptoms and root causes, reduces the time and dosage of used drug and has convenient drug usage, and a preparation method with simple technique and easy implementation. Each 100L injection liquid comprises: 5 to 20 sodium sulfadimidine, 1 to 10kg of lincomycin hydrochloride, 5 to 20kg of doxofylline, 1 to 4kg of trimethoprim, 0.2kg of sodium bisulfite, 0.01kg of EDTA-2Na, 40kg of propylene glycol, 10kg of 95% alcohol and the balance of water for injection. The injection liquid has fast effect for toxoplasmosis of the pig, addresses both the symptoms and root causes, can effectively treat and prevent secondary infection of bacterium, reduces drug resistance of pathogenic microorganism and leads the pig to accelerate restoring. Simultaneously, the compound sodium sulfadimidine injection liquid is an injection liquid and has convenient use.
Owner:TIANJIN SHENGJI GRP CO LTD
Systems and methods for monitoring the use of medications
ActiveUS9155833B2Precise managementAdministration of has been hamperedDrug and medicationsIntravenous devicesControl engineeringPharmaceutical drug
Systems and methods for monitoring the use of a fluid over the lifecycle of the fluid, said systems including a plurality of fluid identification stations, each station having one or more sensors to detect and identify a parameter of a fluid, wherein a each station is operably interconnected thereby permitting each station to access and verify the identity of a fluid as determined by each independent fluid identification station.
Owner:BECTON DICKINSON & CO
Potassium sodium dehydroandroan drographolide succinate enteric dry suspension and preparation method thereof
ActiveCN103479583AAvoid problems with stomach acid damageQuality improvementOrganic active ingredientsAntiviralsOral medicationSuspending Agents
The invention relates to potassium sodium dehydroandroan drographolide succinate enteric dry suspension and a preparation method thereof. The prepared potassium sodium dehydroandroan drographolide succinate enteric dry suspension comprises, by weight percentage, 5%-50% of potassium sodium dehydroandroan drographolide succinate pellets or particles, 1%-10% of suspending agents and 50%-95% of taste-masked agents. According to the potassium sodium dehydroandroan drographolide succinate enteric dry suspension, the potassium sodium dehydroandroan drographolide succinate is creatively changed from previous injection administration preparations to oral administration preparations, the blanks of domestic and oversea potassium sodium dehydroandroan drographolide succinate oral administration preparations are filled, the untoward effect incidence rate caused by potassium sodium dehydroandroan drographolide succinate injection administration is greatly reduced, administration routes and dosage forms of the potassium sodium dehydroandroan drographolide succinate are enriched, and great value and superiority are provided for clinical rational drug usage. The prepared potassium sodium dehydroandroan drographolide succinate enteric dry suspension is stable in quality, takes effect rapidly, and is good in suspension effect and convenience for the old, children and patients with odynophagia to use.
Owner:司鹏 +1
Method, system, and computer program product for determining a narcotics use indicator
ActiveUS8688477B1Rapid assessmentData processing applicationsHealth-index calculationDispensaryDispensing medications
A method, system, and computer program product for determining a narcotics use indicator to enable a physician, or other prescriber, to quickly review a numerical score that reflects a patient's past drug use and is indicative of proper, or improper, future drug use. This score analyzes many aspects of a patient's past activities to determine multiple individual indicator values that may be selectively weighted to create a final narcotics use indicator. Such individual indicator values may include a usage related indicator factoring in the patient's past drug use, particularly the type of narcotics and controlled substances used; an instruction related indicator that may consider the patient's past use of prescribers, quantity of prescriptions, or the number of open prescriptions from different prescribers; a dispensing related indicator that examines a patient's use of pharmacies, in filling prescriptions; or even an auxiliary indicator that may reflect the patient's number of active prescriptions.
Owner:APPRISS
Method of establishing fingerprint spectrum of infant diarrhea stopping drug preparation
ActiveCN106802327AImprove effectivenessImprove securityComponent separationDrugs preparationsPharmaceutical drug
The invention belongs to the field of drug analysis and particularly relates to a method of establishing a fingerprint spectrum of an infant diarrhea stopping drug preparation. According to the method, by taking the infant diarrhea stopping drug preparation as a detection object, the method of establishing the fingerprint spectrum of the infant diarrhea stopping drug preparation is established, common characteristic peaks including peak 1, peak 2 liquiritin, peak 3 ammonium glycyrrhizinate, peak 4, peak 5, peak 6, peak 7 and peak 8 are affirmed, the peak 2 liquiritin is selected as an internal reference peak in the fingerprint spectrum, and a relative retention time of each common characteristic peak is determined; and in addition, with combination of information of a plurality of chromatographic peaks in the fingerprint spectrum, the quality of the infant diarrhea stopping drug preparation can be comprehensively and rapidly detected, so that comprehensive quality detection and whole quality control of the infant diarrhea stopping drug preparation are facilitated, and improvement on the use safety and stability of the drug is facilitated. Simultaneously, the method of establishing the fingerprint spectrum of the infant diarrhea stopping drug preparation has the advantages of high precision, good repeatability, high stability and the like.
Owner:合肥华润神鹿药业有限公司
Patient care management systems and methods
InactiveUS8010379B2Suitable for useDrug and medicationsOffice automationDrug availabilityMedication Prescriber
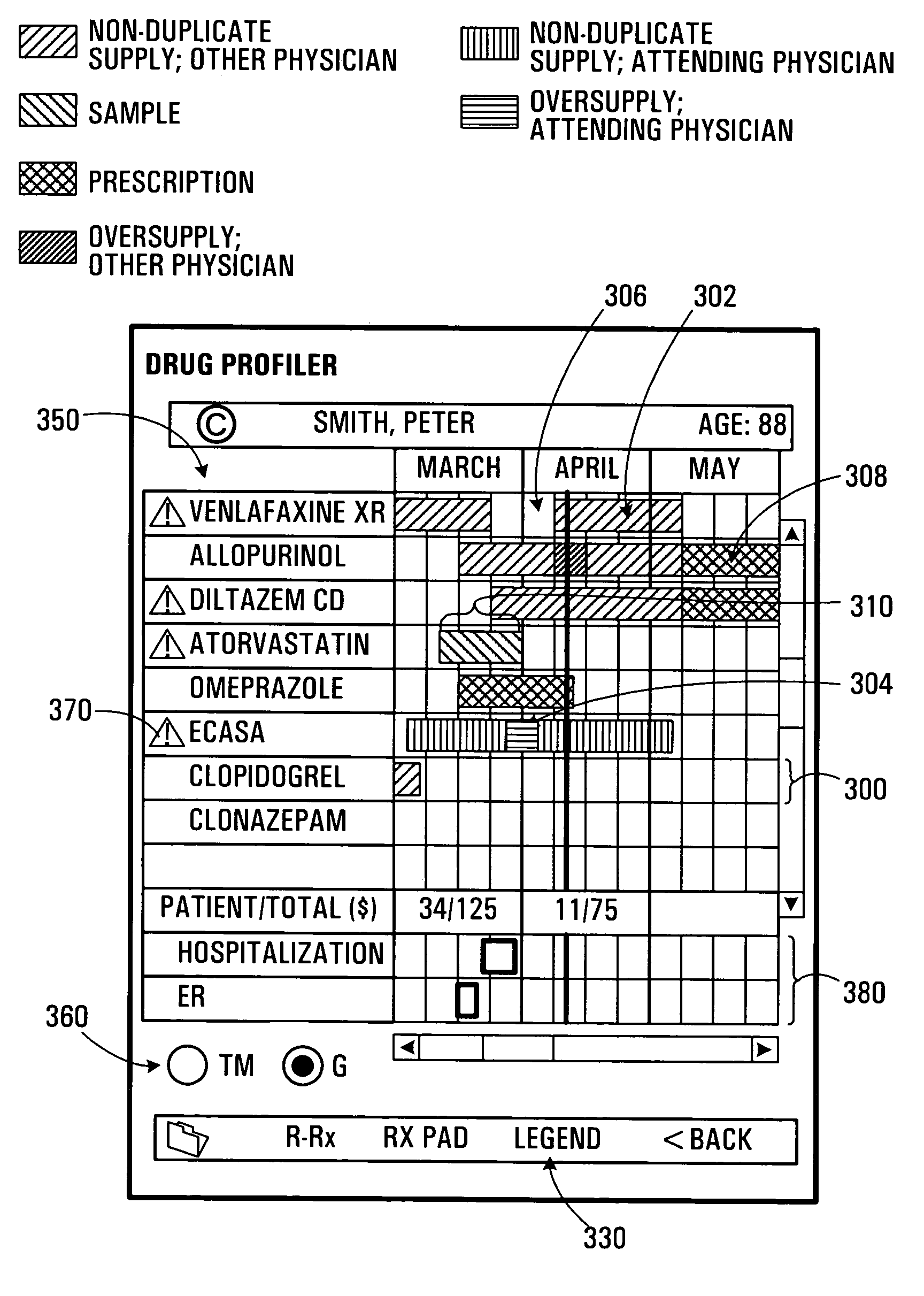
A patient care management system for assisting a physician in monitoring drug use by a patient. The system receives drug dispensation data for a drug, on the basis of which the system determines drug supply availability data. The drug supply availability data is indicative of periods of time during which the drug was available in non-duplicate supply, or in oversupply, or in insufficient supply. The periods of time are displayed with respect to a common time base. Each degree of supply availability is visually displayed via a graphical user interface using, e.g., a color coded scheme, so as to be distinguishable by a user. This allows a physician to rapidly assess over-consumption or compliance problems. Plural drugs may be monitored on a single display screen. The system may also be adapted to allow an prescribing physician to assess refill compliance, hospitalization periods and prescription drug costs.
Owner:MCGILL UNIV
Medication identification and inventory control system
ActiveUS10528911B1Accurate trackingPrecise maintenanceDrug and medicationsHealthcare resources and facilitiesNarcoticPharmaceutical Substances
A system and a method for medication identification and inventory control is disclosed. The disclosed system and method were designed to track specified medications' (e.g., narcotics and controlled substances) inventory from manufacturer's lot number all the way to the use / waste stage of the medications and to provide periodic reports and notifications on the medications' usage, breakage, waste and expiration. Further, the disclosed system and method utilizes tamper evident bags with unique identifier to protect and keep accurate track of the medications.
Owner:LASTER BOBBY J
Adherence monitoring method and device
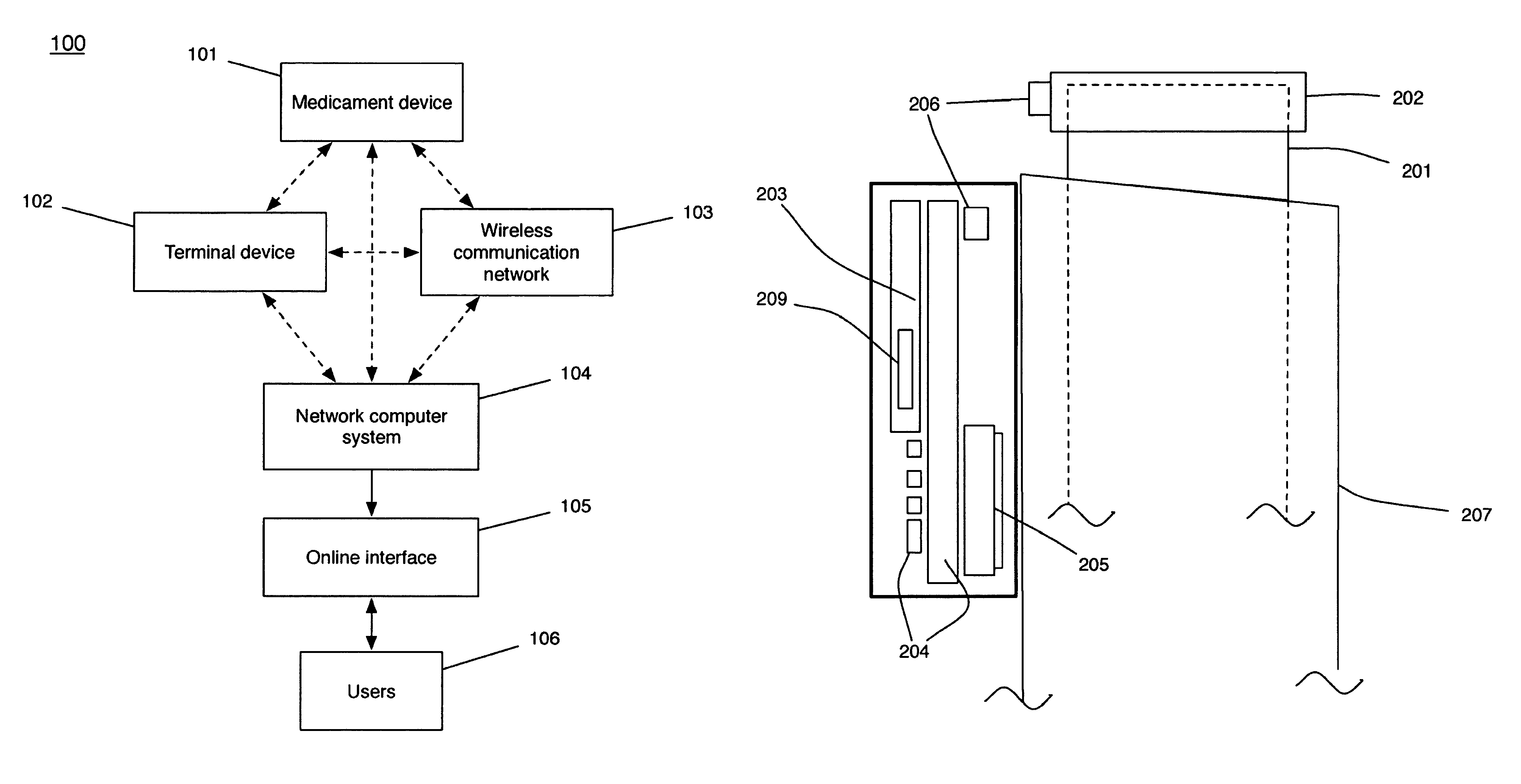
In one aspect the invention provides an adherence monitoring device for a medication delivery device, the monitoring device including at least one inductive coil sensor which includes at least one inductive controller, at least two inductive coils including a first inductive coil coupled to the housing and a second inductive coil coupled to the housing. The first inductive coil is configured to exhibit a response to an inductive change and provide a first change signal in response to an inductive change, and the second inductive coil is configured to exhibit a response to the inductive change and provide a second change signal in response to the inductive change. The monitoring device also includes a processor configured to receive sensor data from the inductive controller representative of the first and second change signals provided by the first inductive coil and second inductive coil and to compare at least one characteristic of the first change signal and the second change signal to detect the occurrence of a medication usage event or a false triggering event.
Owner:ADHERIUM (NZ) LTD
Compound sulfamethoxazole dispersible tablet and preparation method thereof
InactiveCN105769883ASimple processReduce manufacturing costAntibacterial agentsOrganic active ingredientsDrug utilisationTrimethoprim
The invention discloses a compound sulfamethoxazole dispersible tablet and a preparation method thereof. The dispersible tablet is characterized in that the compound sulfamethoxazole dispersible tablet prescription comprises the following components in parts by weight: 360-500 parts of sulfamethoxazole, 50-100 parts of trimethoprim, 360-420 parts of a filler, 80-120 parts of a disintegrating agent, and 3-5 parts of a lubricant. The compound sulfamethoxazole dispersible tablet has the advantages of scientific formula, simple process, rapid disintegration of preparation, good dissolution rate, cheap and easily available auxiliary materials, and low cost; the tablet is suitable for large scale production, and can reduce drug usage burden of patients.
Owner:KAMP PHARMA
Composite insecticidal composition containing dinotefuran and chlopyrifos and purpose thereof
InactiveCN102415408AGood control effectSuppress drug resistanceBiocideAnimal repellantsChlorpyrifosHomoptera
The invention discloses a composite insecticidal composition containing dinotefuran and chlopyrifos and purpose thereof. The insecticidal composition contains main effective components of dinotefuran and chlopyrifos in a mass ratio of 0.1-80:0.5-80. The insecticidal composition of the invention can be used for controlling homoptera insects on crops, especially for controlling rice hopper and aphid, can generate efficient synergism, overcome and delay resistance of insects, and has fast insecticidal speed, long persistent period, reduced drug usage cost and an effect obvious better than that of a single dose.
Owner:NANJING HUAZHOU PHARMA
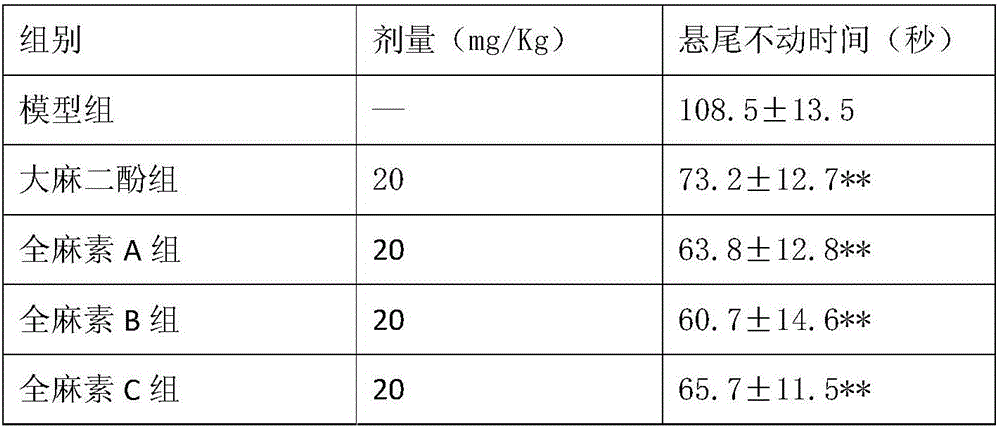
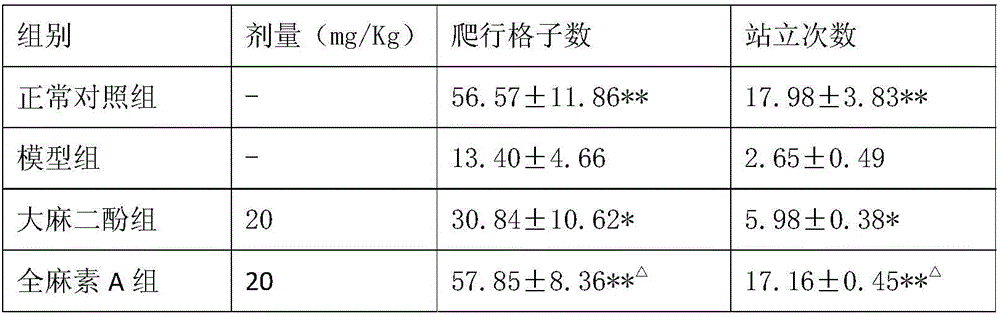
Application of all-cannabinoid in preparation of drugs for treating depression
InactiveCN105963359AShorten immobility timeIncrease the number of voluntary activitiesNervous disorderPlant ingredientsEndogenous depressionReactive Depression
The invention discloses application of all-cannabinoid in preparation of drugs for treating depression. The all-cannabinoid is prepared by uniformly mixing, by weight, 0.3-99.7 parts of an industrial hemp seed extract with 99.7-0.3 parts of industrial cannabinoids. The all-cannabinoid has the obvious relieving effect on the symptoms such as secondary learning memory deterioration of the depression and can be used for preparing the drugs for treating various depression symptoms including endogenous depression, reactive depression, latent depression, secondary depression caused by drugs, climacteric or postnatal depression, depression induced by cerebral trauma or stroke, diabetes-complicated depression and depressive neurosis and used for preparing the drugs for treating the symptoms such as secondary learning memory deterioration and anhedonia which are caused by the depression, and the effect of the all-cannabinoid is better than that of cannabidiol. The all-cannabinoid can be prepared into the clinical drugs of various dosage forms through a conventional preparation method and is convenient to use.
Owner:云南瑞酚生物科技有限公司
Drug for treating insomnia and dreaminess
InactiveCN103989918AGood effectEasy to useNervous disorderInanimate material medical ingredientsSide effectCodonopsis pilosula
The invention provides a drug for treating insomnia and dreaminess, and the drug comprises codonopsis pilosula, rhizoma corydalis, radix ophiopogonis, dens draconis, polygala root, cortex albiziae and caulis polygoni multiflori. The beneficial effects of the drug are that: due to use of the drug of the technical scheme, the insomnia and dreaminess can be radically treated, and the drug has the advantages of being convenient to use, obvious in effect, short in taking period, free of side effect and high in security.
Owner:TIANJIN PACIFIC PHARMA
Broiler chicken breeding method
InactiveCN106665487APromote formationHigh activityFood processingAnimal feeding stuffFeed conversion ratioFodder
The invention discloses a broiler chicken breeding method. The breeding method comprises the steps that a broiler chicken breeding base is built, wherein the broiler chicken breeding base at least comprises a breeding shed with the area of 1 mu, the breeding density is 4-6 / m<2> according to the breeding shed, the breeding shed is divided into two parts, the two parts are connected through a trapezoidal slope, the height of the trapezoidal slope is at least 2 m, the gradient of the slope is 30-45 degrees, a feeding area is arranged on the trapezoidal top surface, the area of the trapezoidal top surface is 1 / 4 of that of the breeding shed, and facilities in the feeding area comprise a feed feeding trough and a water feeding trough. According to the broiler chicken breeding method, the breeding local area is reasonably planned, the breeding shed and the feeding area are arranged, scientific managing and raising and intensive breeding are conducted, the exercise amount of broiler chickens is increased, the appetite and the feed conversion rate of the broiler chickens can be increased, the immune function of the broiler chickens can be improved, chemical drug usage and drug residues are reduced, and the chicken meat quality of the broiler chickens is improved.
Owner:QINZHOU KANGLYUBAO AGRI CO LTD
Methods, dosing regimens and medications using anti-progestational agents for the treatment of disorders
ActiveUS8173626B2Avoid and lessen occurrenceAvoid and lessen and severityElcosanoid active ingredientsAntineoplastic agentsDiseaseRegimen
Owner:LAB HRA PHARMA SA
Eyewear that simulates impairment and method
InactiveUS20150194067A1Safely experience effectRealistic and economical to produceCosmonautic condition simulationsEducational modelsDrugFresnel lens
Impairment simulating eyewear is disclosed. The eyewear simulates impairment, such as sleep deprivation, or the effects of drug use, through the use of specialized distorting lenses, such as lenticular prism lenses and Fresnel lenses. The lens may be provided with an array of light restricting patterns. The eyewear may include a colored transparent cover.
Owner:DRUNK BUSTERS OF AMERICA L L C
Method, device and system applied to drug box for providing drug information for user
InactiveCN107088162AKeep healthyEnsure proper medicationOral administration deviceDomestic articlesUse medicationDisplay device
The invention discloses a method, device and system applied to a drug box for providing drug information for a user. By the adoption of the method, double drug-taking prompts can be provided for the user, not only can the user be reminded of the conditions of drug usage through voice, but also the amount of drugs taken by the user can be displayed through a display device, so that it is ensured that the user can receive prompt information accurately, the situations can not occur that the drugs are taken wrongly or taken in delay time, the health of the user is ensured, at the same time, a son or a daughter can preset setting information and the prompt information through external equipment, the supervision function is achieved, and the situation can be further ensured that the user takes the drugs correctly.
Owner:HANGZHOU LIANLUO INTERACTIVE INFORMATION TECH CO LTD
Compound kanamycin sulfate injection liquid for pig and preparation method thereof
InactiveCN101606946ADelay drug resistanceIncreased sensitivityAntibacterial agentsAntipyreticDoxofyllineTreatment effect
The invention discloses a compound kanamycin sulfate injection liquid for a pig and a preparation method thereof, aiming at providing a compound kanamycin sulfate injection liquid which has fast effect for treating pant disease of the pig, addresses both the symptoms and root causes, reduces the time and dosage of taking drug and has convenient drug usage, and a preparation method with simple technique and easy implementation. Each 100L injection liquid comprises: 5 to 20 billion units of kanamycin monosulfate, 1 to 10kg of lincomycin hydrochloride, 0.1 to 2kg of doxofylline, 0.05kg of dexamethasone sodium phosphate, 0.2kg of sodium bisulfite, 0.01kg of EDTA-2Na, 10kg of dimethyl acetamide and the balance of water for injection. The injection liquid reasonably mixes usage of all the components by a plurality of types of drugs, has fast effect for treating pant disease of the pig, addresses both the symptoms and root causes, reduces drug resistance of pathogenic microorganism and leads the pig to accelerate restoring. Simultaneously, the compound kanamycin sulfate injection liquid has convenient use, and can achieve the treatment effect of a plurality of types of drugs.
Owner:TIANJIN SHENGJI GRP CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com