Anti-pulmonary fibrosis composition with improved dissolution property
A technology for pulmonary fibrosis and composition, which is applied in the field of anti-pulmonary fibrosis composition, can solve the problems of increasing drug dissolution, unexamined formation of dispersion stability, carrier interaction, recrystallization, etc., and achieves the effect of high solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Nintedanib: Preparation of HPMCAS Salt
[0046] Add nintedanib free base and HPMCAS into a mixed solvent of methanol and dichloromethane (1:1 volume ratio) to form a solution, and an ionic bond is formed between the basic nintedanib and the acidic polymer HPMCAS.
[0047] Dissolve 1.2 g of HPMCAS in a certain volume of mixed solvent by magnetic stirring, then add 0.8 g of nintedanib free base and allow it to dissolve.
[0048] The resulting nintedanib containing 40% nintedanib (mass ratio):HPMCAS salt was passed through a Büchi micro spray dryer B290 equipped with an inert cycle B295 ( Labortechnik AG, Switzerland) spray-dried separation. A high-performance cyclone separator is used for separation, and the 50mL blue cap flask can be directly mounted to the cyclone separator for product collection. The parameter settings of the spray drying process are shown in Table 1.
[0049] Table 1
[0050] parameter Settings Suction 40kg / h In...
Embodiment 2
[0052] Embodiment 2: Nintedanib: Preparation of HPMCP salt
[0053] Add nintedanib free base and HPMCP into a mixed solvent of methanol and dichloromethane (1:1 volume ratio) to form a solution, and an ionic bond is formed between the basic nintedanib and the acidic polymer HPMCP.
[0054] 1.0 g of HPMCP was dissolved in a certain volume of mixed solvent by magnetic stirring, then 1.0 g of nintedanib free base was added and allowed to dissolve.
[0055] The resulting nintedanib containing 50% nintedanib (mass ratio): HPMCP salt was passed through a Büchi micro-spray dryer B290 equipped with an inert cycle B295 ( Labortechnik AG, Switzerland) spray-dried separation. A high-performance cyclone separator is used for separation, and the 50mL blue cap flask can be directly mounted to the cyclone separator for product collection. The parameter settings of the spray drying process are shown in Table 2.
[0056] Table 2
[0057] parameter Settings Suction 40kg / ...
Embodiment 3
[0059] Embodiment 3: Preparation of nintedanib amorphous
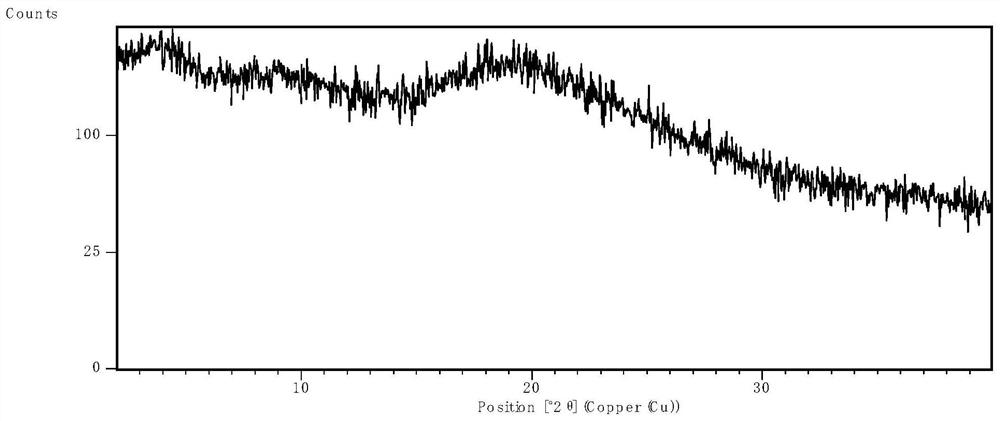
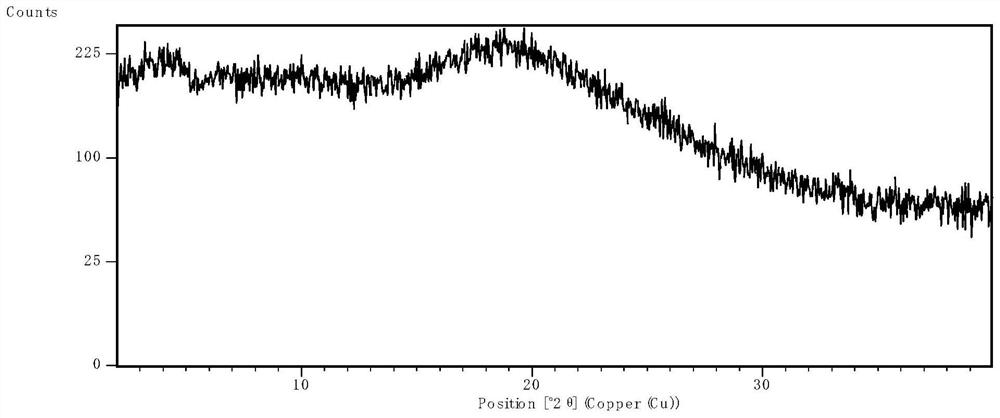
[0060] Nintedanib free base was added into a certain volume of dichloromethane to form a solution, which was then passed through a Büchi miniature spray dryer B290 equipped with an inert cycle B295 ( Labortechnik AG, Switzerland) spray-dried separation. A high-performance cyclone separator is used for separation, and the 50mL blue cap flask can be directly mounted to the cyclone separator for product collection. The parameter settings of the spray drying process are shown in Table 3.
[0061] table 3
[0062] parameter Settings Suction 40kg / h Inlet temperature 85℃ output temperature 60℃ Injection rate 5mL / min Atomizing airflow 0.5kg / h Inert loop cooling temperature -20℃
[0063] After spray drying, the product was placed in an oven at 50°C for 1 hour to remove excess solvent, and then XRPD was used to determine the physical state. The results were as follows: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com