Application of nintedanib in preparing medicament for preventing and treating liver fibrosis and hepatocirrhosis
A technology of nintedanib and medicine, which is applied in the field of application of nintedanib in the preparation of drugs for the prevention and treatment of liver fibrosis and liver cirrhosis, can solve the problems that have not been revealed that nintedanib has anti-hepatic cirrhosis and anti-hepatic fibrosis. the role of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
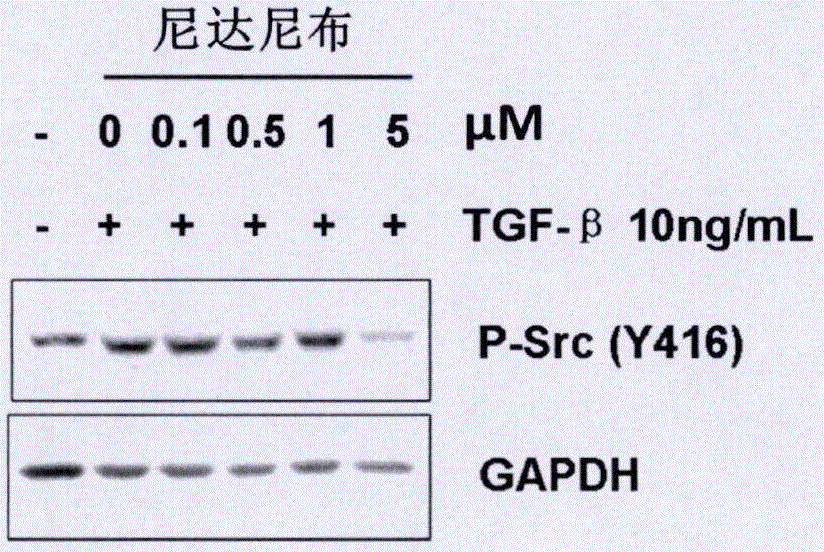
[0041] Example 1 Effect of nintedanib on Src kinase activity in human hepatic stellate cells LX-2
[0042] 1. Experimental materials
[0043] LX-2 cells were provided by Capital Medical University; Nintedanib was provided by Nanjing Chia Tai Tianqing Pharmaceutical Co., Ltd.; Phospho-Src Family (Tyr416) primary antibody was purchased from Cell Signaling Technology Company, Cat. No. #6943; GAPDH- Antibody was purchased from Beijing Zhongshan Jinqiao Biotechnology Co., Ltd., the article number was TA-08; TGF-β was purchased from R&D Systems Company, the article number was 240-B-002.
[0044] 2. Experimental method
[0045] The LX-2 cells were divided into 5×10 5 Each well was inoculated in a 6-well plate, cultured overnight, and then replaced with serum-free DMEM medium, starved for 24 hours (h), and replaced with serum-free dilution concentrations of 0 μm, 0.1 μm, 0.5 μm, 1 μm, respectively. Treat the cells with 5 μm nintedanib for 4 hours, and then stimulate the cells wit...
Embodiment 2
[0048] Example 2 Effect of nintedanib on the production of α-SMA by human hepatic stellate cells LX-2
[0049] 1. Experimental materials
[0050] Anti-α-SMA primary antibody was purchased from Abcam Company, the product number is ab5694.
[0051] 2. Experimental method
[0052] The LX-2 cells were divided into 5×10 5 Each well was inoculated in a 6-well plate, cultured overnight, and then replaced with serum-free DMEM medium. After 24 hours of starvation culture, replaced with 2% serum, 10ng / mL TGF-β and concentrations of 0μm, 0.1μm, 0.5 The treated cells were incubated with DMEM medium of μm, 1 μm, and 5 μm nintedanib, washed twice with PBS at room temperature after 12 hours, and 120 μL of protein lysate was added to collect the cells to obtain samples. The samples were subjected to western blot to detect the level of α-SMA.
[0053] 3. Experimental results
[0054] like figure 2 As shown, nintedanib can inhibit TGF-β-induced α-SMA production in human hepatic stellate ...
Embodiment 3
[0055] Example 3 Effect of nintedanib on proliferation activity of human hepatic stellate cell LX-2 and rat hepatic stellate cell HSC-T6
[0056] 1. Experimental materials
[0057] Cell Counting Kit 8 (Cell Counting Kit-8, CCK-8) was purchased from Dongren Chemical Technology Co., Ltd., the article number is CK04.
[0058] 2. Experimental method
[0059] Inoculate LX-2 cells and HSC-T6 cells in 96-well plates at 3000 cells / well, culture overnight, and then replace with serum-free DMEM medium. After 24 hours of starvation culture, replace with 2% serum, 10ng / mL TGF -β and concentrations of 0 μm, 0.1 μm, 0.5 μm, 1 μm, and 5 μm nintedanib were used to incubate the treated cells in DMEM medium, and the “control” group was cultured in DMEM medium with 2% serum (without adding TGF-β nor ganidanib treatment). At 0h, 24h, 48h and 72h after adding the drug, measure the cell viability and density with CCK-8: suck out the medium, add 100 μL of CCK-8 solution diluted 10 times with the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com