Preparation method of nintedanib
A nintedanib and compound technology, applied in the field of drug synthesis, can solve the problems of long process route, unfavorable environment, complicated process operation, etc., and achieve the effect of simplifying the operation process, improving production efficiency and shortening the operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
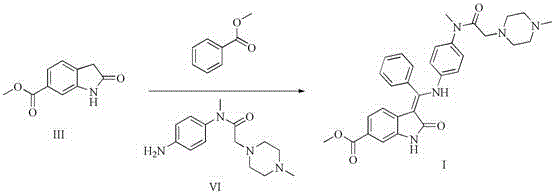
[0030] Preparation of Compound IV
[0031]
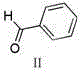
[0032] Add 28.7g of methyl 2-oxindole-6-carboxylate and 130ml of ethanol to a 250ml reaction flask, start stirring, then add 16.8ml (17.6g) of benzaldehyde, 2.97ml of piperidine, heat to 70°C—80 ℃, reacted for 2 hours, cooled naturally to 20℃-30℃, filtered the precipitate, washed the filter cake with absolute ethanol, and dried in vacuum at 50℃ for 5 hours to obtain 40.2g of yellow solid (IV), yield: 96.0%.
[0033] Preparation of Compound V
[0034]
[0035] Add 30g of compound IV and 360ml of dichloromethane into a 500ml reaction flask, cool to 0-5°C with ice water, add 9.6ml (29.9g) of bromine dropwise, raise the temperature to 20-30°C after dropping, and react for 3 hours. After the reaction was completed, the reaction solution was washed once with 150ml of water, and the dichloromethane layer was concentrated and dried to obtain an oily substance, which was crystallized by adding 200ml of absolute ethanol, filtered, and ...
Embodiment 2
[0042] Preparation of Compound IV
[0043] Add 28.7g of methyl 2-oxindole-6-carboxylate and 130ml of ethanol to a 250ml reaction bottle, start stirring, then add 30.3ml (31.8g) of benzaldehyde, 2.97ml of piperidine, heat to 70°C—80 After reacting at ℃ for 2 hours, cool naturally to 20℃-30℃, filter the precipitate, wash the filter cake with absolute ethanol, and dry in vacuum at 50℃ for 5 hours to obtain 38.7g of yellow solid (IV), yield: 92.4%.
[0044] Preparation of Compound V
[0045] Add 30g of compound IV and 360ml of dichloromethane into a 500ml reaction flask, cool to 0-5°C with ice water, add 3.1ml (9.7g) of bromine dropwise, raise the temperature to 20-30°C after dropping, and react for 3 hours , the reaction solution was washed once with 150ml of water, and the dichloromethane layer was concentrated and dried to obtain an oily substance, which was crystallized by adding 200ml of absolute ethanol, filtered, and vacuum-dried at 60°C to obtain 36.1g of off-white solid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com