Application of nintedanib to prevention and treatment of ocular diseases
A technology for nintedanib and eye diseases, which is applied in the field of medicine to achieve good prevention and treatment effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of nintedanib ethanesulfonate eye ointment:
[0034]
[0035]
[0036] Preparation method: In a clean bench (aseptic cabinet) in a class-C clean area, add the prescribed amount of micronized nintedanib ethanesulfonate and benzalkonium chloride into a suitable container according to the aseptic method, and add the prescribed amount The liquid paraffin that has been sterilized, filtered and cooled is ground into a fine paste, passed through a No. 6 sieve, and then gradually added to the sterilized filtered lanolin and vaseline base, stirred evenly, and cooled.
Embodiment 2
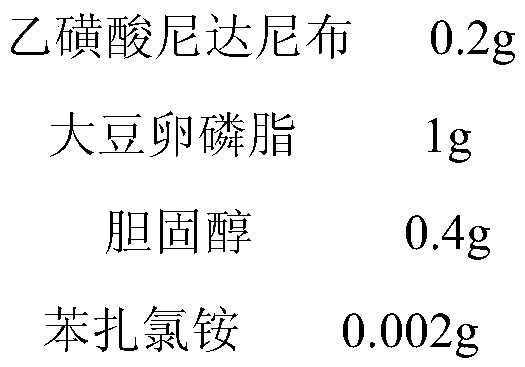
[0038] Preparation of nintedanib ethanesulfonate liposome eye drops:
[0039]
[0040] Preparation method: in a clean bench (aseptic cabinet) in a class C clean area, dissolve the prescribed amount of micronized nintedanib ethanesulfonate, soybean lecithin, cholesterol and benzalkonium chloride in 10ml according to the aseptic method ether, as the oil phase. Prepare 10 ml of 0.9% sodium chloride solution, adjust the pH to 6.8 with hydrochloric acid or sodium hydroxide, and use it as the water phase. The above two solutions were mixed and ultrasonicated intermittently for 30 minutes until a stable water / oil (W / O) emulsion was formed. Evaporate the emulsion under reduced pressure on a rotary evaporator to remove the organic solvent (water bath temperature 30°C, rotating speed 75r / min). After the gel is formed on the bottle wall, continue rotary evaporation to make the gel fall off and hydrate to obtain uniform liposomes. The suspension was sterilized by filtration with a 0....
Embodiment 3
[0042] Preparation of nintedanib ethanesulfonate emulsion eye drops:
[0043]
[0044]
[0045] Adjust the pH to 7.5 with hydrochloric acid or sodium hydroxide, and add water for injection to 100 mL.
[0046] Preparation method: In a clean bench (aseptic cabinet) in a class-C clean area, heat and stir the prescribed amount of medium-chain triglycerides, soybean lecithin, glycerin and one-half of the prescribed amount of polyoxyethylene castor oil , and then add cholesterol to dissolve it, and filter it as the oil phase. Dissolve the prescribed amount of micronized nintedanib ethanesulfonate in the oil phase according to the aseptic method, add the prescribed amount of vitamin E, the prescribed amount of benzalkonium chloride and one-half of the prescribed amount of polyoxyethylene Add castor oil to the prescribed amount of water, filter and use it as the water phase, homogeneously emulsify the water phase at 1000 rpm for 10 minutes, then quickly add the oil phase to the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com