Preparation method of nintedanib key intermediate
A nintedanib and intermediate technology, which is applied in the field of medicinal chemistry, can solve the problems of high death risk, environmental pollution, and hidden safety hazards, and achieves the effects of process safety and environmental protection, simple technical operation, and improved feasibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
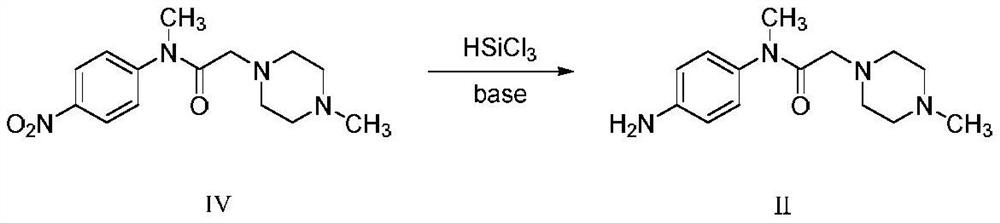
[0055] Embodiment 1: Preparation of N-(4-aminophenyl)-N-methyl-2-(4-methylpiperazin-1-yl)acetamide (compound II)
[0056] 100ml of dichloromethane was added to the reaction flask, and 38.5g (131.7mmol) of N-(4-aminophenyl)-N-methyl-2-(4-methylpiperazin-1-yl)acetamide was added ( Compound IV), 62g trichlorosilane (460mmol) and 90ml triethylamine (647mmol), reacted at room temperature until the reaction was detected by TLC, added 300ml methyl tert-butyl ether to the filtrate, stirred and crystallized, suction filtered, and vacuum-dried at 50°C. 28.4 g of off-white solid was obtained, yield 82.2%, HPLC purity: 99.66%.
Embodiment 2
[0057] Embodiment 2: Preparation of N-(4-aminophenyl)-N-methyl-2-(4-methylpiperazin-1-yl)acetamide (compound II)
[0058] 100ml of chloroform was added to the reaction flask, and 38.5g (131.7mmol) of N-(4-aminophenyl)-N-methyl-2-(4-methylpiperazin-1-yl)acetamide was added ( Compound IV), 62g of trichlorosilane (460mmol) and 87ml of N,N-diisopropylethylamine (526.8mmol), reacted at room temperature until the reaction was detected by TLC, added 300ml of n-hexane, stirred and crystallized, suction filtered, 50°C After vacuum drying, 28.1 g of off-white solid was obtained, yield 81.3%, HPLC purity: 99.67%.
Embodiment 3
[0059] Embodiment 3: Preparation of N-(4-aminophenyl)-N-methyl-2-(4-methylpiperazin-1-yl)acetamide (compound II)
[0060] 100ml of chloroform was added to the reaction flask, and 38.5g (131.7mmol) of N-(4-aminophenyl)-N-methyl-2-(4-methylpiperazin-1-yl)acetamide was added ( Compound IV), 53.5g trichlorosilane (395.1mmol) and 90ml triethylamine (647mmol), react at room temperature until TLC detects that the reaction is complete, add 300ml cyclohexane, stir and crystallize, filter with suction, and dry under vacuum at 50°C to obtain White solid 28.9g, yield 83.6%, HPLC purity: 99.62%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com