Impurity compound of Nintedanib, as well as preparation method, application and detection method of impurity compound
A technology of nintedanib and its compounds, which is applied in the field of medicinal chemistry, can solve the problems of public reporting without impurities and achieve the effect of improving quality standards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
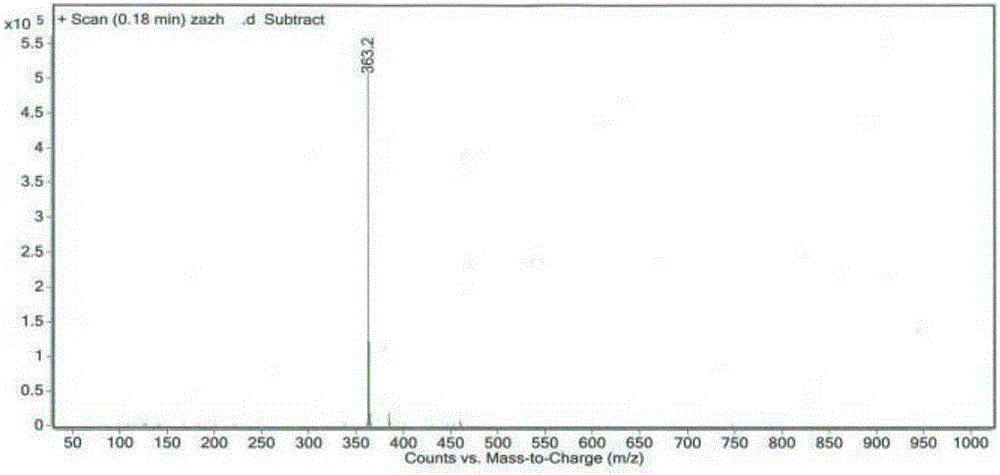
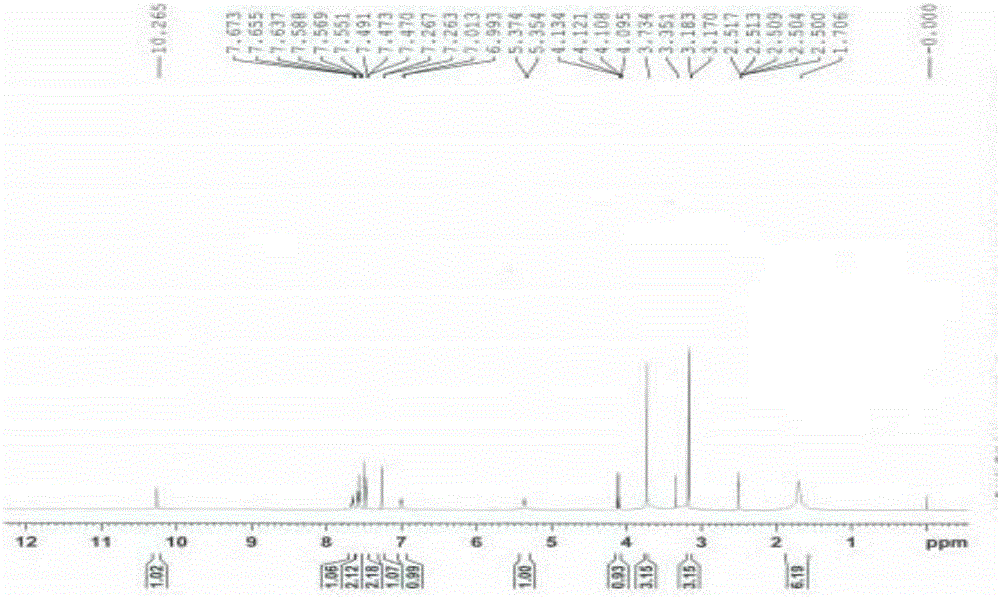
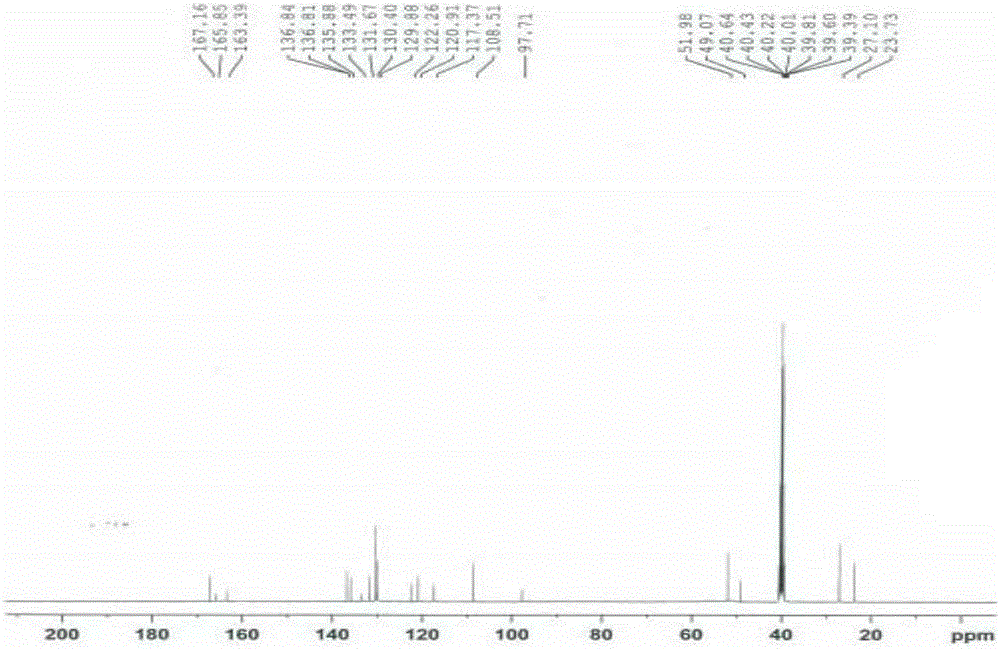
[0052] Add 3-[methoxy(phenyl)methylene]-2-oxoindoline-6-carboxylic acid methyl ester (8.8g, 28mol) and piperidine (4.8g, 57mmol) into methanol 80mL , The reaction was stirred at 60°C, and the reaction was completed in 2 to 5 hours. The reaction solution was cooled, and a solid precipitated out. The solid was filtered and rinsed with methanol to obtain 8.5 g of an impurity compound as a yellow solid.
[0053] HPLC detection, product purity 97.1%.
[0054] The HPLC method is: take 5 mg of the impurity compound, add it to a 25 mL volumetric flask, and use an appropriate amount of mobile phase A-mobile phase B (45:55) to sonicate to dissolve and dilute to the mark as the test solution.
[0055] The chromatographic conditions are:
[0056] (1) Chromatographic column: octadecylsilane bonded silica gel.
[0057] (2) Mobile phase: mobile phase A is 20mmol / L potassium dihydrogen phosphate solution (adjust pH value to 3.0 with phosphoric acid), mobile phase B is acetonitrile, and car...
Embodiment 2
[0062]Methyl N-acetyl-3-[methoxy(phenyl)methylene]-2-oxoindoline-6-carboxylate (10 g, 28 mmol) and piperidine (4.8 g, 57 mmol) Add 40mL of methanol and 10mL of N,N-dimethylformamide, and reflux at 68°C to complete the reaction in 4 to 7 hours. The reaction solution was cooled, added with 100 mL of water, extracted with 80 mL of dichloromethane, washed with 50 mL of saturated sodium chloride solution x 2. The organic phase was dried over anhydrous sodium sulfate, filtered, and the filtrate was distilled under reduced pressure. The residue was crystallized by adding 30 mL of methanol to obtain 8.1 g of the impurity compound as a yellow solid. HPLC detection showed that the purity of the impurity compound was 98.46%.
Embodiment 3
[0064] Methyl N-chloroacetyl-3-[methoxy(phenyl)methylene]-2-oxoindoline-6-carboxylate (10 g, 28 mmol) and piperidine (4.8 g, 57 mmol ) into 30 mL of N,N-dimethylformamide, react at 80°C, and complete the reaction in 2 to 4 hours. The reaction solution was cooled, added with 100 mL of water, extracted with 100 mL of dichloromethane, and washed with 50 mL of saturated sodium chloride solution x 3. The organic phase was dried over anhydrous sodium sulfate, filtered, and dichloromethane was distilled off from the filtrate under reduced pressure. The residue was crystallized by adding 50 mL of isopropanol, and filtered to obtain 7.4 g of an impurity compound as a yellow solid. HPLC detection showed that the purity of the impurity compound was 99.13%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com