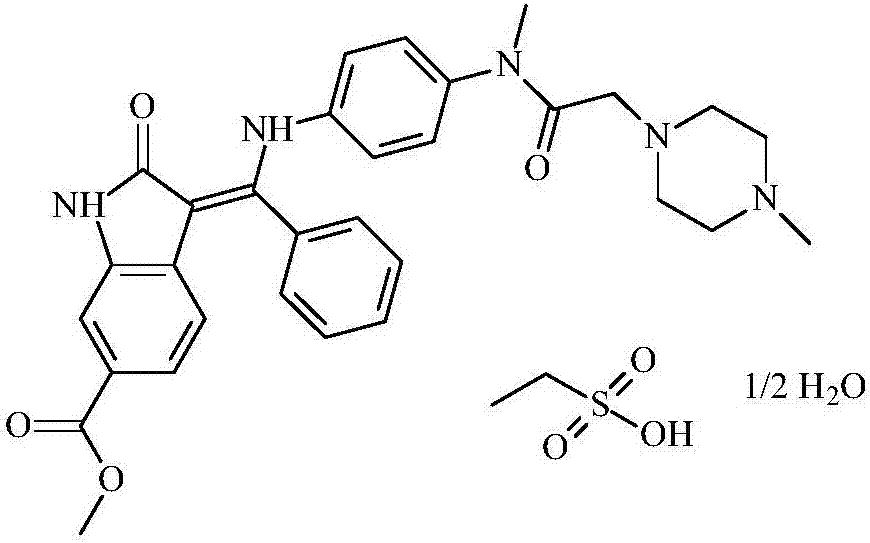
Preparation method of nintedanib ethyl sulfonate
A technology of nintedanib ethanesulfonate and nintedanib, which is applied in the field of chemical drug synthesis, can solve the problems of unreported quality of nintedanib ethanesulfonic acid, the need for metal catalysts, long synthesis steps, etc., and achieve The effect of reducing the introduction of solvent types, reducing post-treatment operations, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of Compound C: Put 100g of methyl 2-oxindole-6-carboxylate and 100g of acetic anhydride into a 1L round-bottomed flask, stir and heat up to 110°C, and keep it warm for 1h; cool down to 76°C, add 340ml of triethyl orthobenzoate, Insulate and stir for 8 hours; turn off the heating, remove excess acetic anhydride by distillation under reduced pressure, and precipitate a large amount of reddish-brown solid. After the residue is cooled to room temperature, add 1.6L methyl tert-butyl ether at room temperature (30°C) for beating for 1 hour, and then filter with 1L methanol The tert-butyl ether was repeatedly beaten once, and fully pumped and air-dried at 80°C for 4 hours to obtain 139.5 g of a reddish-brown solid. The purity of compound C was 96%, and the molar yield was 70.4%.
[0029] Preparation of nintedanib free base (compound F): add 130g of compound C and 89.9g of material D (converted into molar ratio in line with the feeding ratio, compound C has been puri...
Embodiment 2
[0041] The preparation reaction of compound C is consistent with that of Example 1, and will not be repeated here.
[0042]Preparation of compound F: 10.0 g of compound C and 7.2 g of compound D were added to 2 groups of 250 ml reaction devices, 8V methanol (i.e. 138 ml of methanol) was added to one group, and 10 V methanol (i.e. 170 ml of methanol) was added to the other group; after nitrogen protection , the first group was slightly difficult to stir but the reaction could occur, the second group was stirred normally, and the temperature was raised to reflux temperature of 67°C. After 5 hours, the reaction feed liquid was detected by HPLC, and the remaining raw materials were 47.1% and 48.2% respectively. After the reaction, the molar yield of nintedanib free base (compound F) was 87.3%, the purity was 99.7%, and the largest single impurity was less than 0.1%.
[0043] Preparation of nintedanib ethanesulfonate (compound G): consistent with Example 1, no further details are g...
Embodiment 3
[0045] The preparation reaction of compound C is consistent with that of Example 1, and will not be repeated here.
[0046] Preparation of Compound F: 10.0g of Compound C, 7.2g of Compound D and 170ml of methanol were added to a 250ml reaction device, stirred under nitrogen protection, heated and refluxed for 7.5h, and then the liquid was detected by HPLC, containing 76.5% of Compound F and only 15.2% of Compound C %.
[0047] Under the same conditions, but the solvent was changed to DMF, ethanol or 1,4-dioxane as the solvent, the temperature was raised to 75-77°C for the reaction, and samples were taken at 7.5h and 9.5h respectively. The product compound F was tested at 7.5h and 9.5h respectively. For: (3.2%, 4.5%), (4.7%, 6.3%), (7.5%, 8.6%), the rest is mainly compound E.
[0048] Examples 2 and 3 are mainly to illustrate that the use of solvent methanol in the preparation of compound F can directly react compounds C and D to compound F in one step, while other solvents ne...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com