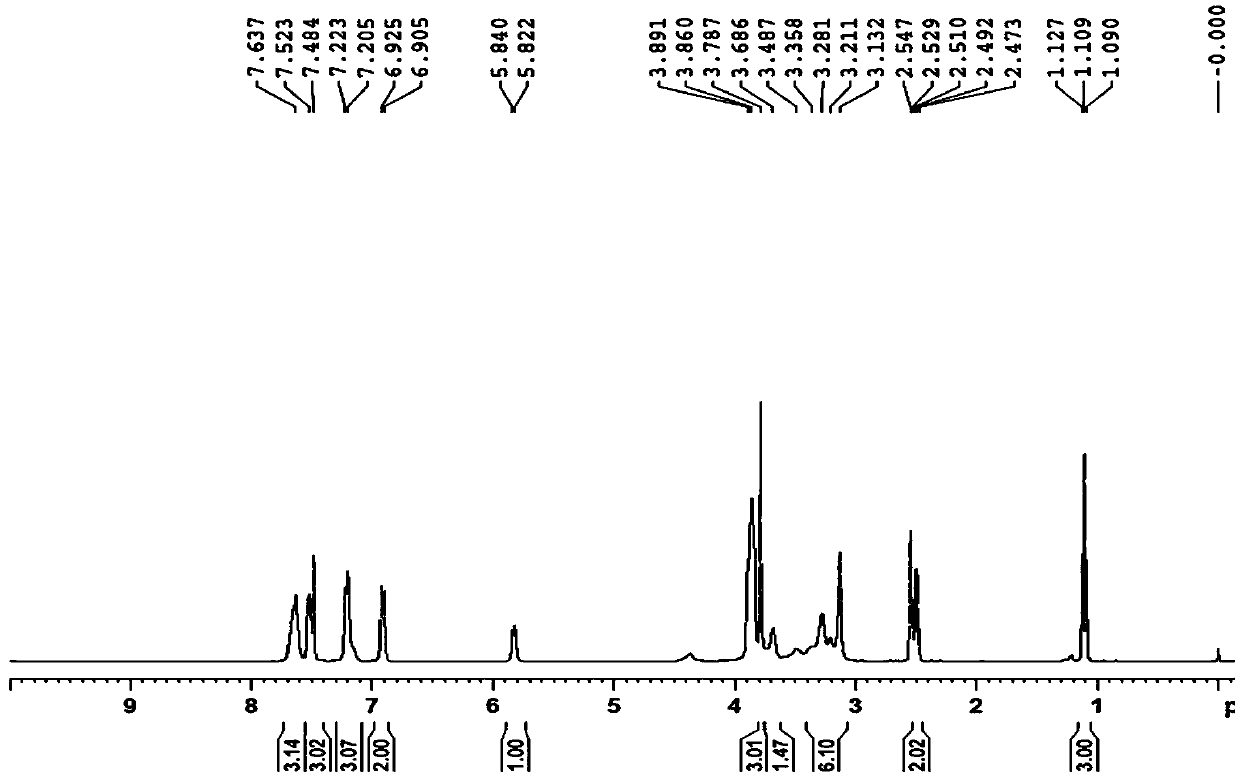
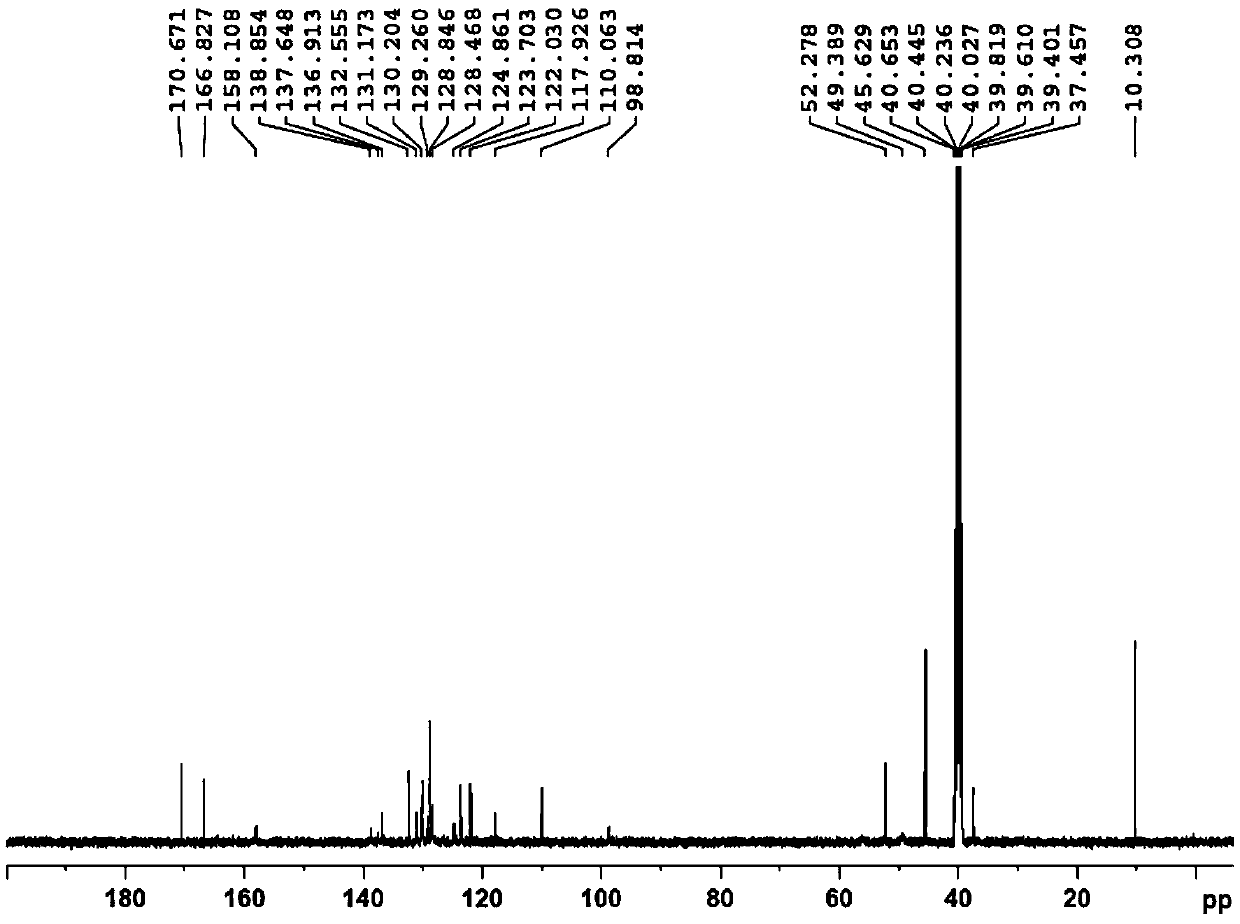
Nintedanib esilate impurity as well as preparation method and application thereof
A technology of nintedanib ethanesulfonate and ethanesulfonic acid is applied in the field of nintedanib ethanesulfonate impurity and its preparation, and can solve the problem of low yield, difficult purification of nintedanib ethanesulfonate impurity, and difficulty in obtaining Nintedanib ethanesulfonate and other problems, to achieve the effect of high yield, optimized solvent screening and good purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] 1, preparation formula III compound
[0070]
[0071] Add 2-chloro-N-methyl-(4-nitrophenyl)acetamide (30g, 131.6mmol) and methyl tert-butyl ether (450mL) into the reaction flask, stir, add piperazine (5.4g, 62.7mmol) and cesium carbonate (204g, 627mmol), heated to reflux, reacted for 7 hours, cooled, filtered, washed with dichloromethane, concentrated and dried under reduced pressure at 40°C, added 100ml isopropanol for crystallization, filtered, and dried at 40°C After 5 hours, 27.5 g of the compound of formula III was obtained, the molar yield: 92.8% (based on piperazine), HPLC: 99.41%.
[0072] 2. Nintedanib ethanesulfonate impurity shown in the preparation formula II
[0073]
[0074] The compound of formula III (20g, 42.5mmol) was added to 1000mL ethyl formate, ferric chloride hexahydrate (0.9g, 3.4mmol) and activated carbon (1.6g, 127.5mmol) were added successively, heated to 40°C, batchwise Add 85% hydrazine hydrate (11.8 g, 199.8 mmol). After the reacti...
Embodiment 2
[0079] 1, preparation formula III compound
[0080] Add 2-chloro-N-methyl-(4-nitrophenyl)acetamide (28.7g, 125.8mmol) and acetonitrile (620mL) into the reaction flask, stir, add piperazine (5.4g, 62.7mmol) and Triethylamine (87g, 627mmol), heated to reflux, reacted for 6 hours, cooled, filtered, washed with dichloromethane, concentrated and dried under reduced pressure at 40°C, added 100ml of isopropanol for crystallization, and obtained 24.1g of the compound of formula III. Yield: 81.2% (based on piperazine), HPLC: 99.25%.
[0081] 2. Nintedanib ethanesulfonate impurity shown in the preparation formula II
[0082] The compound of formula III (20g, 42.5mmol) was added to 1000mL ethyl acetate, ferric chloride hexahydrate (0.57g, 2.13mmol), activated carbon (2.6g, 212.5mmol) were added successively, heated to 50°C, batchwise Add 85% hydrazine hydrate (12.5 g, 212.5 mmol). After the reaction is complete, filter, wash with dichloromethane-methanol (5:1), concentrate the filtrat...
Embodiment 3
[0087] 1, preparation formula III compound
[0088] Add 2-chloro-N-methyl-(4-nitrophenyl)acetamide (31.5g, 137.9mmol) and tetrahydrofuran (450mL) into the reaction flask, stir, add piperazine (5.4g, 62.7mmol) and Potassium carbonate (60.6g, 438.9mmol), heated to reflux, reacted for 7 hours, cooled, filtered, washed with dichloromethane, concentrated and dried under reduced pressure at 40°C, added 100ml of isopropanol for crystallization, filtered, and dried at 40°C for 5 hours , to obtain 24.5 g of compound of formula III, molar yield: 82.5% (based on piperazine), HPLC: 98.37%.
[0089] 2. Nintedanib ethanesulfonate impurity shown in the preparation formula II
[0090] The compound of formula III (20g, 42.5mmol) was added to 1000mL of isopropyl acetate, ferric chloride hexahydrate (2.3g, 8.5mmol), activated carbon (5.0g, 425mmol) were added successively, heated to 50°C, batchwise Add 85% hydrazine hydrate (25 g, 425 mmol). After the reaction is complete, filter, wash with d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com