Derivatives containing 2, 5-substituted heterocyclic radical sulphone and synthesis method and application thereof
A technology of heterocyclic groups and derivatives, applied in the field of sulfone derivatives and their synthesis, can solve the problems of low cost, unsatisfactory activity of soil-borne diseases and migratory diseases, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] Example 1: Preparation of 2,5-substituent-1,3,4-oxadiazole compounds
[0114] Series I compounds of the present invention contain 2,5-substituent-1,3,4-oxadiazole derivatives preparation process steps and conditions with 2-(methylsulfonyl)-5-phenyl-oxadiazole as For example, others can refer to synthesis.
[0115]
[0116] (1) preparation of methyl benzoate intermediate
[0117] Throw benzoic acid (37.8 g, 0.31 mol) and anhydrous methanol (198.0 g, 6.2 mol) into a 500 mL three-neck flask, slowly add concentrated sulfuric acid (61 g, 0.62 mol) dropwise at room temperature and then raise the temperature to reflux, and the reaction ends for 8 hours. After demethanolization under reduced pressure, it was washed with saturated sodium bicarbonate solution until neutral. Liquid separation obtained 39.1 g of methyl benzoate, the refractive index: n20 / D 1.516 (lit.), and the yield was 92.8%. 1 H NMR (500MHz, CDCl 3 )δ: 8.04-7.31 (m, 5H, benzyl-H), 3.81 (s, 3H, CH 3 );
...
Embodiment 2
[0138] Example 2: Preparation of 2-(methylsulfonyl)-5-p-chlorophenyl-1,3,4-thiadiazole
[0139] The preparation process steps and conditions of series II compounds of the present invention containing 2,5-substituent-1,3,4-thiadiazole derivatives are based on 2-(methylsulfonyl)-5-p-chlorophenyl-thiadiazole Azole is taken as an example, others can be synthesized with reference. The preparation of methyl p-chlorobenzoate and p-chlorobenzohydrazide intermediate is completed with reference to Example 2.
[0140] (1) Preparation of 2-mercapto-5-p-chlorophenyl-1,3,4-thiadiazole
[0141]Weigh p-chlorobenzohydrazide (8g, 0.047mol) into a 500mL three-neck round bottom flask, add 200mL of absolute ethanol, and then potassium hydroxide (2.6g, 0.047mol), stir to dissolve. Control the temperature to room temperature, add carbon disulfide (5.3 g, 0.07 mol) dropwise, and stir rapidly for 5 h. Filtrate with suction and wash with absolute ethanol to obtain potassium chlorobenzoyl hydrazide d...
Embodiment 3
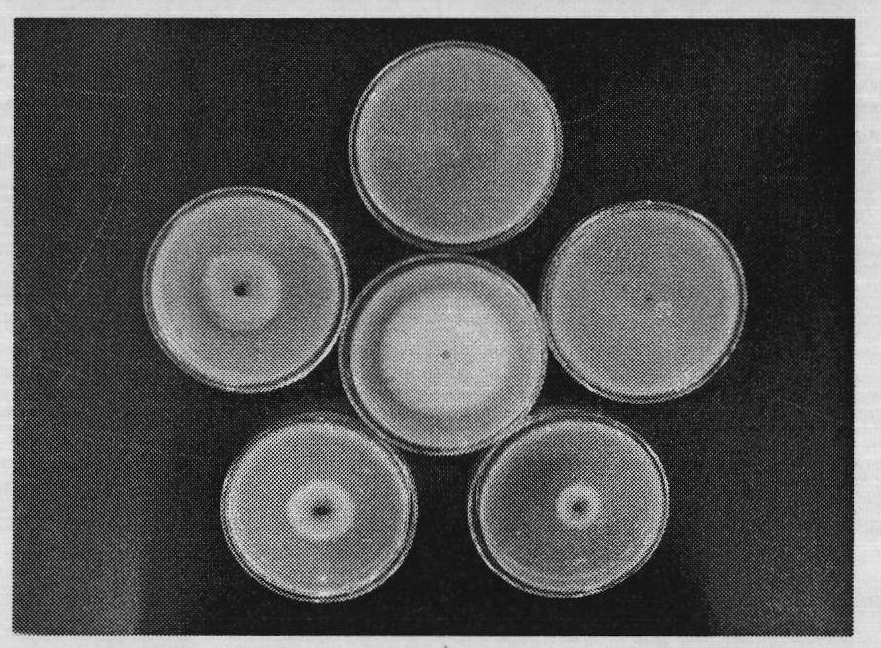
[0154] Embodiment three: the bacteriostatic activity test of compound
[0155] The antibacterial activity of the compounds was determined by the in vitro growth rate method. Heat potato dextrose agar medium (PDA medium: 200g potato, 20g agar, 20g glucose, 1000mL distilled water) to a molten state (40-60°C), pour 10mL of the drug solution (10 times the final concentration of the drug solution) into 90mL PDA In the culture medium, shake well, evenly pour into the petri dish with a diameter of 9cm, place it horizontally, and let it cool and solidify. Use a puncher to punch a 4mm-diameter fungus dish at the edge of the fresh pathogenic bacteria colony that has been cultured for 4 days, put the fungus dish upside down in the center of the PDA plate containing the drug, and then place it in a constant temperature and humidity incubator at 27°C for upside-down culture, and wait for the blank control When the colony grows to nearly two-thirds of the plate, observe it, measure the dia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com