Alginate hard capsule disintegrable at different positions in gastrointestinal tract
An alginate, gastrointestinal tract technology, applied in the directions of capsule delivery, food forming, medical preparations of inactive ingredients, etc., can solve the problems of delayed disintegration, low ionic strength, insufficient degree of de-crosslinking, etc. Achieve the effects of low cost, simple preparation method and good damp-heat stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Colon Dissolving Hard Capsule 1
[0029] Table 1
[0030]
[0031] (1) Glue preparation: slowly dissolve 10g of high-viscosity sodium alginate and 100g of glycerin in 890g of water, and let it stand to remove air bubbles after the dissolution;
[0032] (2) Glue dipping: slowly pour the glue into the dipping tank, and automatically dip the glue through the hard capsule shell mold;
[0033] (3) Preparation and curing of the curing liquid: the curing liquid is an aluminum chloride aqueous solution with a concentration of 30% by mass, spray the curing liquid and rinse the mold for 6 seconds, so that the dipping liquid in the mold is cured into alginate gel capsules;
[0034] (4) Post-processing: the alginate gel capsule is subjected to the conventional hard capsule shell process of drying, shelling, cutting, fastening, light inspection, and sterilization to obtain the alginate hard capsule shell;
[0035] (5) Capsule filling: Fill 0.5 g of starch as an activ...
Embodiment 2
[0038] Example 2 Colon Dissolving Hard Capsule 2
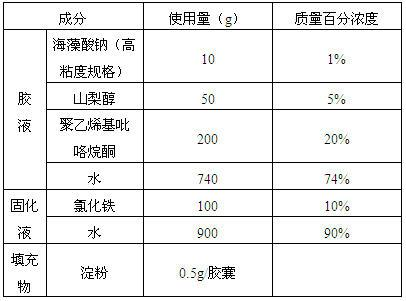
[0039] Table 2
[0040]
[0041](1) Glue preparation: slowly dissolve 10g of high-viscosity sodium alginate, 50g of sorbitol and 200g of polyvinylpyrrolidone in 740g of water, and decompress to remove air bubbles after the dissolution;
[0042] (2) Glue dipping: slowly pour the glue into the dipping tank, and automatically dip the glue through the hard capsule shell mold;
[0043] (3) Preparation and curing of the curing liquid: the curing liquid is an aqueous solution of ferric chloride with a concentration of 10% by mass. Spray and rinse the mold for 30 seconds with the curing liquid, so that the dipping liquid in the mold is solidified into alginate gel capsules;
[0044] (4) Post-processing: the alginate gel capsule is subjected to the conventional hard capsule shell process of drying, shelling, cutting, fastening, light inspection, and sterilization to obtain the alginate hard capsule shell;
[0045] (5) Capsule fil...
Embodiment 3
[0048] Example 3 Enteric Dissolving Hard Capsules
[0049] table 3
[0050]
[0051] (1) Glue preparation: Slowly dissolve 100g of ultra-low viscosity sodium alginate, 50g of glycerin and 100g of polyethylene glycol 6000 in 750g of water, and let stand to remove air bubbles after dissolution;
[0052] (2) Glue dipping: slowly pour the glue into the dipping tank, and automatically dip the glue through the hard capsule shell mold;
[0053] (3) Preparation and curing of the curing liquid: the curing liquid is an aqueous solution of ferric chloride with a mass percent concentration of 2%, and the pH is adjusted to 3.0 with hydrochloric acid, and the curing liquid is soaked in the mold for 1 minute, so that the dipping glue in the mold is cured into Alginate gel capsules;
[0054] (4) Post-processing: the alginate gel capsule is subjected to the conventional hard capsule shell process of drying, shelling, cutting, fastening, light inspection, and sterilization to obtain the al...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com