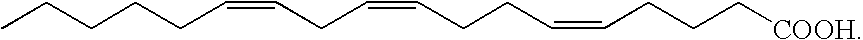Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
212 results about "Gelatin capsule" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
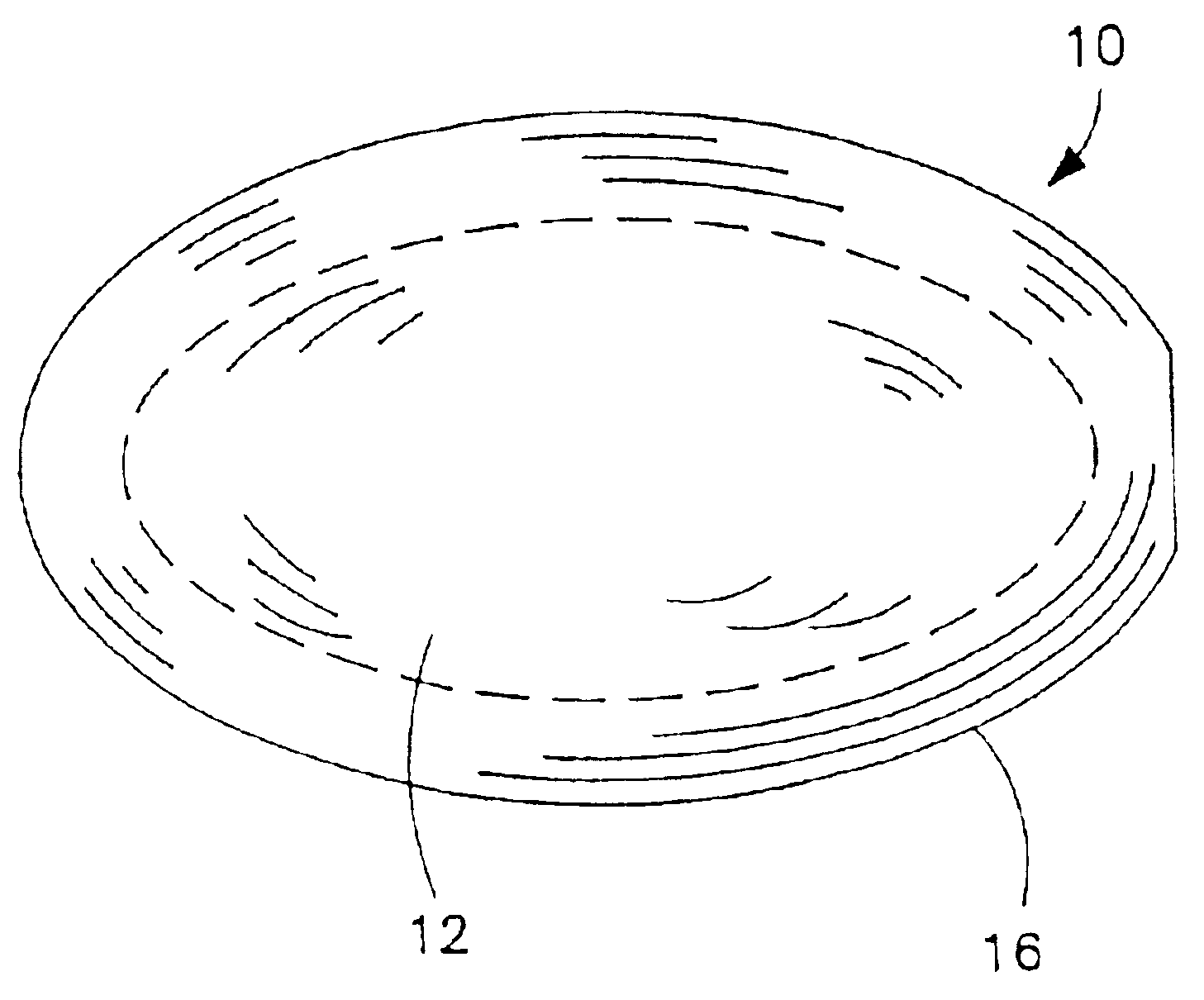
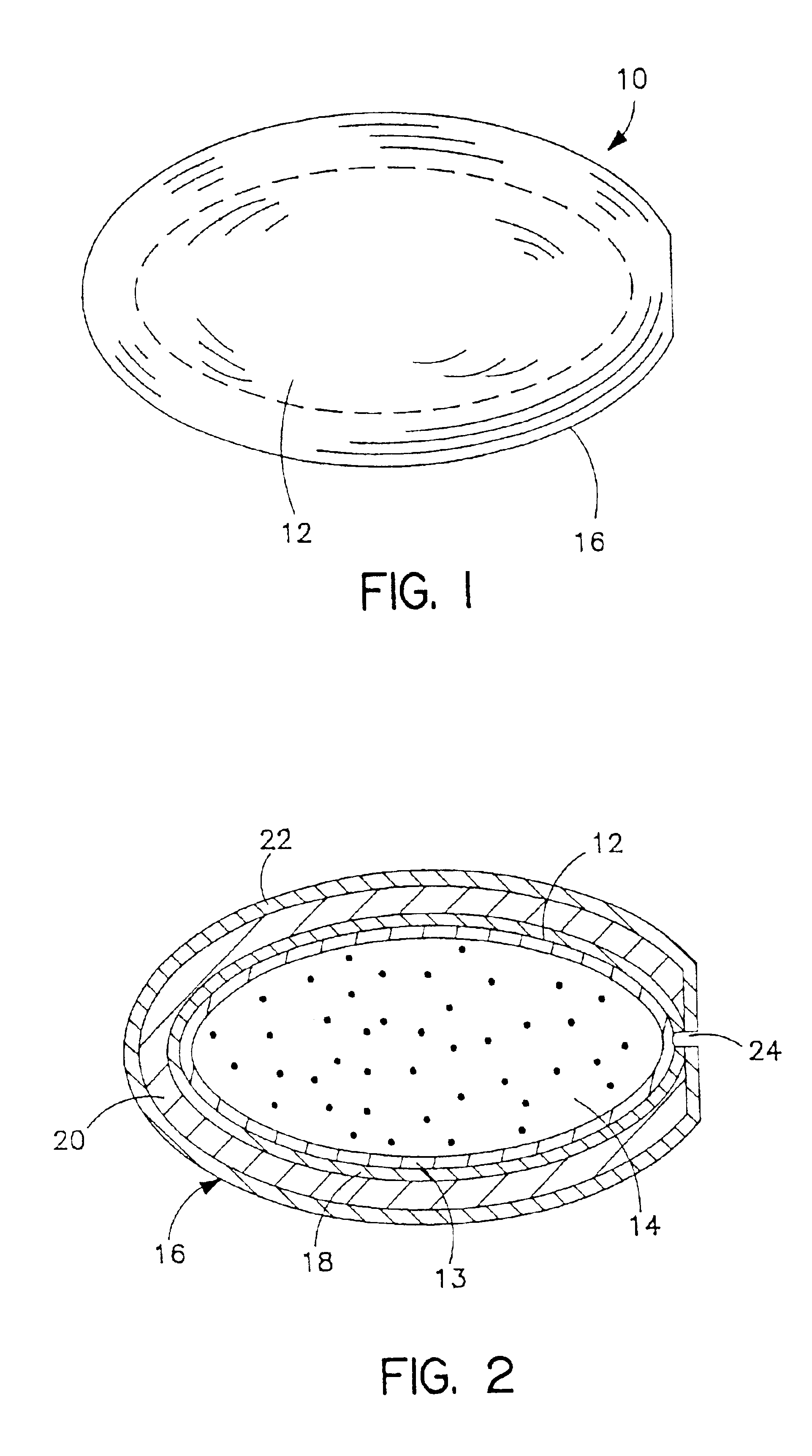
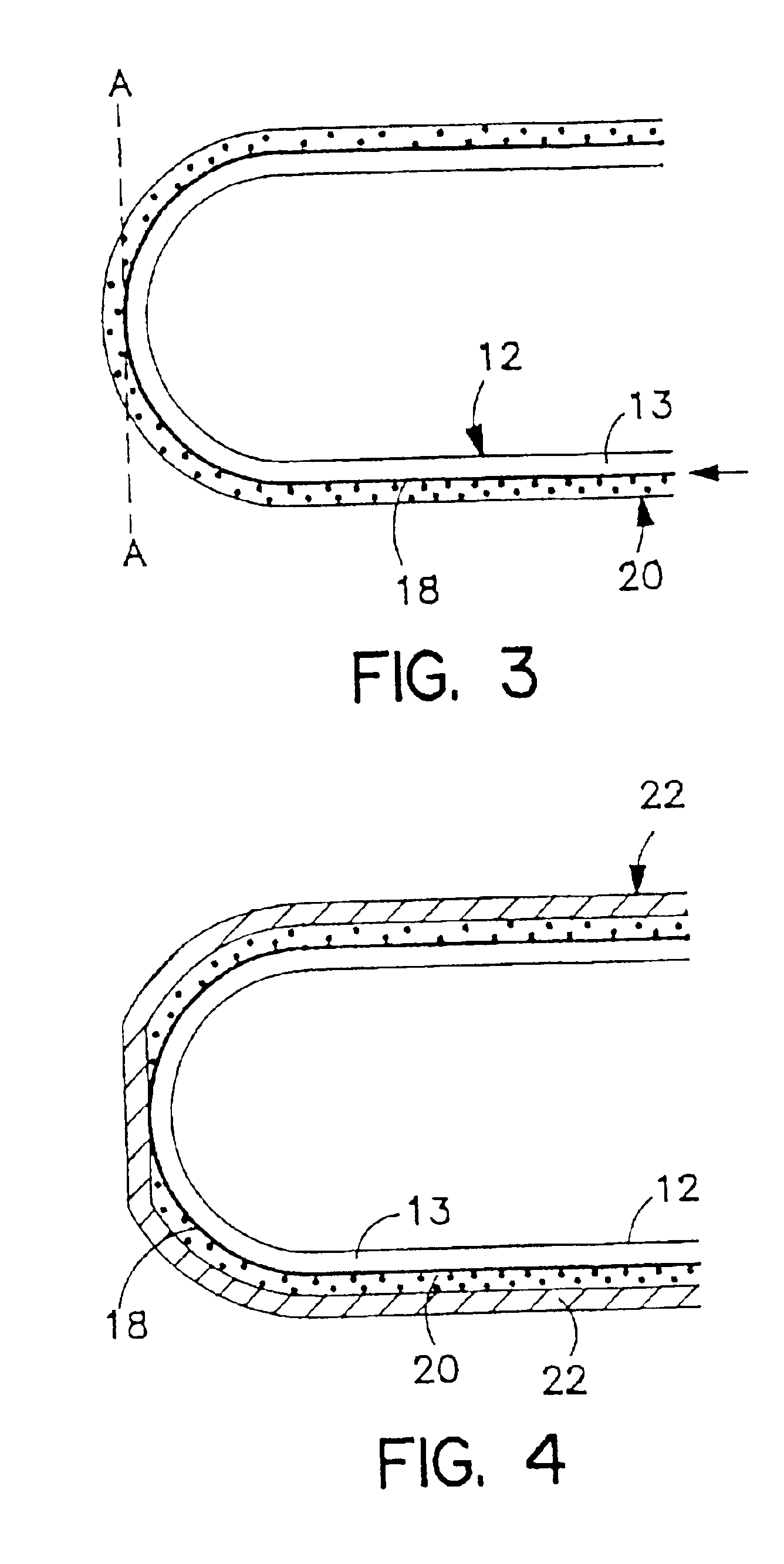
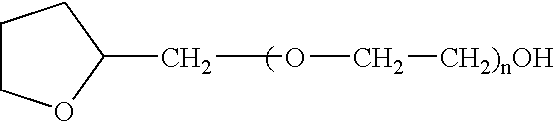
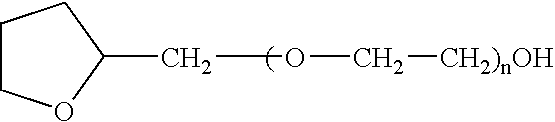
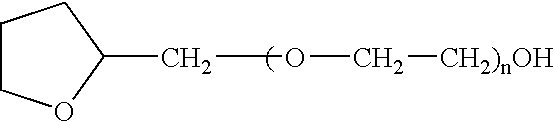
Non-gelatin substitutes for oral delivery capsules, their composition and process of manufacture
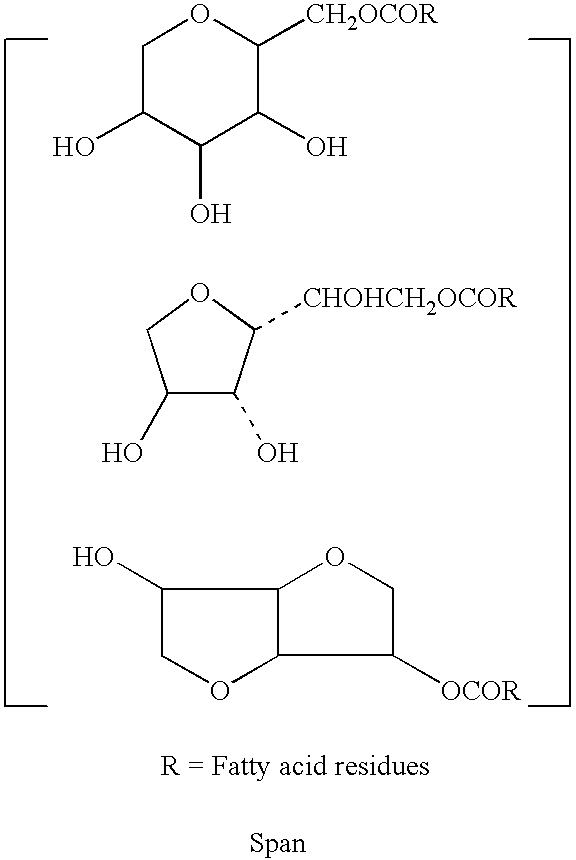
Gelatin-free capsule for use in oral administration of medicines, cosmetic or bath applications, or dietary supplements can be prepared from compositions comprisinga) 8-50% by weight of water-dispersible or water-soluble plasticizer,b) 0.5 to 12% by weight kappa-carrageenan,c) 0 to 60% dextrins, andd) 1% to 95% by weight water,with the kappa-carrageenan comprising at least 50% by weight of all gums forming or contributing to formation of thermoreversible gels in the composition. A capsule for oral administration or cosmetic application may comprise a fill material to be administered to a patient or subject and a capsule, the capsule comprising an aqueous based film comprisinga) water-dispersible or water-soluble plasticizer, andb) carrageenan,with the carrageenan comprising at least 50% or 75% by weight of kappa-carrageenan, and the carrageenan comprising at least 50% or 75% by weight of all gums which form or contribute to the formation of thermoreversible gels. A process for forming the capsules may comprise heating the composition, casting or extruding the composition into a film, gelling the composition by cooling, associating a fill material with the gelled composition (usually as a film) and sealing the film about the fill material.
Owner:PATHEON SOFTGELS INC
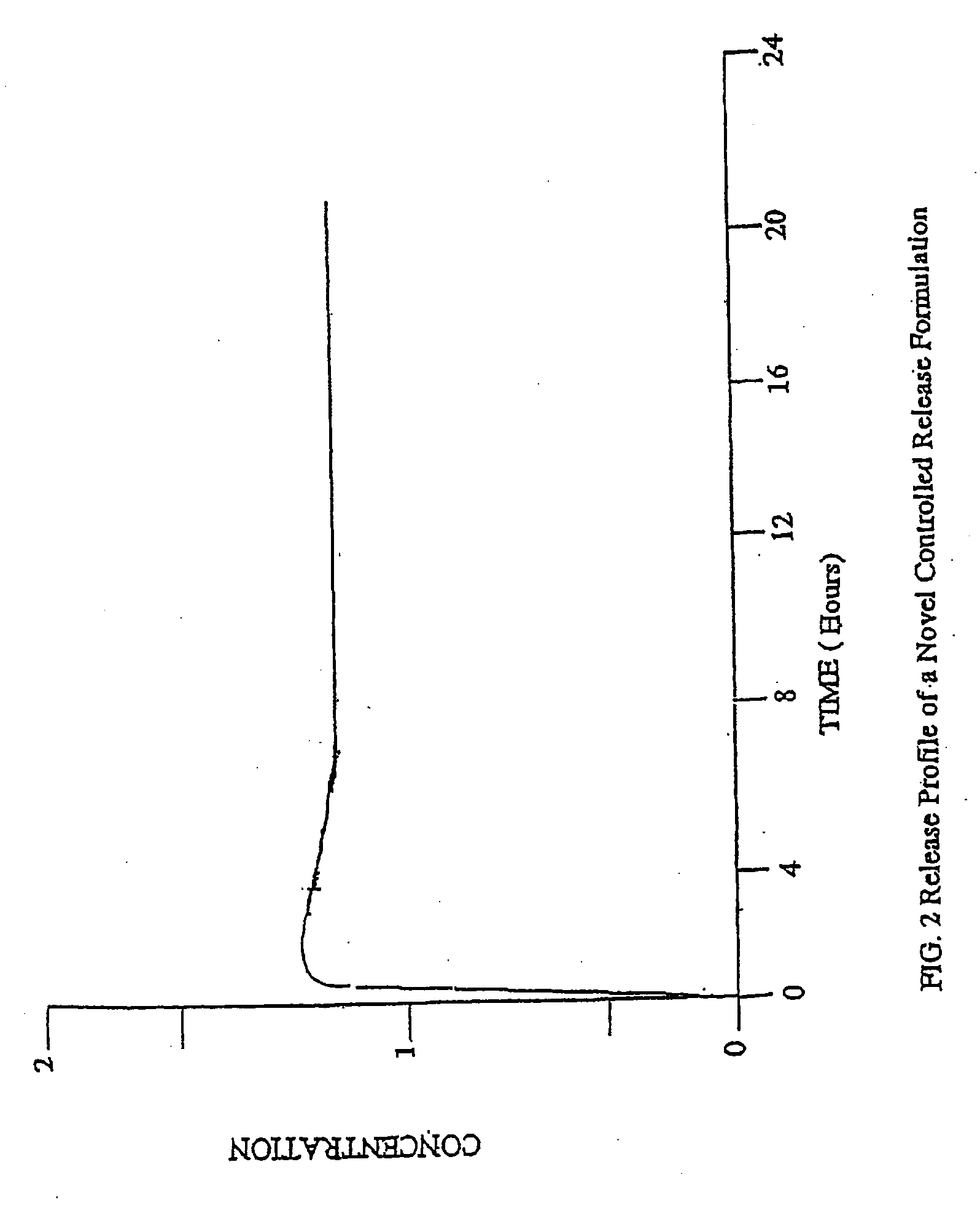
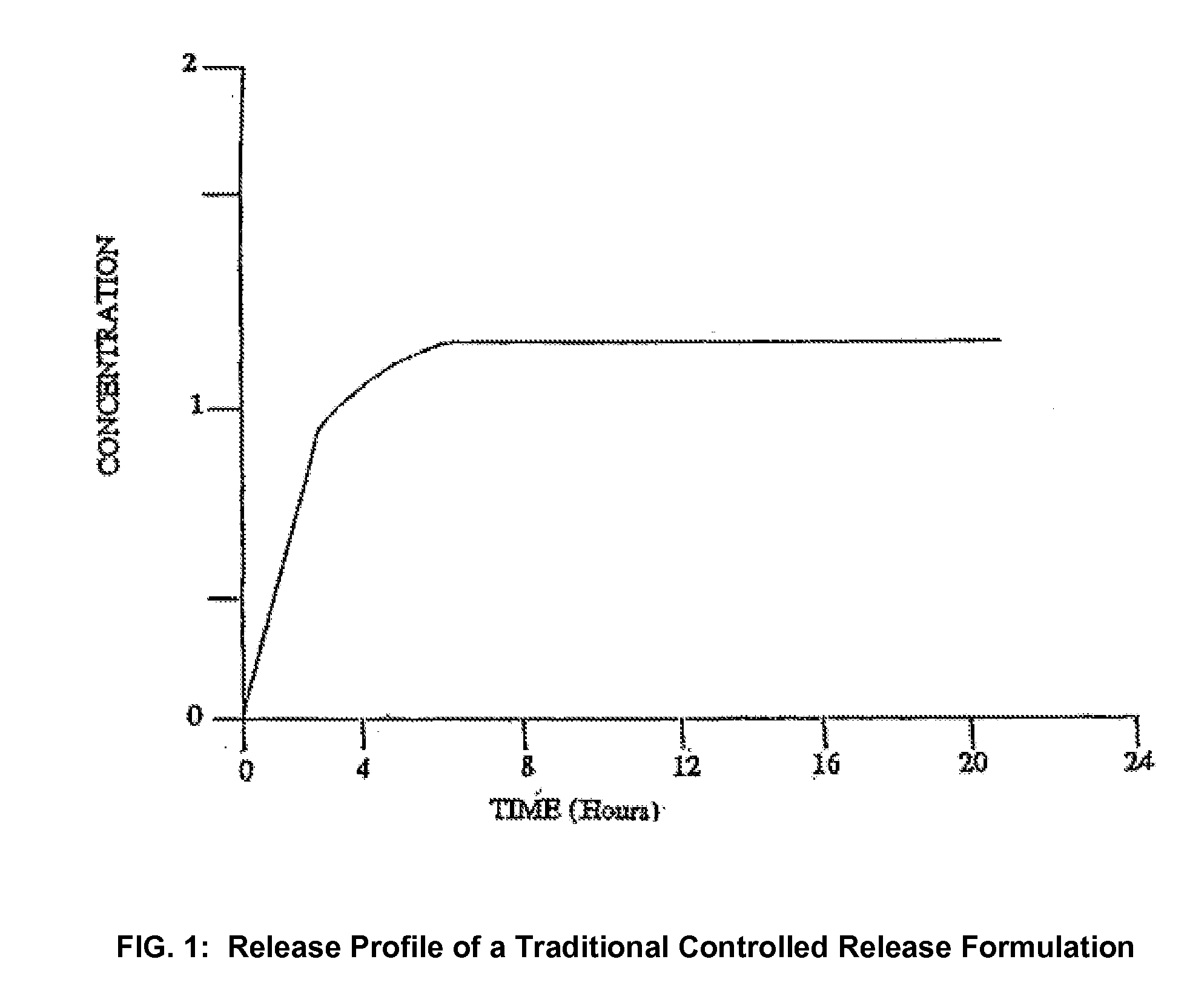
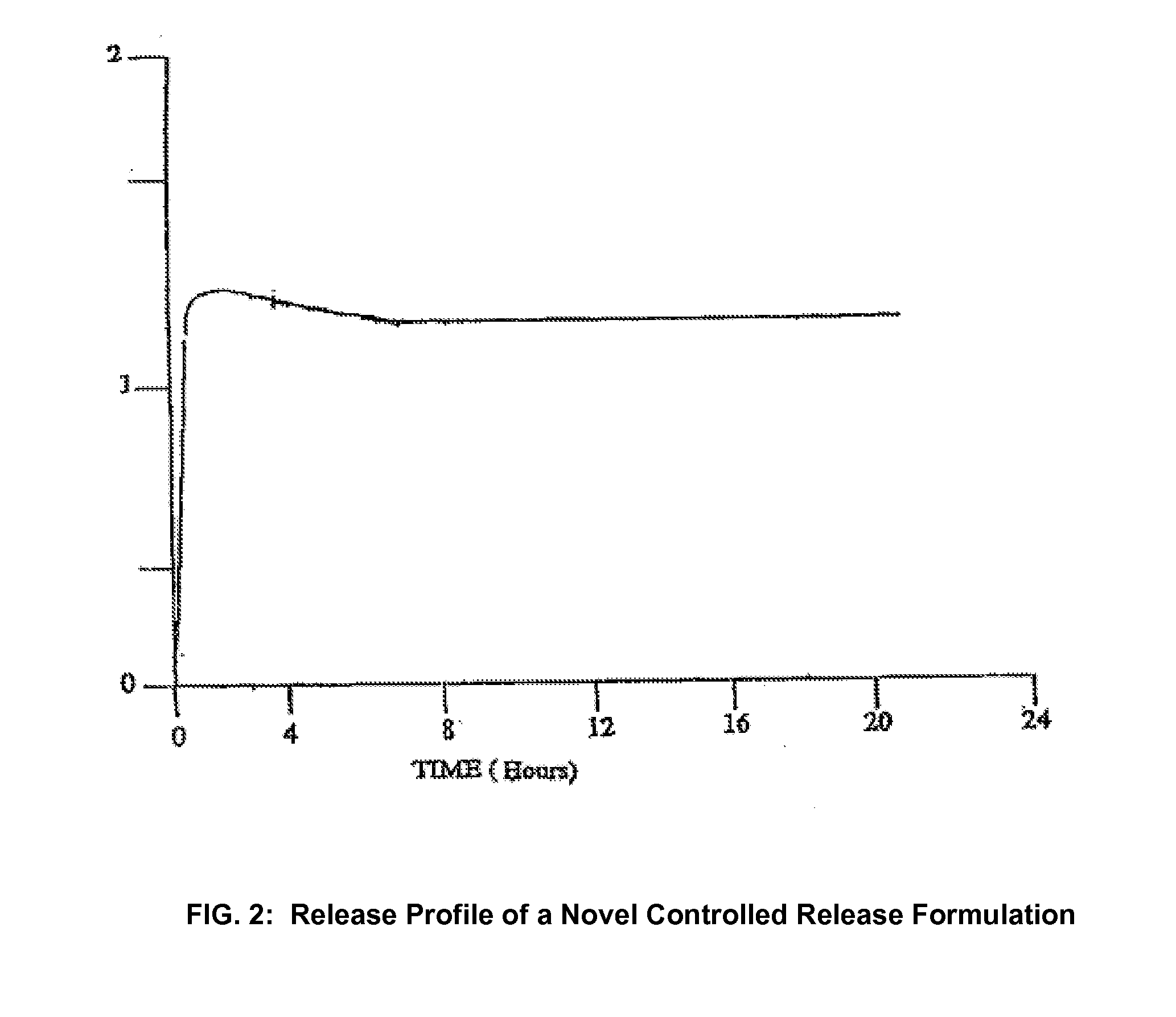
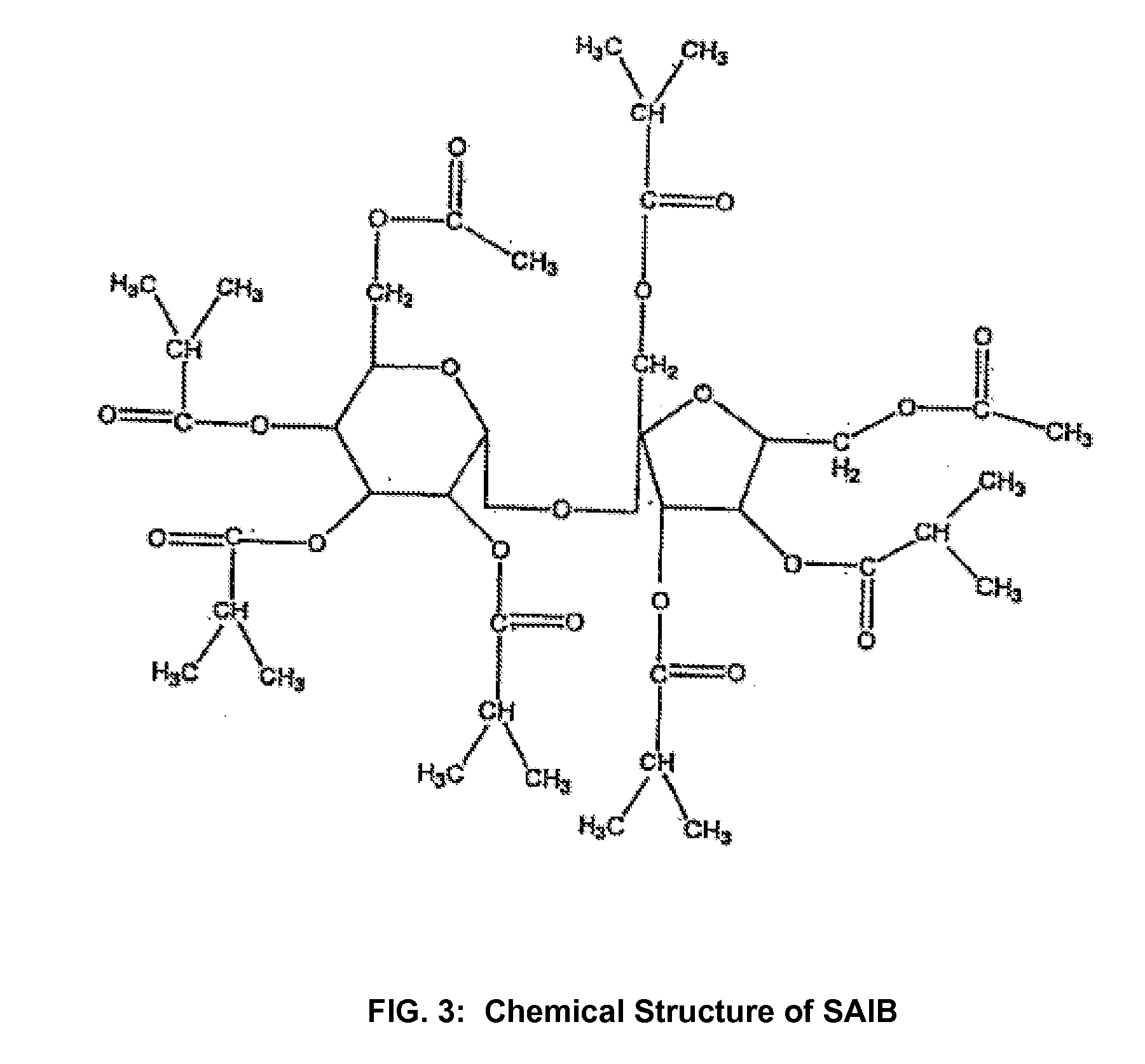
Conversion of liquid filled gelatin capsules into controlled release systems by multiple coatings
InactiveUS6929803B2Pretreated surfacesMacromolecular non-active ingredientsControl releaseActive agent
A dosage form comprising a gelatin capsule formed with a composite wall and containing a liquid, active agent formulation where the wall comprises a barrier layer formed over the external surface of the gelatin capsule, an expandable layer formed over the barrier layer and a semipermeable layer formed over the expandable layer is described. The dosage forms and methods provide for the conversion of standard gelatin, liquid formulation capsules into controlled, release dosage forms that permit the controlled release of the active agent into the environment of use over time.
Owner:ENCINAL PHARMA INVESTMENTS
Non-gelatin capsule shell formulation
A film-forming hydrocolloid composition comprising kappa carrageenan, iota carrageenan, a bulking agent, plasticizer and water is described. The ratio of bulking agent to total carrageenan is from about 1:1 to 20:1. Kappa carrageenan is present in an amount of less than or equal to 50% by weight of total carrageenan present. To form the composition, all dry materials are mixed and added to a heated mixture of all liquid materials. The final mixture is heated until a composition free of particulate materials is formed. The formed composition can be cast or extruded into ribbons, films, sheets, tubes or the like, for encapsulating wet or dry materials including medicinal dosage forms, nutritional supplements, cosmetics, bath oils and gels, and paint balls.
Owner:PATHEON SOFTGELS INC
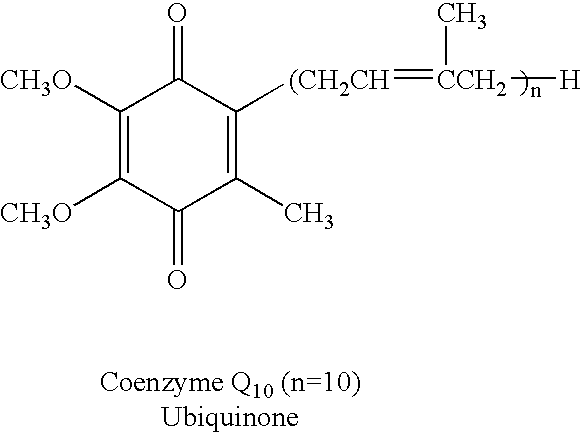
Reduced form of Coenzyme Q in high bioavailability stable oral dosage form
InactiveUS6740338B1Improve bioavailabilityReduced form requirementsBiocideEther/acetal active ingredientsOral medicationBioavailability
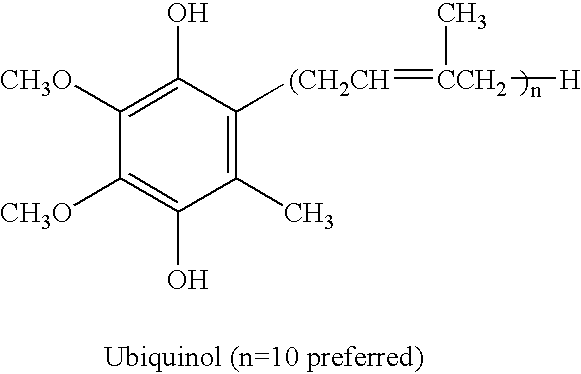
The present invention relates to a reduced form of Coenzyme Q also known as ubiquinol in oral dosage form such as a gelatin capsule, preferably a soft gelatin capsule. Compositions according to the present invention include storage stable compositions comprising effective amounts of ubiquinol in combination with an amount of a reducing agent effective to maintain ubiquinol in its reduced state when formulated in capsules, tablets and other orally administrable form. Methods of use are also disclosed.
Owner:QUTEN RES INST LLC
Oral Dosage Forms with Therapeutically Active Agents In Controlled Release Cores and Immediate Release Gelatin Capsule Coats
InactiveUS20080026052A1Increase release rateIncrease ratingsBiocideNervous disorderActive agentGelatin capsule
The present invention relates to oral dosage form with active agents in controlled release cores and in immediate release gelatin capsule coats.
Owner:SCHOENHARD GRANT L
Non-gelatin capsule shell formulation
Owner:FONKWE LINUS G +2
Oral dosage forms with therapeutically active agents in controlled release cores and immediate release gelatin capsule coats
InactiveUS20110287093A1Increase release rateOrganic active ingredientsNervous disorderControlled releaseImmediate release
The present invention relates to oral dosage form with active agents in controlled release cores and in immediate release gelatin capsule coats.
Owner:SCHOENHARD GRANT L
Effects of probiotics on humans and animals under environmental or biological changes
A dry, stable and viable probiotic composition comprising, a probiotic microorganism and a dried plant powder, with the proviso that said composition is not a blended mixture of at least one biologically pure Pediococcus acidilactici probiotic culture and dried tomato powder at a weight ratio of 1:4 encapsulated in an effective amount in a gelatin capsule.
Owner:IMAGILIN TECH LLC
Non-gelatin soft capsule system
ActiveUS20060099246A1Improve stabilityImprove solubilityBiocidePharmaceutical non-active ingredientsHigh resistanceCarrageenan
A non-gelatin encapsulation system for liquid filled soft capsules, by nature of the carrier, the cationic-ionic balance of the carrier and the active ingredients, or the concentration of the active ingredients and excipients, are difficult or impossible to commercially encapsulate in gelatin capsules. In particular, the system is adapted for the encapsulation of highly basic, or alkaline, fills. The system provides for a predominantly starch and gelling carrageenan based shell, which displays high resistance to both concentrated fills and to alkaline fills, in particular, to those fills which contain the salt or salts of weak acids and strong bases.
Owner:R P SCHERER TECH INC
Chewable soft gelatin capsules
InactiveUS20070292501A1Improve overall utilizationOrganic active ingredientsCapsule deliveryMedicineActive agent
The present invention is directed to compositions and methods of delivery of fill materials containing active agents, optionally dissolved or suspended in a suitable carrier, encapsulated in a chewable soft gelatin capsule.
Owner:SOFT GEL TECHNOLGIES
Pullulan polysaccharide hollow hard capasule and its preparing method
InactiveCN101069677ASmall range of molecular weight variationProduct quality is easy to controlPharmaceutical non-active ingredientsCapsule deliveryPullulanHard Capsule
The present invention discloses a hollow hard capsule and its preparation method. It is made up by adopting prolanpolysaccharide as main body material and adding gelling agent, coagulant aids, surfactant and humectant through a certain preparation process. Said invention also provides the concrete steps of its preparation method.
Owner:广东强基药业有限公司
Soft gelatin capsules containing particulate material
Disclosed are suspensions suitable for encapsulation in gelatin capsules, comprising a solid phase consisting of solid particles having a mean diameter of at least about 149 mum, and a liquid phase capable of suspending the solid phase, the suspension having a predetermined rheology at a temperature suitable for encapsulation into gelatin capsules.
Owner:CATALENT USA WOODSTOCK INC +3
Non-gelatin soft capsule system
ActiveUS8231896B2Increase resistanceLarge doseBiocidePharmaceutical non-active ingredientsCarrageenanSoftgel
A non-gelatin encapsulation system for liquid filled soft capsules, by nature of the carrier, the cationic-ionic balance of the carrier and the active ingredients, or the concentration of the active ingredients and excipients, are difficult or impossible to commercially encapsulate in gelatin capsules. In particular, the system is adapted for the encapsulation of highly basic, or alkaline, fills. The system provides for a predominantly starch and gelling carrageenan based shell, which displays high resistance to both concentrated fills and to alkaline fills, in particular, to those fills which contain the salt or salts of weak acids and strong bases.
Owner:R P SCHERER TECH INC
Method of sterilizing
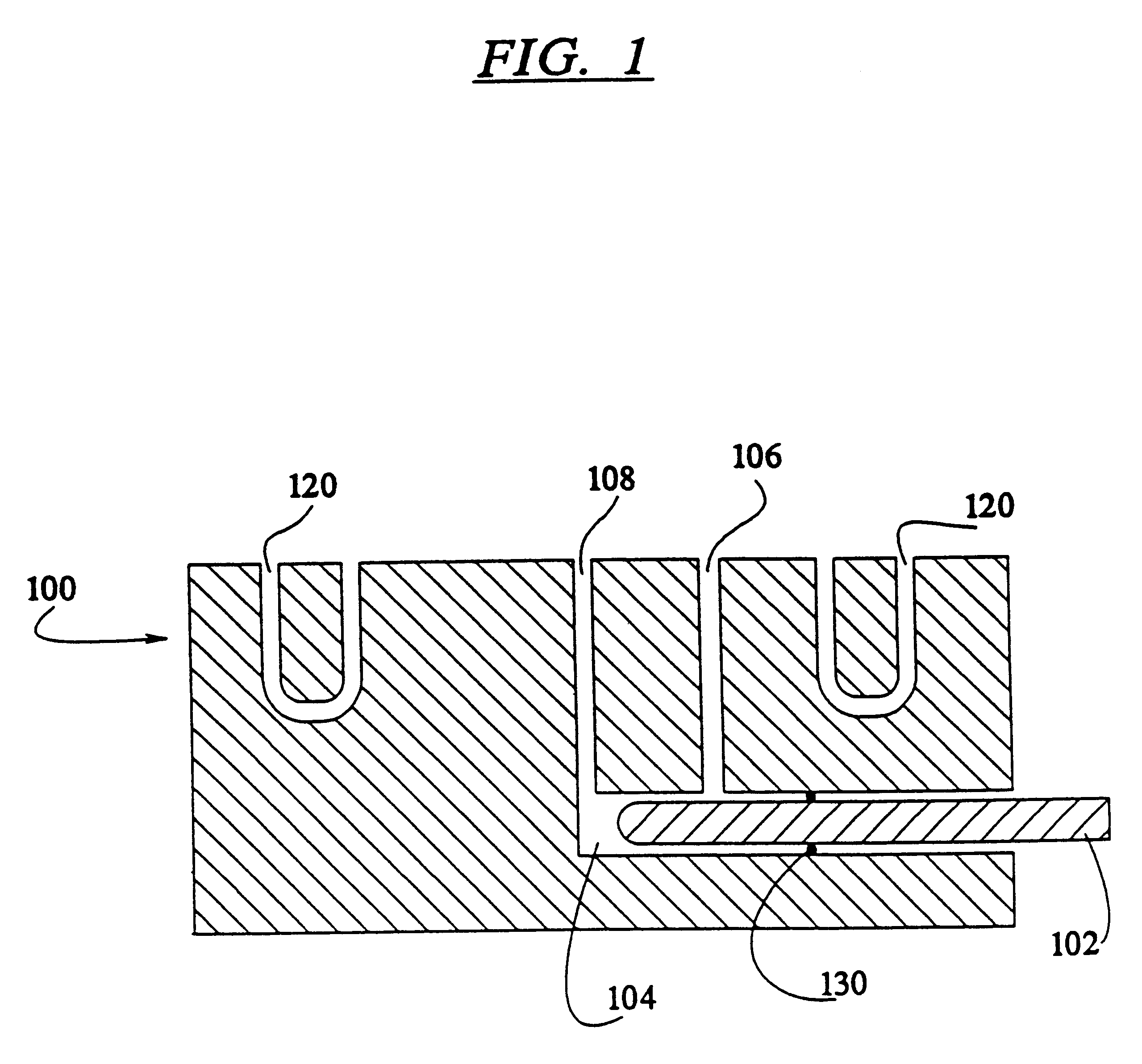
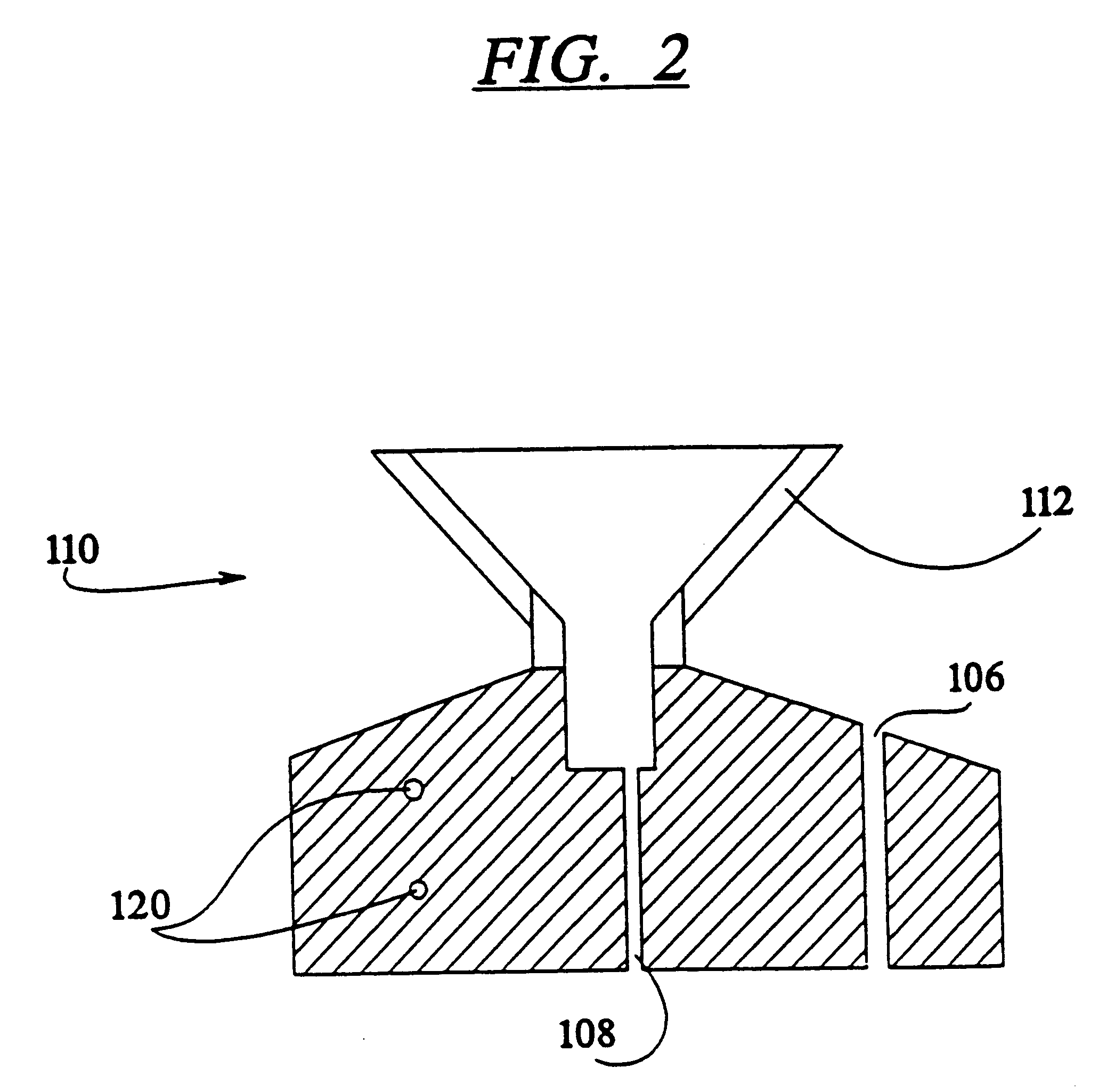
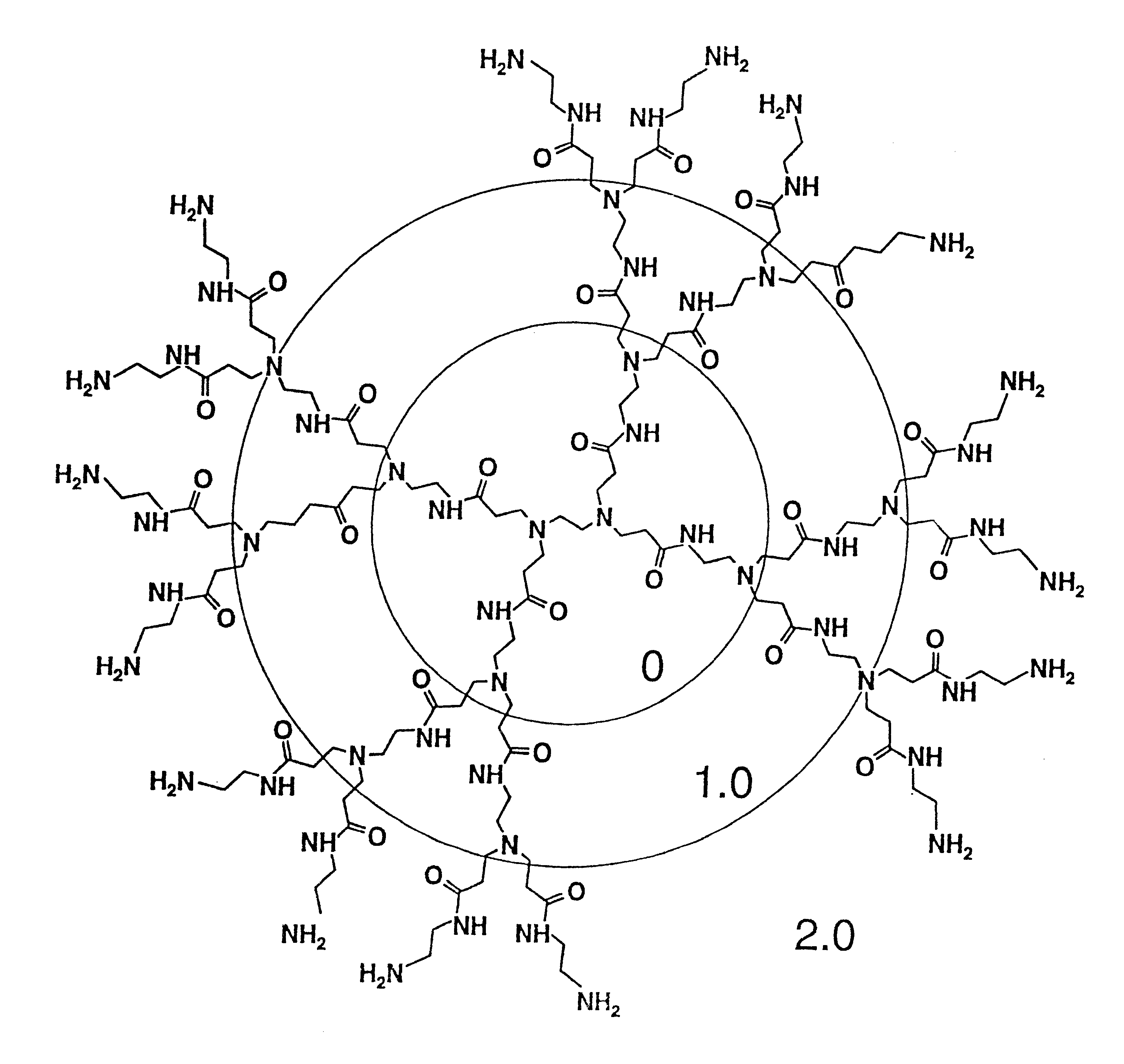
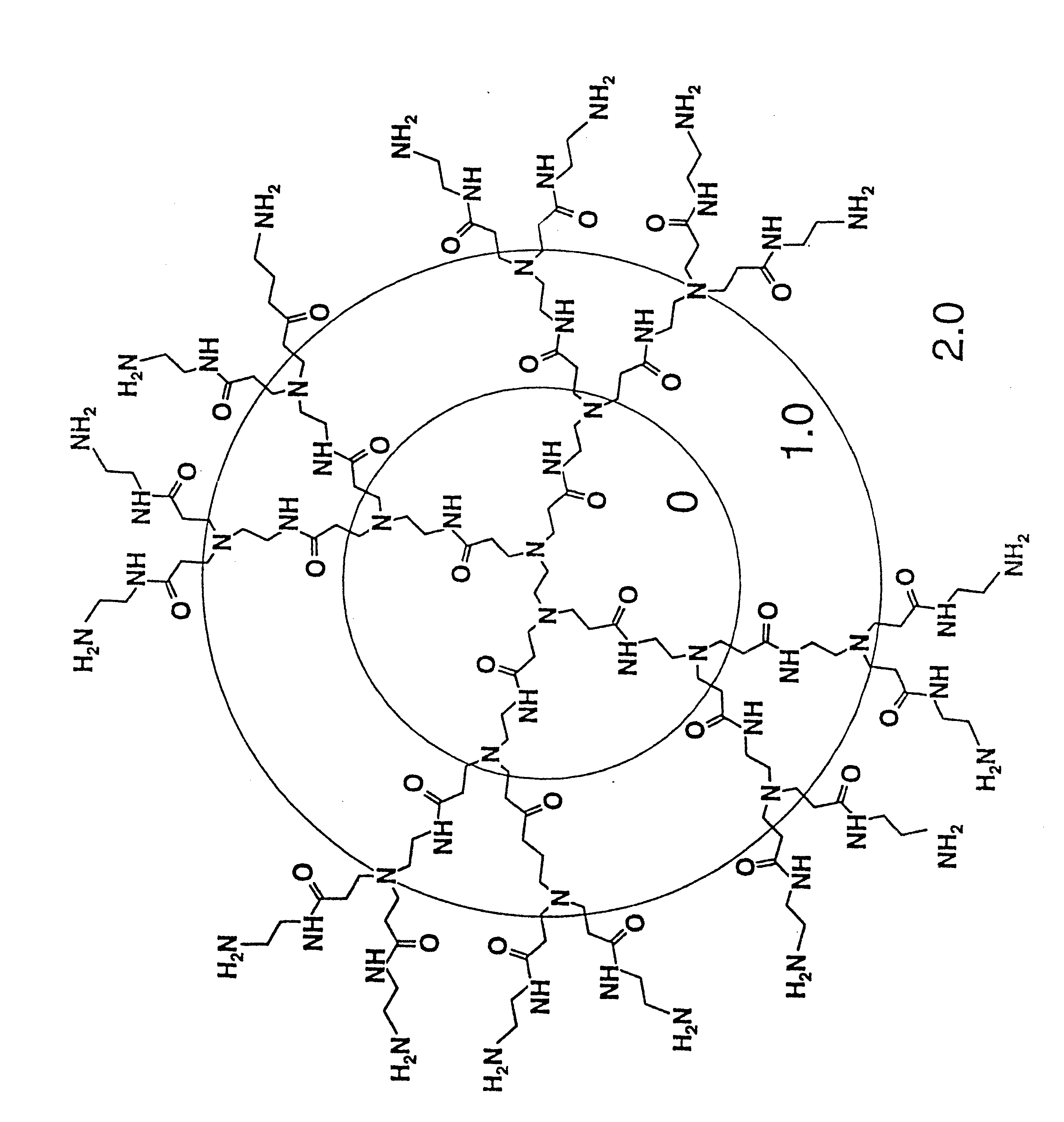
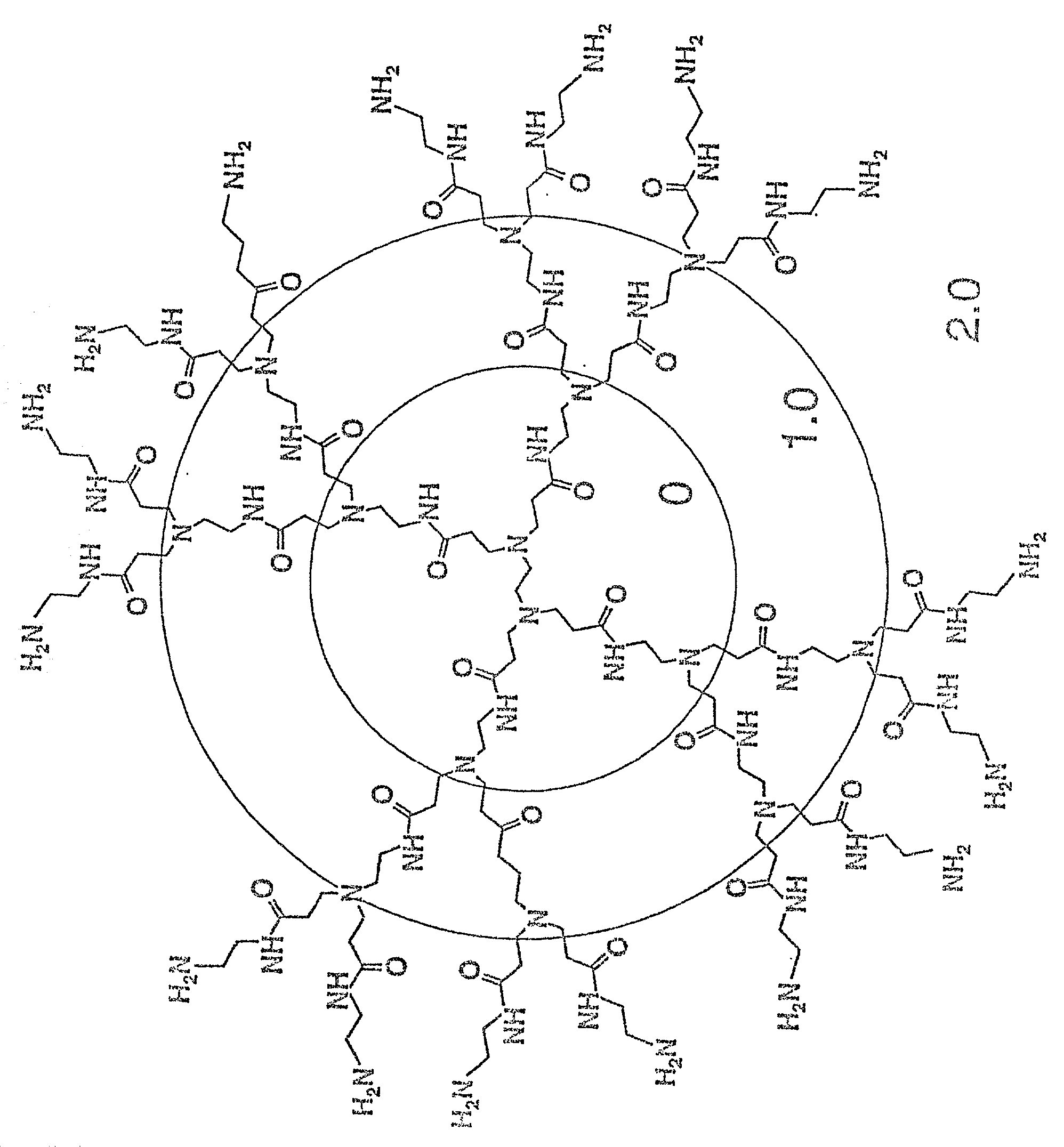
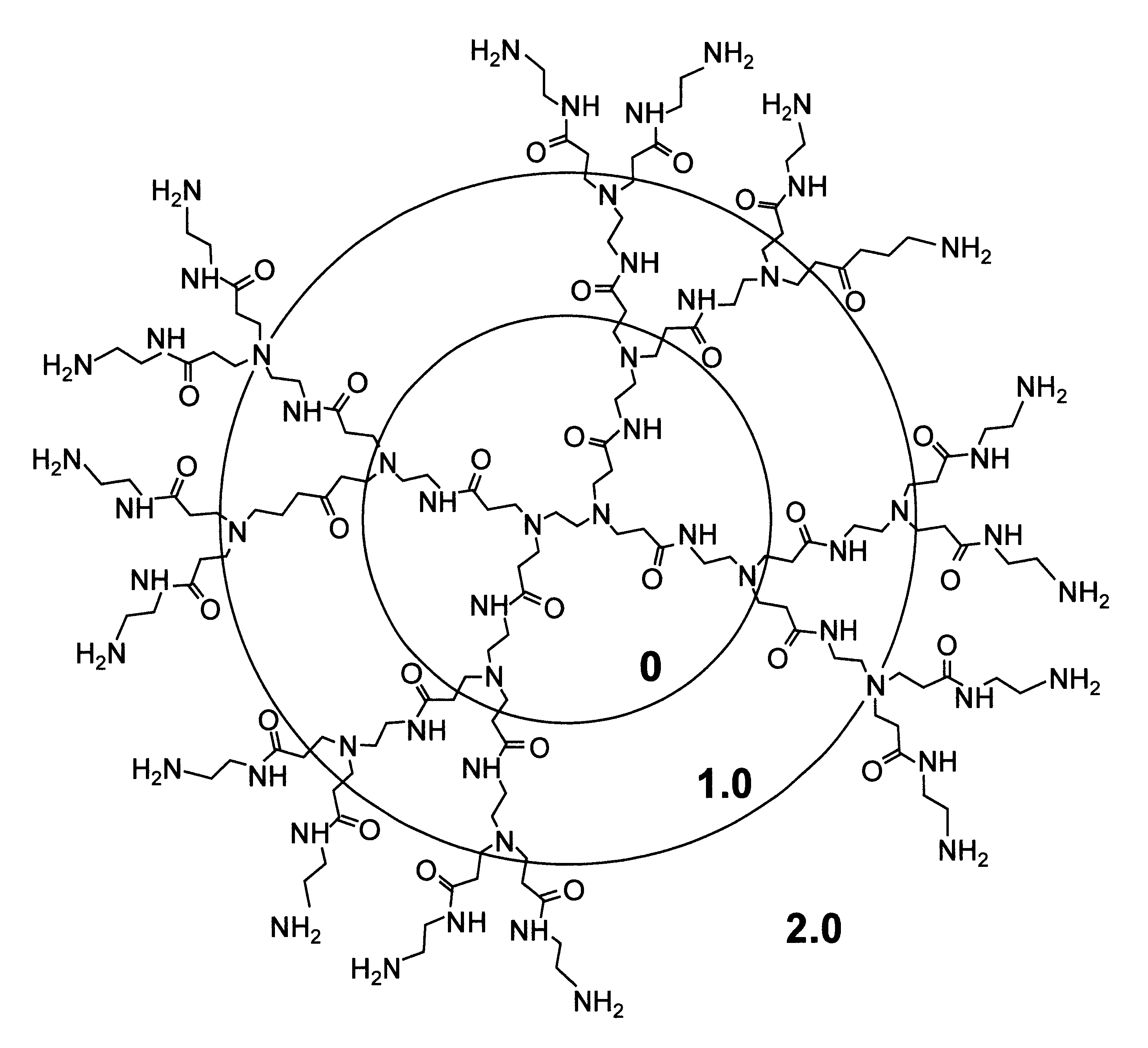
A method of sterilizing objects as well as the sterilized objects obtained from the method are disclosed. The method involves contacting an object such as a medical device to be reused with polycationic dendrimer under conditions which result in rendering a conformationally altered protein (e.g. a prion) non-infectious. A disinfecting agent or surgical scrub composition which comprises the dendrimers is also disclosed as are gelatin capsules treated with polycationic dendrimers.
Owner:RGT UNIV OF CALIFORNIA
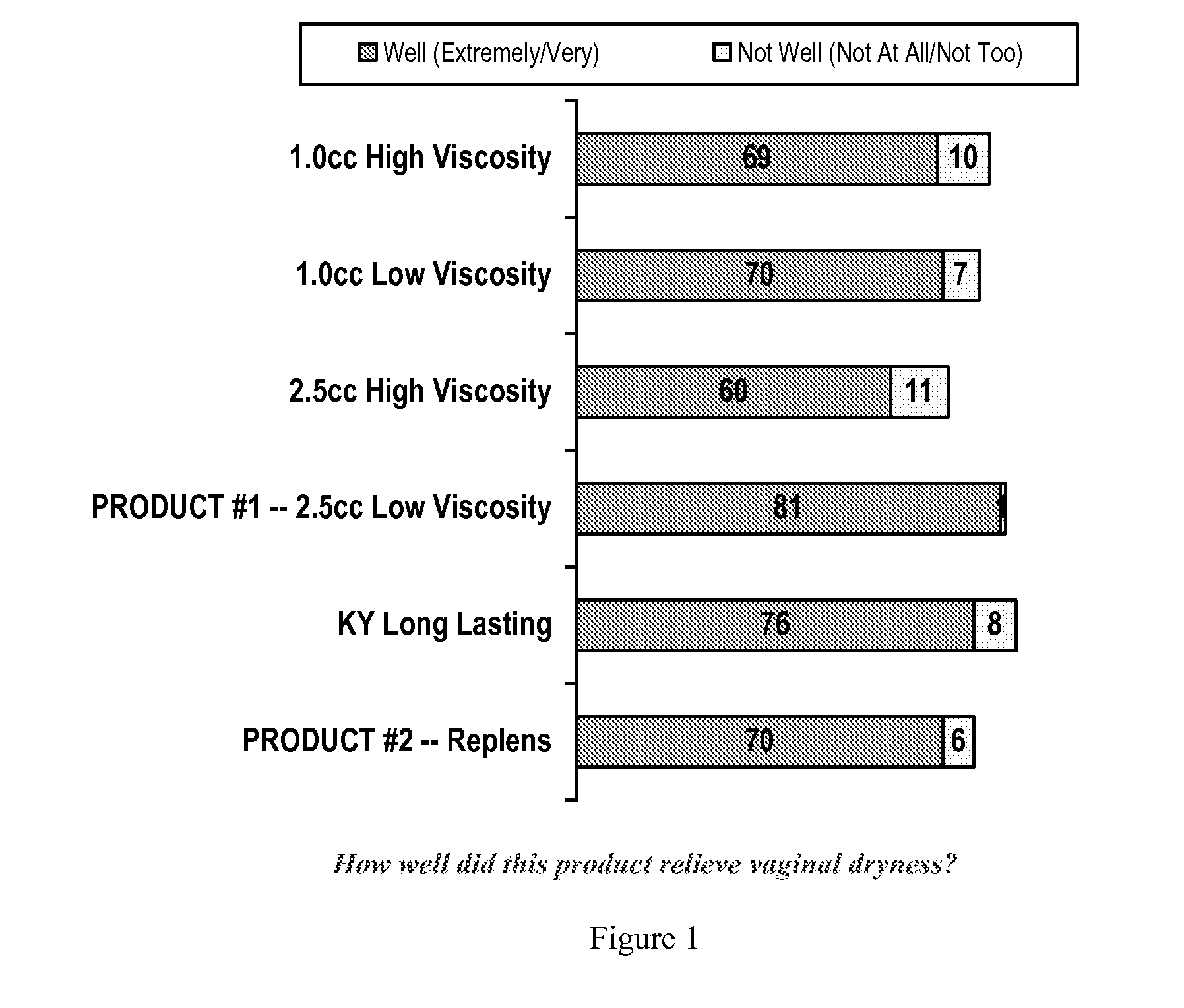
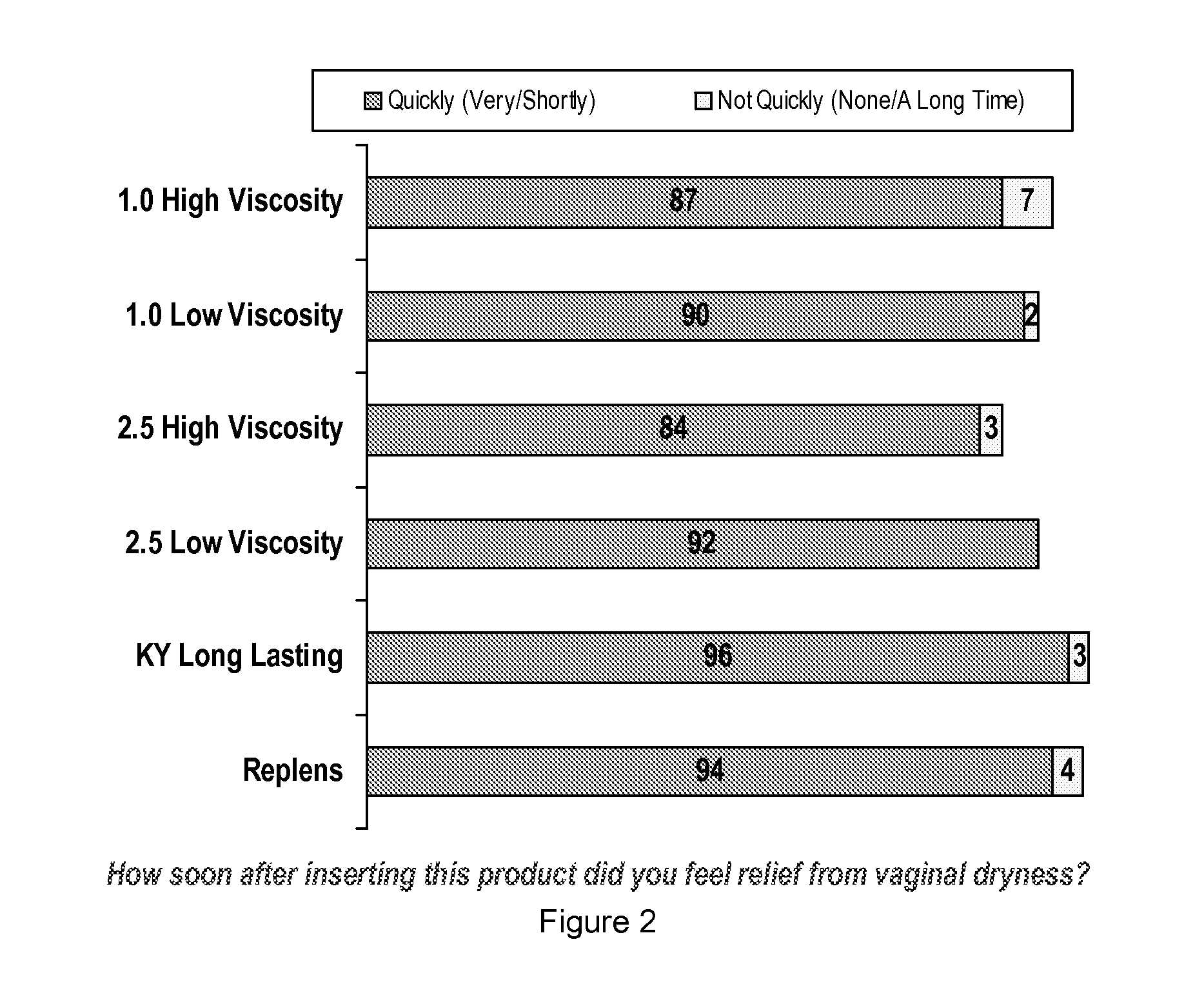
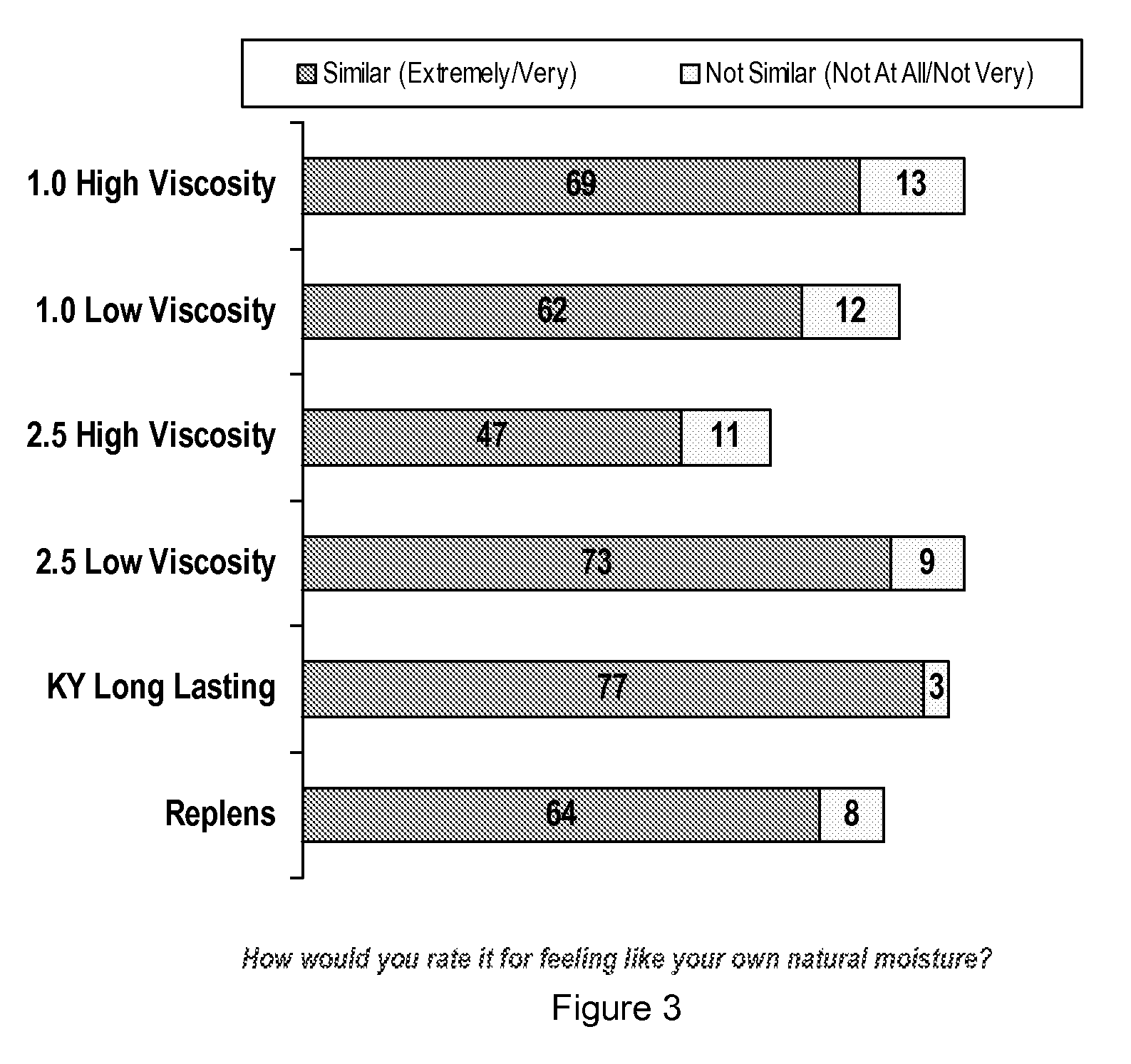
Personal lubricant compositions and kits for providing personal lubrication
This invention relates to body orifice moisturizing compositions and kits and methods of their use. More particularly, it relates to vaginal moisturizing compositions and kits and methods of their use. The vaginal moisturizing compositions of this invention are preferably in the form of a soft gelatin capsule containing therein a lubricating fluid, said soft gelatin capsule being capable of insertion into a body orifice. It will provide sustained lubrication to the body orifice.
Owner:LIN SHUN Y +2
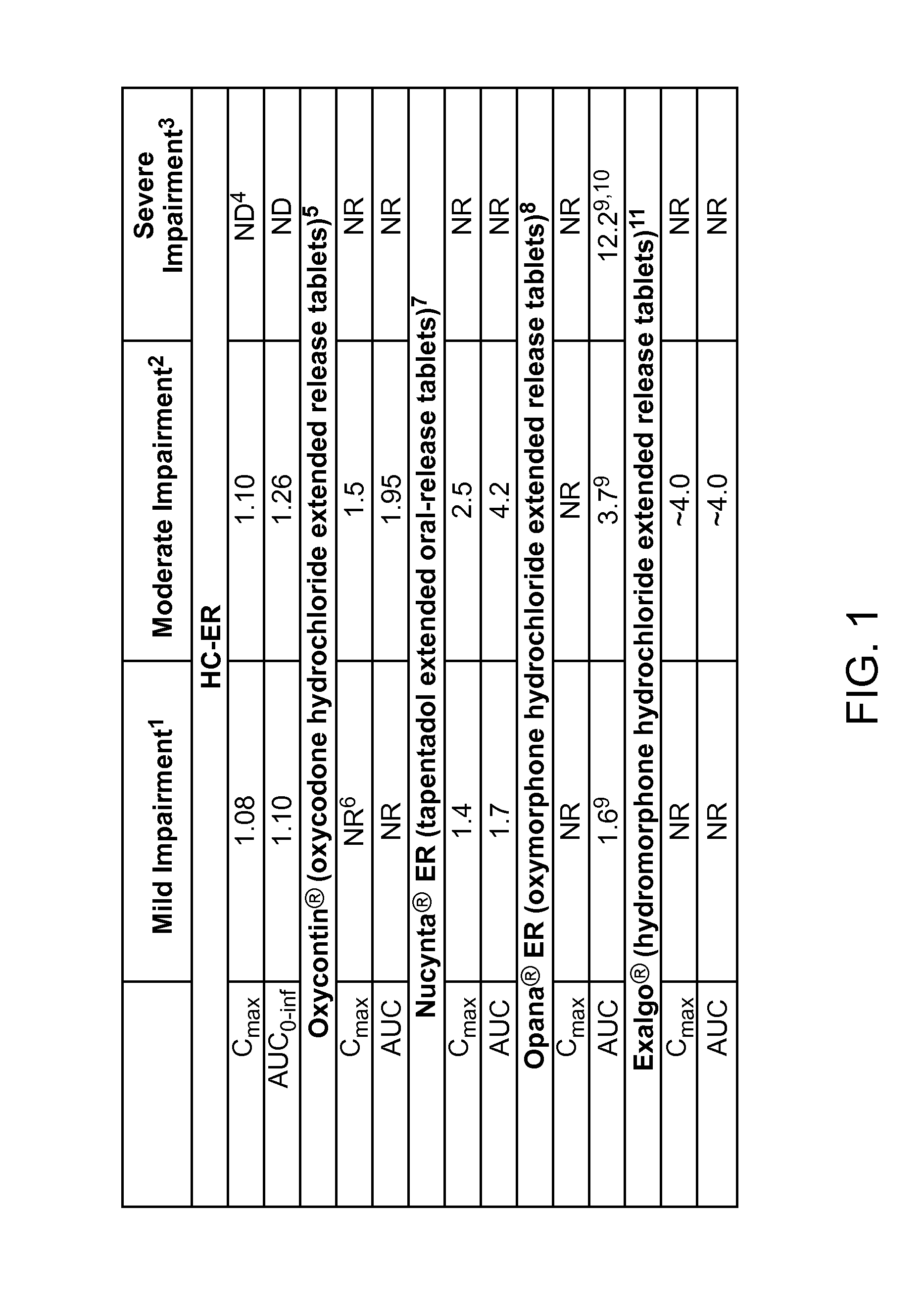
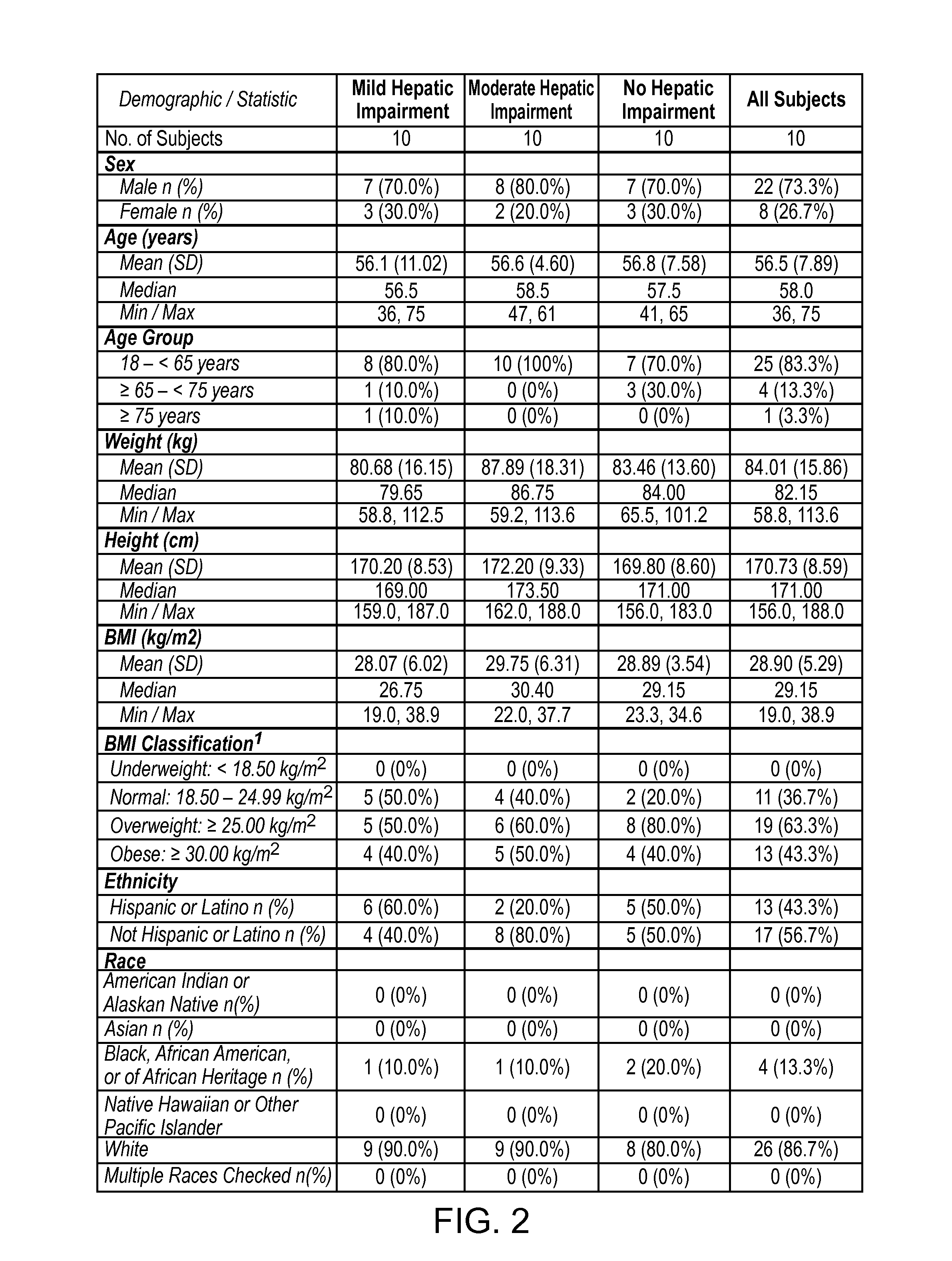
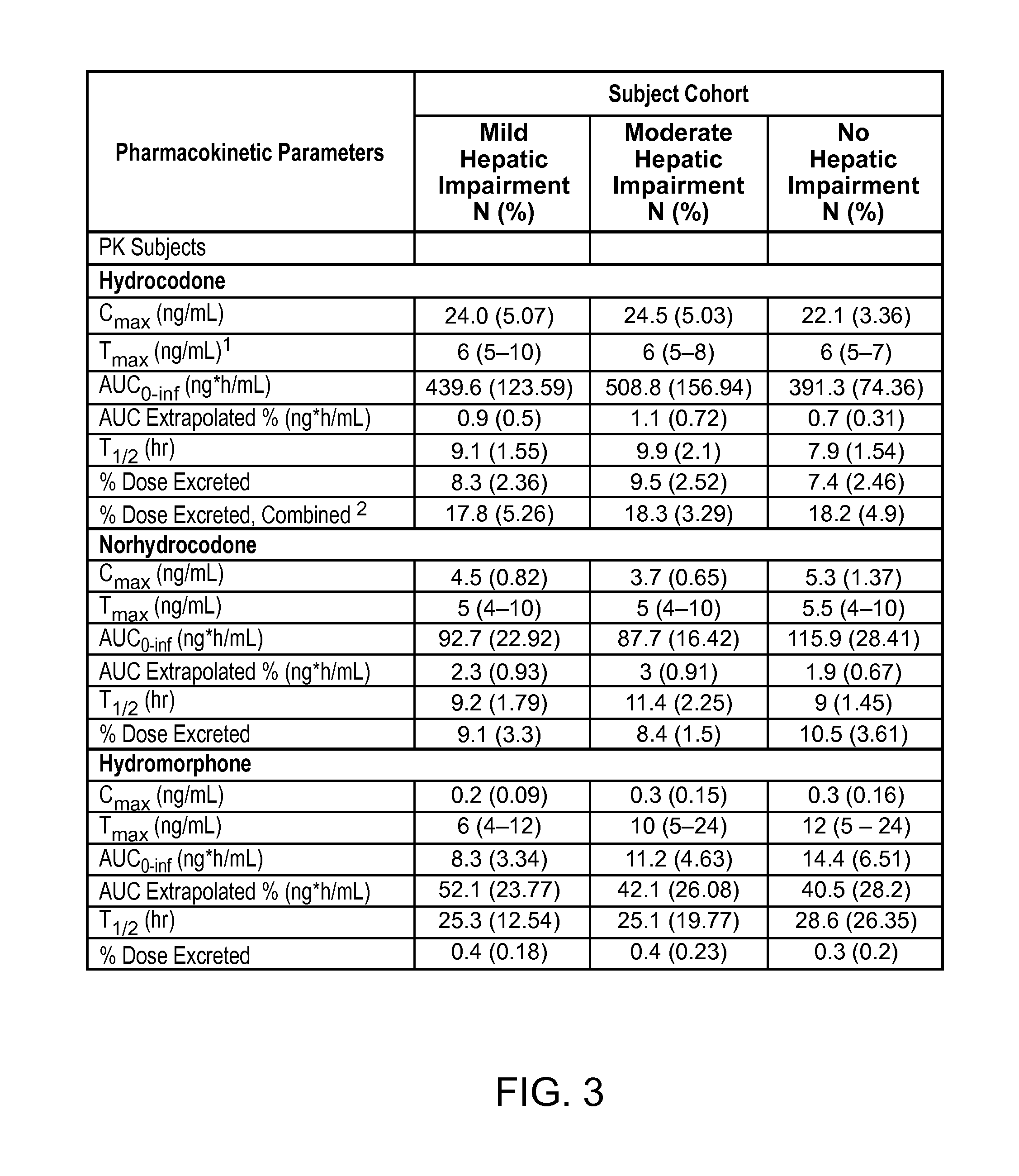
Treating pain in patients with hepatic impairment
An extended release composition for an analgesic active pharmaceutical ingredient which may be an opioid, preferably hydrocodone as the only active ingredient. The extended release composition comprises a multiparticulate modified release composition which may be in the form of beads contained in an oral dosage form such as gelatin capsules as the primary package. The oral dosage units are supplied as part of a kit, which also includes a package insert all sold as a commercially marketed product. The primary package and package insert are contained in an optional secondary package and the package insert does not contain a warning, a dosing instruction, or a dosing table specifically directed to patients suffering from mild, moderate or severe hepatic impairment.
Owner:PERSION PHARMA LLC
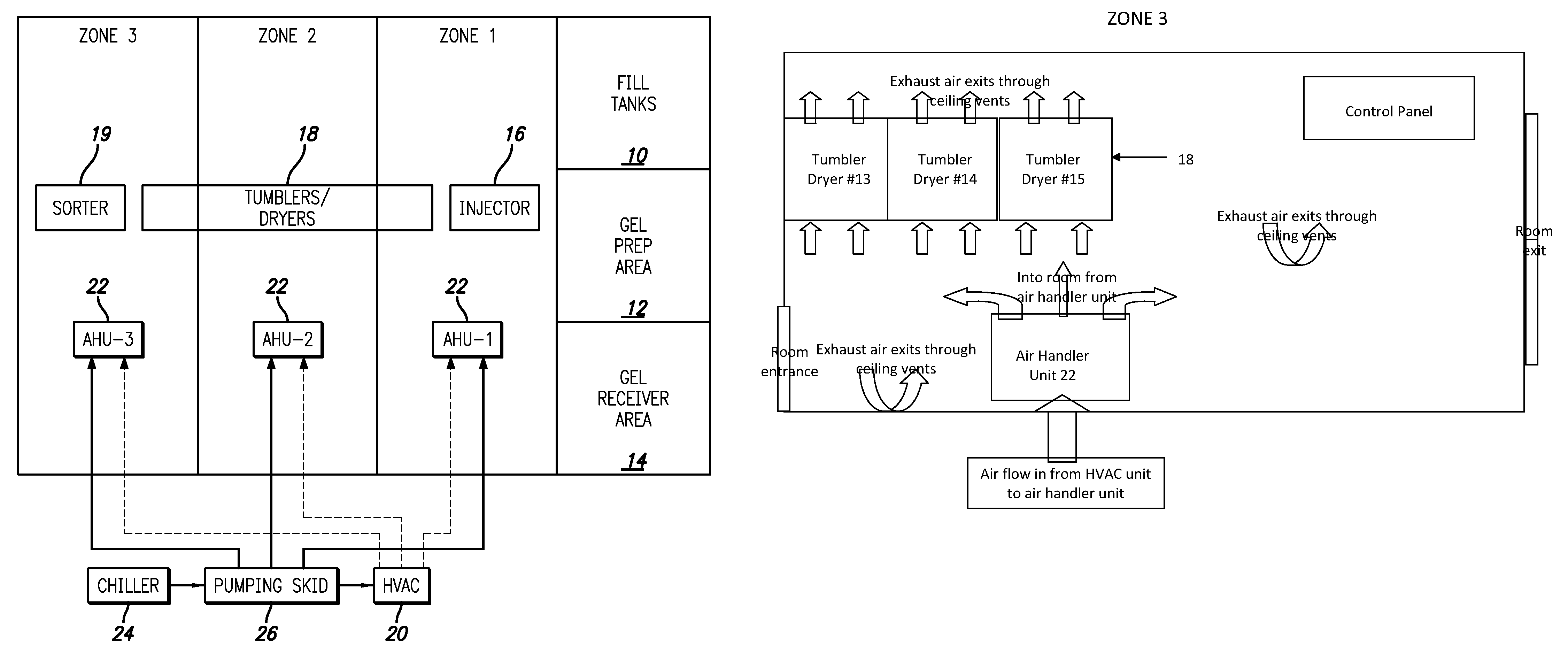
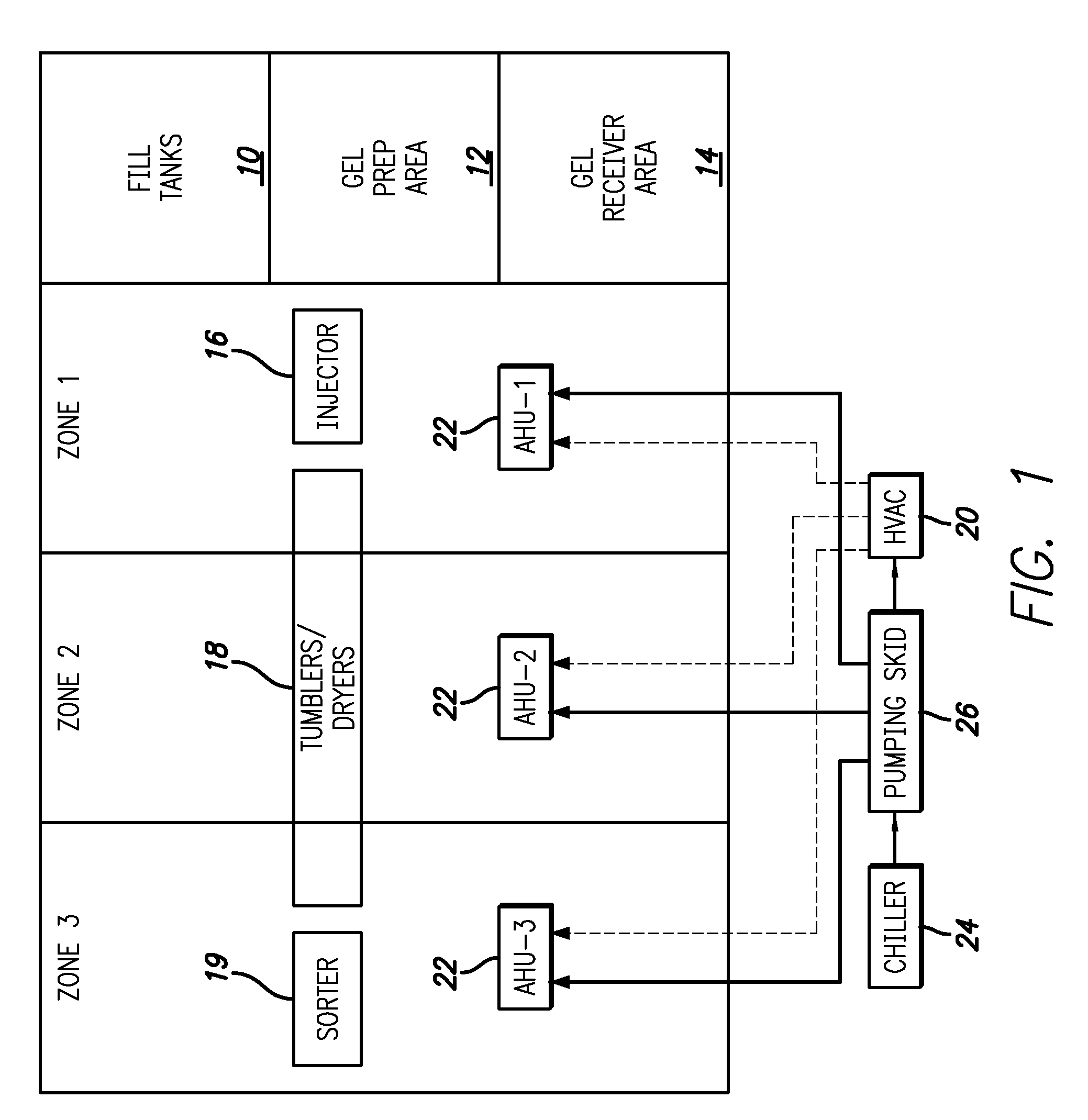
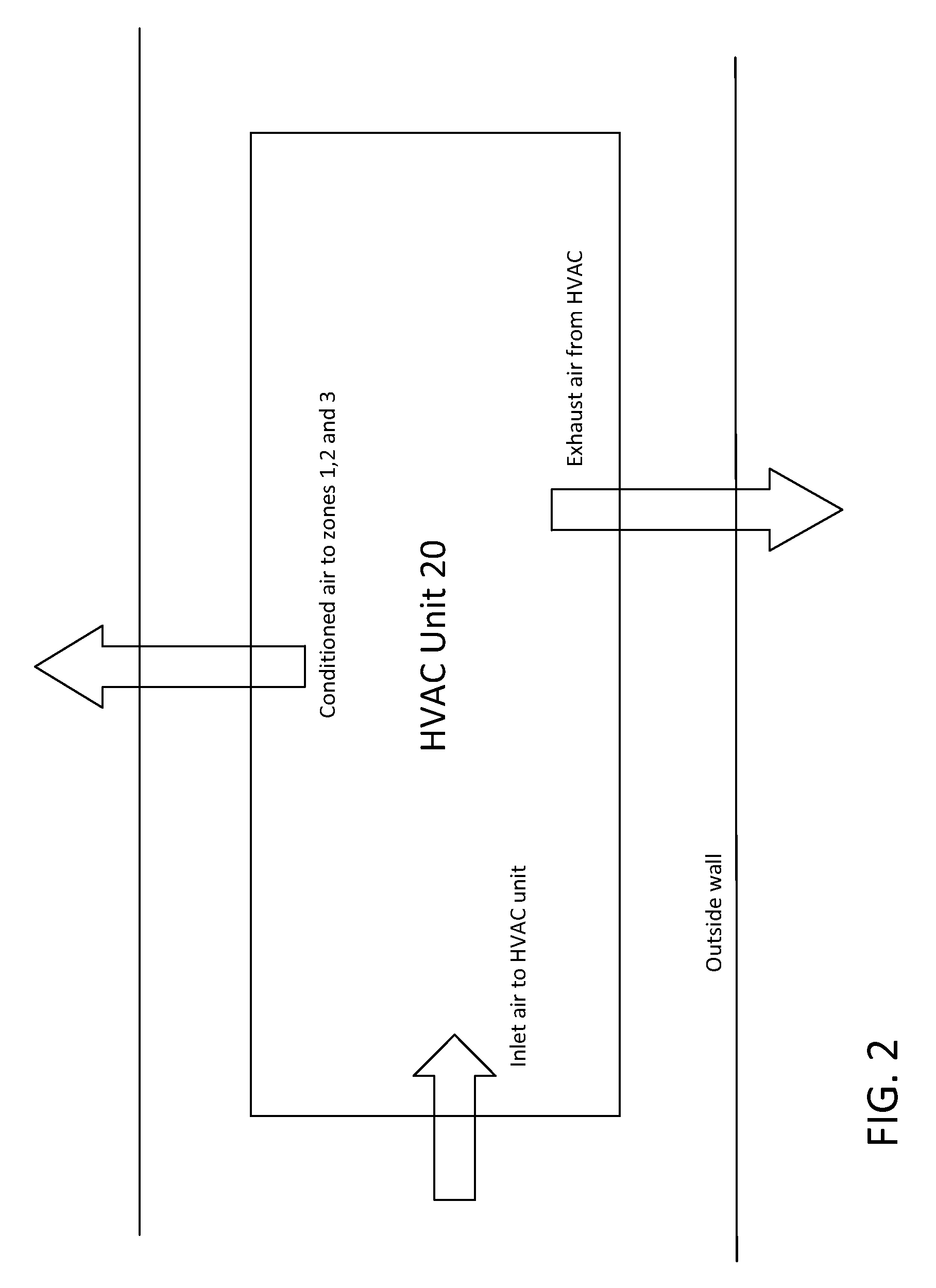
Gelatin capsule formulation and drying system
ActiveUS8621764B2Drying solid materials with heatDomestic cooling apparatusAir handlerEnvironmental engineering
A gelatin capsule drying system that includes a structure divided into first, second and third zones, a first air handler unit positioned to discharge air into the first zone, a second air handler unit positioned to discharge air into the second zone and a third air handler unit positioned to discharge air into the third zone. The system further includes a series of tumble dryers that extend from the first zone, through the second zone and into the third zone, and an HVAC unit that provides air to the first, second and third air handler units.
Owner:GEL CAP TECH LLC
Soft shell gelatin capsules containing non-steroidal anti-inflammatories
InactiveUS6689382B2Rapid onset of pharmacologic actionAccurate and uniform deliveryBiocideCapsule deliveryFenamic acidSoftgel
Owner:PROCAPS
Method for preparing novel medicine capsule and plant capsule
InactiveCN101023934AImprove performanceReduce moisture contentPharmaceutical non-active ingredientsCapsule deliveryBiotechnologyDrug capsule
The present invention relates to a preparation method of new-type medicine capsule-plant capsule. It is characterized by that it adopts conventional capsule production method, uses the gelatin capsule production machine and utilizes natural plant cellulose derivative-cellulose ether as raw material to produce the invented plant capsule.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Softgel formulations of bisphosphonates bone resorption inhibitors
InactiveUS20050142185A1Improve bioavailabilityInhibiting bone resorptionBiocidePhosphorous compound active ingredientsSoftgelGlycerol
The present invention provides a pharmaceutical formulation suitable for filling softgel capsules comprising: (a) from about 1% to about 90% by weight of a bisphosphonic acid or a pharmaceutically acceptable salt; and (b) from about 40% to about 80% by weight of a liquid carrier comprising 50% to 80% by weight polyethylene glycol; 5% to 15% by weight of glycerin; and 5% to 20% by weight water. The invention also describes a method for preparing alendronate or its pharmaceutical acceptable salts in encapsulated therapeutic dosage form, comprising the steps of reducing the size of alendronate particles to an average size no larger than about 80 microns, then mixing the micronized particles of alendronate with a solvent essentially consisting predominantly of polyethylene glycol of a molecular weight no greater than about 1000, and heating the mixture at a temperature of from about 40° C. to about 50° C. until the alendronate is dissolved in the solvent, and then encapsulating therapeutic doses of the dissolved alendronate in gelatin capsules soluble in water but insoluble in said solvent.
Owner:BELENO ALFREDO BERTHEL +1
Fatty acids for use as a medicament
ActiveUS20100113387A1Clear and significant laxative effectAntibacterial agentsBiocideDiseasePharmaceutical formulation
The invention relates to fatty acid stimulation of rectal mucosa initiating the process of defecation, acting as a laxative. Furthermore, the invention relates to the usage of free fatty acids, fatty acid mixtures and fatty acid extracts from marine lipids in pharmaceutical formulations such as suppositories, ointments, tablets and gelatin capsules for treatment and prevention of multiple disorders like constipation, hemorrhoids, bacterial infections (e.g. helicobacter pylori), viral infections (e.g. herpes simplex virus infections) and inflammations, as well as against fissura ani and pruritus ani.
Owner:LIPID PHARMA EHF
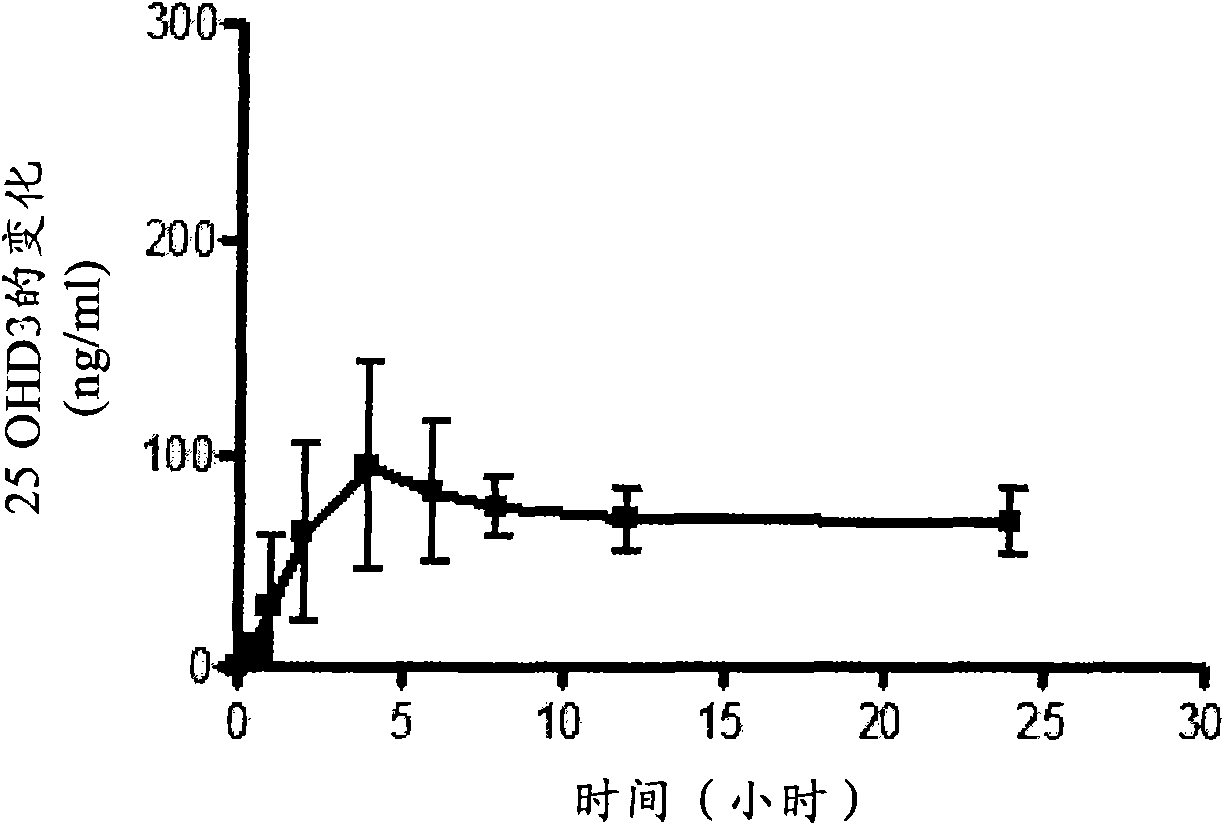
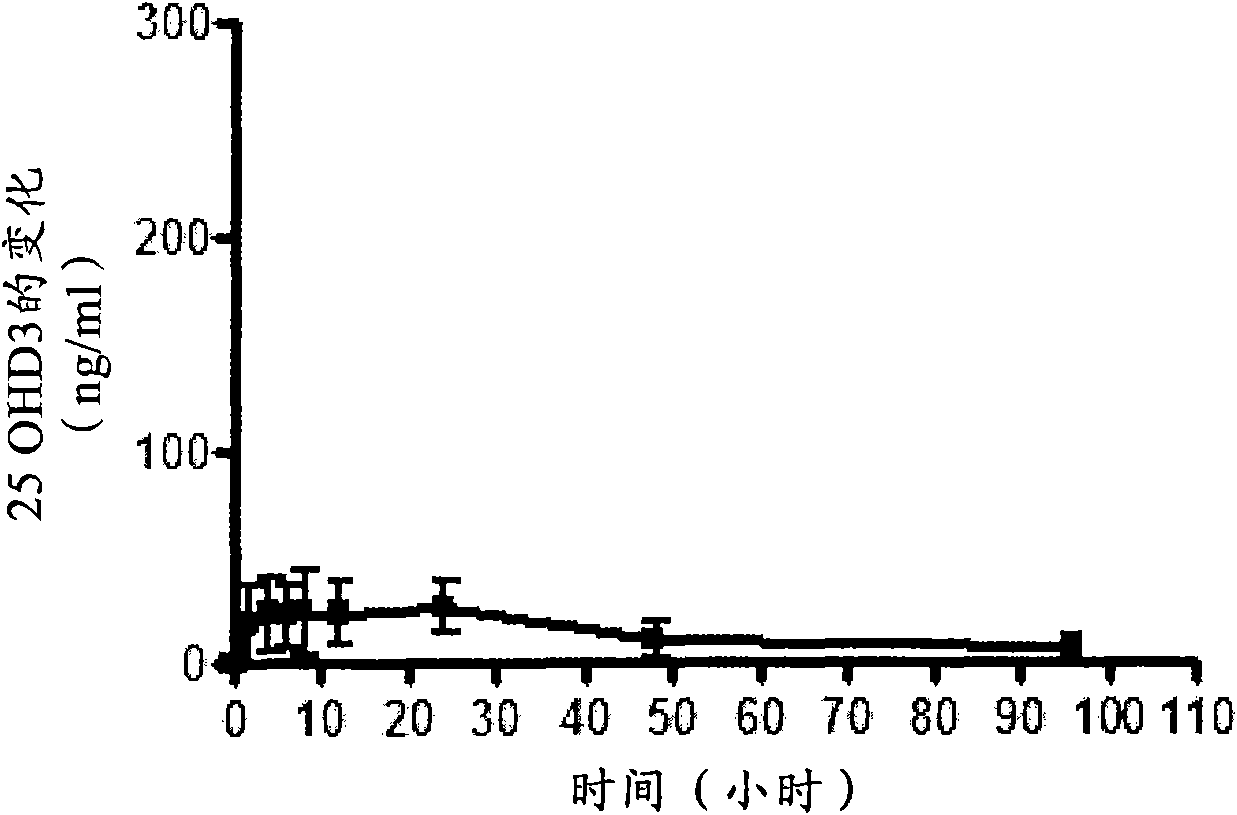
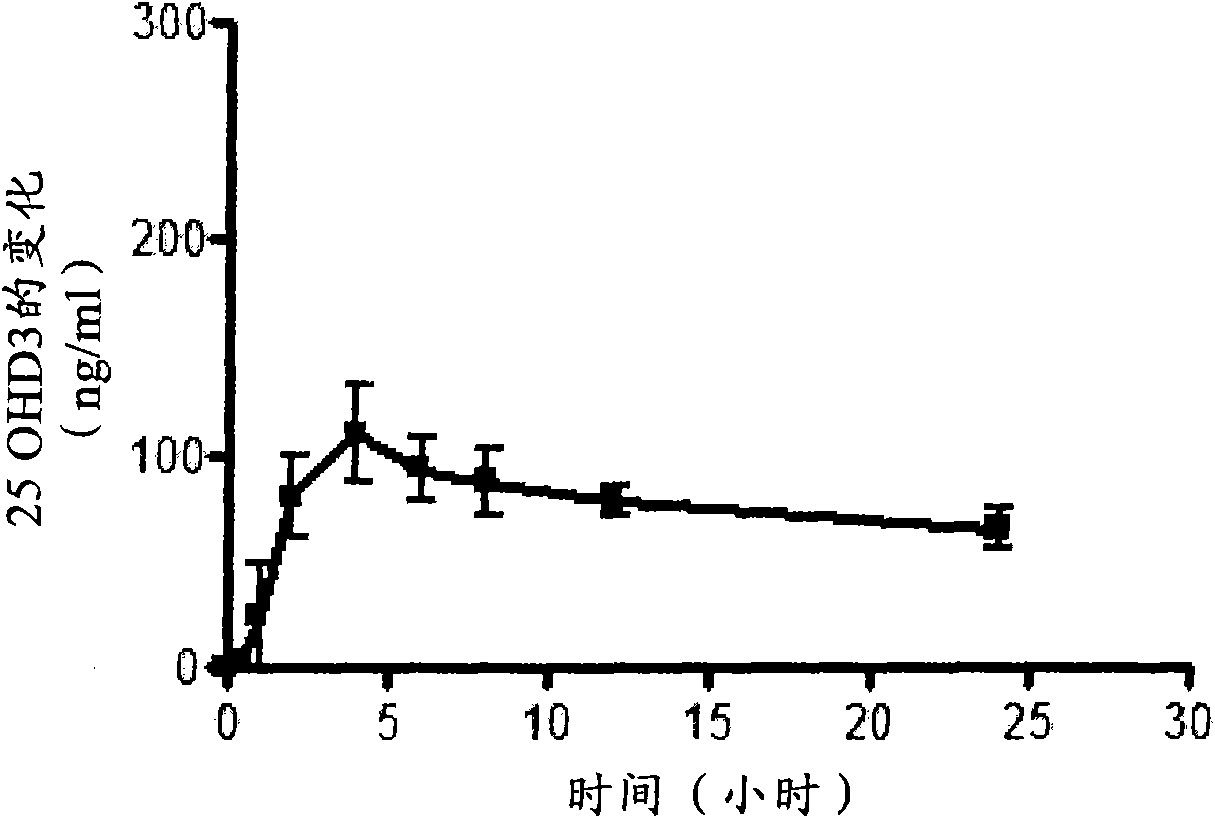
Oral controlled release compositions comprising vitamin d compound and waxy carrier
Owner:OPKO IP HLDG II INC +1
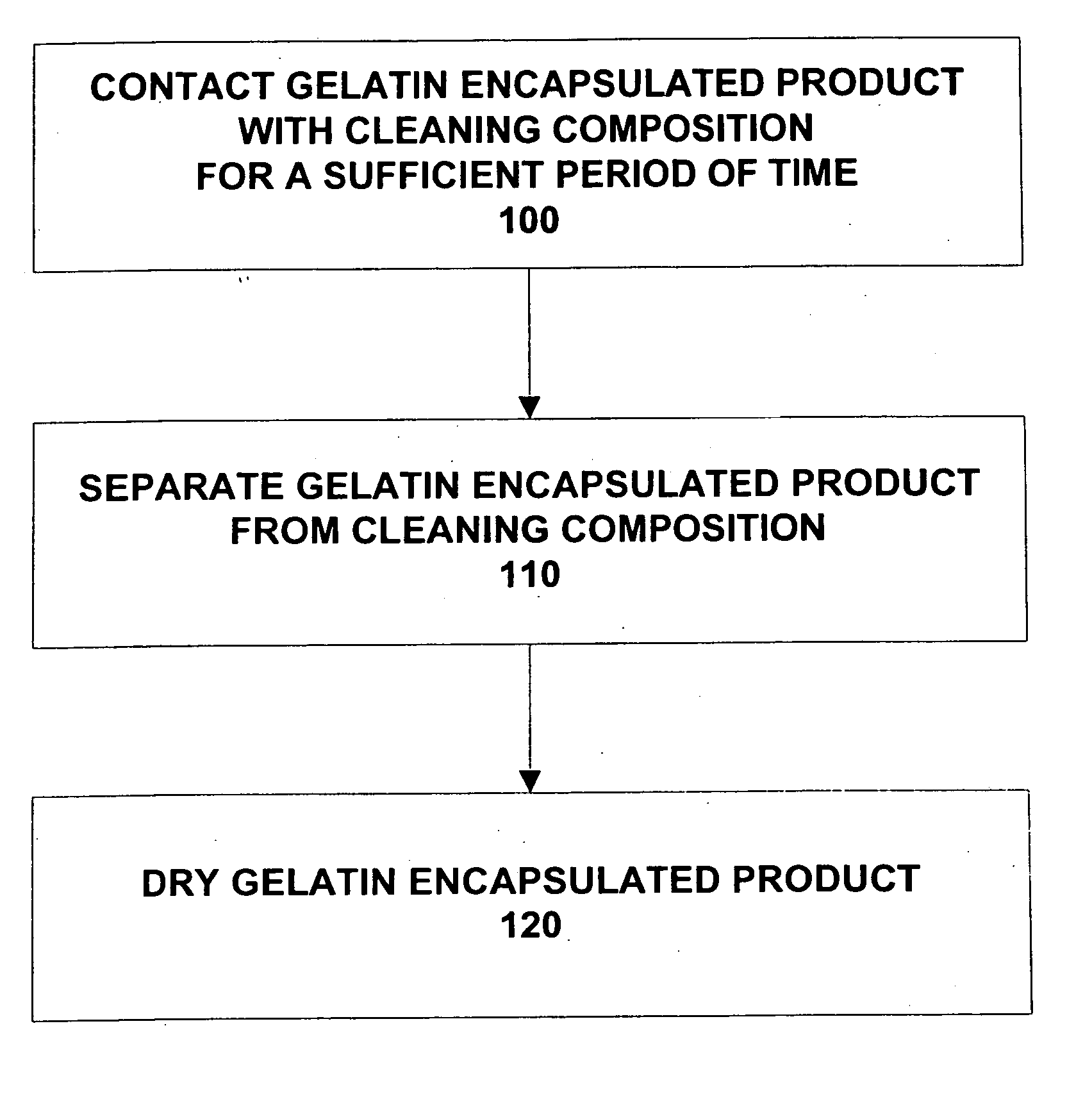
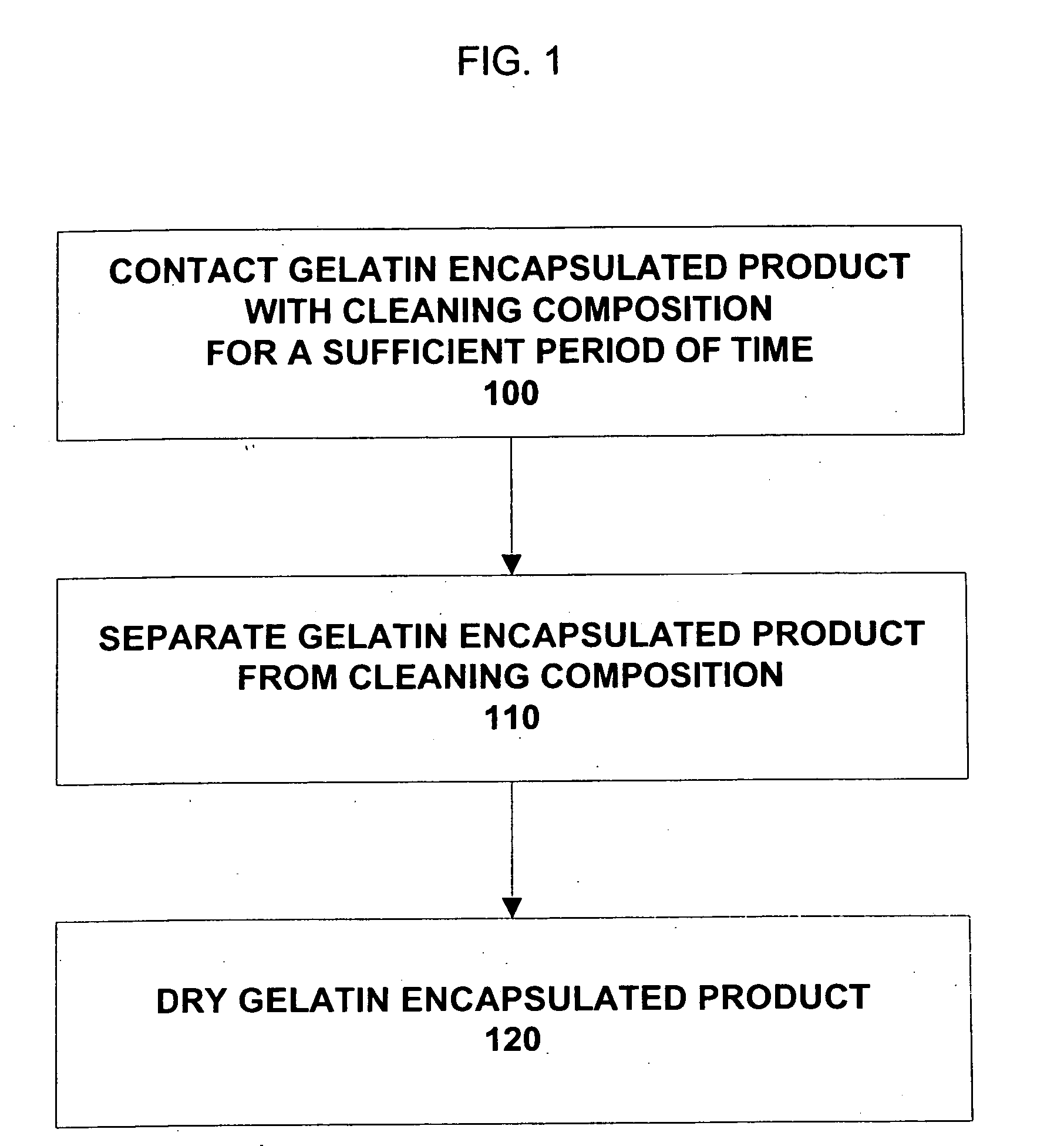
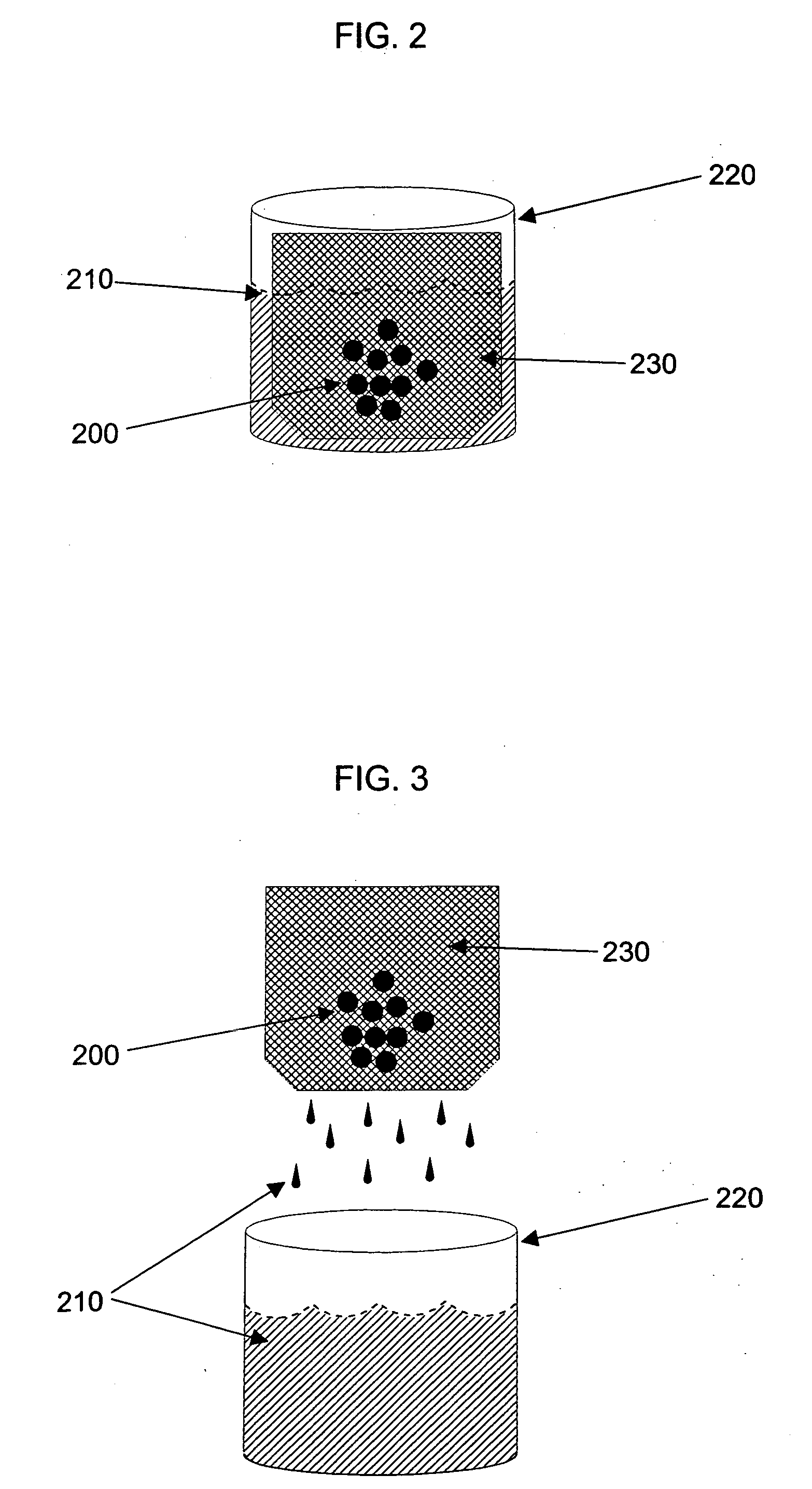
Composition and method for cleaning gelatin encapsulated products comprising comprising a non-volatile silicone/volatile silicone mixture
ActiveUS20050277574A1Increase the number ofPrevents excessive swelling of the gelatin outer shellInorganic/elemental detergent compounding agentsCosmetic preparationsAlcoholWater soluble
A composition and method is provided capable of removing contaminants from the surface of a gelatin capsule, such as a paint ball, to allow the gelatin capsules to be used for their intended use. The composition comprising, by weight, about 70 to 99.9 percent of a water-soluble alcohol, about 0.1 to 30 percent water, about 0.1 to 10 percent of a volatile silicone, and about 0.1 to 1 percent of a non-volatile silicone. Furthermore, the composition prevents excessive swelling of the gelatin outer shell of the gelatin encapsulated product. A method is also provided for cleaning a gelatin encapsulated product comprising the steps of contacting the gelatin encapsulated product with a cleaning composition, separating the gelatin encapsulated product from the cleaner, and drying the gelatin encapsulated product. The gelatin capsule may be a paintball. The composition may include additives to improve the performance of the gelatin capsules.
Owner:NIEDBALA CARL +1
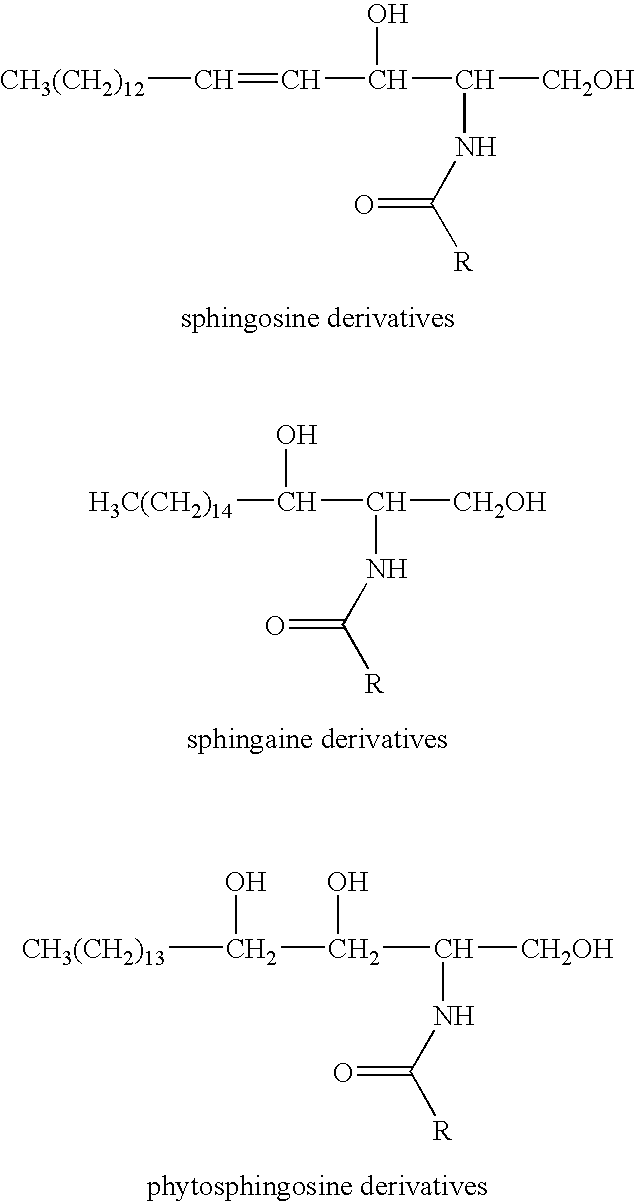
Gelatin capsules
InactiveUS20050058703A1Connective tissue peptidesPeptide/protein ingredientsGelatin filmGelatin capsule
The invention relates to recombinant gelatins, and to recombinant gelatins useful in gelatin capsule manufacture, and to compositions, gelatin capsules, and gelatin films comprising these, as well as methods of production.
Owner:FIBROGEN INC
Pharmaceutical Composition Comprising A Plurality of Mini-Tablets Comprising A Factor XA Inhibitor
A modified release pharmaceutical composition for oral administration comprising plural mini-tablets, comprising a therapeutically effective amount of a Factor Xa inhibitor within a matrix of polymer(s). The mini-tablets are suitably encapsulated within a gelatin capsule. A manufacturing process and method of use are also described.
Owner:GLAXO GROUP LTD
Stable capsule preparation
InactiveUS20070141137A1High mechanical strengthLow moisture stateBiocideOrganic chemistryPullulanWater soluble polysaccharides
A capsule in which an unstable active ingredient has been stabilized is obtained by lowering the moisture content of a solid preparation (granules, subtilis, tablets, etc.) containing a chemical unstable to moisture such as an imidazole PPI compound by drying or the like, and then filling in a capsule comprising a water-soluble polysaccharide such as pullulan as the main component or a PEG-containing gelatin capsule. For the further stabilization, the capsule per se may be dried. The capsule preparation thus obtained is a stable capsule preparation with the use of a capsule being stable at a low moisture content which contains a chemical unstable to moisture such as an imidazole compound.
Owner:TAKEDA PHARMA CO LTD
Traditional Chinese medicine capsule for repairing dysinsulinism function to balance blood sugar
InactiveCN101306171APromote blood circulationImprove nutritional statusMetabolism disorderCapsule deliveryAdditive ingredientRadix Rehmanniae Preparata
The invention relates to a Chinese traditional hypoglycemic medicine, in particular a thirty-ingredient hypoglycemic gelatin capsule, which is prepared from thirty ingredients, including red ginseng, astragali, ganoderma lucidum, magnolia vine fruit, kudzu root, radix salviae miltiorrhizae, hemlock parsley, symbranchoid eels, dodder, black sesame seed, radix rehmanniae, radix rehmanniae preparata, blackfungus and so on, through extraction.
Owner:王跃进
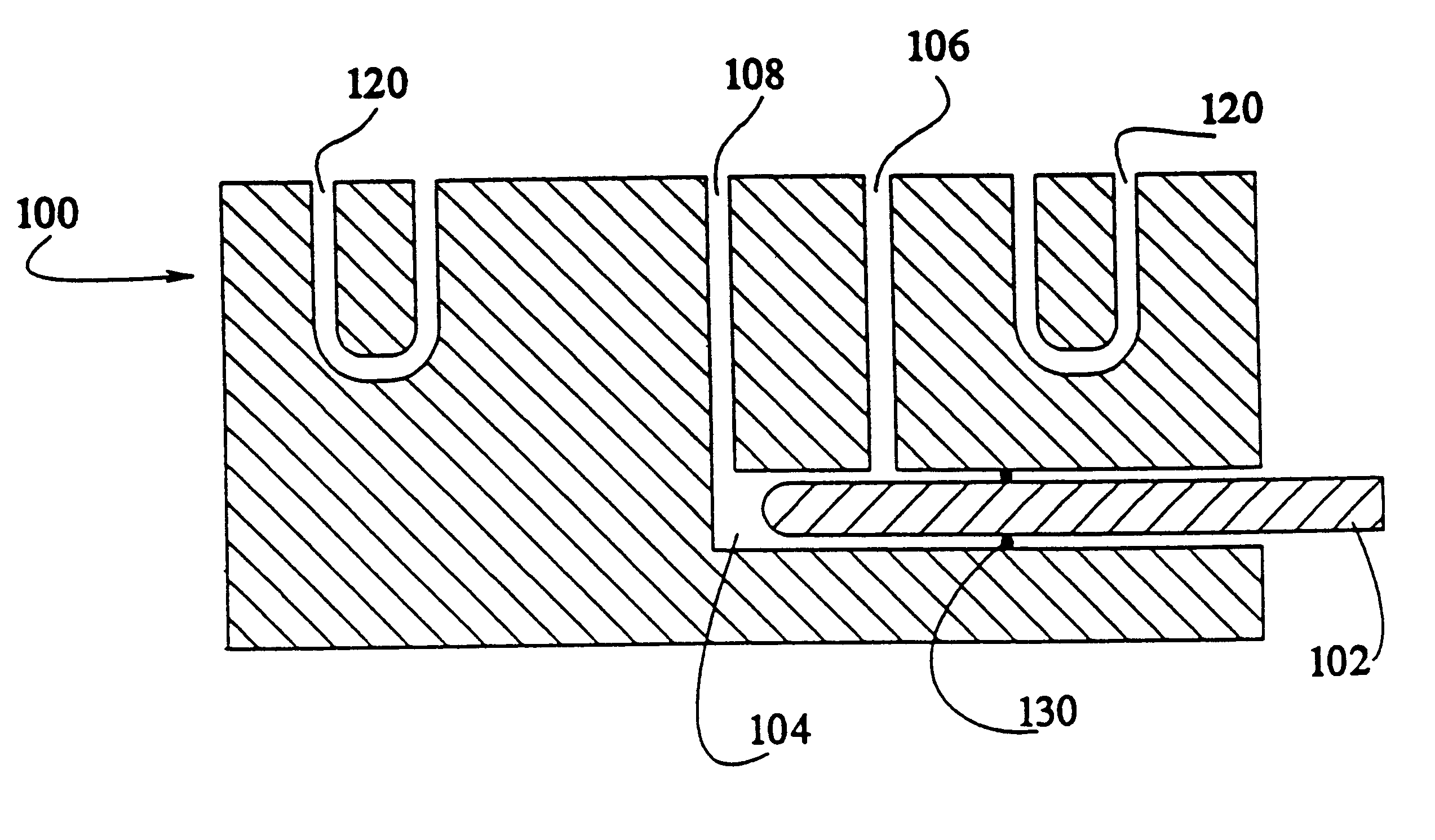
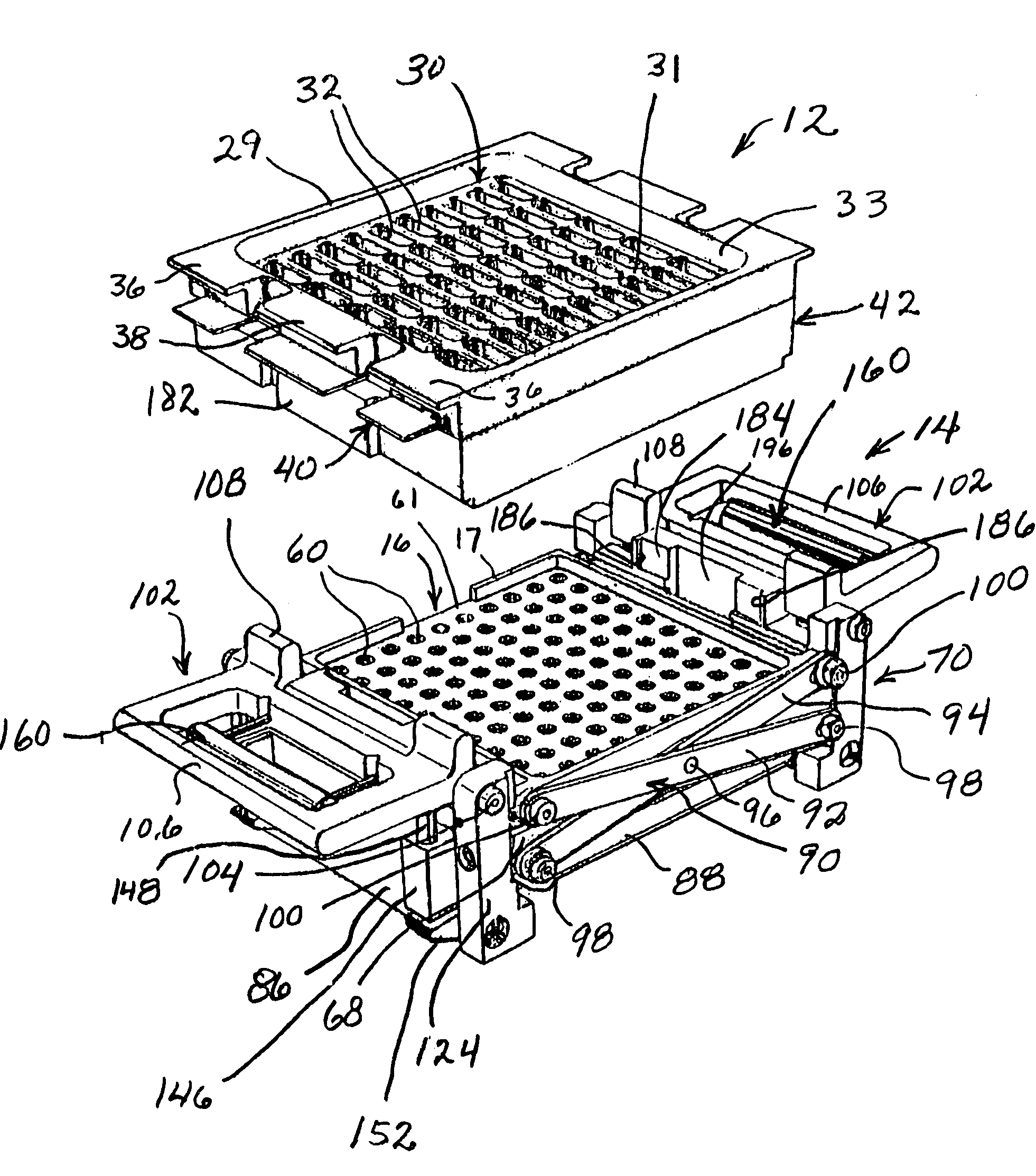
Manual capsule loading machine and method
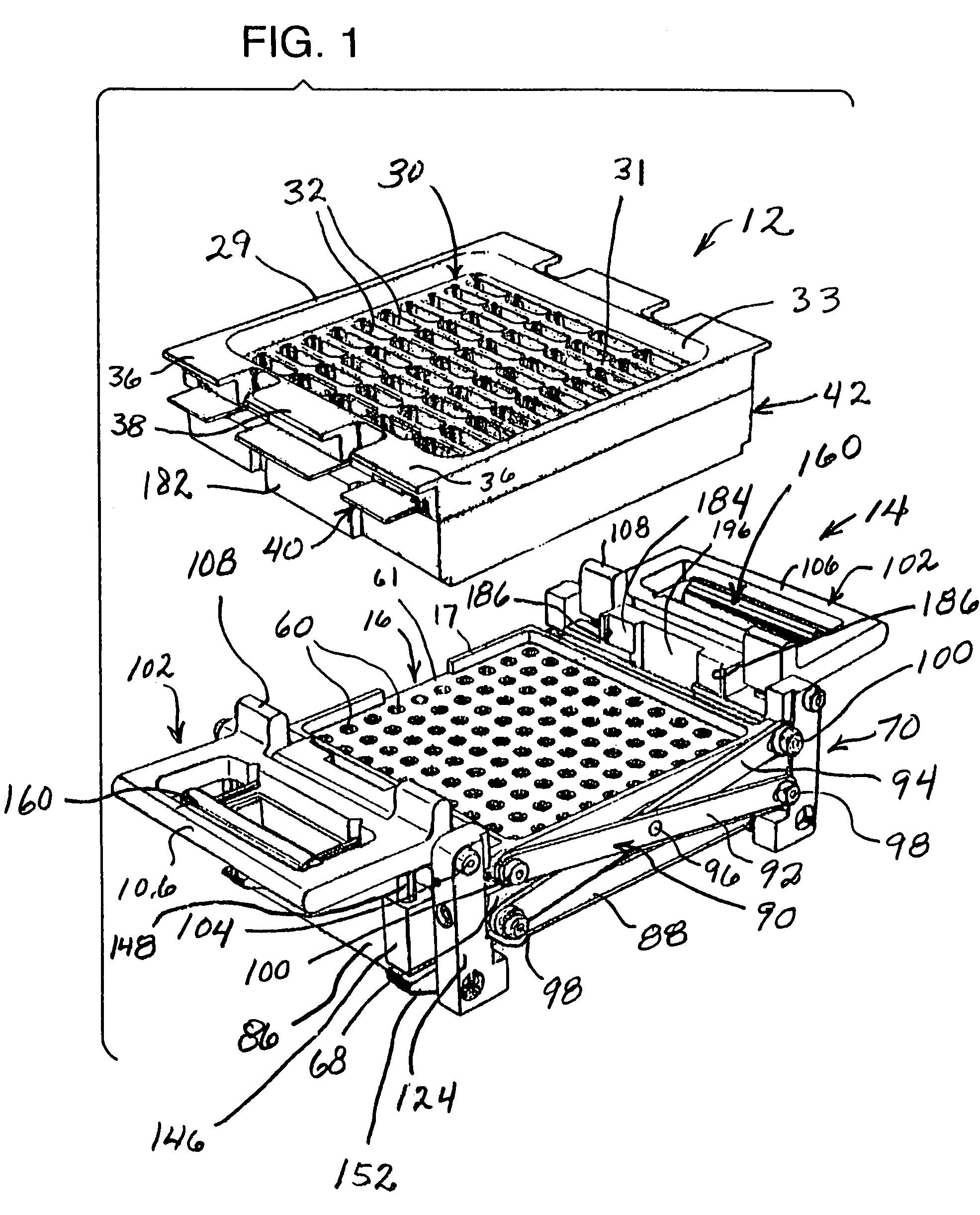
InactiveUS7337596B2Easy to operateQuick alignmentOpening closed containersCapsGelatin capsuleBiomedical engineering
A capsule orienting and separation apparatus is provided which allows a multiplicity of gelatin capsule shells to be uniformly oriented in a separable condition and for the caps of those shells to be separated from the capsule bodies. The apparatus allows a multiplicity of capsule shells all to be concurrently oriented in an upright disposition by a manually operated mechanism. The capsule caps are all uniformly oriented atop the capsule bodies and are exposed for gripping by a capsule cap lift assembly. The capsule caps are concurrently lifted from the capsule bodies while the capsule bodies remain in position in a matrix of openings in a capsule receiving tray. The capsules can then be filled with capsule filler material and tamped down as appropriate. The capsule caps are then all concurrently replaced on the capsule bodies and the capsule caps and capsule bodies are then concurrently permanently sealed together. All of the steps of orientation, filling, and sealing are performed with a manually operable mechanism.
Owner:BARRETT DENE
Method of sterilizing
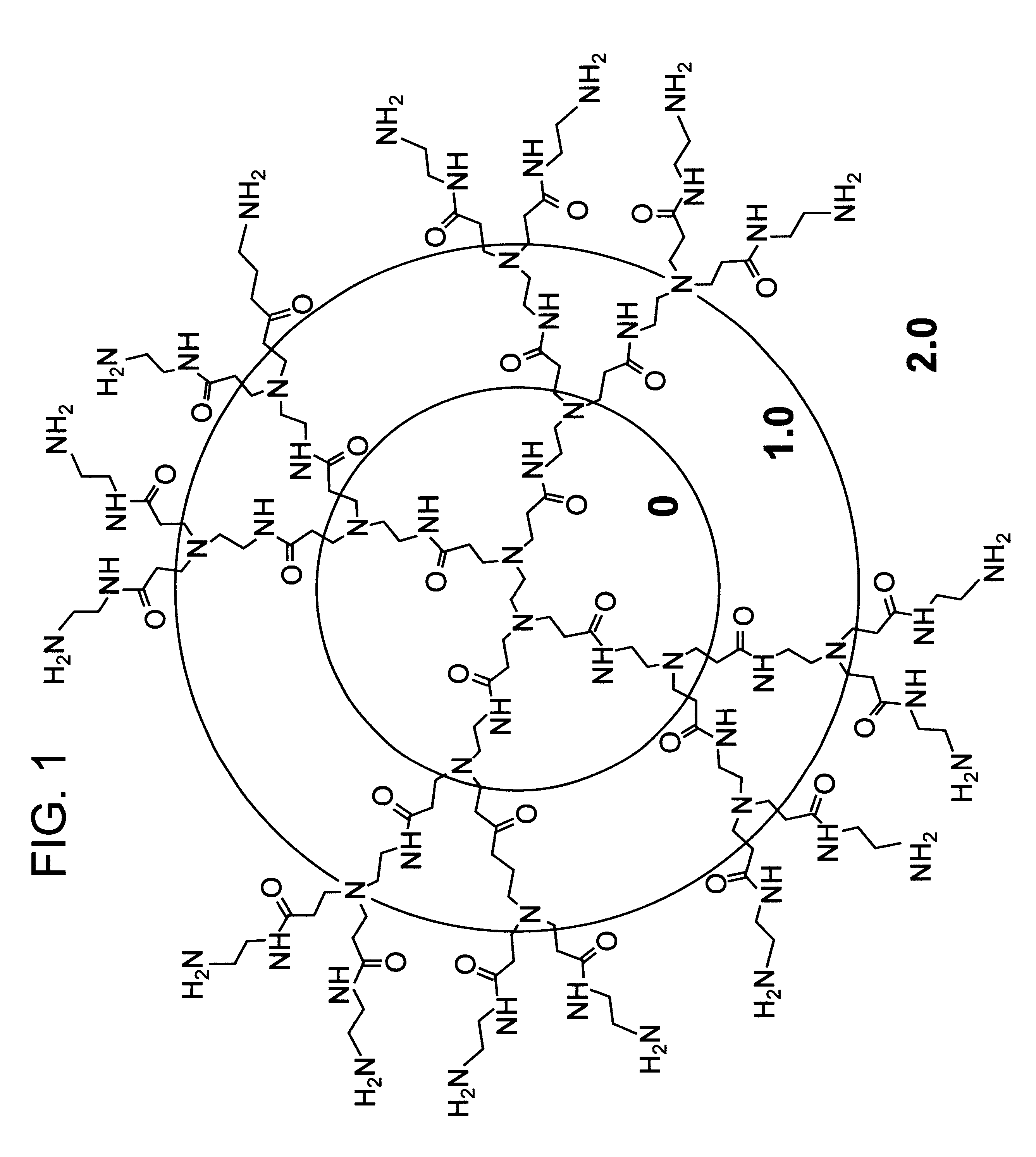
InactiveUS20020041862A1Reduce concentrationBiocideCosmetic preparationsDendrimerDisinfection methods
A method of sterilizing objects as well as the sterilized objects obtained from the method are disclosed. The method involves contacting an object such as a medical device to be reused with polycationic dendrimer under conditions which result in rendering a conformationally altered protein (e.g. a prion) non-infectious. A disinfecting agent or surgical scrub composition which comprises the dendrimers is also disclosed as are gelatin capsules treated with polycationic dendrimers.
Owner:RGT UNIV OF CALIFORNIA
Method of sterilizing
A method of sterilizing objects as well as the sterilized objects obtained from the method are disclosed. The method involves contacting an object such as a medical device to be reused with polycationic dendrimer under conditions which result in rendering a conformationally altered protein (e.g. a prion) non-infectious. A disinfecting agent or surgical scrub composition which comprises the dendrimers is also disclosed as are gelatin capsules treated with polycationic dendrimers.
Owner:RGT UNIV OF CALIFORNIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com