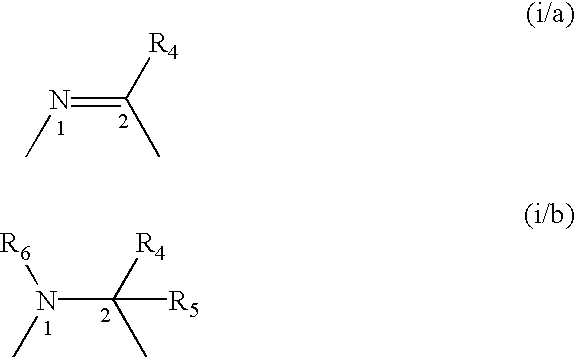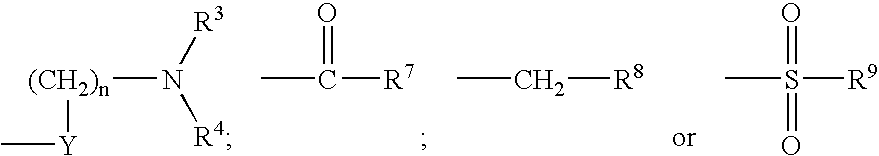Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
56 results about "Azabicyclo Compounds" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
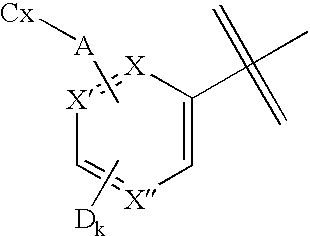
Bicyclic bridged compounds that contain a nitrogen which has three bonds. The nomenclature indicates the number of atoms in each path around the rings, such as [2.2.2] for three equal length paths. Some members are TROPANES and BETA LACTAMS.
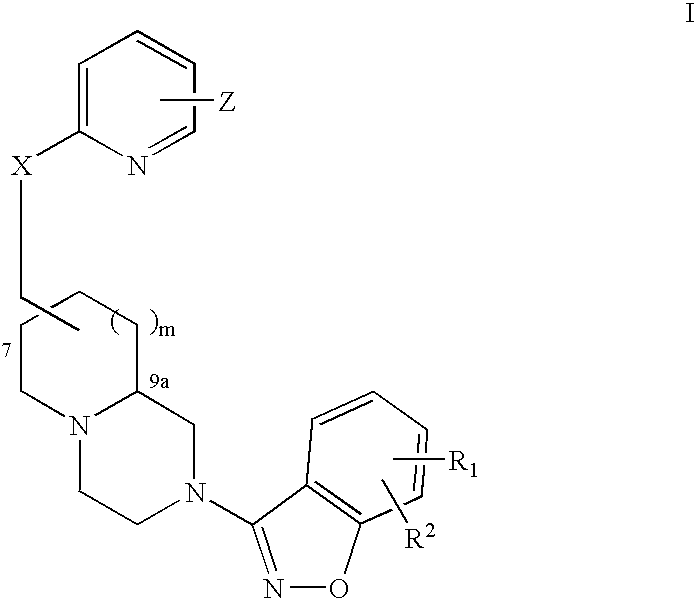
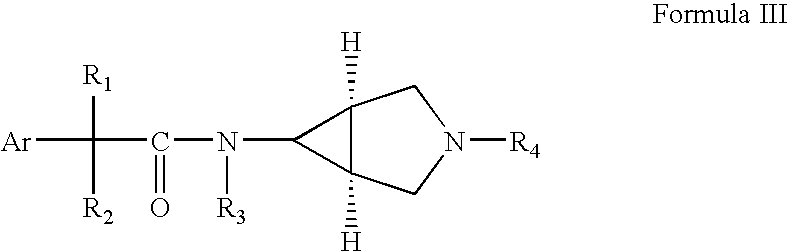
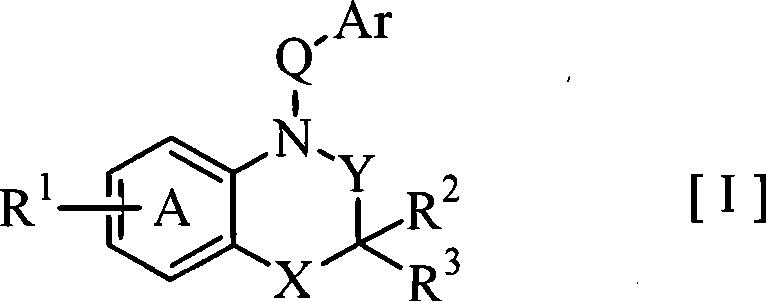
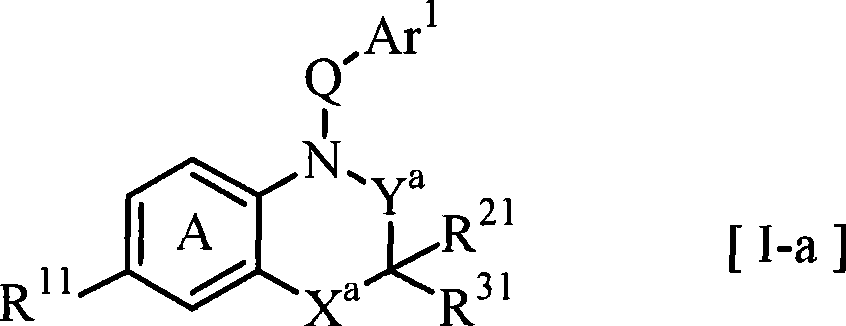
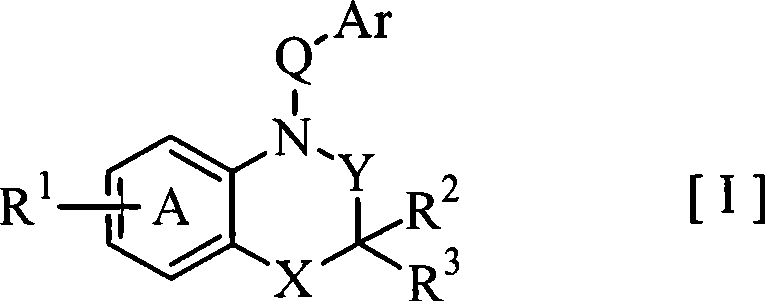
Fused azabicyclic compounds that inhibit vanilloid receptor subtype 1 (VR1) receptor
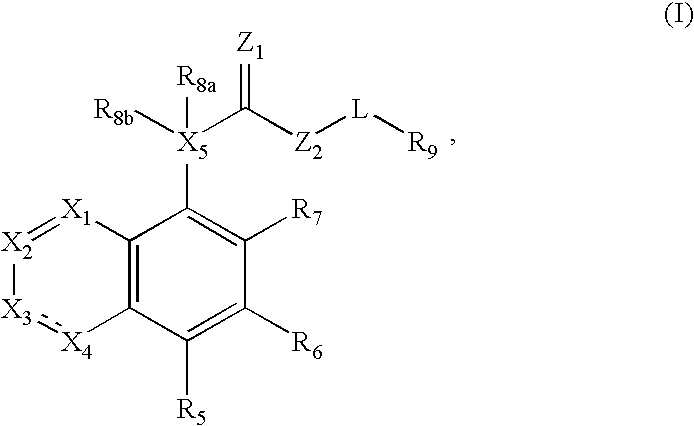
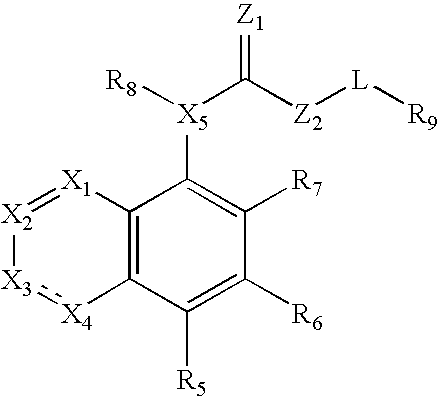
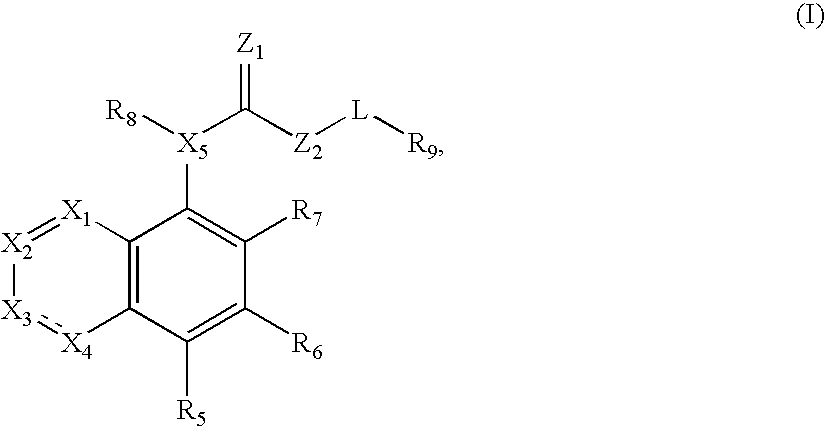
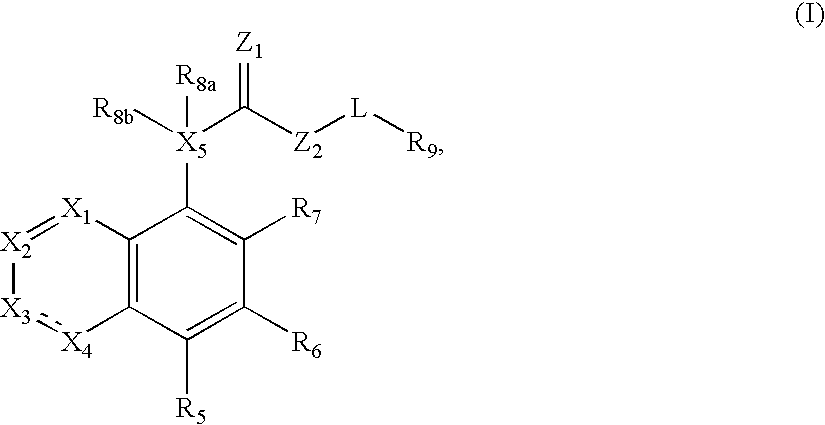
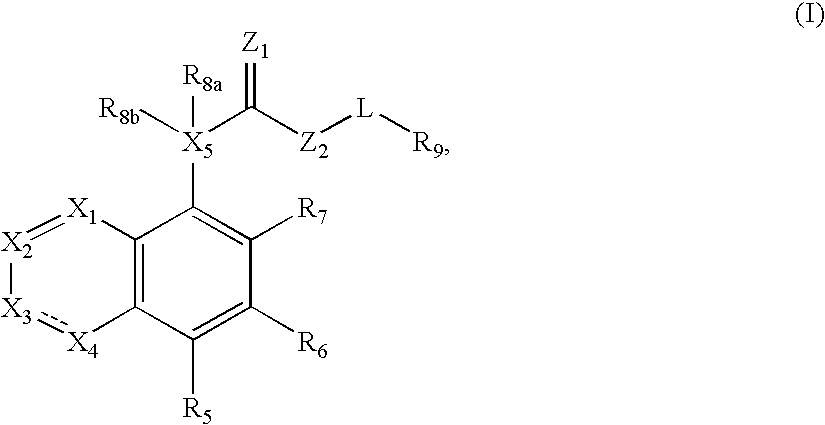
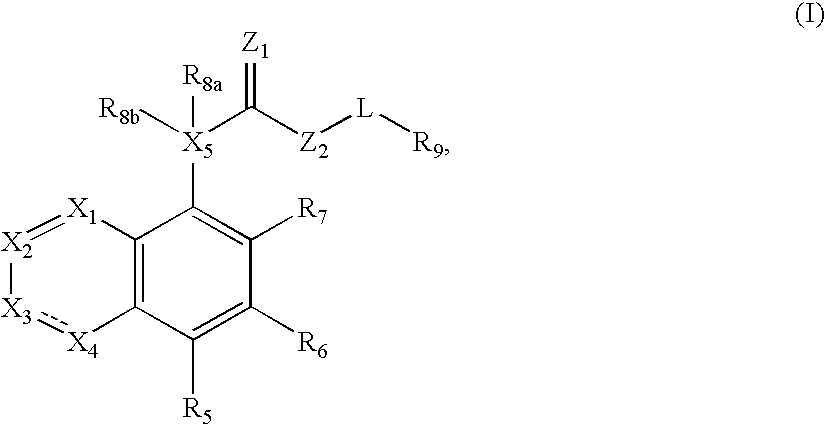
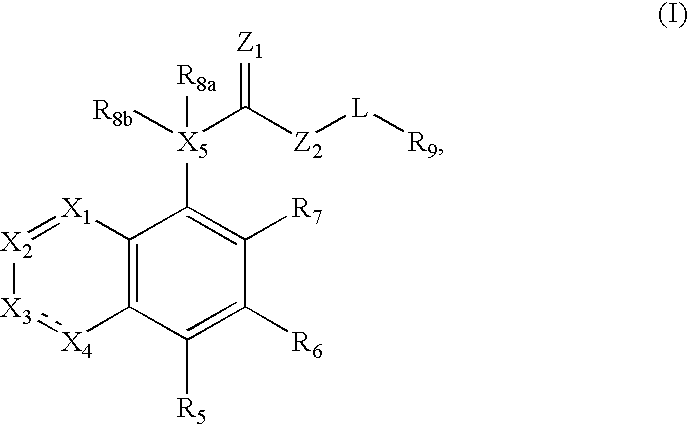
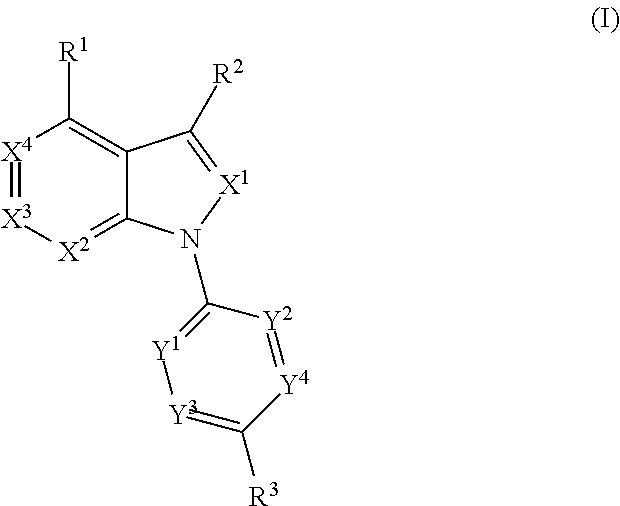
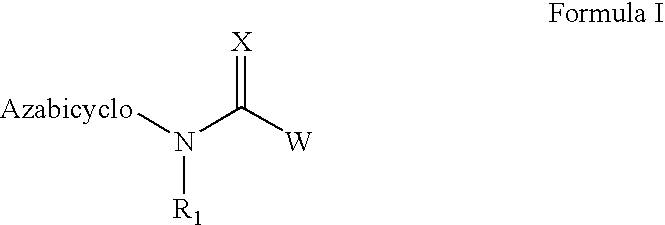
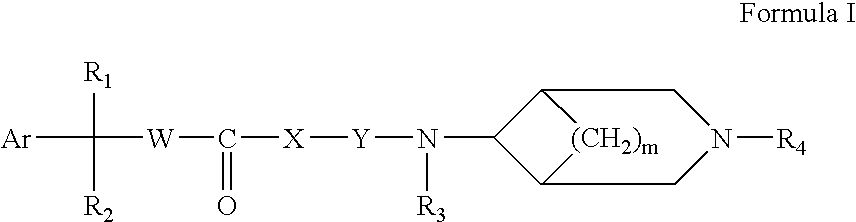
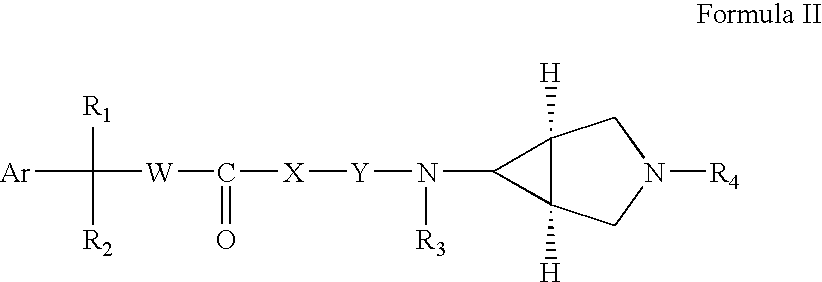
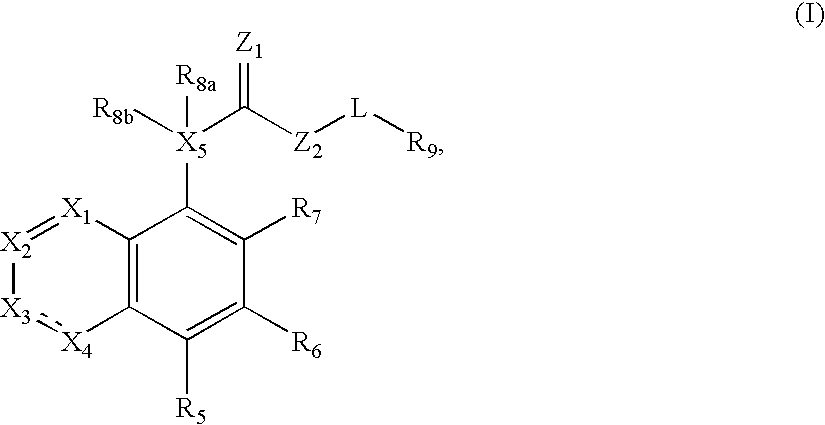
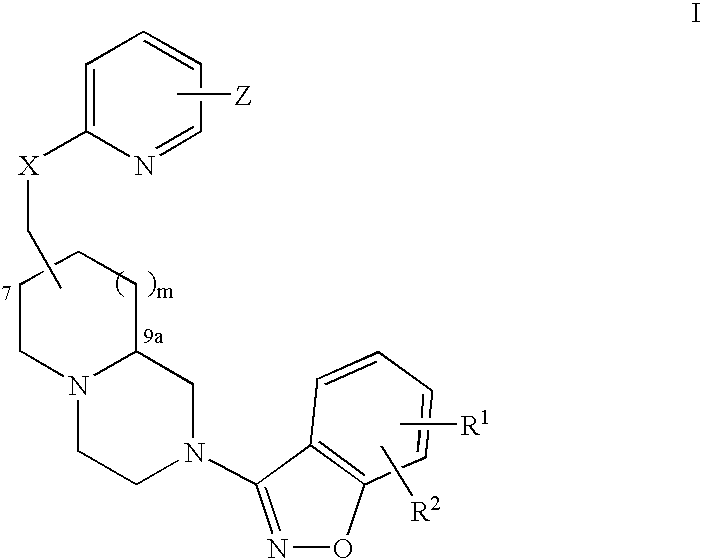
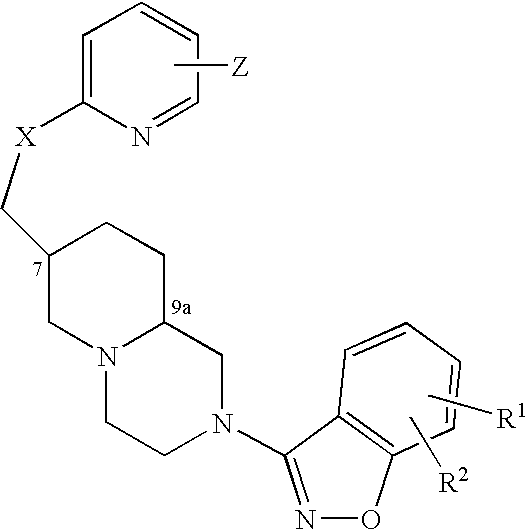
Compound of formula (I) are novel VR1 antagonist that are useful in treating pain, inflammatory thermal hyperalgesia, urinary incontinence and bladder overactivity.
Owner:ABBVIE INC
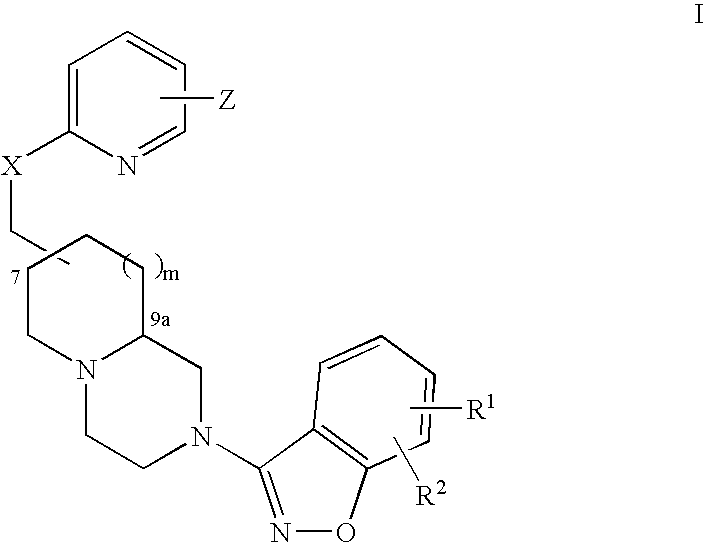
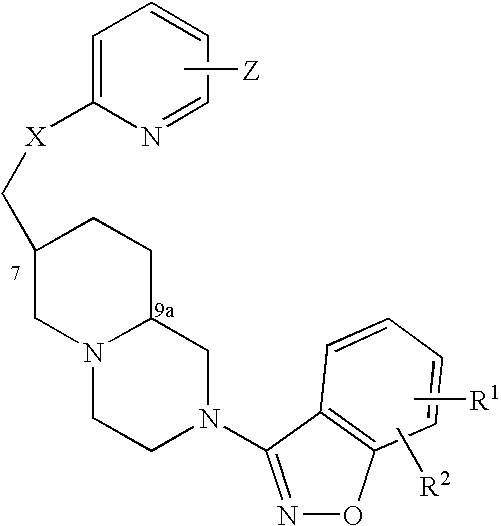
Azabicyclic compounds for the treatment of disease
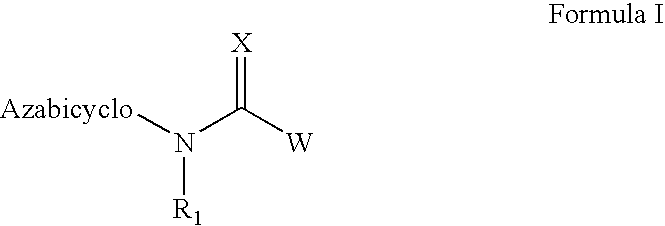
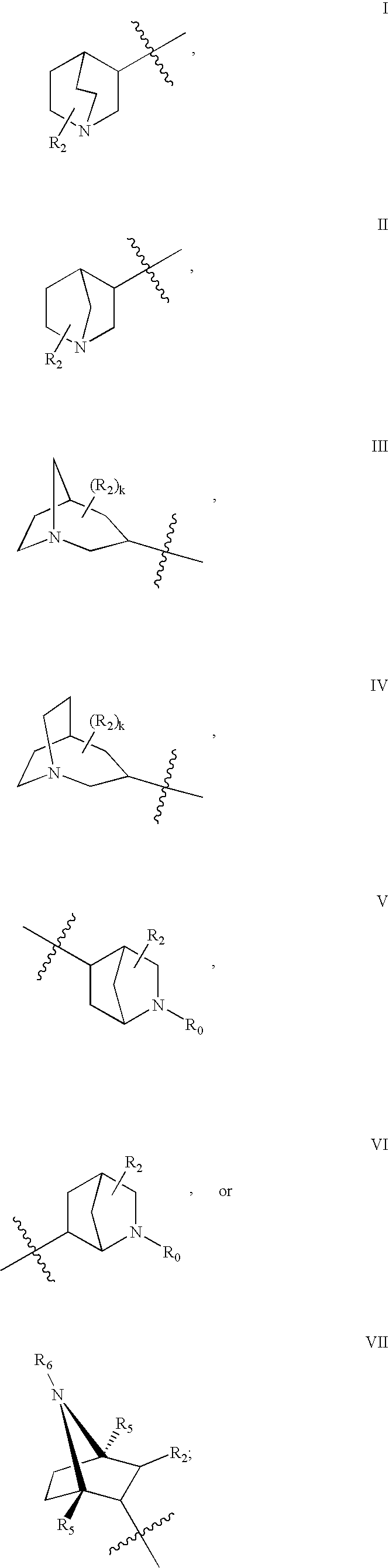
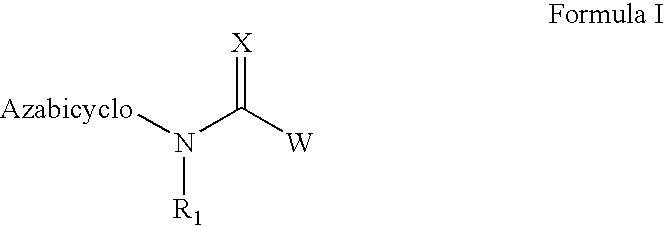
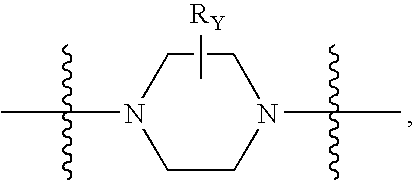
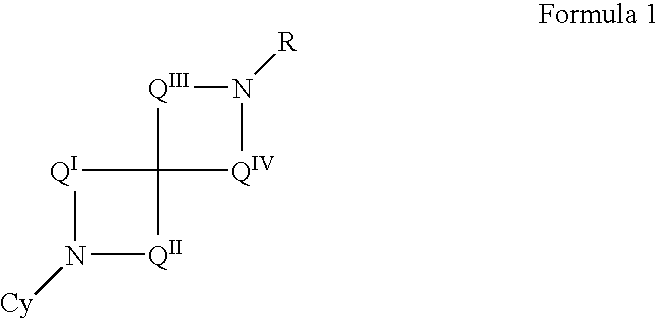
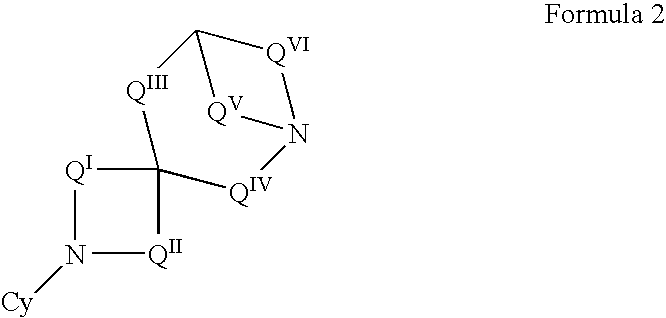
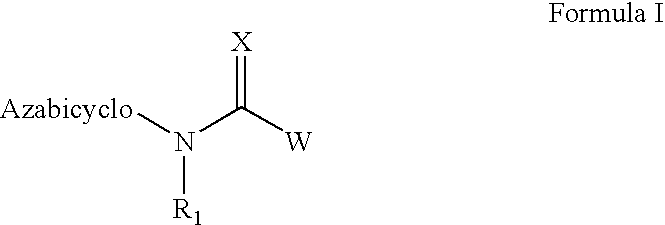
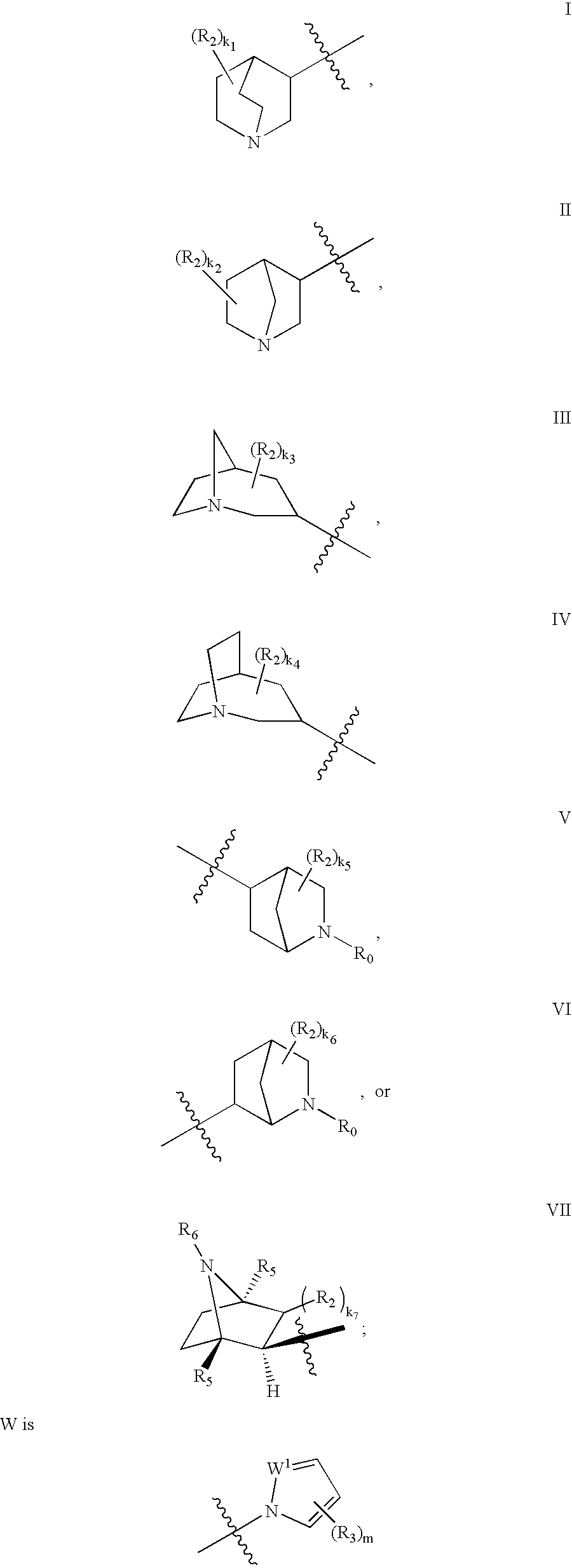
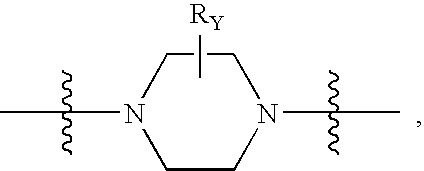
The invention provides compounds of Formula I: wherein Azabicyclo is W is a six-membered heterocyclic ring system having 1–2 nitrogen atoms or a 10-membered bicyclic-six-six-fused-ring system having up to two nitrogen atoms within either or both rings, provided that no nitrogen is at a bridge of the bicyclic-six-six-fused-ring system, and further having 1–2 substitutents independently selected from R3.These compounds may be in the form of pharmaceutical salts or compositions, may be in pure enantiomeric form or racemic mixtures, and are useful in pharmaceuticals to treat diseases or conditions in which α7 is known to be involved.
Owner:PHARMACIA & UPJOHN CO
Fused azabicyclic compounds that inhibit vanilloid receptor subtype 1 (VR1) receptor
Compounds of formula (I) are novel VR1 antagonists that are useful in treating pain, inflammatory thermal hyperalgesia, urinary incontinence and bladder overactivity.
Owner:ABBOTT LAB INC
Fused azabicyclic compounds that inhibit vanilloid receptor subtype 1 (VR1) receptor
Compounds of formula (I) are novel VR1 antagonists that are useful in treating pain, inflammatory thermal hyperalgesia, urinary incontinence and bladder overactivity.
Owner:ABBVIE INC
Azabicyclic compounds are central nervous system active agents
Compounds of formula (I)are novel CNS active agents that are useful for treating pain and for treating other disorders associated with the cholinergic system.
Owner:ABBVIE INC
Fused azabicyclic compounds that inhibit vanilloid receptor subtype 1 (VR1) receptor
InactiveUS20050113576A1Utility in overactivityUtility in treating painOrganic chemistryAzabicyclo CompoundsThermal Hyperalgesia
Compounds of formula (I) are novel VR1 antagonists that are useful in treating pain, inflammatory thermal hyperalgesia, urinary incontinence and bladder overactivity.
Owner:ABBOTT LAB INC
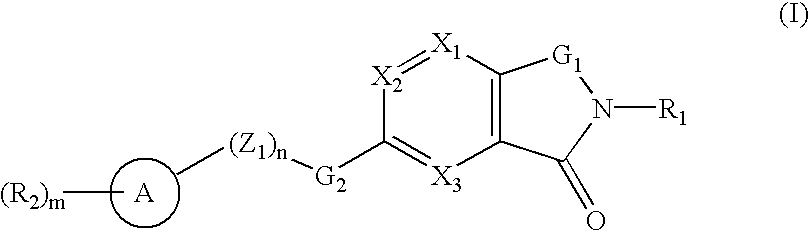
Oxo-azabicyclic compounds
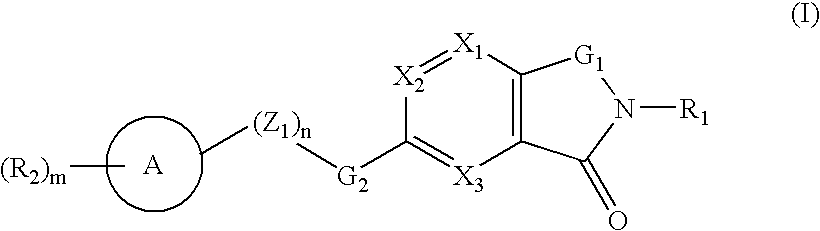
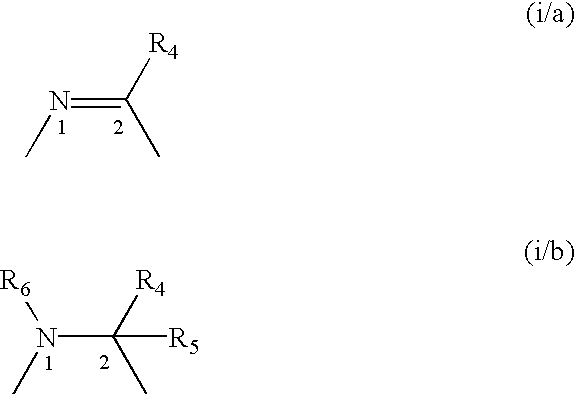
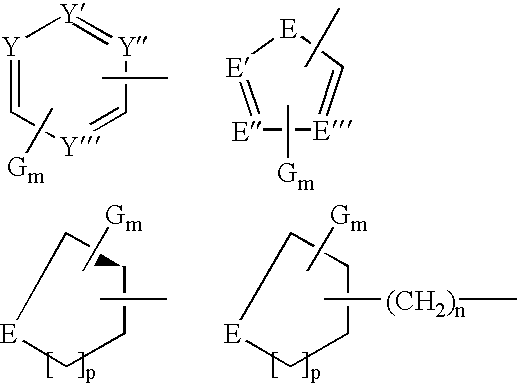
A compound selected from those of formula (I): wherein: X1, X2, and X3, represent N or -CR3 in which R3 is as described in the description, G1 represents a group selected from those of formulae (i / a) and (i / b): in which R4, R5, and R6 are as defined in the description, G2 represents a group selected from carbon-carbon triple bond, -CH=C=CH-, C=O, C=S, S(O)n1 in which n1 represents an integer from 0 to 2 inclusive, or a group of formula (i / c): in which Y1 represents O, S, -NH or -Nalkyl, and Y2 represents O, S, -NH or -Nalkyl, n is an integer from 0 to 6 inclusive, and m is an integer from 0 to 7 inclusive, Z1 represents -CR9R10, wherein R9 and R10 are as defined in the description, A represents a ring system, R1 represents a group selected from H, alkyl, alkenyl, alkynyl, optionally substituted and the group of formula (i / d): in which p, Z2, B, q and G3 are as defined in the description and optionally, its optical isomers, N-oxide, and addition salts thereof with a pharmaceutically-acceptable acid or base, and medicinal products containing the same are useful as specific inhibitors of type-13 matrix mettaloprotease.
Owner:WARNER-LAMBERT CO
Compositions for treating CNS disorders
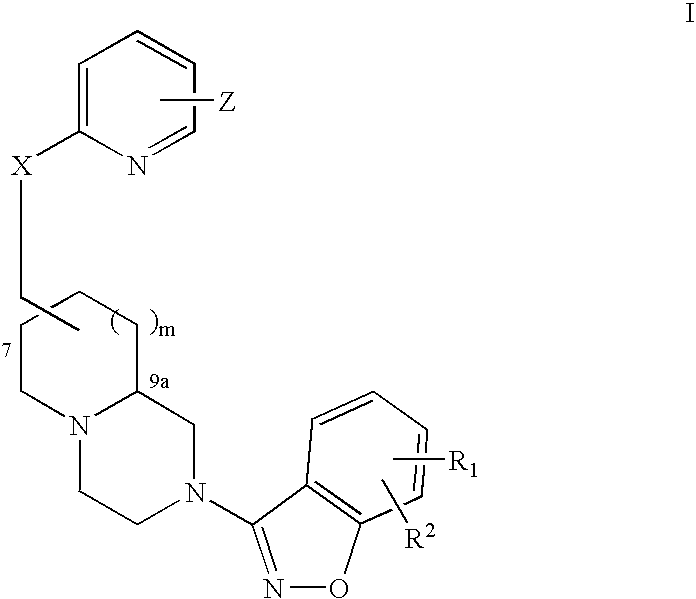
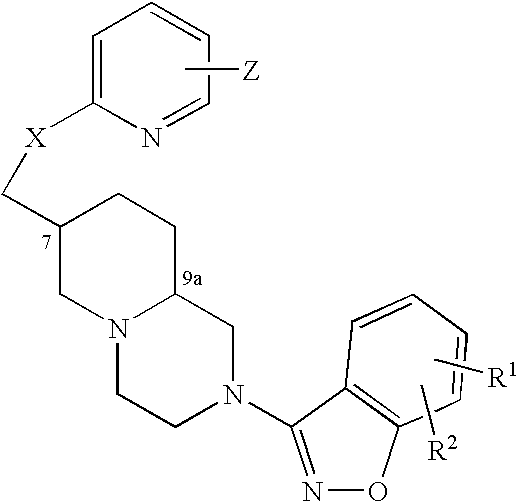
An aminomethylpyridyloxymethyl / benzisoxazole substituted azabicyclic compound, a pharmaceutical composition comprising same, and a method of treating one or more CNS or other disorders, including concurrent treatment of disorders such as schizophrenia and depression.
Owner:PFIZER INC
Oxo-azabicyclic compounds
A compound selected from those of formula (I): wherein: X1, X2, and X3, represent N or -CR3 in which R3 is as described in the description, G1 represents a group selected from those of formulae (i / a) and (i / b): in which R4, R5, and R6 are as defined in the description, G2 represents a group selected from carbon-carbon triple bond, -CH=C=CH-, C=O, C=S, S(O)n1 in which n1 represents an integer from 0 to 2 inclusive, or a group of formula (i / c): in which Y1 represents O, S, -NH or -Nalkyl, and Y2 represents O, S, -NH or -Nalkyl, n is an integer from 0 to 6 inclusive, and m is an integer from 0 to 7 inclusive, Z1 represents -CR9R10 wherein R9 and R10 are as defined in the description, A represents a ring system, R1 represents a group selected from H, alkyl, alkenyl, alkynyl, optionally substituted and the group of formula (i / d): in which p, Z2, B, q and G3 are as defined in the description and optionally, its optical isomers, N-oxide, and addition salts thereof with a pharmaceutically-acceptable acid or base, and medicinal products containing the same are useful as specific inhibitors of type-13 matrix mettaloprotease.
Owner:WARNER-LAMBERT CO
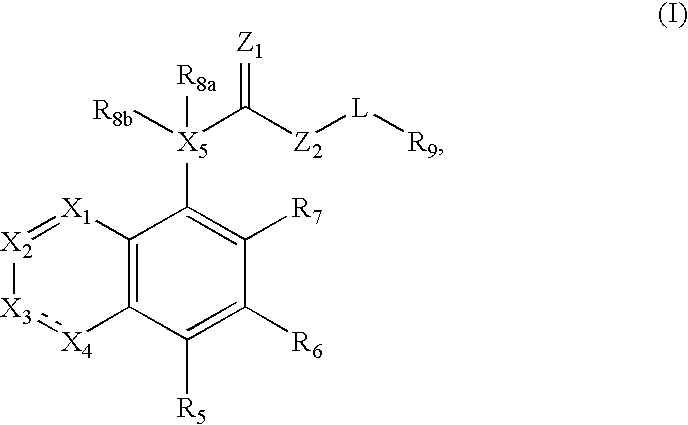
Fused azabicyclic compounds that inhibit vanilloid receptor subtype 1 (VR1) receptor
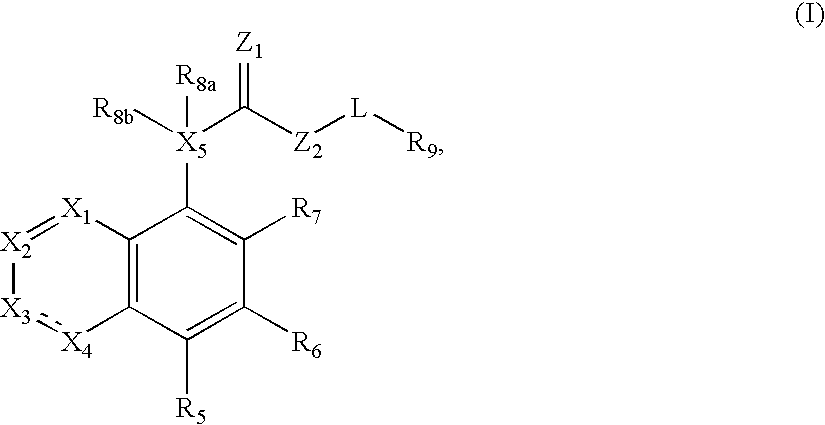
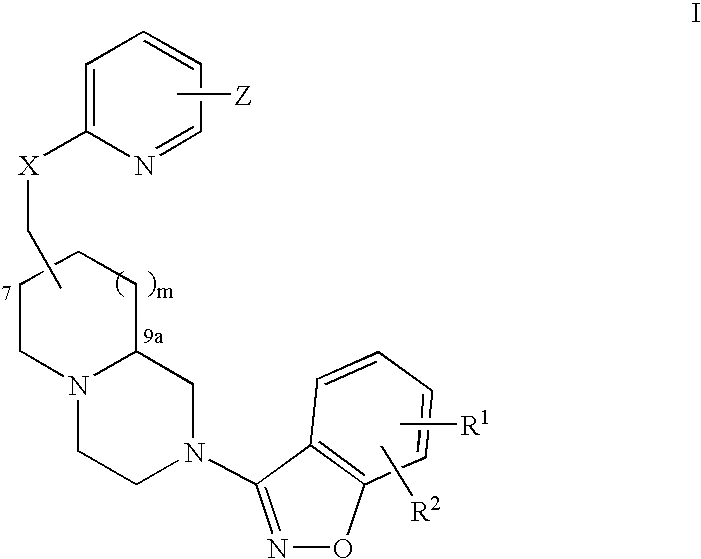
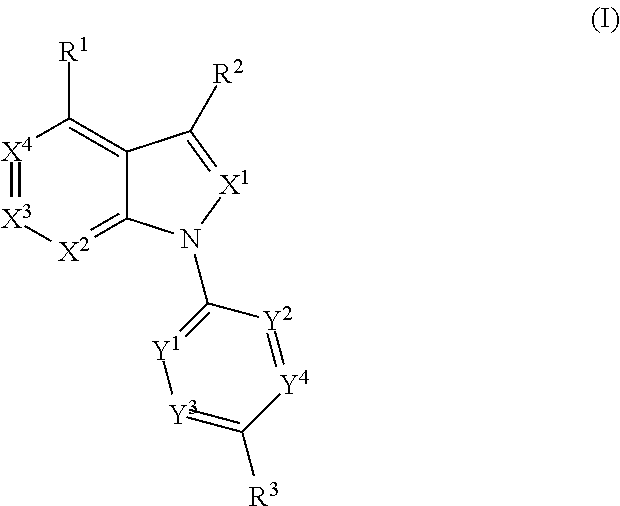
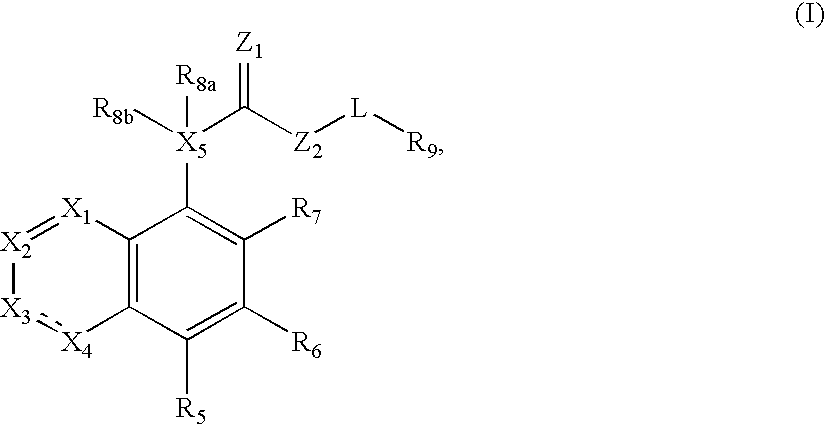
Compounds of formula (I) are novel VR1 antagonists that are useful in treating pain, inflammatory thermal hyperalgesia, urinary incontinence and bladder overactivity, wherein X1, X2, X3, X4, X5, R5, R6, R7, R8a, R8b, R9, Z1, Z2 and L are as defined in the description.
Owner:ABBVIE INC
Pyridyloxymethyl and benzisoxazole azabicyclic derivatives
Owner:PFIZER INC
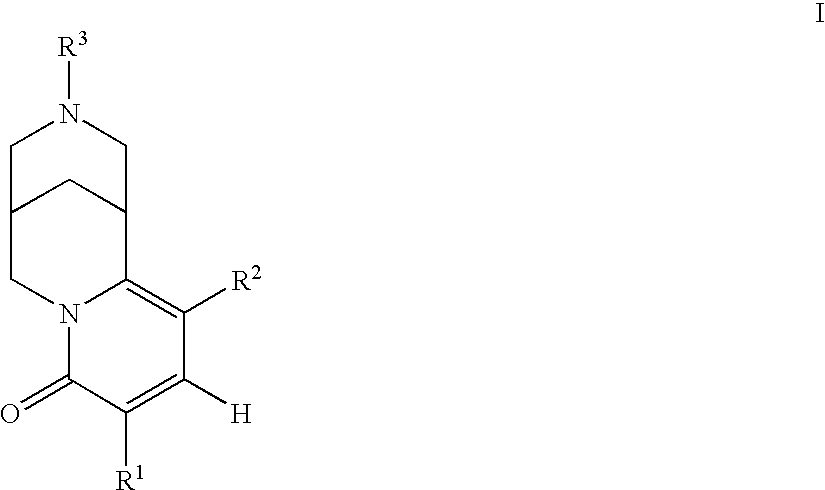
Azabicyclo compound and salt thereof
ActiveUS20120108589A1Excellent HSP9 inhibitory activityStrong inhibitory activityBiocideOrganic chemistryAzabicyclo CompoundsHeteroatom
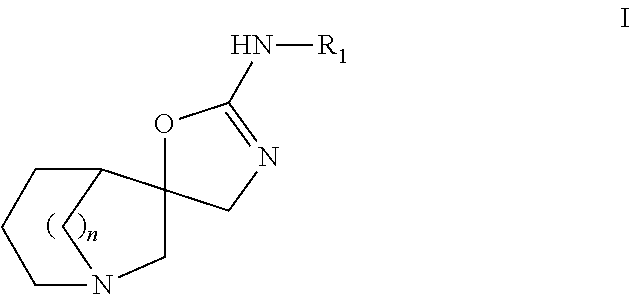
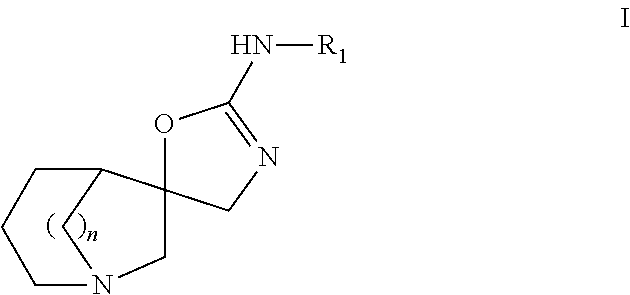
It is intended to provide a novel azabicyclo compound which exhibits both HSP90 inhibitory activity and cell proliferation inhibitory effect. Specifically disclosed is a compound represented by the following general formula (I) or a salt thereof: wherein X1 represents CH or N; any one of X2, X3 and X4 represents N, and the others represent CH; any one or two of Y1, Y2, Y3 and Y4 represent C—R4, and the others are the same or different and represent CH or N; R1 represents an optionally substituted monocyclic or bicyclic unsaturated heterocyclic group having 1 to 4 heteroatoms selected from N, S and O; R2 represents an alkyl group having 1 to 6 carbon atoms, or the like; and R3 and R4 represent —CO—R5 or the like.
Owner:TAIHO PHARMA CO LTD
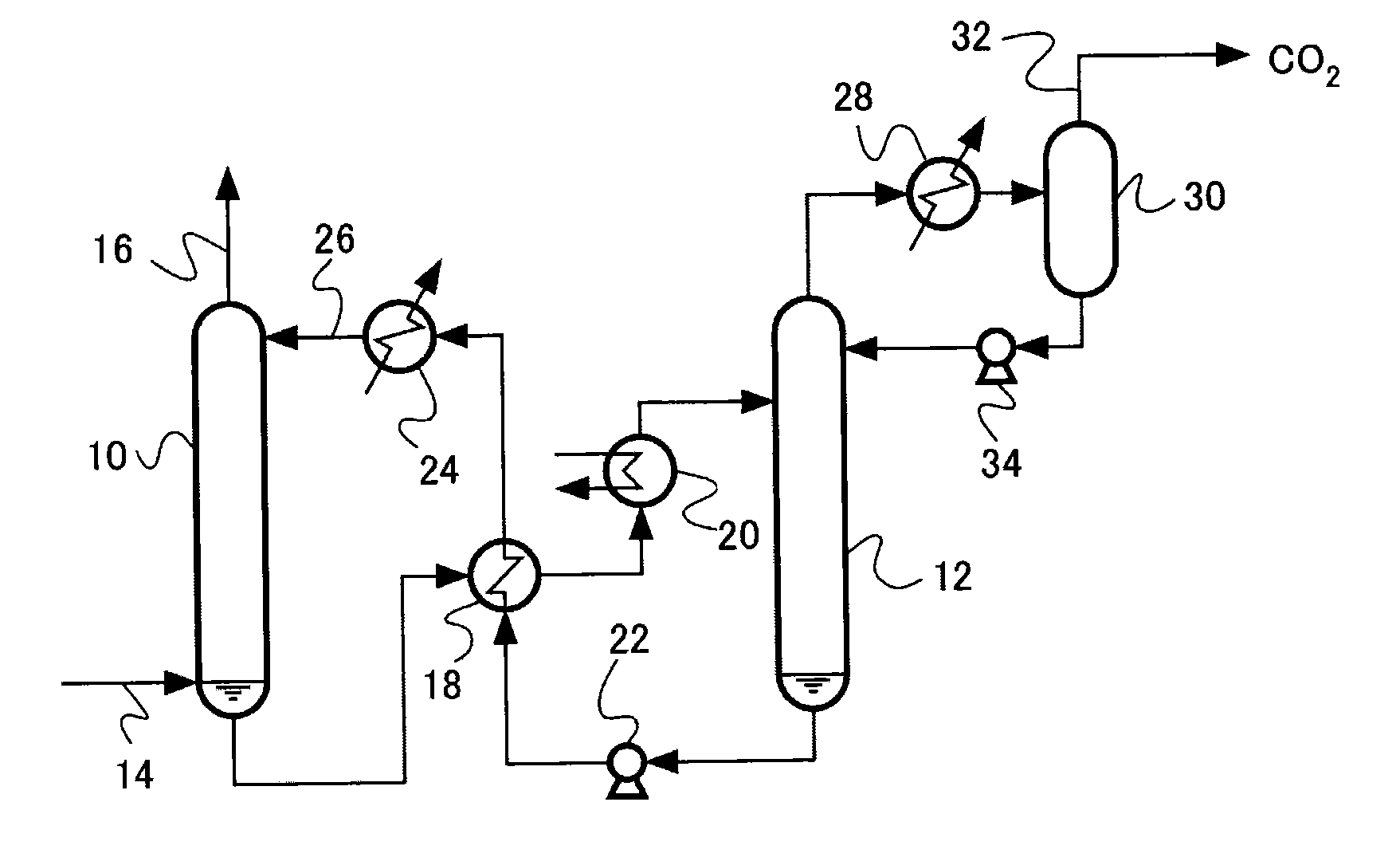
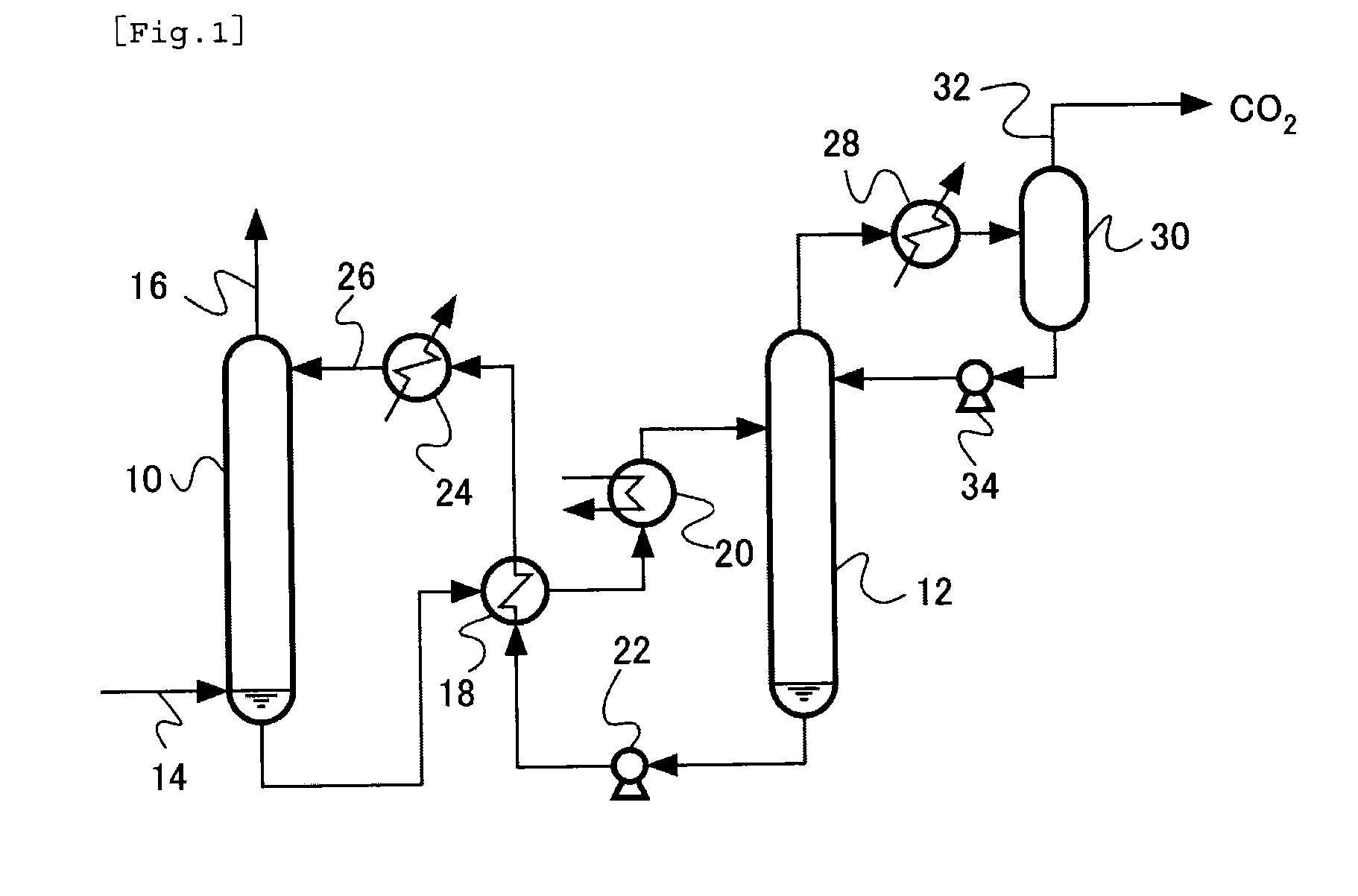
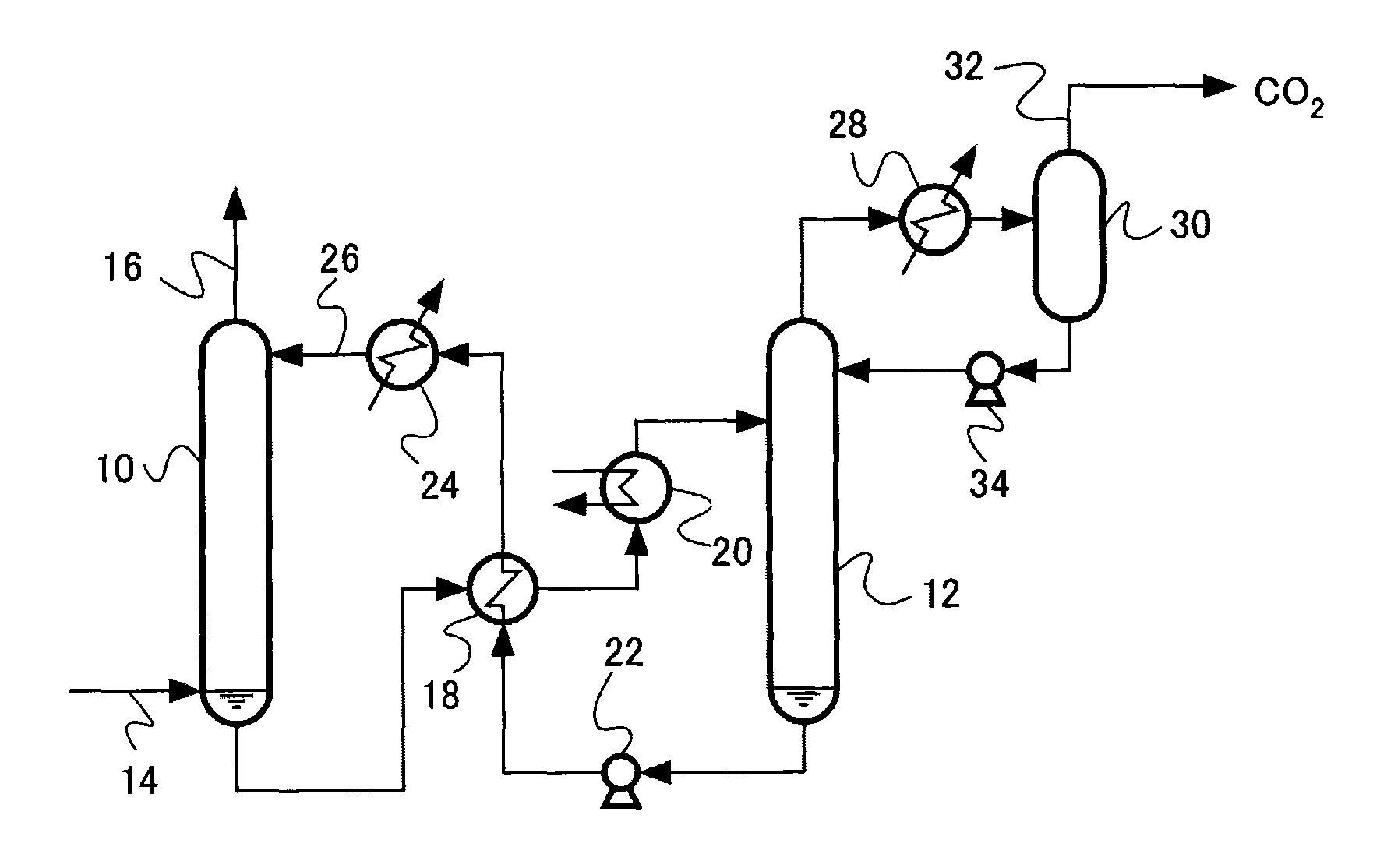
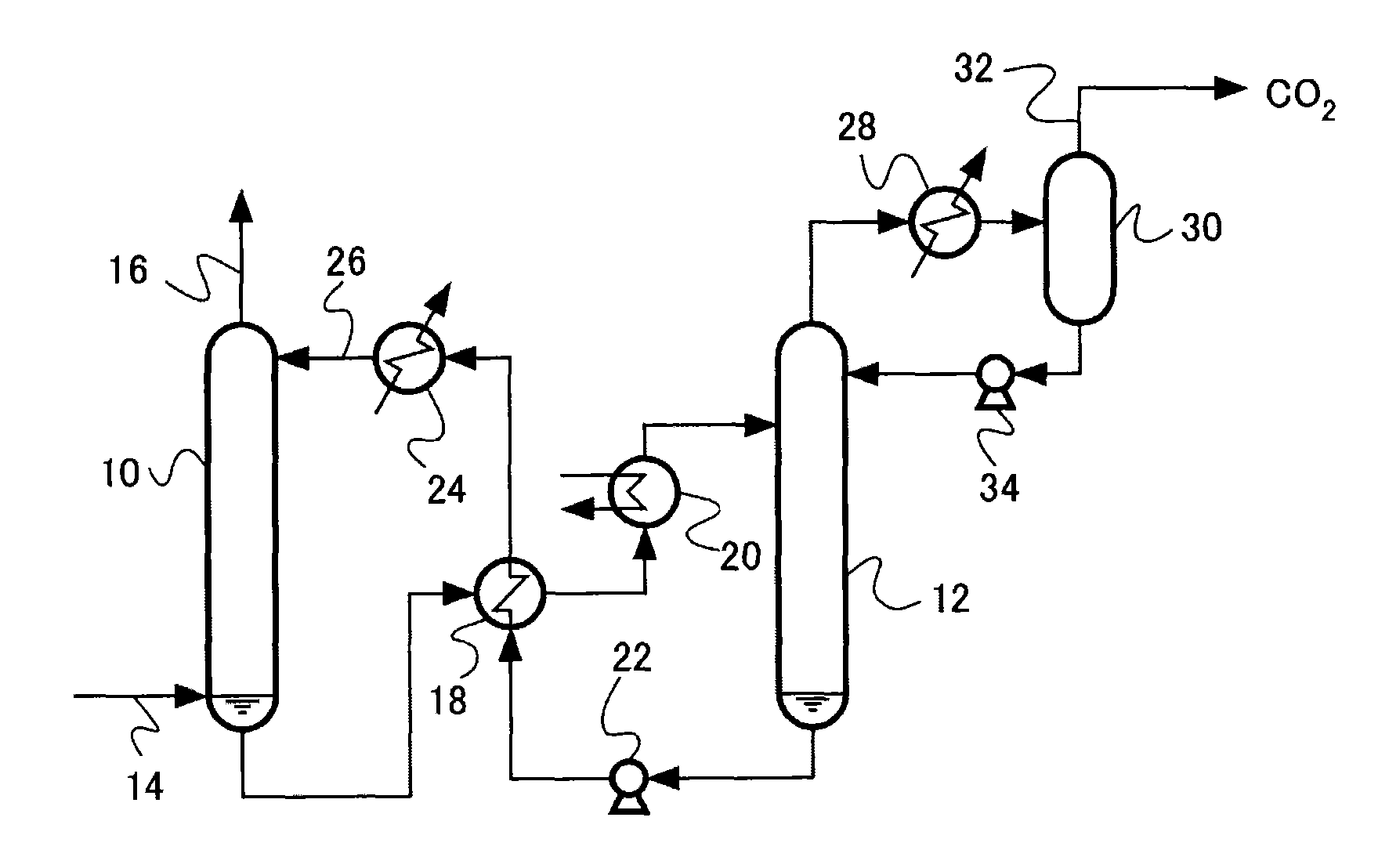
Acidic gas absorbent, acidic gas removal device, and acidic gas removal method
InactiveUS20120294785A1Promote absorptionGaseous chemical processesGas treatmentAbsorption capacityAzabicyclo Compounds
An acidic gas absorbent having a high acidic gas absorption capacity, that is, a high acidic gas absorption amount and a high acidic gas absorption rate, an acidic gas absorption device, and a method for absorbing an acidic gas, are provided. An acidic gas absorbent containing an azabicyclo compound and a primary or secondary amine compound; an acidic gas absorbent containing a heteroaromatic ring compound and a primary or secondary amine compound; an acidic gas removal device using these acidic gas absorbents; and a method for removing an acidic gas are disclosed.
Owner:KK TOSHIBA
Pyridone-fused azabicyclic- or cytisine derivatives, their preparation and their use in addiction therapy
InactiveUS6630467B2Reduce addictionLessing of tobacco useBiocideNervous disorderUlcerative colitisAzabicyclo Compounds
Owner:PFIZER INC
Use of N-aryl diazaspiracyclic compounds in the treatment of addiction
InactiveUS20060058328A1Reduces dopamine releaseReduce releaseBiocideNervous disorderMetaboliteSide effect
Compounds, compositions and methods for treating drug addiction, nicotine addiction, and / or obesity are disclosed. The compounds are N-aryl diazaspirocyclic compounds, bridged analogs of N-heteroaryl diazaspirocyclic compounds, or prodrugs or metabolites of these compounds. The aryl group can be a five- or six-membered heterocyclic ring (heteroaryl). The compounds are effective at inhibiting dopamine production and / or secretion, and accordingly are effective at inhibiting the physiological “reward” process that is associated with ingestion of nicotine and / or illicit drugs. The compounds and compositions can be administered in effective amounts to inhibit dopamine release, without resulting in appreciable adverse side effects (e.g., side effects such as significant increases in blood pressure and heart rate, significant negative effects upon the gastro-intestinal tract, and significant effects upon skeletal muscle).
Owner:BHATTI BALWINDER S +2
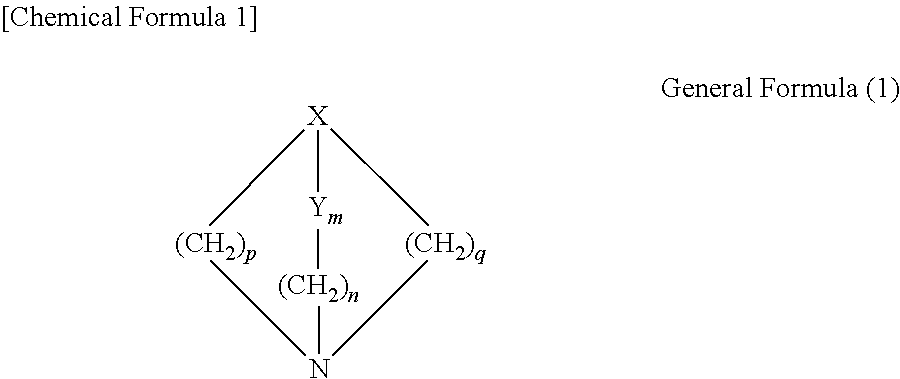
1H-pyrazole and 1H-pyrrole-azabicyclic compounds for the treatment of disease
The invention provides compounds of Formula I:wherein Azabicyclo iswhere the variables have the definitions discussed herein. These compounds may be in the form of pharmaceutical salts or compositions, may be in pure enantiomeric form or racemic mixtures, and are useful in pharmaceuticals to treat a disease or condition in which α7 is known to be involved.
Owner:PFIZER INC
3,6-Disubstituted azabicyclo derivatives as muscarinic receptor antagonists
This invention relates to derivatives of 3,6-disubstituted azabicyclo compounds. The compounds of this invention can function as muscarinic receptor antagonists, and can be used for the treatment of various diseases of the respiratory, urinary and gastrointestinal systems mediated through muscarinic receptors. The invention also relates to pharmaceutical compositions containing the compounds of the present invention and the methods for treating the diseases mediated through muscarinic receptors.
Owner:RANBAXY LAB LTD
Methods of Treating Mood Disorders Using Pyridyloxymethyl and Benzisoxazole Azabicyclic Derivatives
InactiveUS20080318926A1Effective inhibitory activityIncrease in horizontal locomotor activityBiocideNervous disorderAzabicyclo CompoundsAntisocial personality disorder
An aminomethylpyridyloxymethyl / benzisoxazole substituted azabicyclic compound, a pharmaceutical composition comprising same, and a method of treating a mood disorder selected from the group consisting of Somatization Disorder, Borderline Personality Disorder, Narcissistic Personality Disorder, Suicidal Ideation, and Antisocial Personality Disorder.
Owner:PFIZER INC
Azabicyclo compound and salt thereof
ActiveUS8779142B2Excellent HSP9 inhibitory activityBiocideOrganic chemistryAzabicyclo CompoundsHeteroatom
It is intended to provide a novel azabicyclo compound which exhibits both HSP90 inhibitory activity and cell proliferation inhibitory effect. Specifically disclosed is a compound represented by the following general formula (I) or a salt thereof: wherein X1 represents CH or N; any one of X2, X3 and X4 represents N, and the others represent CH; any one or two of Y1, Y2, Y3 and Y4 represent C—R4, and the others are the same or different and represent CH or N; R1 represents an optionally substituted monocyclic or bicyclic unsaturated heterocyclic group having 1 to 4 heteroatoms selected from N, S and O; R2 represents an alkyl group having 1 to 6 carbon atoms, or the like; and R3 and R4 represent —CO—R5 or the like.
Owner:TAIHO PHARMA CO LTD
Fused azabicycic compounds that inhibit vanilloid receptor subtype 1(VR1) receptor
Compounds of formula (I) are novel VR1 antagonists that are useful in treating pain, inflammatory thermal hyperalgesia, urinary incontinence and bladder overactivity.
Owner:ABBVIE INC
Oxo-azabicyclic compounds
A compound selected from those of formula (I): wherein:X1, X2, and X3, represent N or —CR3 in which R3 is as described in the description,G1 represents a group selected from those of formulae (i / a) and (i / b): in which R4, R5, and R6 are as defined in the description,G2 represents a group selected from carbon—carbon triple bond, —CH═C═CH—, C═O, C═S, S(O)n1 in which n1 represents an integer from 0 to 2 inclusive, or a group of formula (i / c): in which Y1 represents O, S, —NH or -Nalkyl, and Y2 represents O, S, —NH or -Nalkyl,n is an integer from 0 to 6 inclusive, and m is an integer from 0 to 7 inclusive,Z1 represents —CR9R10, wherein R9 and R10 are as defined in the description,A represents a ring system,R1 represents a group selected from H, alkyl, alkenyl, alkynyl, optionally substituted and the group of formula (i / d): in which p, Z2, B, q and G3 are as defined in the description and optionally, its optical isomers, N-oxide, and addition salts thereof with a pharmaceutically-acceptable acid or base, and medicinal products containing the same are useful as specific inhibitors of type-13 matrix mettaloprotease.
Owner:WARNER LAMBERT CO LLC
Benzoxazines and related nitrogen-containing heterobicyclic compounds useful as mineralocorticoid receptor modulating agents
The present invention relates to a compound, useful as a mineralocorticoid receptor-modulating agent, of the following formula [I]: wherein Ring A is a benzene ring optionally having a substituent(s) other than R1 etc, R1 is a group of the formula: RaSO2NH- etc, Ra is an alkyl group etc, R2 and R3 are each a hydrogen atom, a phenyl group, an optionally substituted alkyl group etc, X is an oxygen atom etc, Y is a group of the formula: -C(=O)- etc, Ar is an optionally substituted aryl group or an optionally substituted heteroaryl group, Q is a single bond, an alkylene group etc, or a pharmaceutically acceptable salt thereof.
Owner:MITSUBISHI TANABE PHARMA CORP
Methods of Treating Cognitive Disorders Using Pyridyloxymethyl and Benzisoxazole Azabicyclic Derivatives
InactiveUS20070270430A1Effective inhibitory activityIncrease in horizontal locomotor activityBiocideNervous disorderAzabicyclo CompoundsCognitive diseases
An aminomethylpyridyloxymethyl / benzisoxazole substituted azabicyclic compound, a pharmaceutical composition comprising same, and a method of treating a cognitive disorder selected from the group consisting of Asperger's disorder, Autistic Disorder, Oppositional Defiant Disorder, and Conduct Disorder.
Owner:PFIZER INC
Fuel cell separator resin composition and fuel cell separator
InactiveUS20090148775A1Improve conductivityLess impuritiesNon-metal conductorsConductive materialOrganic acidFuel cells
The present invention provides a fuel cell separator resin composition comprising: (A) an epoxy resin; (B) a curing agent; (C) a curing accelerator comprising a salt of a diazabicyclo compound and an organic acid; and (D) a carbon material, wherein the content of the carbon material (D) is 35 to 85% by mass based on the total amount of the composition, wherein the carbon material (D) comprises high crystalline artificial graphite having a particle size of 150 to 500 μm in an amount of 5 to 100% by mass based on the total amount of the carbon material (D), and wherein the content of the curing accelerator (C) is 1 to 20 parts by weight per 100 parts by weight of the curing agent (B).
Owner:NICHIAS CORP
Monoamine oxidase and application thereof in synthesis of chiral azabicyclic compounds
ActiveCN105441401AThe reaction process is simpleSuitable for industrial productionBacteriaEnzymesChemical synthesisMonoamine oxidase A
The invention provides a monoamine oxidase with high catalysis activity, strong enantioselectivity and good substrate survivability, and an enzyme-chemical synthesis method using the above enzyme to carry out enzyme catalyzed synthesis of (1S,2S,5R)-6,6-dimethyl-2-substituted-3-azabicyclo[3.1.0]hexane or (1S,3aR,6aS)-1-substituted-octahydrocyclopenta[c]pyrrole in order to further synthesize (1S,2S,5R)-6,6-dimethyl-2-3-azabicyclo[3.1.0]hexane-2-nitrile and its hydrolysate methyl (1S,2S,5R)-6,6-dimethyl-2-3-azabicyclo[3.1.0]hexane-2-carboxylate or further synthesize (1S,3aR,6aS)-1-octahydrocyclopenta[c]pyrrole-1nitrile The invention also provides a gene for coding the monoamine oxidase, a recombinant expression vector containing the gene, a recombinant expression transformant containing the gene, and efficient preparation methods of the vector and the transformant. The monoamine oxidase can respectively react with 3-5 and 5-5 azacyclopentamine systems with different sizes to generate corresponding imides; the imides undergo cyanide addition, so a cyanide is added to an enzyme catalysis system, and the above obtained addition product can be directly alcoholyzed in a hydrochloric acid-alcohol solution to obtain hydrochloride of amino acid methyl ester, so one-pot feeding is realized, the reaction technology is simplified, and industrial production is facilitated.
Owner:ABIOCHEM BIOTECH CO LTD
Acidic gas absorbent, acidic gas removal device, and acidic gas removal method
InactiveUS8506913B2Promote absorptionGaseous chemical processesGas treatmentAbsorption capacityAzabicyclo Compounds
An acidic gas absorbent having a high acidic gas absorption capacity, that is, a high acidic gas absorption amount and a high acidic gas absorption rate, an acidic gas absorption device, and a method for absorbing an acidic gas, are provided. An acidic gas absorbent containing an azabicyclo compound and a primary or secondary amine compound; an acidic gas absorbent containing a heteroaromatic ring compound and a primary or secondary amine compound; an acidic gas removal device using these acidic gas absorbents; and a method for removing an acidic gas are disclosed.
Owner:KK TOSHIBA
Pyridyloxymethyl and benzisoxazole azabicyclic derivatives
InactiveUS20050026922A1Shown some efficacyIncrease in horizontal locomotor activityBiocideNervous disorderAzabicyclo CompoundsSchizophrenia
An aminomethylpyridyloxymethyl / benzisoxazole substituted azabicyclic compound, a pharmaceutical composition comprising same, and a method of treating one or more CNS or other disorders, including concurrent treatment of disorders such as schizophrenia and depression.
Owner:PFIZER INC
Pharmaceutical compositions and methods for use
InactiveUS6852721B2Prevent and suppress symptomsAppreciable adverse side effectBiocideNervous disorderArylAzabicyclo Compounds
The present invention relates to diazabicyclic compounds, preferably to N-aryl diazabicyclic compounds. Of particular interest are 2-pyridinyl diazabicyclic compounds, such as (1S,4S)-2-(5-(3-methoxyphenoxy)-3-pyridyl)-2,5-diazabicyclo[2.2.1]heptane. Other exemplary compounds of the present invention include (1S,4S)-2-(5-(4-methoxyphenoxy)-3-pyridyl)-2,5-diazabicyclo[2.2.1]heptane, (1S,4S)-2-(5-(3-thienyl)-3-pyridyl)-2,5-diazabicyclo[2.2.1]heptane, (1S,4S)-2-(5-(4-fluorophenoxy)-3-pyridyl)-2,5-diazabicyclo[2.2.1]heptane, and (1S,4S)-2-(5-benzoyl-3-pyridyl)-2,5-diazabicyclo[2.2.1]heptane. The present invention also relates to prodrug derivatives of the compounds of the present invention.
Owner:TARGACEPT INC
Azabicyclic compounds as alpha-7 nicotinic acetylcholine receptor ligands
ActiveUS8507516B2Useful in treatmentBiocideNervous disorderAzabicyclo CompoundsNeuro-degenerative disease
The disclosure provides compounds of formula I, including their salts, as well as compositions and methods of using the compounds. The compounds are ligands for the nicotinic α7 receptor and may be useful for the treatment of various disorders of the central nervous system, especially affective and neurodegenerative disorders.
Owner:BRISTOL MYERS SQUIBB CO
Catalyst system and method for preparation of azoxystrobin or intermediates thereof with the same
ActiveCN109529928AHigh yieldImprove conversion rateOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsAzoxystrobinAzabicyclo Compounds
The invention relates to a catalyst system, which comprises one or more of a methano-containing azabicyclo compound or salt thereof, an aza cage compound or salt thereof. The invention also relates toa method for preparation of azoxystrobin or intermediates thereof with the catalyst system. The efficient catalytic system utilized by the invention and the method for preparation of azoxystrobin orintermediates thereof have the characteristics of easily controllable process, short reaction time, high conversion rate, low energy consumption, etc., and are suitable for large-scale industrial production.
Owner:HEBEI VEYONG BIO CHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com