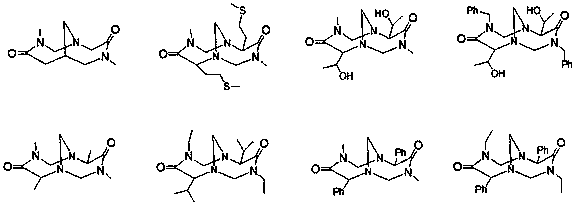
Catalyst system and method for preparation of azoxystrobin or intermediates thereof with the same
A technology of azoxystrobin and intermediates, applied in the field of catalyst systems prepared by azoxystrobin and its intermediates, can solve the problems of difficult control of reaction conditions and low yield, and achieve short reaction time, high yield and conversion rate high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
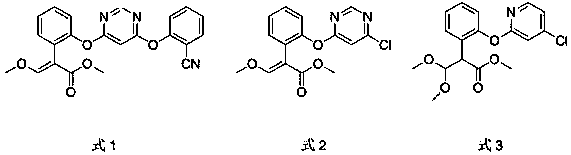
[0066] Example 1 This example is used to illustrate the preparation of azoxystrobin shown in formula 1
[0067] Add dry toluene (100mL), salicylonitrile (0.105mol, 12.50g), compound E-2-{2-[6-chloropyrimidin-4-yloxy]benzene Base}-3-methoxymethyl acrylate (0.1mol, 32.07g), potassium carbonate (0.08mol, 11.06g) and catalyst 2-carbonyl-1,4-diazabicyclo[2.2.1]heptane ( 0.01mol , 1.14g), start stirring, heat to 85°C, keep warm for 4h, HPLC detects that the content of salicylonitrile is less than 0.5%, cool the reaction system to 40~50°C, add 20mL of water, let stand to separate after stirring, separate The water phase and the toluene phase were washed once with 10 mL of water, the water phase was separated again, the toluene phase was evaporated to dryness, and recrystallized to obtain azoxystrobin shown in formula 1, with a content of 98.6% and a yield of 97.9%.
Embodiment 2
[0069] This embodiment is used to illustrate the impact of catalyst dosage on the reaction in the preparation process of azoxystrobin shown in formula 1
[0070] Add dry toluene (100mL), salicylonitrile (0.105mol, 12.50g), compound E-2-{2-[6-chloropyrimidin-4-yloxy]benzene Base}-3-methoxymethyl acrylate (0.1mol, 32.07g), potassium carbonate (0.08mol, 11.06g) and catalyst 2-carbonyl-1,4-diazabicyclo[2.2.1]heptane ( 0.001mol , 0.114g), start stirring, heat to 85°C, keep warm for 4h, HPLC detects that the content of salicylonitrile is less than 0.5%, cool the reaction system to 40~50°C, add 20mL of water, leave to separate after stirring, separate The water phase and the toluene phase were washed once with 10 mL of water, the water phase was separated again, the toluene phase was evaporated to dryness, and recrystallized to obtain azoxystrobin shown in formula 1, with a content of 98.6% and a yield of 97.6%.
Embodiment 3
[0072] Add dry toluene (100mL), salicylonitrile (0.105mol, 12.50g), compound E-2-{2-[6-chloropyrimidin-4-yloxy]benzene Base}-3-methoxymethyl acrylate (0.1mol, 32.07g), potassium carbonate (0.08mol, 11.06g) and catalyst 2-carbonyl-1,4-diazabicyclo[2.2.1]heptane ( 0.0005mol , 0.057g), start stirring, heat to 85°C, keep warm for 8h, HPLC detects that the content of salicylonitrile is less than 0.5%, cool the reaction system to 40~50°C, add 20mL of water, let it stand for stratification after stirring, separate The water phase and the toluene phase were washed once with 10 mL of water, the water phase was separated again, the toluene phase was evaporated to dryness, and recrystallized to obtain azoxystrobin shown in formula 1, with a content of 98.3% and a yield of 97.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com