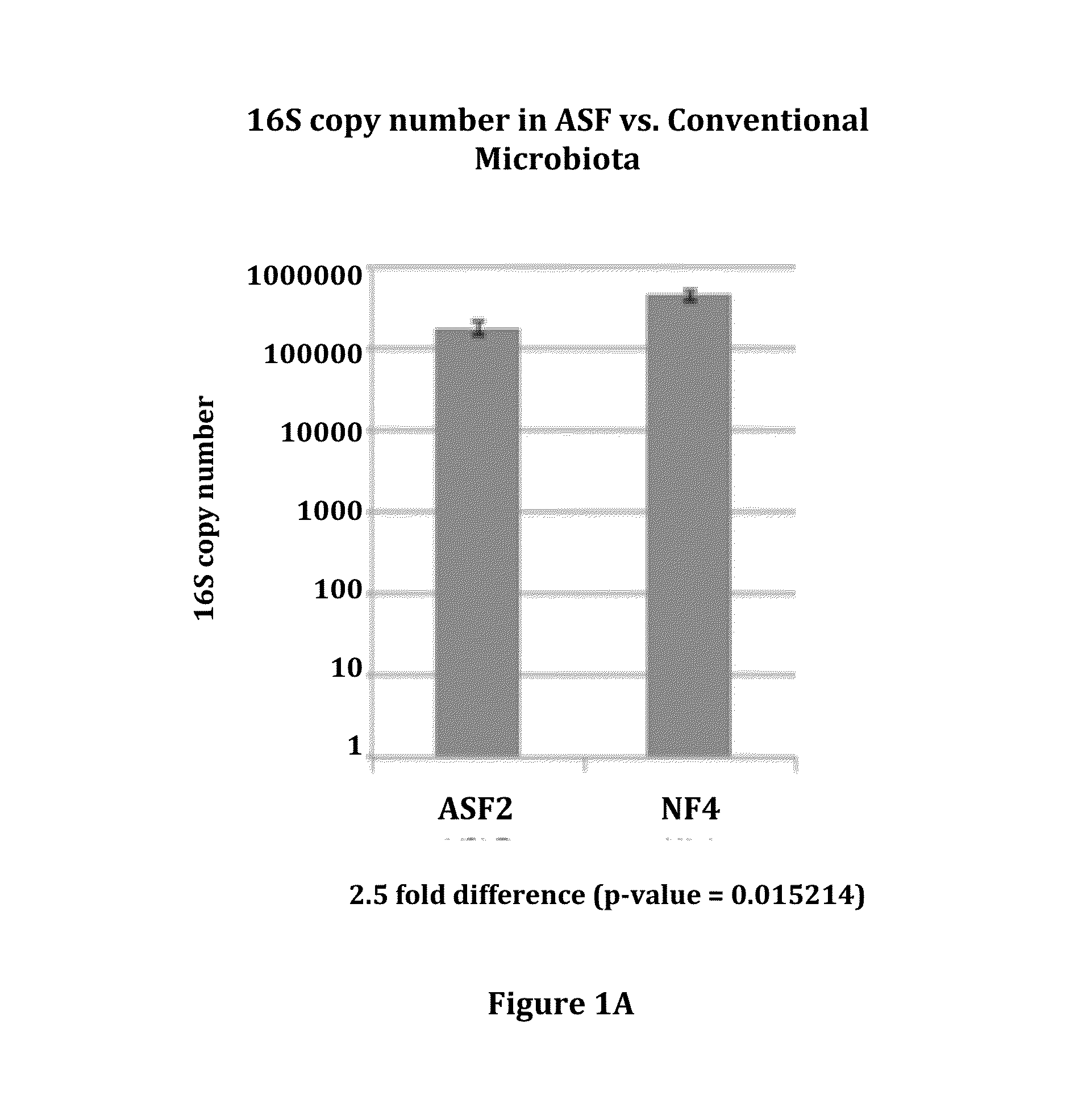
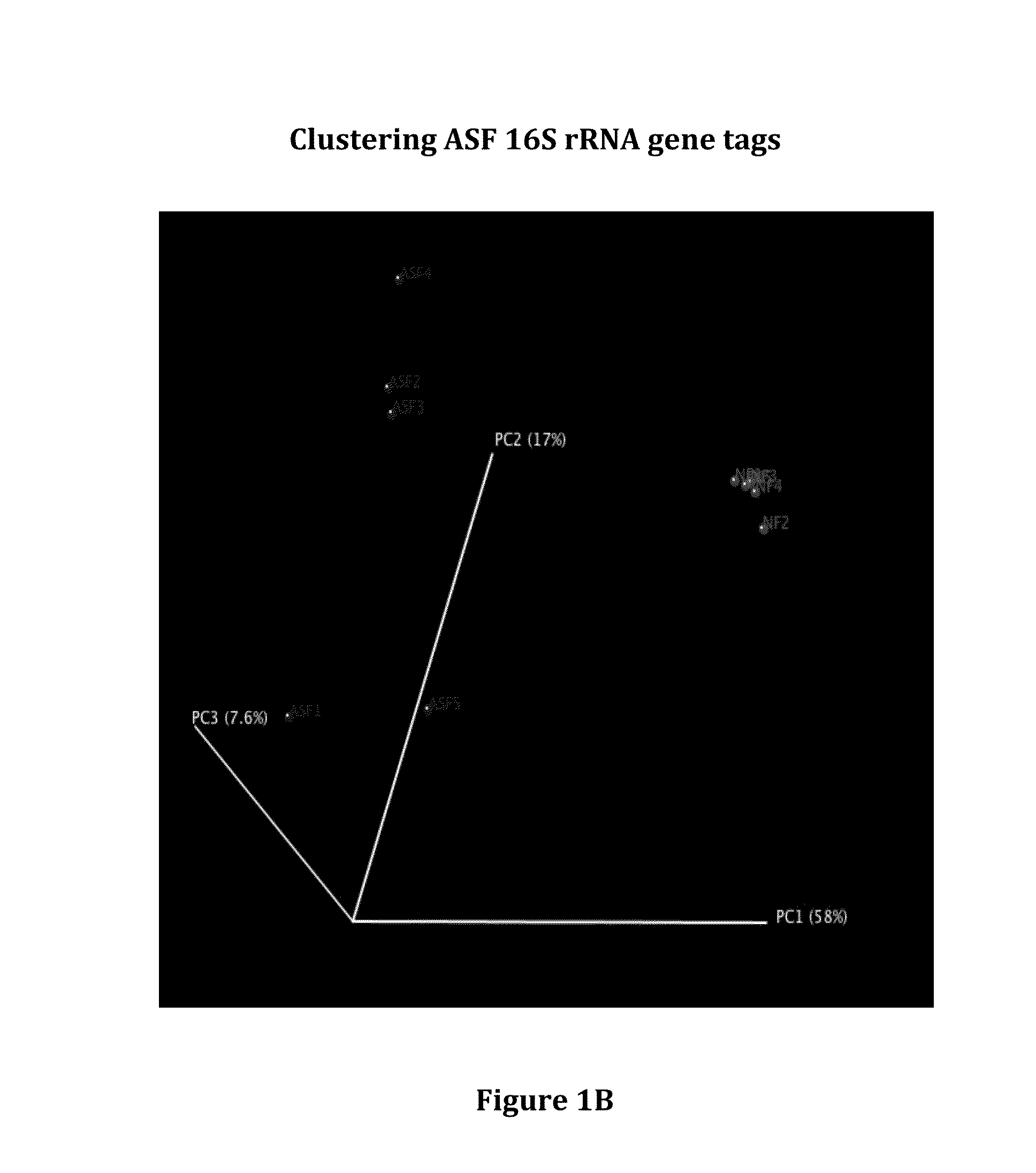
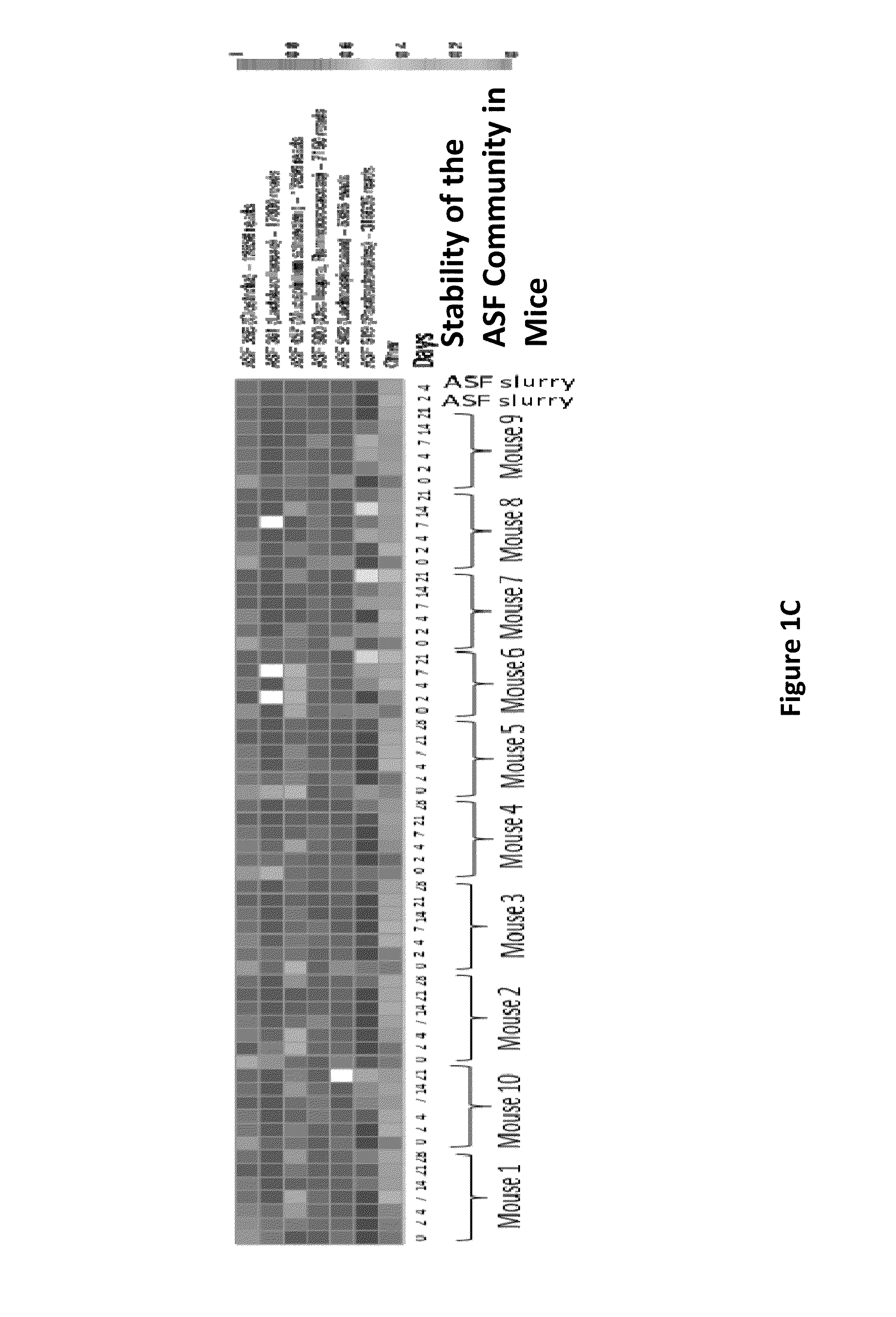
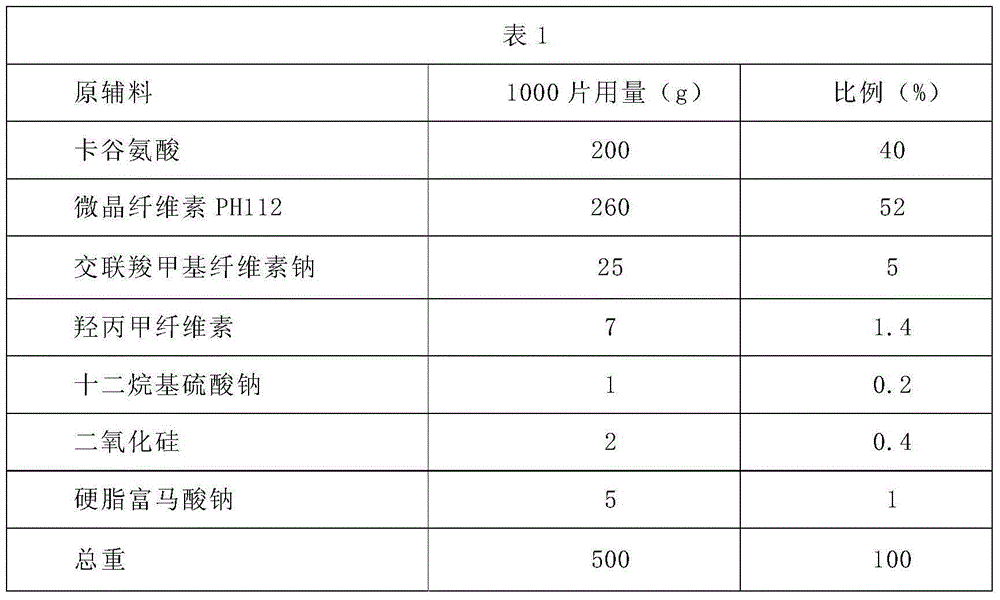
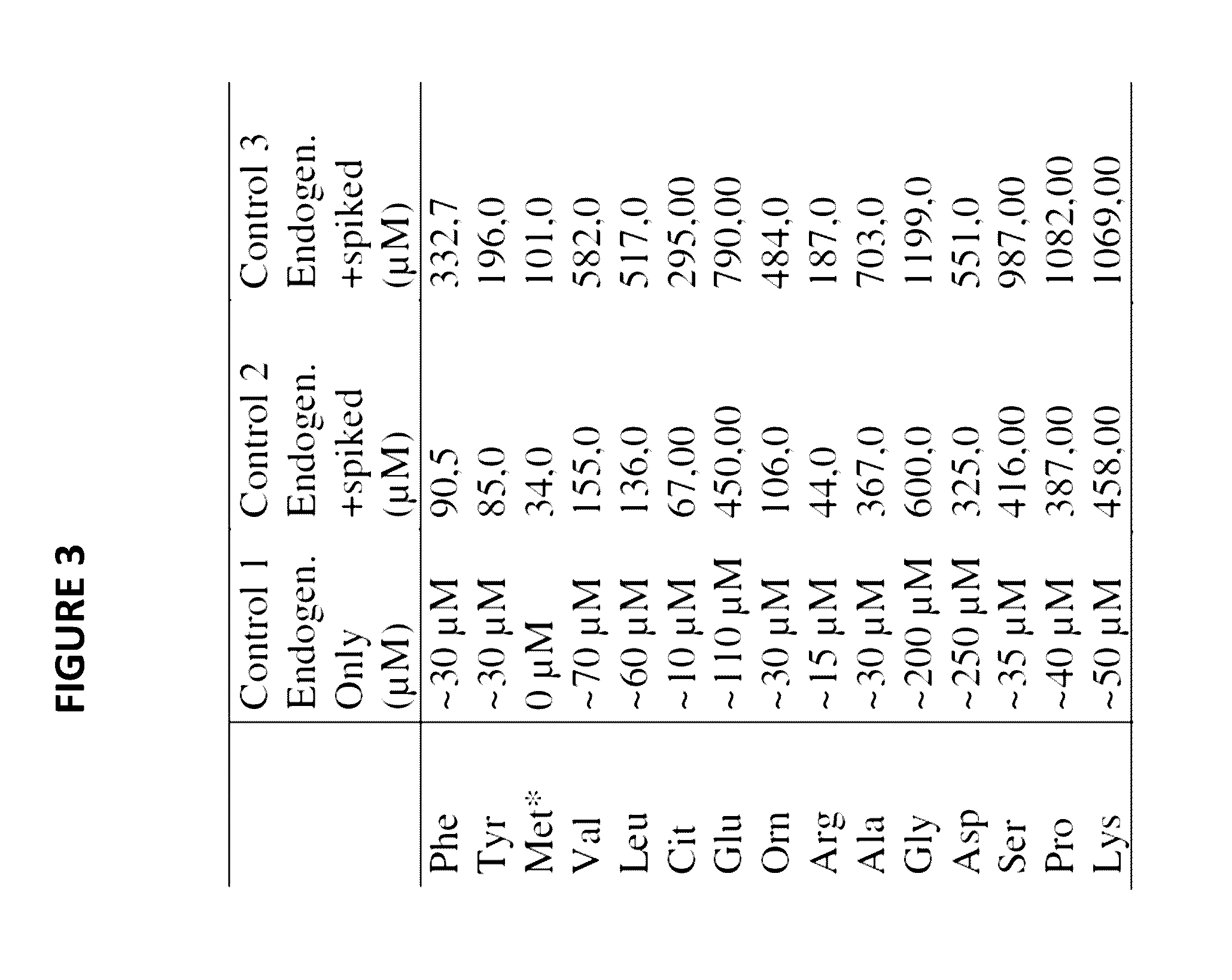
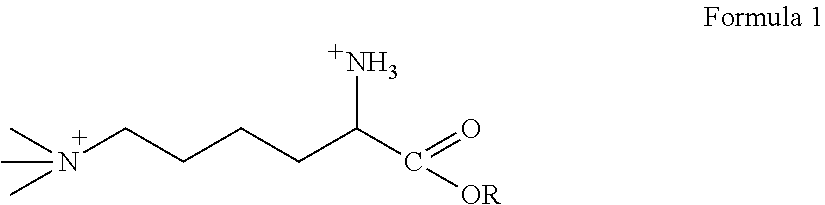
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Hyperammonemic encephalopathy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
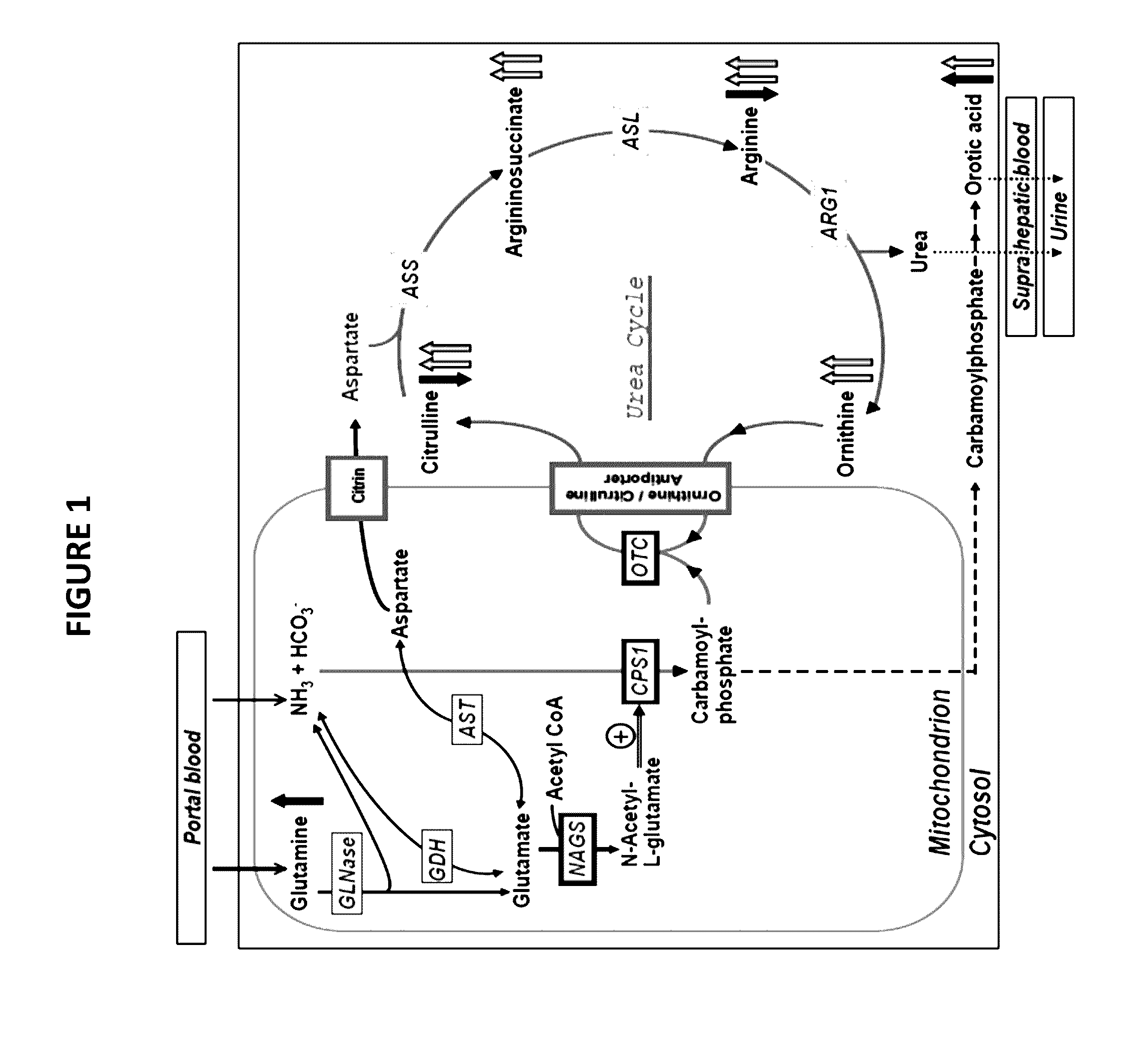
Hyperammonemia is one of the metabolic derangements that contribute to hepatic encephalopathy, which can cause swelling of astrocytes and stimulation of NMDA-receptors in the brain. Overstimulation of NMDA-receptors induces excitotoxicity.
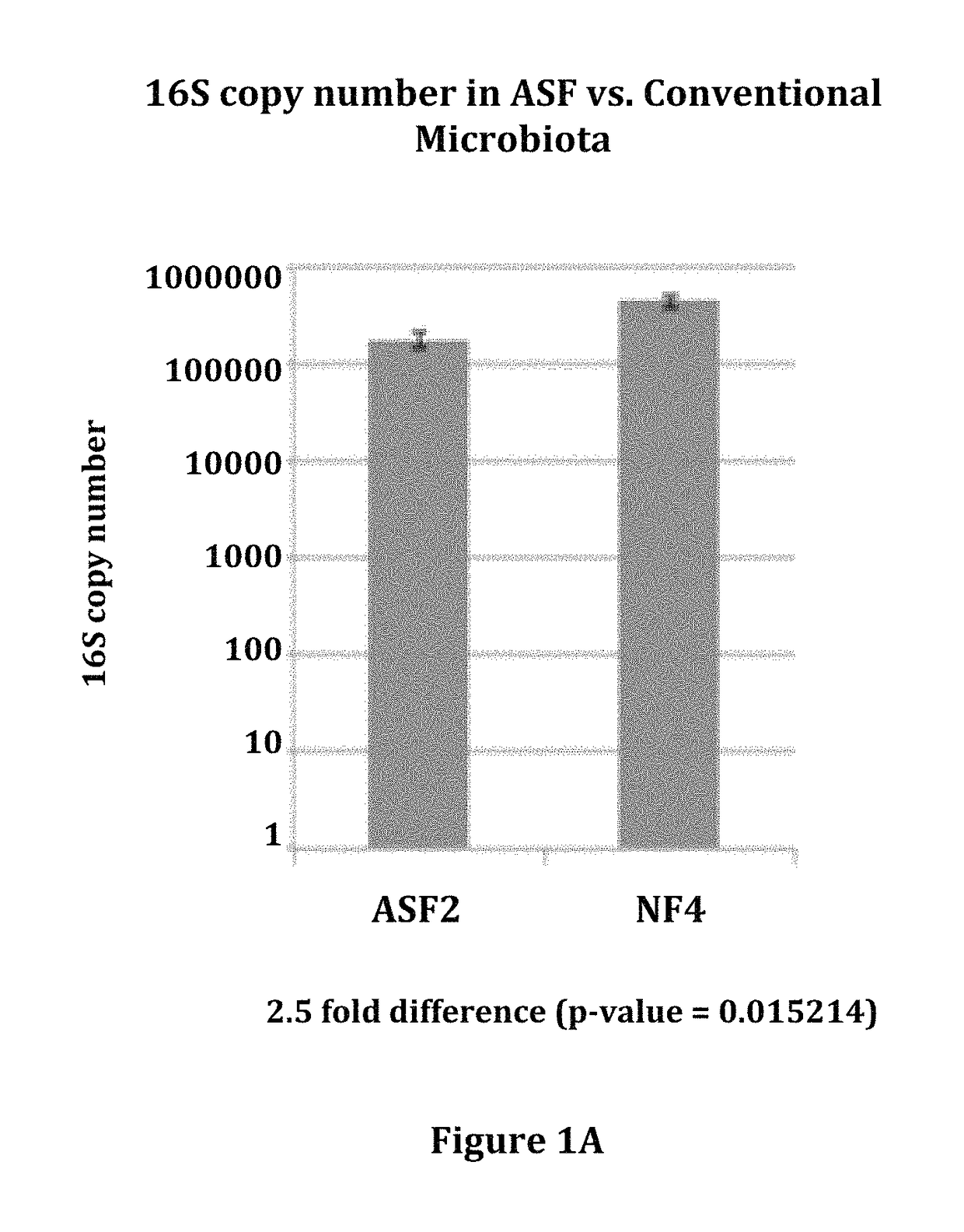
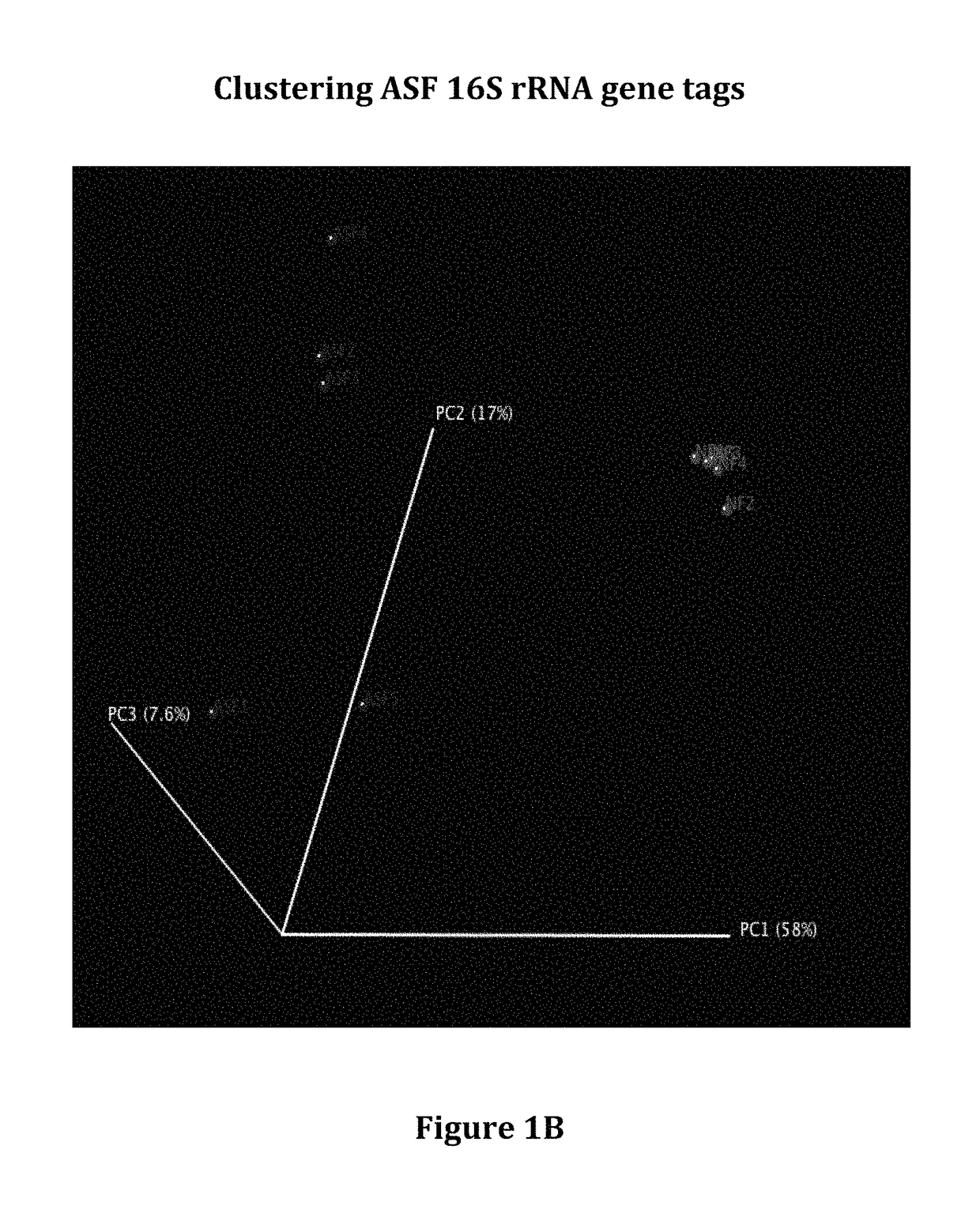
Compositions and methods comprising a defined microbiome and methods of use thereof
ActiveUS20160243175A1Minimal urease activityReduce bacteria countBacteriaBacteria material medical ingredientsMicroorganismInflammatory Bowel Diseases
The invention features the use of a defined microbial consortia for the replacement of a gut microbiome associated with disease. In particular, the invention provides for the treatment of hyperammonemia, Clostridium difficile colitis, hepatic encephalopathy associated with cirrhosis, and inflammatory bowel disease.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Carglumic acid solid composition and preparation method thereof
InactiveCN105056246AOvercome solubilityOvercome the slow dissolution problemOrganic active ingredientsAntinoxious agentsCarglumic acidAdhesive
The invention relates to a carglumic acid solid composition for treating hyperammonemia and a preparation method of the composition. The composition includes carglumic acid, one or more of a filling agent, an adhesive, a disintegrating agent, a surfactant, and a lubricant or a flow agent, and is characterized in that microcrystalline cellulose and lauryl sodium sulfate are added to the tablet prescription, so that the problems of tablet disintegration and slow dissolution of the main drug are solved; the tablets are prepared through a direct powder compression method; the preparation method is simple in operating steps, stable in process parameters, and good in repeatability; meanwhile, the impact of damp and hot on the stability of the raw drug materials of carglumic acid is avoided; compared with the original tablets, the tablets provided by the invention are more stable and reliable in product quality, and are suitable for industrialized production.
Owner:WUHAN WUYAO SCI & TECH
L-onithine and L-aspartic acid composition
InactiveCN1582912AQuality improvementAvoid decompositionPowder deliveryOrganic active ingredientsDiseaseHyperammonemic encephalopathy
A powder injection for preventing and treating hepatitis, the hyperammonemia caused by hepatitis, the disease in central nerve system caused by hepatism and the diseases caused by deficiency of L-ornithin or L-aminosuccinic acid is prepared from L-ornithin and L-aminosuccinic acid or the L-ornithin-L-aminosuccinic acid salt through dissolving in water, filtering and freeze drying.
Owner:WUHAN QR SCI & TECH DEV
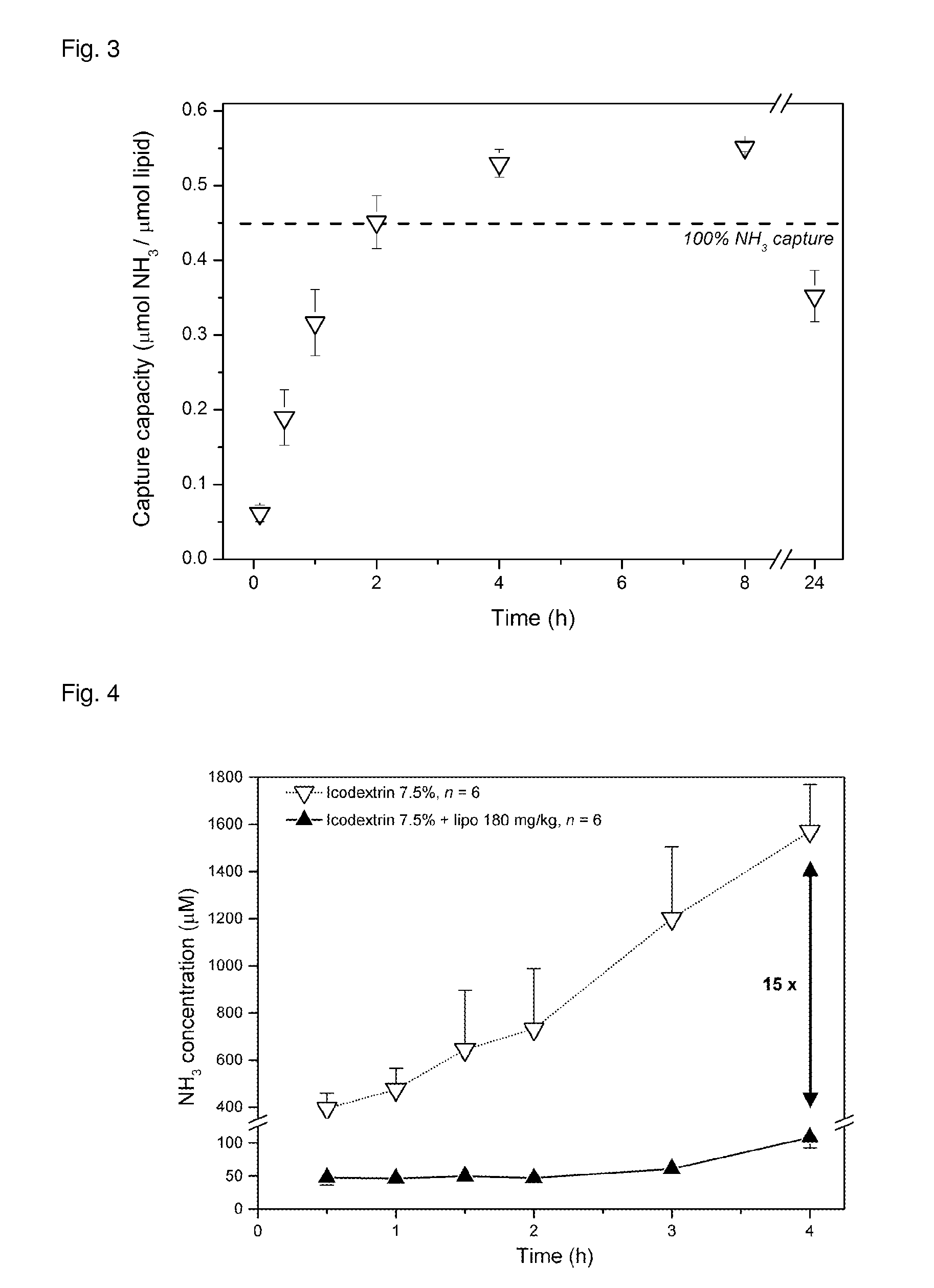
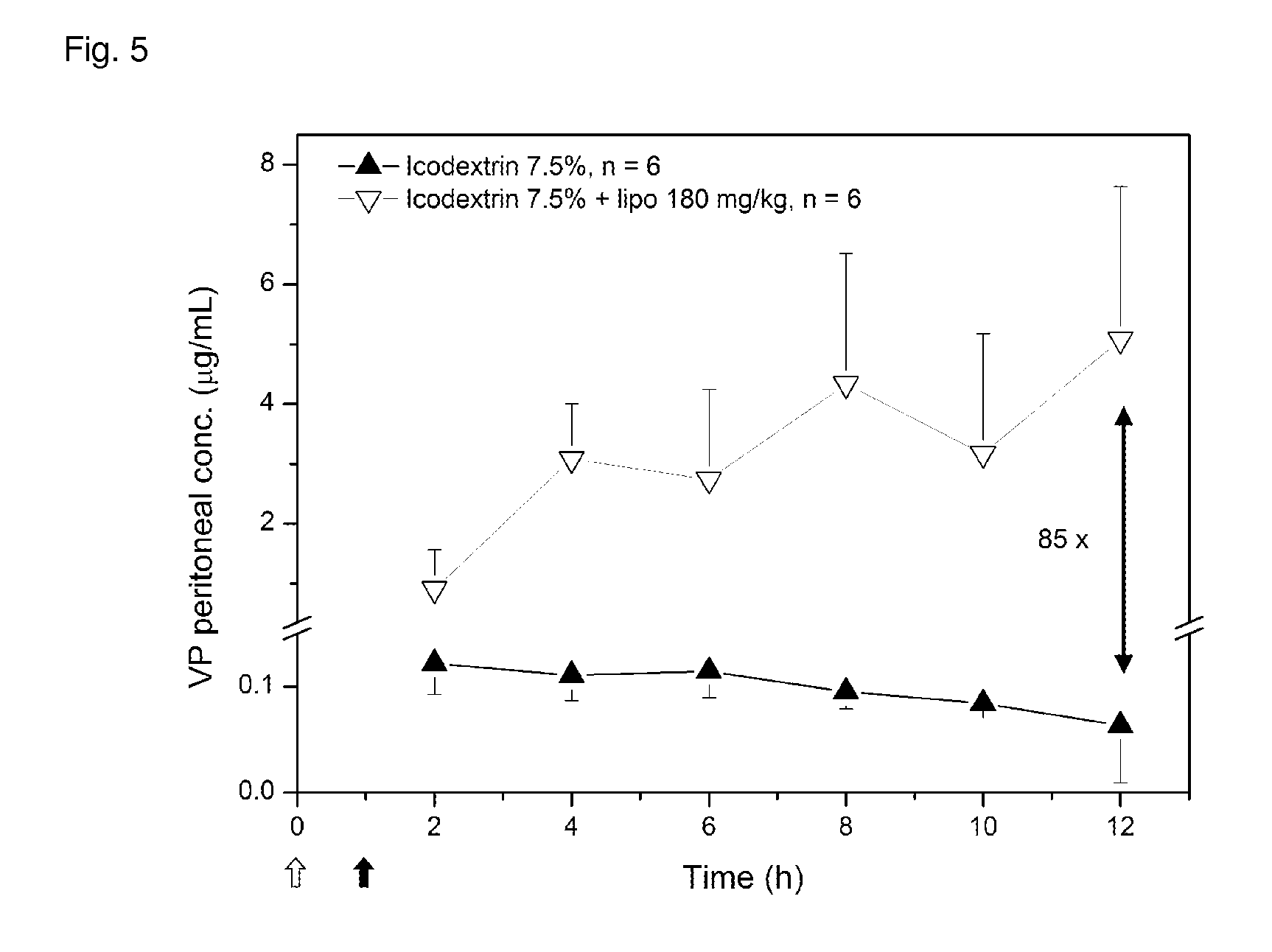
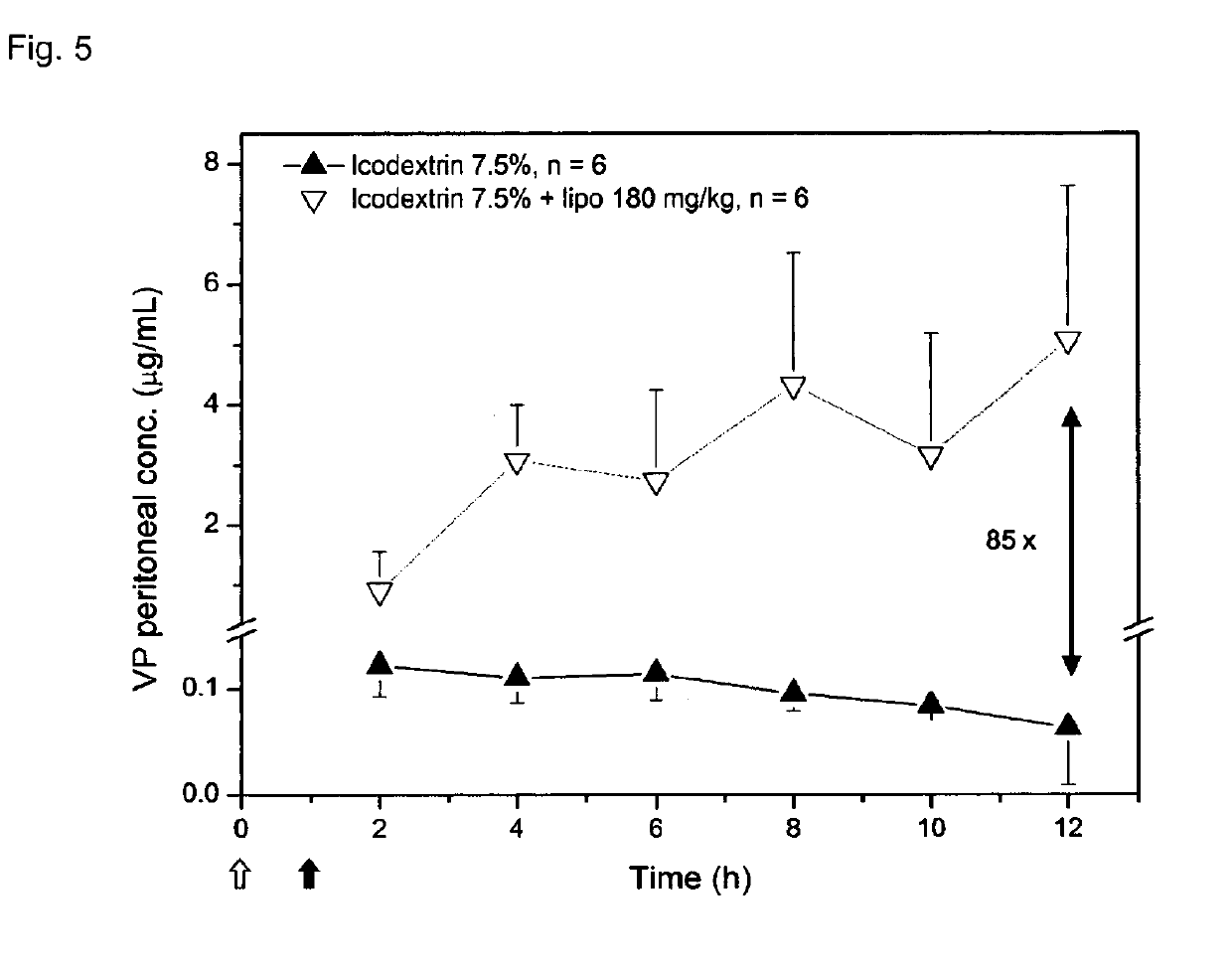
Liposome composition for use in peritoneal dialysis
ActiveUS20150216802A1Reduce concentrationImprove localizationMetabolism disorderSkeletal disorderPeritoneal spaceHyperammonemic encephalopathy
The present invention is directed to a liposome composition for use in the peritoneal dialysis of patients suffering from endogenous or exogenous toxicopathies, wherein the pH within the liposomes differs from the pH in the intraperitoneal cavity and wherein the pH within the liposome results in a liposome-encapsulated charged toxin. The invention also relates to a pharmaceutical composition comprising said liposomes. A further aspect of the present invention relates to a method of treating patients suffering from endogenous or exogenous toxicopathies, preferably selected from drug, metabolite, pesticide, insecticide, toxin, and chemical warfare toxicopathies, more preferably hyperammonemia, comprising the step of administering liposomes of the invention in a therapeutically effective amount into the peritoneal space of a patient in need thereof. Next to human, the present invention is particularly suitable to veterinary aspects.
Owner:ETH ZZURICH
Compositions useful in treatment of ornithine transcarbamylase (OTC) deficiency
ActiveUS9890365B2Metabolism disorderGenetic material ingredientsHyperammonemic encephalopathyRNA Sequence
Non-viral delivery systems comprising engineered hOTC DNA and RNA sequences are provided which when delivered to a subject in need thereof are useful for treating hyperammonemia, ornithine transcarbamylase deficiency and symptoms associated therewith. Also provided are methods of using hOTC for treatment of liver fibrosis and / or cirrhosis in OTCD patients by administering hOTC.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Methods of using zonisamide as an adjunctive therapy for partial seizures
Methods of using zonisamide as an adjunctive therapy for partial seizures are disclosed. In particular, the methods enhance the safety of patients taking pharmaceutical formulations of zonisamide by providing information that increases the awareness of hyperammonemia as a possible side effect; wherein the patients and / or prescribing physicians and other medical care providers are advised to monitor for hyperammonemia and employ methods that will improve the therapeutic outcome in the few patients who experience hyperammonemia associated with zonisamide therapy.
Owner:EISAI INC
Composition and method for treatment of hepatic encephalopathy
InactiveUS20070269403A1Reduce ammonia levelsReduce constipationDigestive systemSynthetic polymeric active ingredientsSide effectPolyethylene glycol
The inventions provide an improved treatment for hepatic encephalopathy characterized by hyperammonemia and / or constipation, comprising the oral administration of polyethylene glycol (PEG) in amounts sufficient to reduce plasma levels of ammonia and / or to alleviate constipation. Preferably, the PEG is administered in combination with lactulose, which provides a palatable composition for the treatment of HE with excellent therapeutic benefits and reduced side effects as compared to lactulose alone.
Owner:HALOW GEORGE M
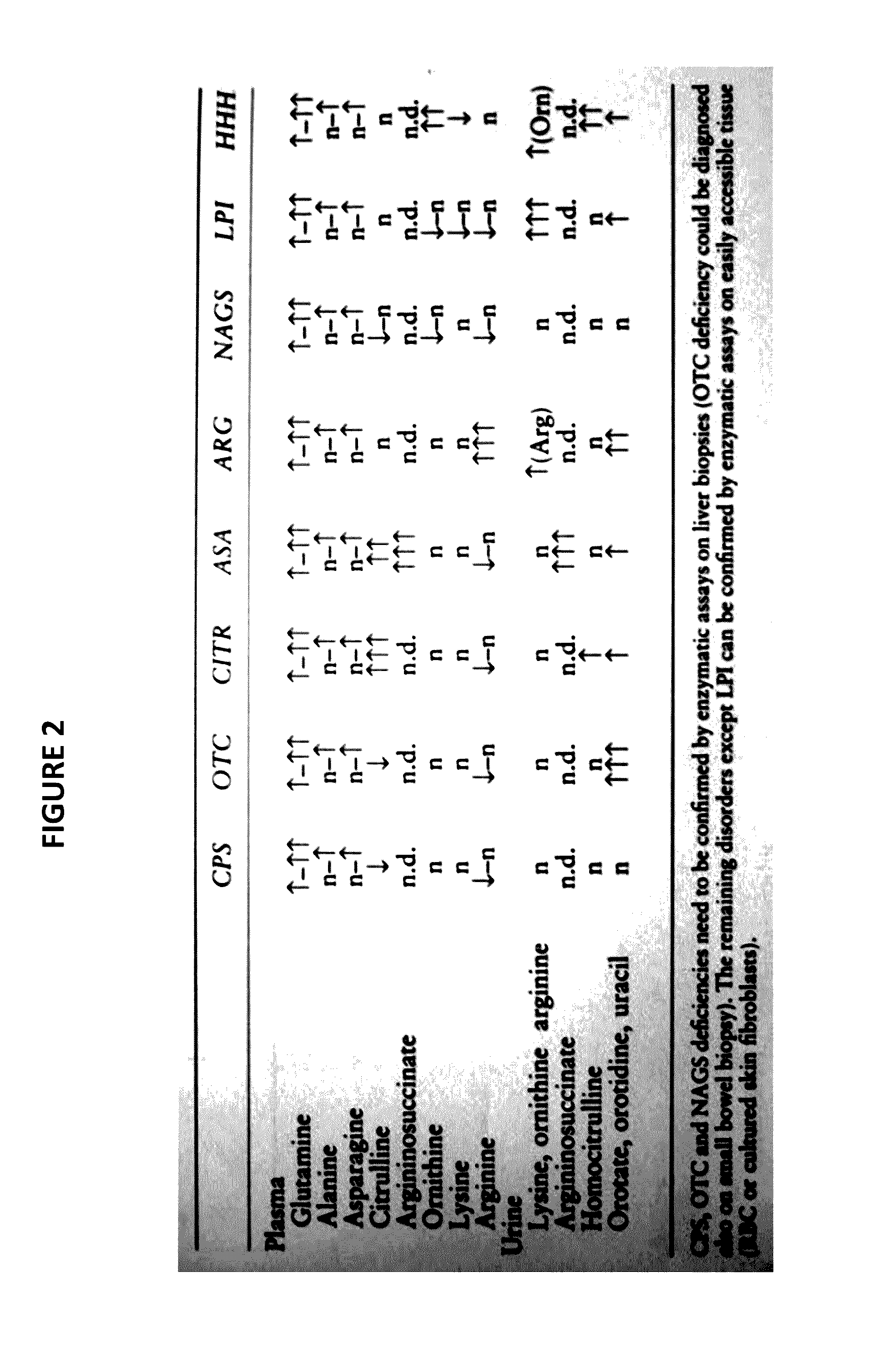
Novel methods and kits for detecting of urea cycle disorders using mass spectrometry
InactiveUS20170059535A1Component separationBiological testingStable Isotope LabelingHigh-Throughput Screening Methods
The present invention relates to newborn screening kits, methods, stable isotopically-labeled internal standards or internal standard solution for high throughput screening and analysis of metabolic disorders using liquid chromatography mass spectrometry (LC-MS) are provided. The metabolic disorders can be amino acid, organic acid or fatty acid oxidation disorders, and particularly urea cycle disorders or deficiencies, hyperammonemia, Hyperornithinemia-hyperammonemia-homocitrullinuria (HHH), and / or argininosuccinic aciduria. The newborn screening kits, methods, stable isotopically-labeled internal standards or internal standard solution are particularly useful for newborn screening (NBS) of metabolic disorders.
Owner:LABSYST DIAGNOSTICS OY
Methods of using zonisamide as an adjunctive therapy for partial seizures
Methods of using zonisamide as an adjunctive therapy for partial seizures are disclosed. In particular, the methods enhance the safety of patients taking pharmaceutical formulations of zonisamide by providing information that increases the awareness of hyperammonemia as a possible side effect; wherein the patients and / or prescribing physicians and other medical care providers are advised to monitor for hyperammonemia and employ methods that will improve the therapeutic outcome in the few patients who experience hyperammonemia associated with zonisamide therapy.
Owner:EISAI INC
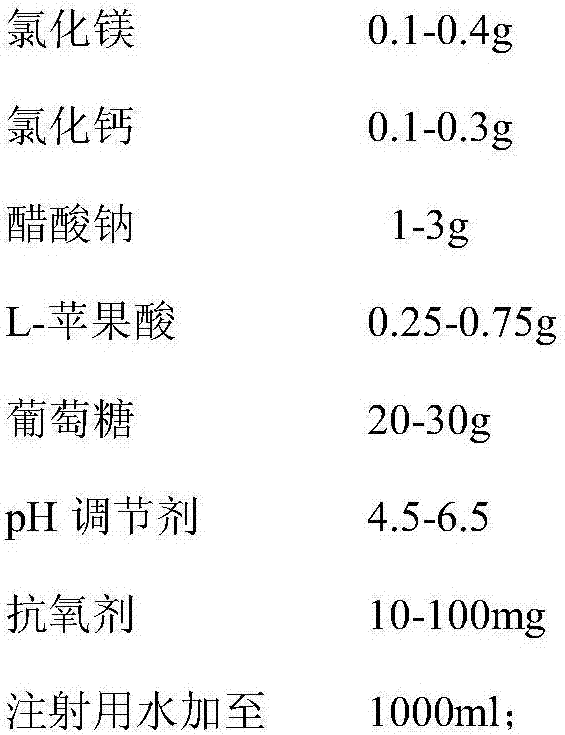
Compound electrolyte glucose injection and preparation method thereof
InactiveCN107468705AReduce oxygen consumptionAdjust extracellular fluidMetabolism disorderInorganic non-active ingredientsSodium acetateDisease
The invention belongs to the field of a medicine preparation, and particularly relates to a compound electrolyte glucose injection and a preparation method thereof. The injection is prepared from the following ingredients in parts by weight: 2.5 to 5.5g of sodium chloride, 0.1 to 0.4g of magnesium chloride, 0.1 to 0.3g of calcium chloride, 1 to 3g of sodium acetate, 0.25 to 0.75g of L-malic acid, 20 to 30g of glucose, 4.5 to 6.5g of pH regulators, 10 to 100mg of antioxidizers and injection water for reaching the total volume of 1000ml. The invention also provides a preparation process of the sterile injection. The injection prepared by the method is colorless and clear; the side reactions are few; the medication adaptability and the safety of patients are high; the injection is applicable to supplementation of water, electrolyte and carbohydrate after the operation or injury, and is particularly applicable to the patients with low immunity and the patients with diseases of hyperammonemia, angiocardiopathy, anemia, uremia, hypertension, burn wound and the like.
Owner:JINAN KANGHE MEDICAL TECH
Compound containing structure of o-naphthaquinone and application
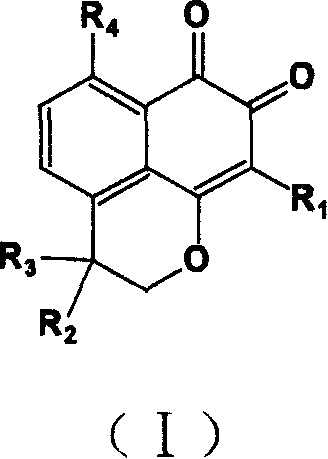
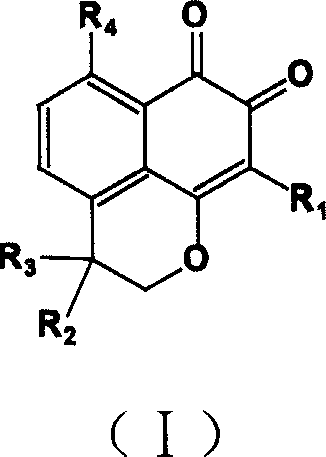
InactiveCN1660833AHigh speedImprove convenienceOrganic active ingredientsNervous disorderQuinoneHyperammonemic encephalopathy
A compound containing o-naphthalene quinone structure for preparing medicines to prevent and treat hyperammonemia and hepatic encephalopathy is prepared from natural Masonate F through structure reformation and optimization.
Owner:SUN YAT SEN UNIV
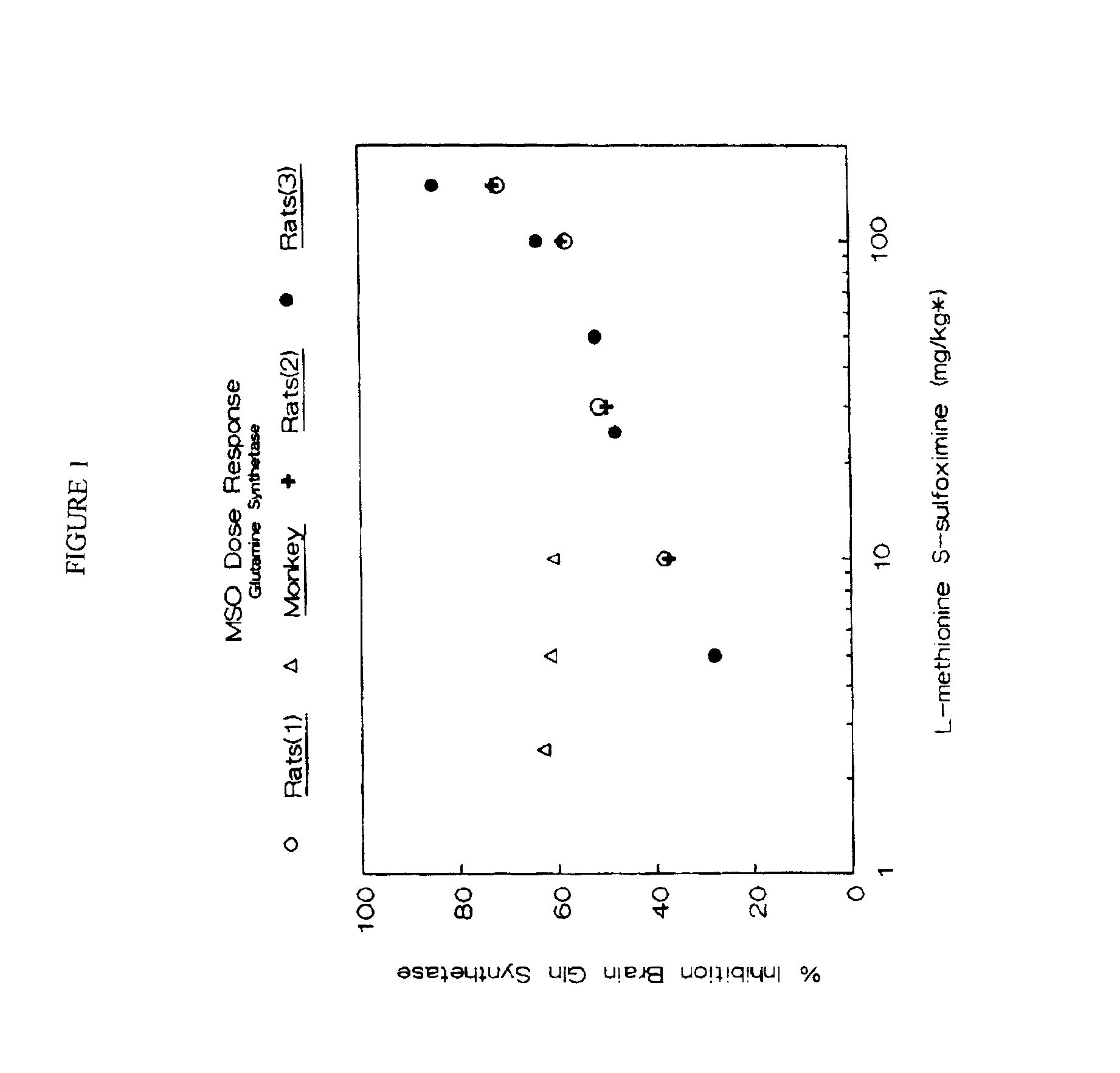
Dosage form of L-Methionine S-Sulfoximine
InactiveUS6875792B2Suppression problemLower Level RequirementsBiocideNervous disorderDiseaseHyperammonemic encephalopathy
Methods for the treatment of both diseases susceptible to the inhibition of mammalian glutamine synthetase and progressive hyperammonemic encephalopathy comprising administering L-methionine S-sulfoxime at a dose not to exceed 10 mg / kg body weight are disclosed. Preferably, the dose should not exceed 8 mg / kg; more preferably, the dose should not exceed 5 mg / kg; most preferably, the dose should not exceed 2.5 mg / kg.
Owner:BURTCH PHARMA LLC
Compositions useful in treatment of ornithine transcarbamylase (OTC) deficiency
ActiveUS20170021037A1Preventing and treating fibrosisMetabolism disorderGenetic material ingredientsHyperammonemic encephalopathyRNA Sequence
Non-viral delivery systems comprising engineered hOTC DNA and RNA sequences are provided which when delivered to a subject in need thereof are useful for treating hyperammonemia, ornithine transcarbamylase transcarbamylase deficiency and symptoms associated therewith. Also provided are methods of using hOTC for treatment of liver fibrosis and / or cirrhosis in OTCD patients by administering hOTC.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Hyperammonemia therapy for children suffering from urea cycle disorders
A method and composition for treating or preventing the progression of hyperammonemia caused by a Urea Cycle Disorder, the method comprising administration of an effective amount of porous activated carbon particles, wherein the porous activated carbon particles are enteric-coated in order to control their release and adsorption properties. The porous activated carbon particles or microspheres can initially be coated with lactulose, followed by enterically coating the lactulose-covered carbon particles. The inventive method and composition provides a safe and uncomplicated reduction of ammonia levels in affected children.
Owner:CT DEV ONE LLC
Composition and method for treatment of hepatic encephalopathy
InactiveUS7256202B2Reduce ammonia levelsReduce constipationBiocideDigestive systemSide effectHyperammonemic encephalopathy
The inventions provide an improved treatment for hepatic encephalopathy characterized by hyperammonemia and / or constipation, comprising the oral administration of polyethylene glycol (PEG) in amounts sufficient to reduce plasma levels of ammonia and / or to alleviate constipation. Preferably, the PEG is administered in combination with lactulose, which provides a palatable composition for the treatment of HE with excellent therapeutic benefits and reduced side effects as compared to lactulose alone.
Owner:HALOW GEORGE M
Remedies for hyperammonemia
InactiveUS6852707B1Reduce ammonia levelsOrganic active ingredientsBacteriaDiabetes mellitusSide effect
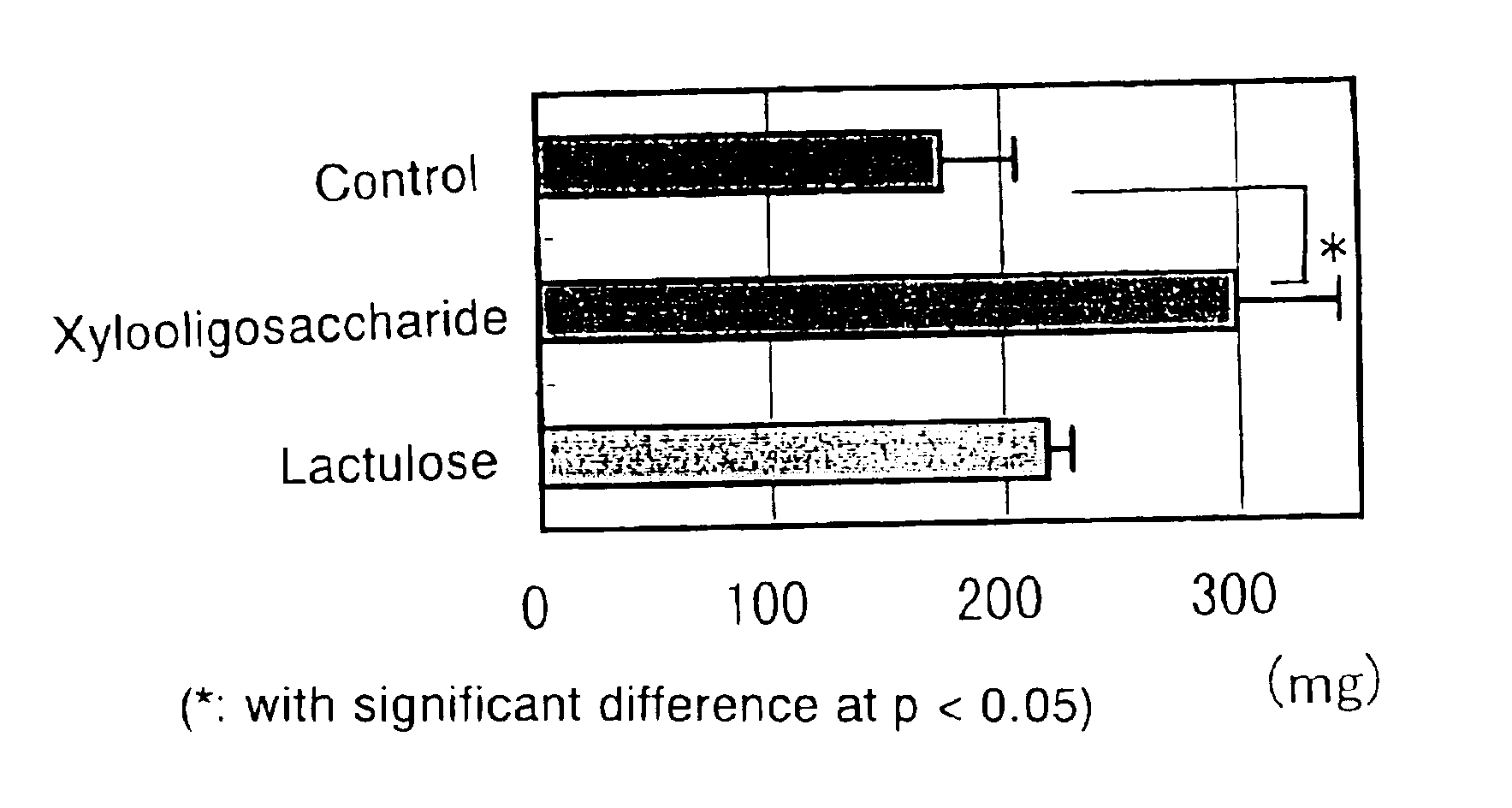
By using xylobiose or xylooligosaccharide containing xylobiose as a main ingredient in place of lactulose, there is provided a blood ammonia lowering agent, a therapeutic agent of hyperammonemia or a therapeutic agent of hepatic encephalopathy that need be adminstered in smaller doses and which have no concern over side effects.Lactulose conventionally used as such drugs has to be administered in high doses and involves a safety problem when administered to patients with galactosemia or diabetes mellitus. The drug of the invention which contains xylobiose as a main ingredient solves these problems.
Owner:SUNTORY HLDG LTD
Compositions and methods comprising a defined microbiome and methods of use thereof
ActiveUS10058576B2Minimal urease activityReduce bacteria countPowder deliveryBacteriaHyperammonemic encephalopathyGut microbiome
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
L-ornithine glutamic acid double salt and preparation method and application thereof
ActiveCN103833564AShort processEmission reductionOrganic active ingredientsNervous disorderHepatic comaHyperammonemic encephalopathy
The invention belongs to the field of medicinal chemistry, biochemistry and medicines, and discloses an L-ornithine glutamic acid double salt and a preparation method and an application thereof. The structural formula of the L-ornithine glutamic acid double salt is shown as the formula (I) described in the specification. The L-ornithine glutamic acid double salt can be used for preparing a medicament for treating hyperammonemia caused by acute and chronic liver diseases, and particularly for medicaments for removing symptoms of the central nervous system caused by liver diseases and salvaging hepatic coma. The preparation method provided by the invention is simple, easy to operate, high in yield, low in cost, small in environmental pollution and convenient for industrialized production, and the double salt is perfect in crystalline form, greater in granularity and good in crystallization effect.
Owner:NANJING UNIV OF TECH
Liposome composition for use in peritoneal dialysis
InactiveUS20190105270A1Metabolism disorderAntinoxious agentsPeritoneal dialysis catheterPeritoneal space
The present invention is directed to a liposome composition for use in the peritoneal dialysis of patients suffering from endogenous or exogenous toxicopathies, wherein the pH within the liposomes differs from the pH in the intraperitoneal cavity and wherein the pH within the liposome results in a liposome-encapsulated charged toxin. The invention also relates to a pharmaceutical composition comprising said liposomes. A further aspect of the present invention relates to a method of treating patients suffering from endogenous or exogenous toxicopathies, preferably selected from drug, metabolite, pesticide, insecticide, toxin, and chemical warfare toxicopathies, more preferably hyperammonemia, comprising the step of administering liposomes of the invention in a therapeutically effective amount into the peritoneal space of a patient in need thereof. Next to human, the present invention is particularly suitable to veterinary aspects.
Owner:ETH ZZURICH
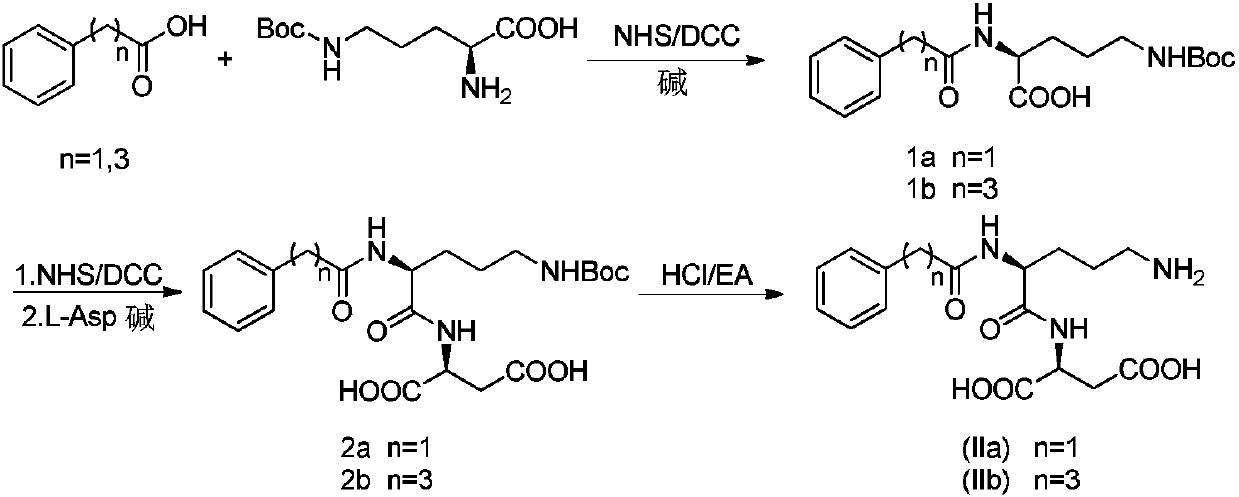
Acylated derivative for ornithine and aspartate dipeptide compound and application thereof
ActiveCN107619428ALower blood ammonia levelsQuick effectNervous disorderDipeptide ingredientsRat liverDipeptide
The invention provides an acylated derivative for an ornithine and aspartate dipeptide compound or an officinal salt thereof, a preparation method thereof, a medicine composition containing the derivative or the officinal salt thereof, and the application of the derivative or the officinal salt thereof for preparing medicines for preventing or curing hyperammonemia or liver diseases, particularlyhepatic encephalopathy. Experimental results definitely indicate that the concentration of blood ammonia can be obviously reduced after administration for the acylated derivative for the ornithine andaspartate dipeptide compound, the acylated derivative takes effect quickly by preferably compared with LOLA, subsequent dysmnesia after TAA-induced rat liver injury can be obviously improved, and theacylated derivative for the dipeptide has a certain treatment effect on hyperammonemia or liver diseases, particularly hepatic encephalopathy.
Owner:NANJING YOUKE BIOLOGICAL MEDICAL RES +3
Device and methods of using device for detection of hyperammonemia
ActiveUS20180143210A1Organic active ingredientsMaterial analysis by observing effect on chemical indicatorPoint of careGas phase
The present disclosure relates to a biosensor capable of measuring the total concentration of one or a plurality of ammonia or ammonium ions with the use of indophenol reagents in the presence of an ionomer. In some embodiments, the biosensor comprises a perflurinated membrane that comprises an ionomer in contact with an alkali buffer in a vessel configured to receive a sample, such as whole blood. The disclosure also relates to a method of detecting or quantifying the ammonia or ammonium ion concentration in whole blood in a point of care bio sensor without reliance on gas chromatography or any measurement that takes more than about twenty minutes.
Owner:UNIV OF MARYLAND +1
Arginine glutamate injection pharmaceutical composition and preparation method thereof
InactiveCN108451942AEasy to control main impuritiesHigh content of impuritiesOrganic active ingredientsMetabolism disorderHyperammonemic encephalopathyImpurity
The invention relates to an arginine glutamate injection pharmaceutical composition and a preparation method thereof. The injection is prepared from arginine glutamate, water for injection and an acidifying or alkalizing agent; the pH value of the injection is 5.2 to 6.0; the concentration of the injection is 0.01 to 1g / ml. The preparation method comprises the following steps: dissolving the arginine glutamate with the water for injection; adjusting the pH value with the acidifying or alkalizing agent; decoloring with active carbon; performing volume fixing, split charging and sterilization. The injection is used for treating hyperammonemia caused by acute and chronic liver diseases such as liver cirrhosis, fatty liver and hepatitis. The injection provided by the invention has the characteristics of low impurity content, easiness in controlling quality, higher safety in clinical application and the like.
Owner:LIAONING HAISCO PHARMACEUTICAL CO LTD
Medicament composition for treating hyperammonemia and preparation method thereof
InactiveCN101596179ABest Prescription TechnologyOrganic active ingredientsDigestive systemHepatic comaFatty liver
The invention provides a medicament composition for treating hyperammonemia and a preparation method thereof. The medicament composition uses an extractive glutamic acid as an active component and is an extractive glutamic acid injection used for treating the hyperammonemia caused by acute and chronic liver diseases, such as cirrhosis, fatty liver and hepatitis, and is specially suitable for eliminating central nervous system symptoms caused by the liver diseases and the emergency treatment of hepatic coma.
Owner:FUREN PHARMA GROUP
Application of L-arabinose to preparation of medicine or health care products for preventing or curing hyperammonemia
The invention discloses the application of L-arabinose to the preparation of medicine or health care products for preventing or curing hyperammonemia, and particularly discloses the application of L-arabinose to the preparation of medicine or health care products for reducing blood ammonia and / or endotoxin. The applicant finds, in experiments, that L-arabinose can effectively reduce the level of blood ammonia and endotoxin of a mouse (1 to 4 grams / kg / day), and body health is not damaged while the function is achieved.
Owner:唐传生物科技(厦门)有限公司
Method of treating a human being for a class of metabolic defects and energy production disorders
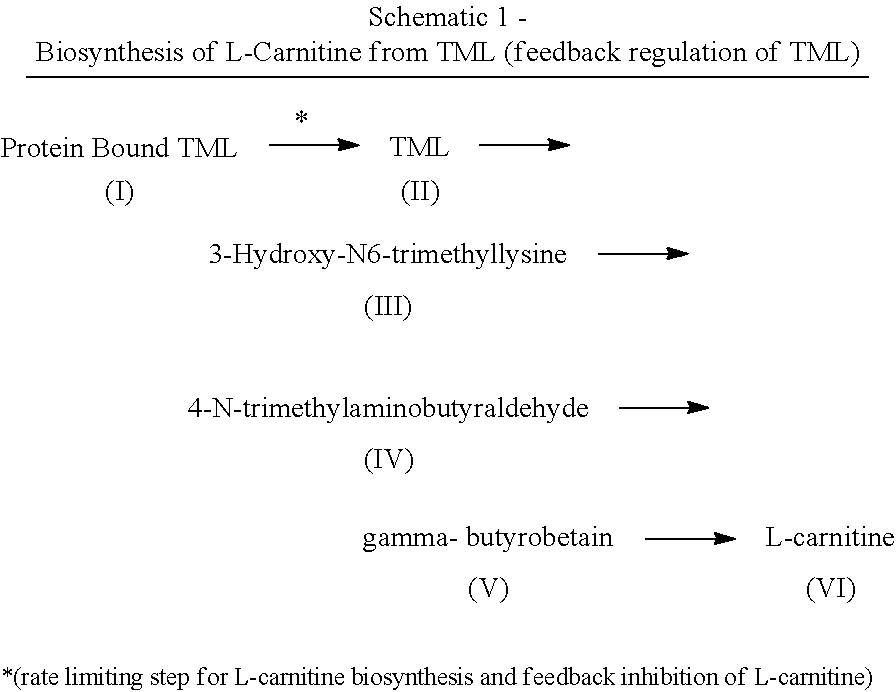
The invention involves various embodiments of a method for treating a human being for a condition associated with (1) a clinical state of impairment of carnitine or carnitine esters, or decreased fatty acid metabolism, (2) low energy production or lower ATP production, (3) clinical hyperammonemia, and (4) clinically high pyruvate levels resulting from a deficiency in the biosynthesis of carnitine. The method involves administering a therapeutically effective salt of N-6-trimethyl-L-lysine.
Owner:CHEMGENES CORP
Application of l-arabinose in the preparation of medicines or health products for preventing or treating hyperammonemia
The invention discloses the application of L-arabinose to the preparation of medicine or health care products for preventing or curing hyperammonemia, and particularly discloses the application of L-arabinose to the preparation of medicine or health care products for reducing blood ammonia and / or endotoxin. The applicant finds, in experiments, that L-arabinose can effectively reduce the level of blood ammonia and endotoxin of a mouse (1 to 4 grams / kg / day), and body health is not damaged while the function is achieved.
Owner:唐传生物科技(厦门)有限公司
Formulations of L-ornithine phenylacetate
Some embodiments of the present application are directed to oral formulations of L- ornithine phenylacetate and methods of preparing the same. These oral formulations offer altemative administration route than the standard intravenous administration of L-ornithine phenylacetate for treating hyperammonemia in patients having various acute and chronic liver diseases and disorders, for example, acuteliver failure, liver cirrhosis, liver decompensation, portal hypertension, hepatic encephalopathy, or patients with urea cycle disorders.
Owner:OCERA THERAPEUTICS INC
Hyperammonemia model of affecting apoptosis gene expression in rat brains and construction method of hyperammonemia model
InactiveCN106727684ATotal swimming distance increasedImprove learning and memory abilityMicrobiological testing/measurementChlorine active ingredientsHyperammonemic encephalopathyBlood plasma
The invention discloses a hyperammonemia model of affecting apoptosis gene expression in rat brains and a construction method of the hyperammonemia model. The hyperammonemia model of affecting the apoptosis gene expression in the rat brains is as follows: 40 male rats are equally divided into two groups randomly, namely a normal control group and a hyperammonemia model group; the learning and memorizing functions of the rats are evaluated through a water maze test; the rats are narcotized and then plasma is separated to detect blood ammonia; one part of rat brain tissues is fixed through 4% paraformaldehyde and the other part is stored at 80 DEG C below zero; the rat brain apoptosis condition is detected through a Tunel kit; and the expression of the rat brain tissue Bcl-2 and Bax genes is detected through RT-qPCR. Neuronal apoptosis of the rat brain tissues employing the hyperammonemia model is increased. Due to the multifactor action result in the brain tissues, disequilibrium of Bcl-2 and Bax apoptosis gene expression is an important mechanism.
Owner:THE SECOND HOSPITAL AFFILIATED TO WENZHOU MEDICAL COLLEGE
L-ornithine glutamic acid double salt and its preparation method and application
ActiveCN103833564BShort processEmission reductionOrganic active ingredientsNervous disorderHepatic comaHyperammonemic encephalopathy
The invention belongs to the field of medicinal chemistry, biochemistry and medicines, and discloses an L-ornithine glutamic acid double salt and a preparation method and an application thereof. The structural formula of the L-ornithine glutamic acid double salt is shown as the formula (I) described in the specification. The L-ornithine glutamic acid double salt can be used for preparing a medicament for treating hyperammonemia caused by acute and chronic liver diseases, and particularly for medicaments for removing symptoms of the central nervous system caused by liver diseases and salvaging hepatic coma. The preparation method provided by the invention is simple, easy to operate, high in yield, low in cost, small in environmental pollution and convenient for industrialized production, and the double salt is perfect in crystalline form, greater in granularity and good in crystallization effect.
Owner:NANJING TECH UNIV
Method of treating a human being for a class of metabolic defects and energy production disorders
The invention involves various embodiments of a method for treating a human being for a condition associated with (1) a clinical state of impairment of carnitine or carnitine esters, or decreased fatty acid metabolism, (2) low energy production or lower ATP production, (3) clinical hyperammonemia, and (4) clinically high pyruvate levels resulting from a deficiency in the biosynthesis of carnitine. The method involves administering a therapeutically effective salt of N-6-trimethyl-L-lysine.
Owner:CHEMGENES CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com