Medicament composition for treating hyperammonemia and preparation method thereof
A technology for hyperammonemia and drug activity, applied in the field of arginine glutamate injection, can solve problems such as abnormal ion state, achieve the effects of relieving hyperammonemia, restoring liver function, and avoiding serious organ damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
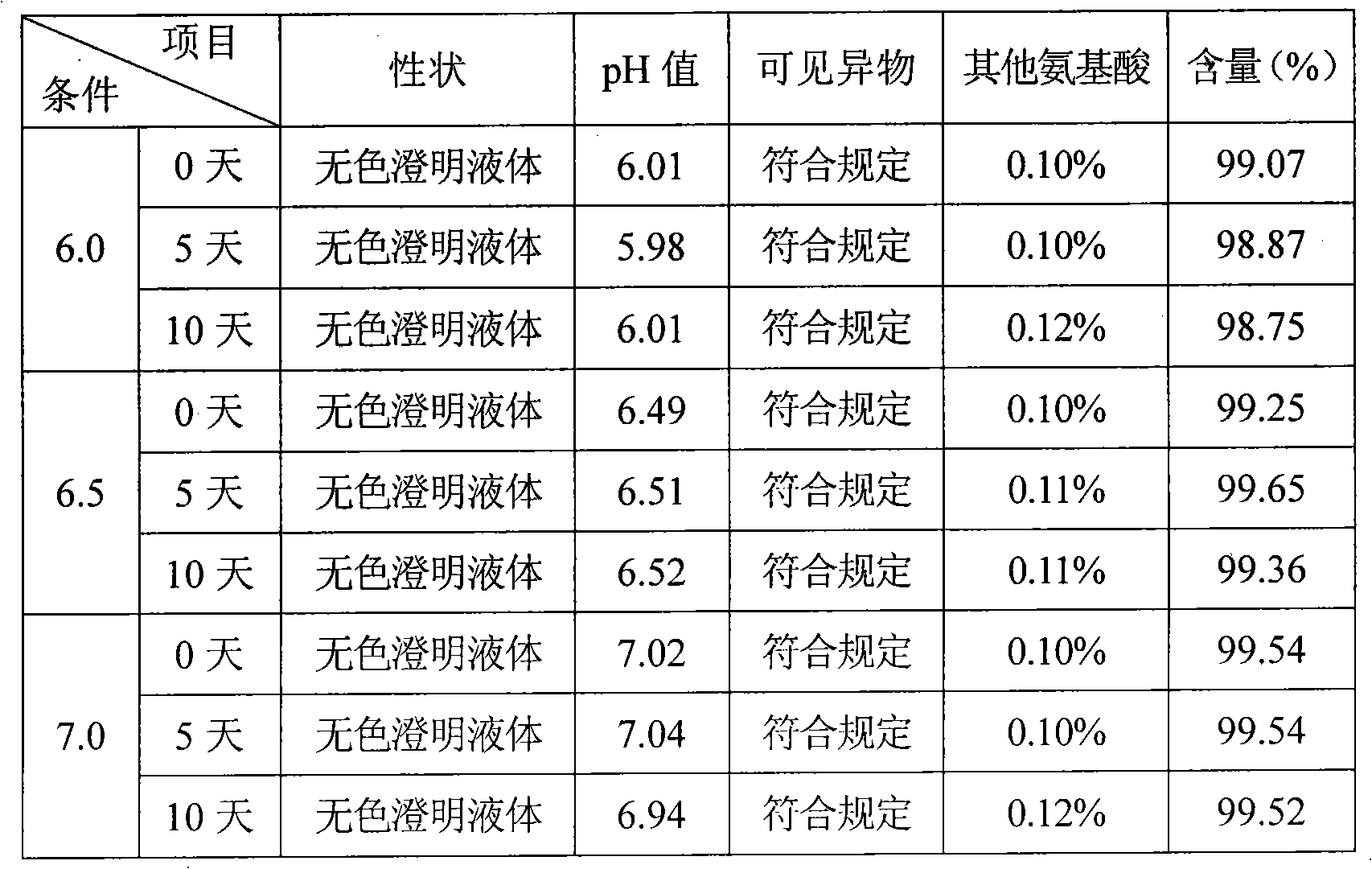
[0031] Embodiment 1, put the refined glutamic acid in the liquid preparation tank, add 9 / 10 prescription water for injection, stir to dissolve, add acid-base buffer solution to adjust the pH value to 6.2-6.5, replenish the water for injection to the full amount, add 0.05% (g / v) Activated carbon, stirred and adsorbed for 15 minutes, decarbonized, finely filtered with 0.45 μm and 0.22 μm microporous membranes, potted, sterilized by circulating steam at 115°C for 10 minutes, leak detection, light inspection, sampling full inspection, Package.
[0032] Functions and Indications: Indications: It is used to treat hyperammonemia caused by acute and chronic liver diseases such as liver cirrhosis, fatty liver and hepatitis, especially for the relief of central nervous system symptoms and rescue of hepatic coma caused by liver diseases.
[0033] Usage and dosage: depending on the patient's condition and rescue situation, 2-20g each time, diluted with glucose injection and injected intra...
Embodiment 2
[0034] Example 2, put refined glutamic acid in the liquid preparation tank, add 9 / 10 prescription water for injection, stir to dissolve, add acid-base buffer to adjust the pH value to 6.2-7.0, replenish the water for injection to the full amount, add 0.1% (g / v) Activated carbon, stirred and adsorbed for 15 minutes, decarbonized, finely filtered with 0.45 μm and 0.22 μm microporous membranes, potted, sterilized by circulating steam at 115°C for 30 minutes, leak detection, light inspection, sampling full inspection, Package.
[0035] Functions and Indications: Indications: It is used to treat hyperammonemia caused by acute and chronic liver diseases such as liver cirrhosis, fatty liver and hepatitis, especially for the relief of central nervous system symptoms and rescue of hepatic coma caused by liver diseases.
[0036] Usage and dosage: depending on the patient's condition and rescue situation, 2-20g each time, diluted with glucose injection and injected intravenously.
Embodiment 3
[0037] Example 3, put the refined glutamic acid in the liquid preparation tank, add 9 / 10 prescription water for injection, stir to dissolve, add acid-base buffer solution to adjust the pH value to 6.2-7.0, replenish the water for injection to the full amount, add 0.1% (g / v) Activated carbon, stirred and adsorbed for 15 minutes, decarbonized, finely filtered with 0.45μm and 0.22μm microporous membranes, potted, sterilized by circulating steam at 115°C for 60 minutes, leak detection, light inspection, sampling full inspection, Package.
[0038] Functions and Indications: Indications: It is used to treat hyperammonemia caused by acute and chronic liver diseases such as liver cirrhosis, fatty liver and hepatitis, especially for the relief of central nervous system symptoms and rescue of hepatic coma caused by liver diseases.
[0039] Usage and dosage: depending on the patient's condition and rescue situation, 2-20g each time, diluted with glucose injection and injected intravenous...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com