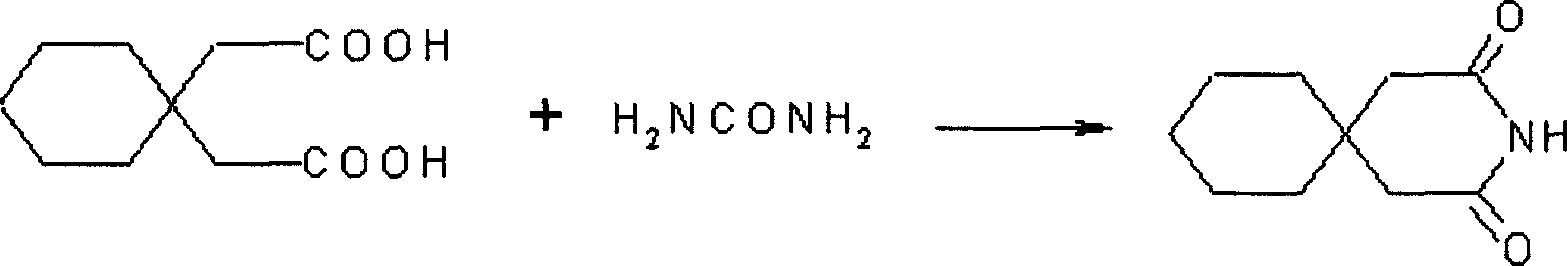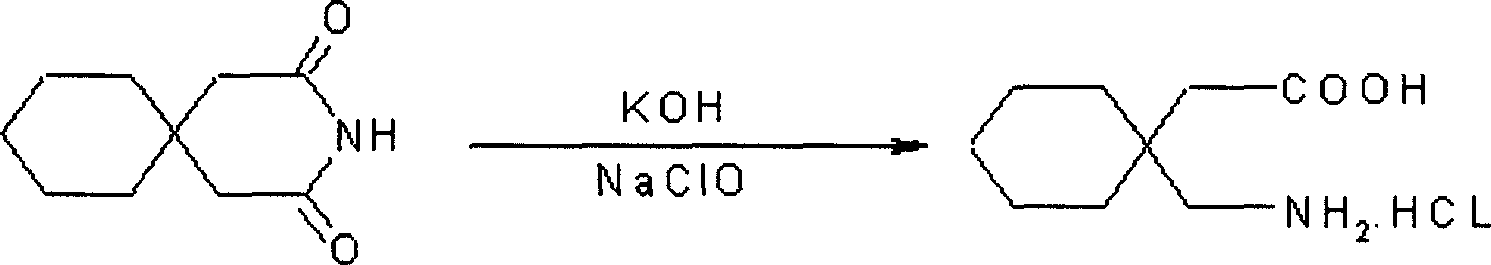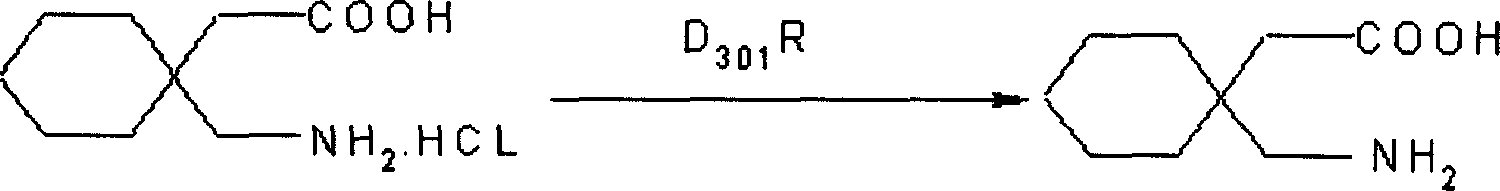Gabapentin hydrochloride and its intermediate preparation method
A technology of gabapentin hydrochloride and hydrochloric acid, which is applied in the preparation of organic compounds, chemical instruments and methods, and cyanide reaction preparation, etc., can solve problems such as being unsuitable for large-scale industrial production, and achieve the advantages of large-scale production, use and reduction in industrialization. Subsequent treatment process, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Mix 200g of 1,1-cyclohexyldiacetic acid and 120g of urea, heat to 150°C, and react for 2h. Slightly cool to room temperature, without filtering, directly add 1500ml of ethanol, water (1:1), heat and reflux for 30 minutes, decolorize activated carbon, filter while it is hot, cool the filtrate, filter, and dry to obtain 3,3-pentamethylene pentamethylene diamide. Yield 95%, mp: 168-172°C.
[0024]Dissolve 180g of 3,3-pentamethylene glutaramide in a 20% solution prepared by 110g of potassium hydroxide and water. It is not necessary to heat when dissolving, leave it at room temperature for 24 hours, cool at 0°C, and add it to the pre-cooled Add 940ml of 10% sodium hypochlorite solution at 0°C, stir for 15 minutes, add 300ml of 40% KOH solution dropwise at the same temperature, continue stirring for 30 minutes, then raise the temperature to 45°C, stir for 2h, cool, and neutralize with 38% hydrochloric acid to pH 2. Filter, dry, and crystallize with isopropanol to obtain ga...
Embodiment 2
[0027] Mix 200g of 1,1-cyclohexyldiacetic acid and 30g of urea, heat to 180°C, react for 3h, cool down to room temperature slightly, add 1500ml of methanol and water (1:1), heat and reflux for 30 minutes, decolorize activated carbon, take advantage of After hot filtration, the filtrate was cooled, filtered, and dried to obtain 3,3-pentamethylene glutaramide.
[0028] Dissolve 180g of 3,3-pentamethylene glutaramide in a 25% solution prepared by 50g of potassium hydroxide, leave it at room temperature for 30 hours, cool to 5°C, add to 940ml of 8% In the sodium hypochlorite solution, stir for 10 minutes, add 420ml of 35% KOH solution dropwise at the same temperature, continue stirring for 40 minutes, then raise the temperature to 70°C, stir for 3 hours, cool, and neutralize to pH 1 with 36% hydrochloric acid. Filter, dry, and crystallize with isopropanol to obtain gabapentin hydrochloride. Yield 70%, mp: 117-118°C.
Embodiment 3
[0030] Mix 200g of 1,1-cyclohexyldiacetic acid and 60g of urea, heat to 140°C, react for 3h, cool down to room temperature slightly, add 1500ml of ethanol, water (2:1) mixed solution, heat and reflux for 30 minutes, activated carbon decolorization , Filtrate while hot, the filtrate is cooled, filtered, and dried to obtain 3,3-pentamethylene glutaramide.
[0031] Dissolve 180g of 3,3-pentamethyleneglutaramide in a 20% solution prepared by 250g of sodium hydroxide, leave it at room temperature for 26 hours, cool, and add to 940ml of 12% pre-cooled to 0°C at 5°C In the sodium hypochlorite solution, stir for 20 minutes, add 420ml of 30% sodium hydroxide solution dropwise at the same temperature, continue stirring for 40 minutes, then raise the temperature to 45°C, stir for 3h, cool, and neutralize to pH 1 with 40% hydrochloric acid. Filter, dry, and crystallize with isopropanol to obtain gabapentin hydrochloride. Yield 80%, mp: 117-118°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com