Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
75results about How to "Significant variability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Controlled regional oral delivery
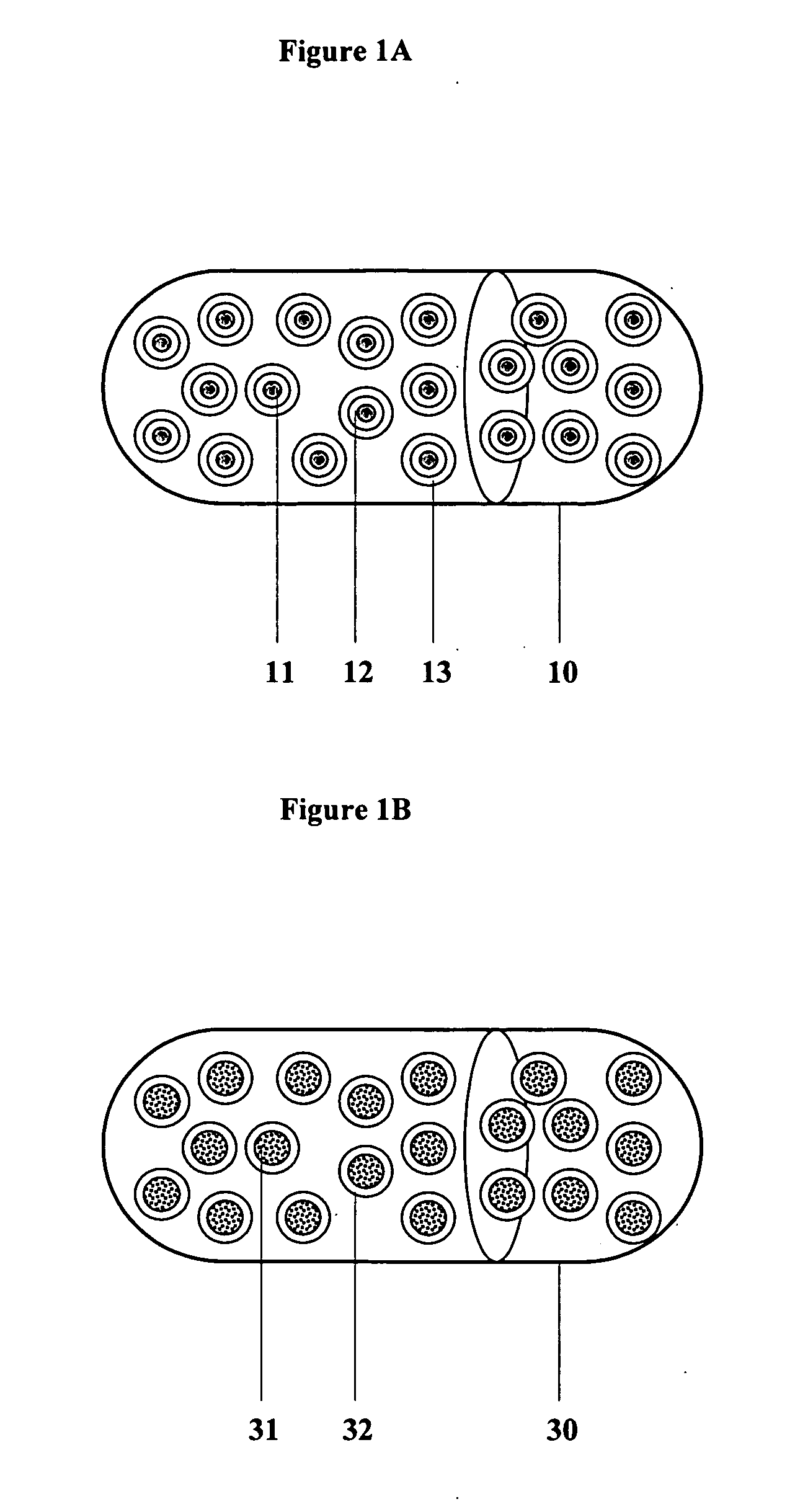
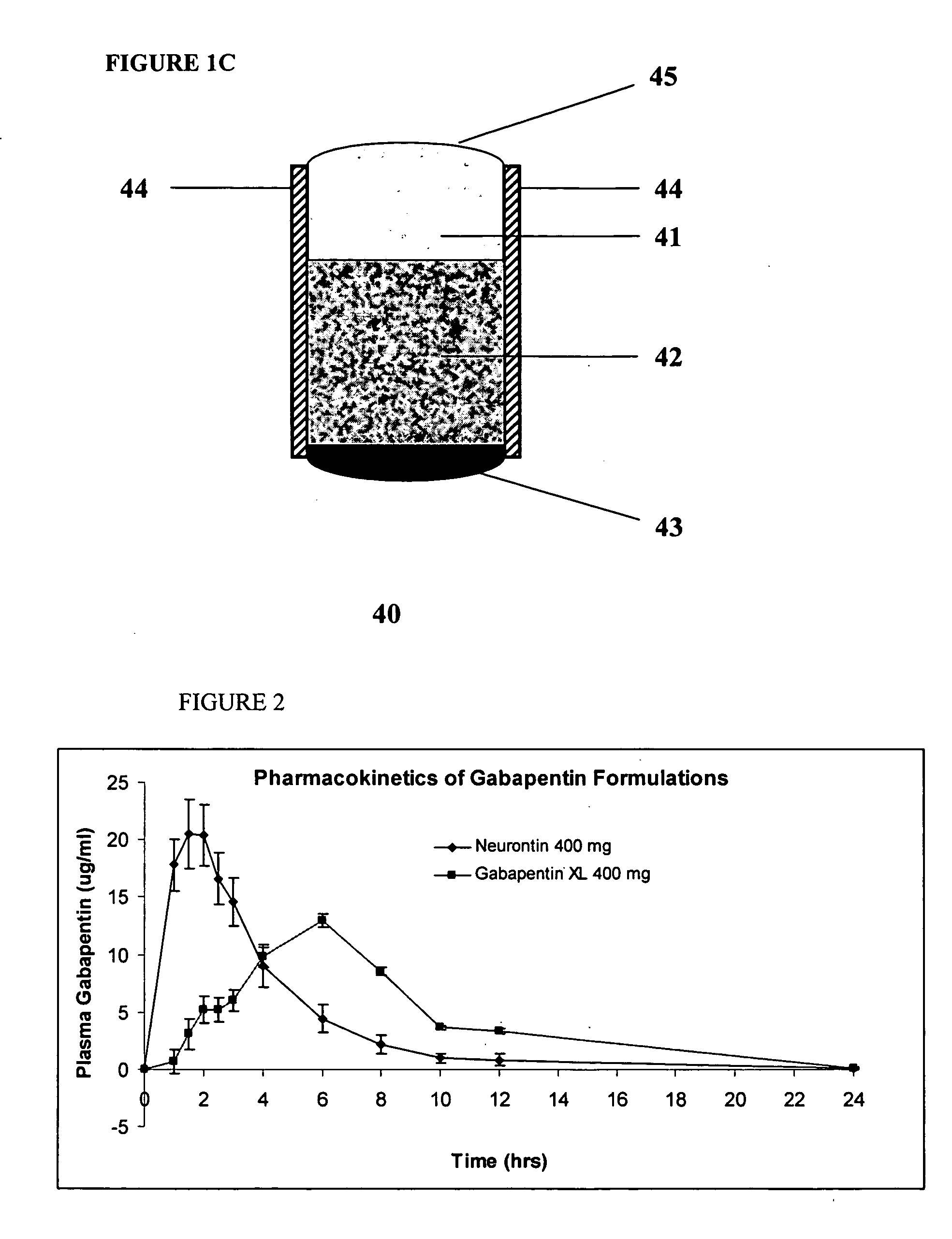
InactiveUS20060045865A1Significant variabilityLow variabilityPill deliveryGranular deliverySolubilityGabapentin
A composite formulation has been developed for selective, high efficacy delivery to specific regions of the mouth and gastrointestinal tract. The formulation is typically in the form of a tablet or capsule, which may include microparticles or beads. The formulation uses bioadhesive and controlled release elements to direct release to specific regions, where the drug is absorbed in enhanced amounts relative to the formulation in the absence of the bioadhesive and / or controlled release elements. This is demonstrated by an example showing delivery of gabapentin with a greater area under the curve (“AUC”) relative to the FDA reference immediate release drug, i.e., the AUC of the composite bioadhesive formulation is greater than 100% of the AUC of the immediate release drug. In the preferred embodiments, the formulation includes drug to be delivered, controlled release elements, and one or more bioadhesive elements. The bioadhesive polymer may be either dispersed in the matrix of the tablet or applied as a direct compressed coating to the solid oral dosage form. The controlled release elements are selected to determine the site of release. The bioadhesive components are selected to provide retention of the formulation at the desired site of uptake and administration. By selecting for both release and retention at a specific site, typically based on time of transit through the gastrointestinal tract, one obtains enhanced efficacy of uptake of the drug. This is particularly useful for drugs with narrow windows of absorption, and drugs with poor solubility such as the BCE class III and class IV drugs.
Owner:VAUNNEX
Pedestrian Detection
InactiveUS20070230792A1Improve discriminationImprove abilitiesCharacter and pattern recognitionMachine learningPedestrian detection
Owner:MOBILEYE TECH
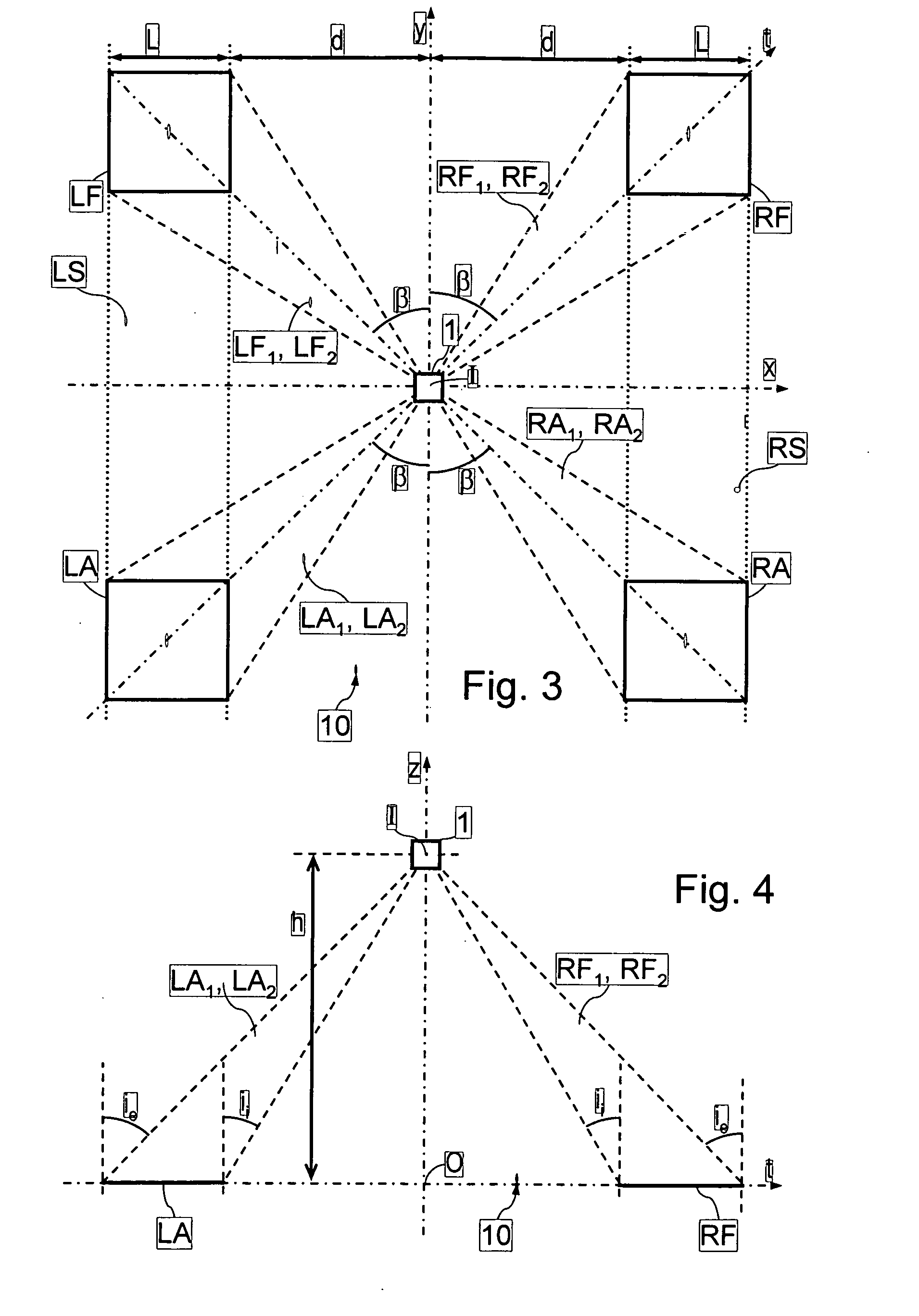
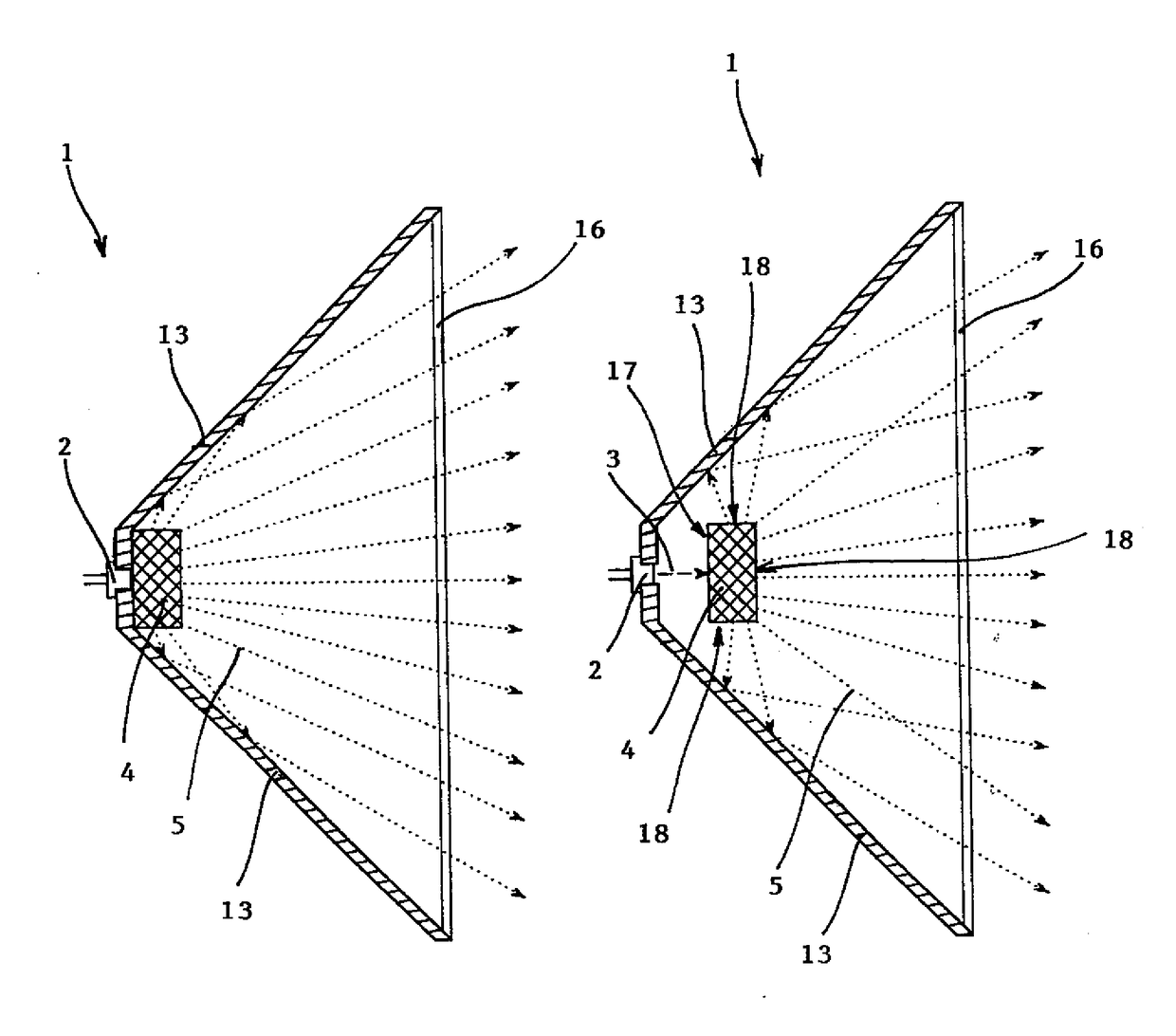
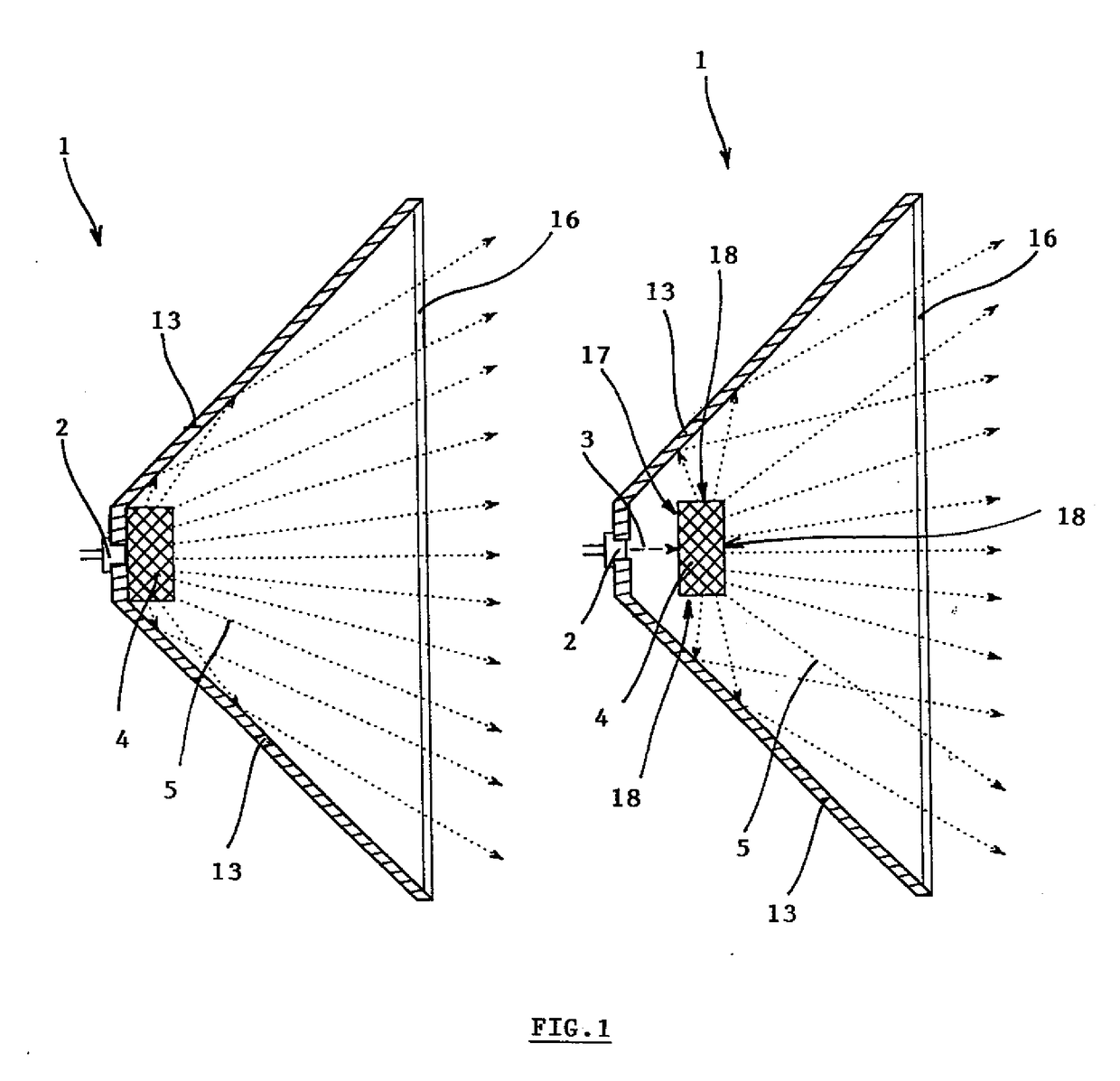
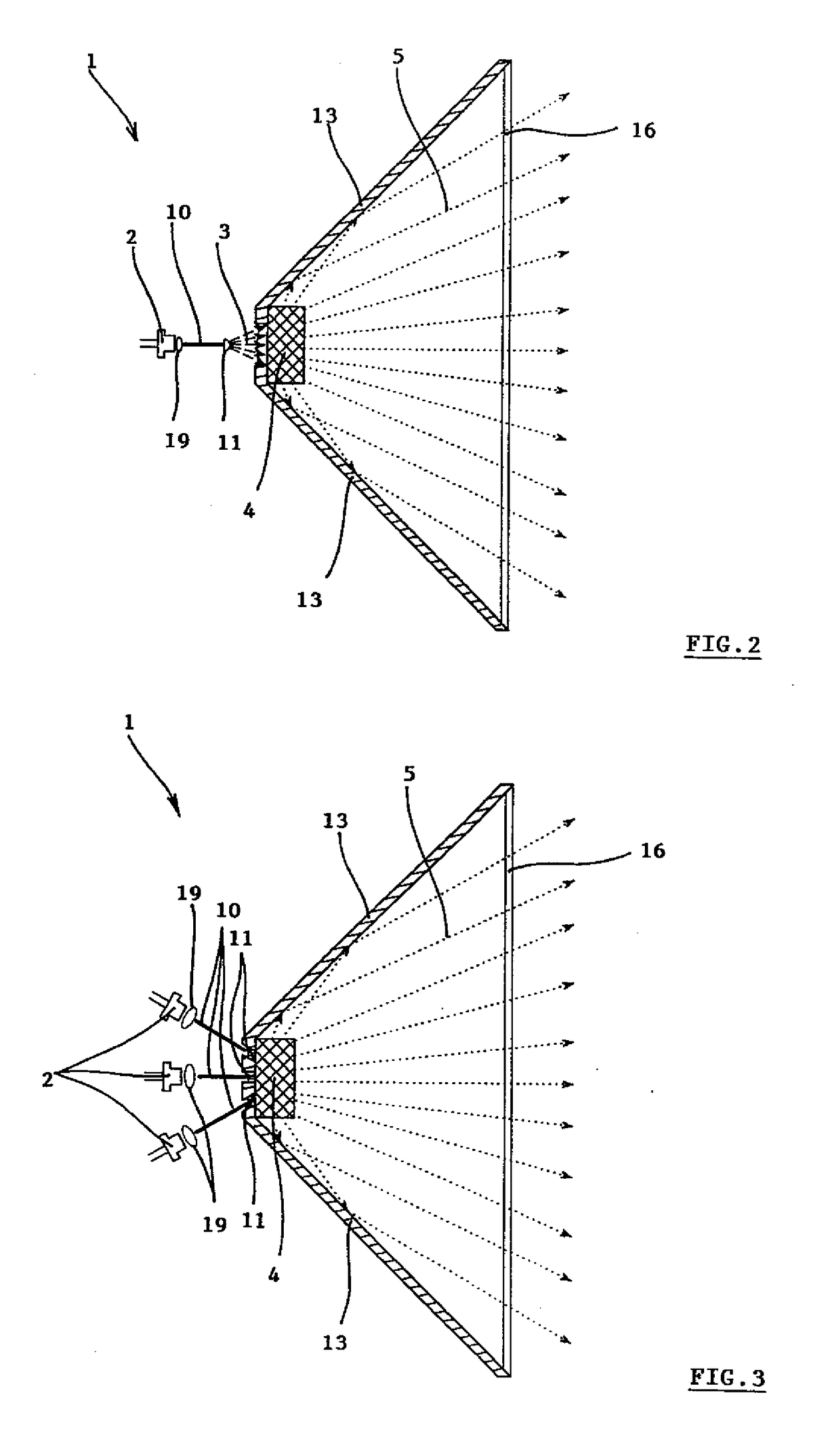
Dielectric antenna
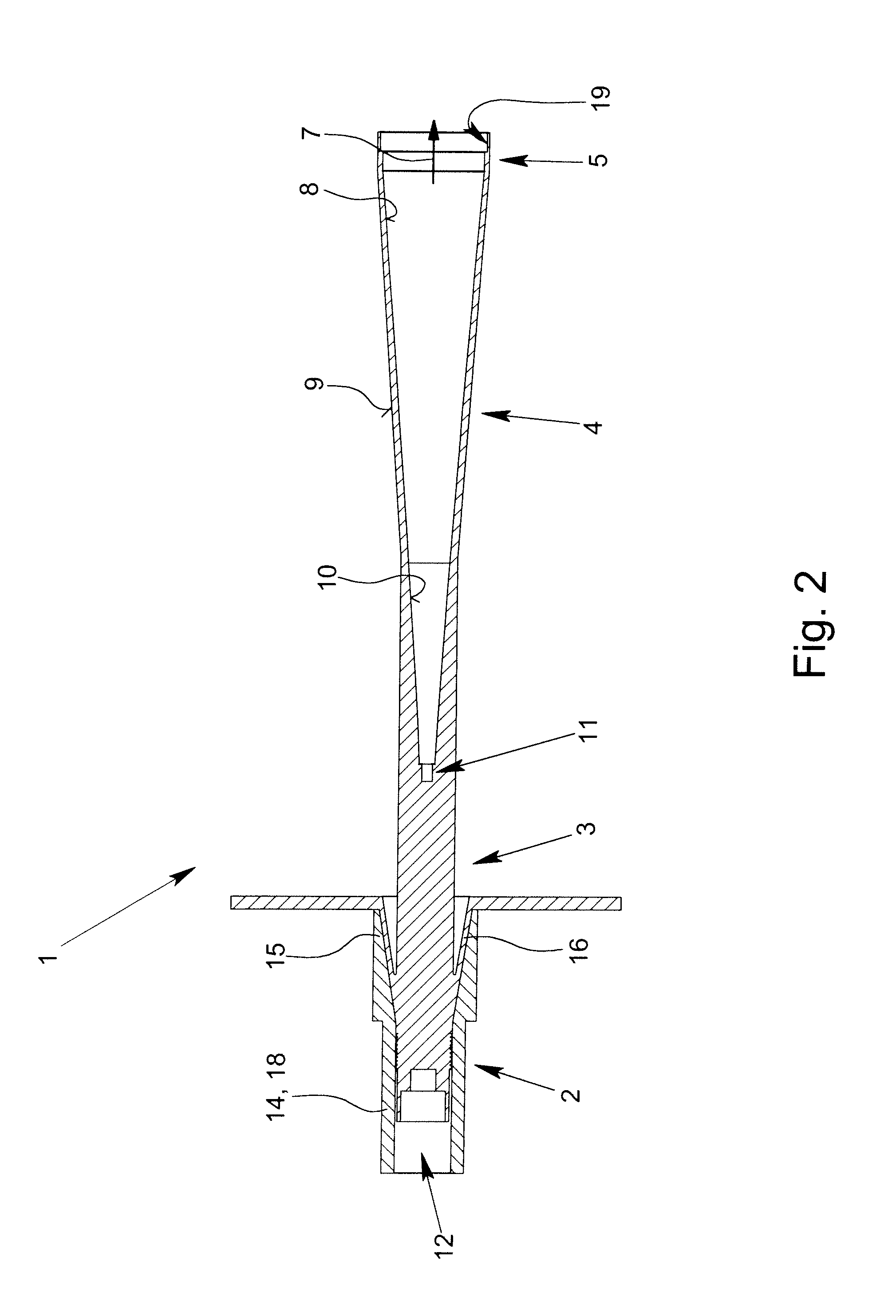
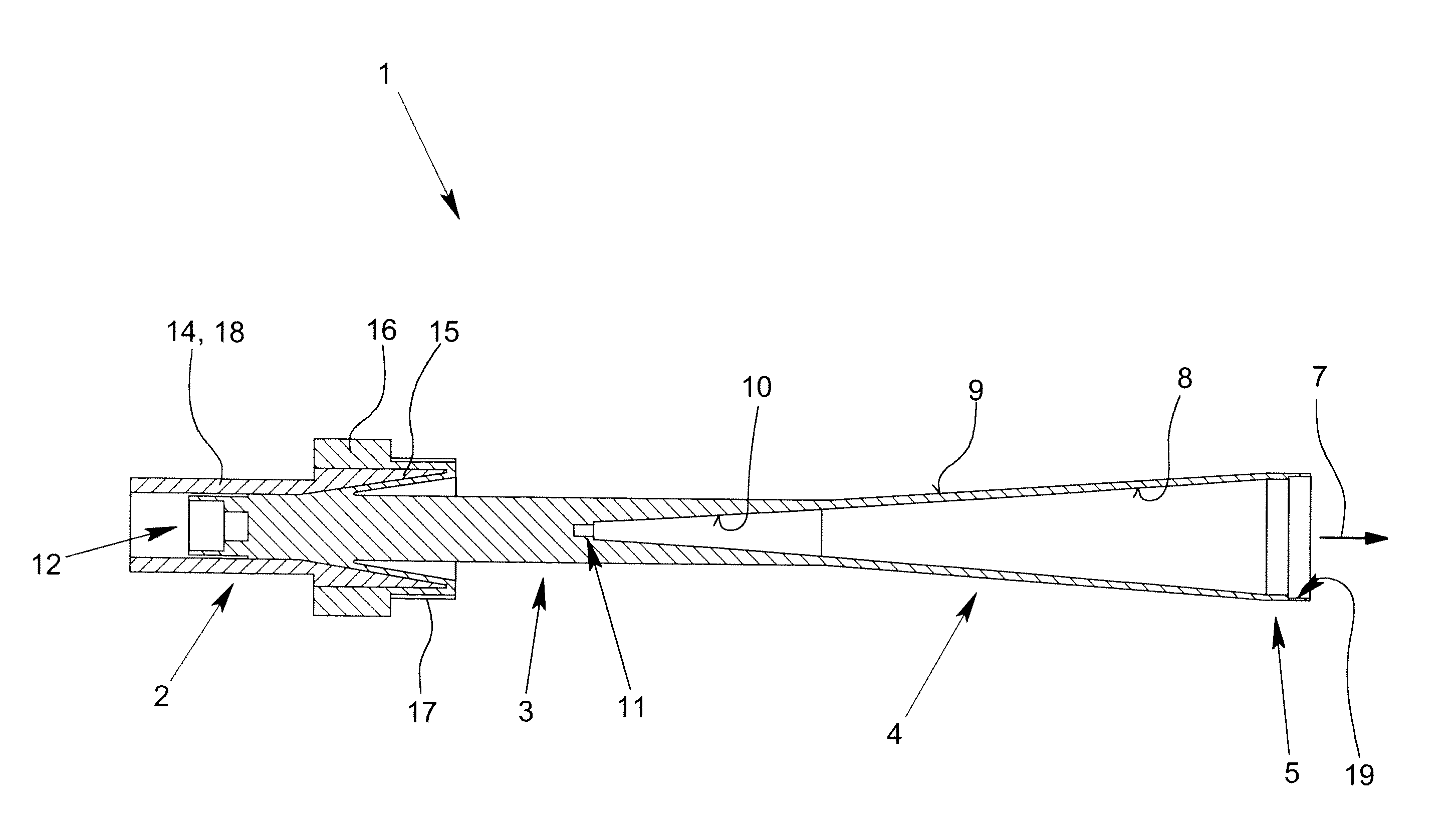
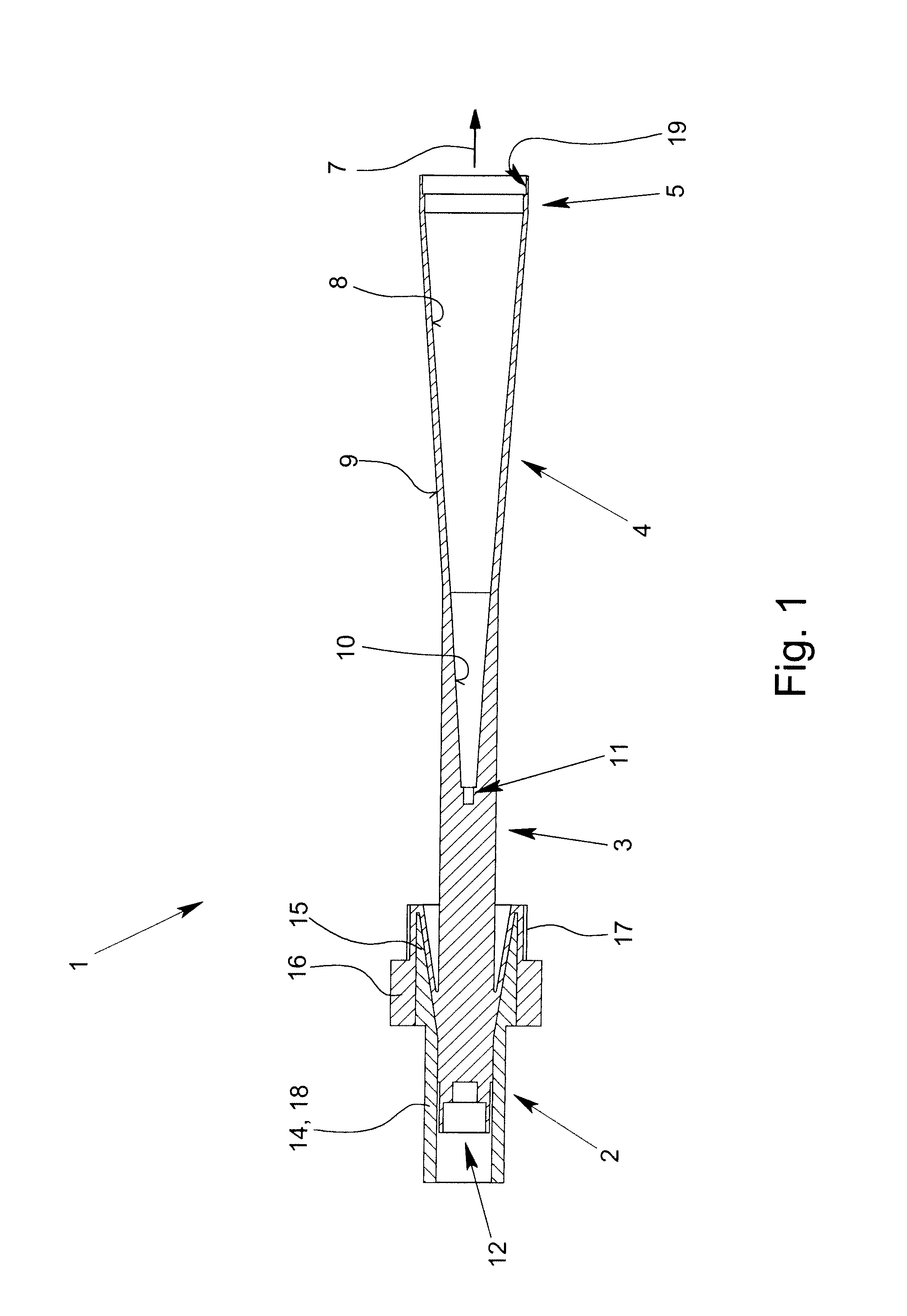
ActiveUS8354970B2Highly bundlingSignificant variabilityWaveguide hornsAntenna detailsElectromagnetic radiationDielectric tube
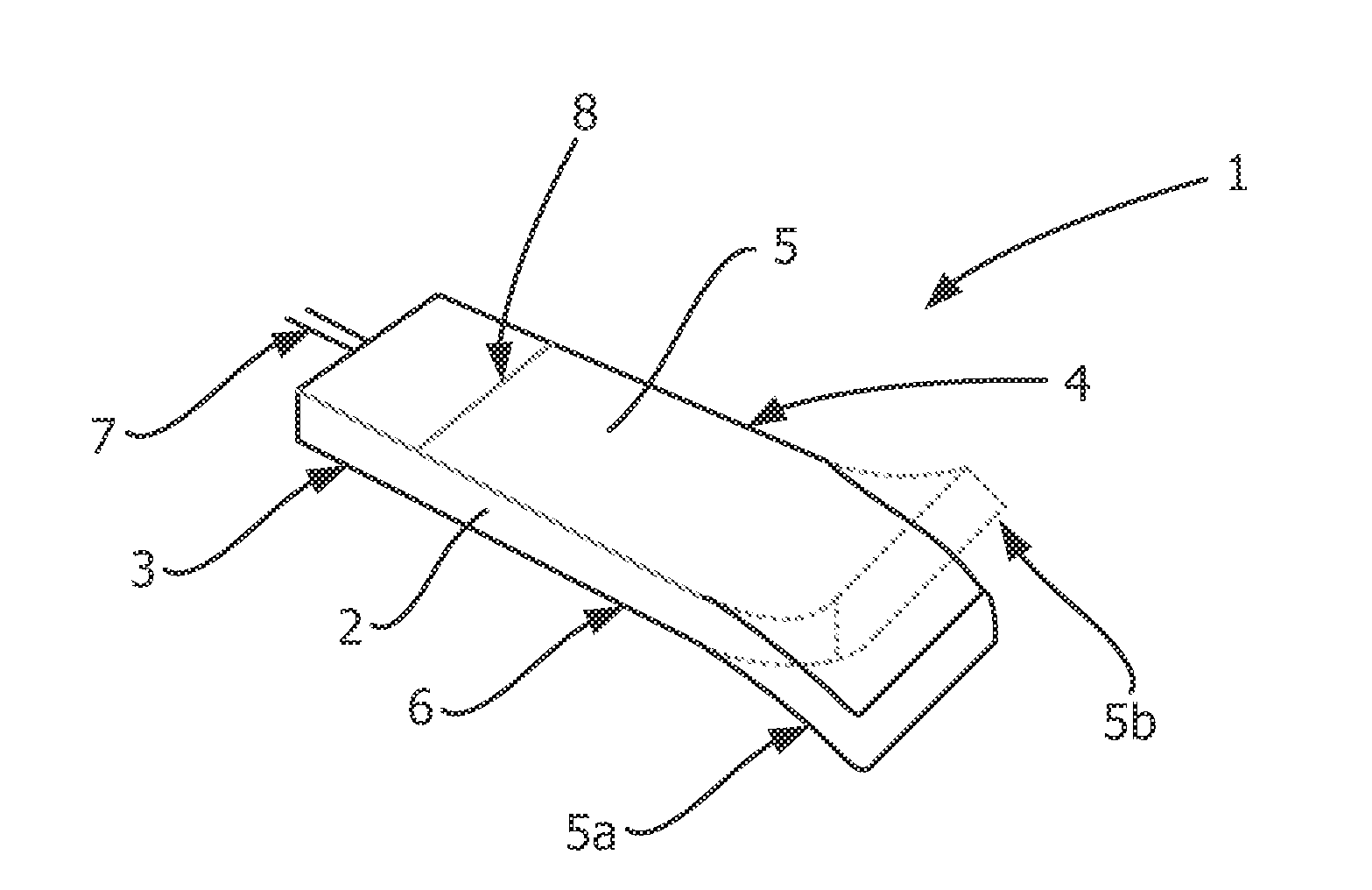
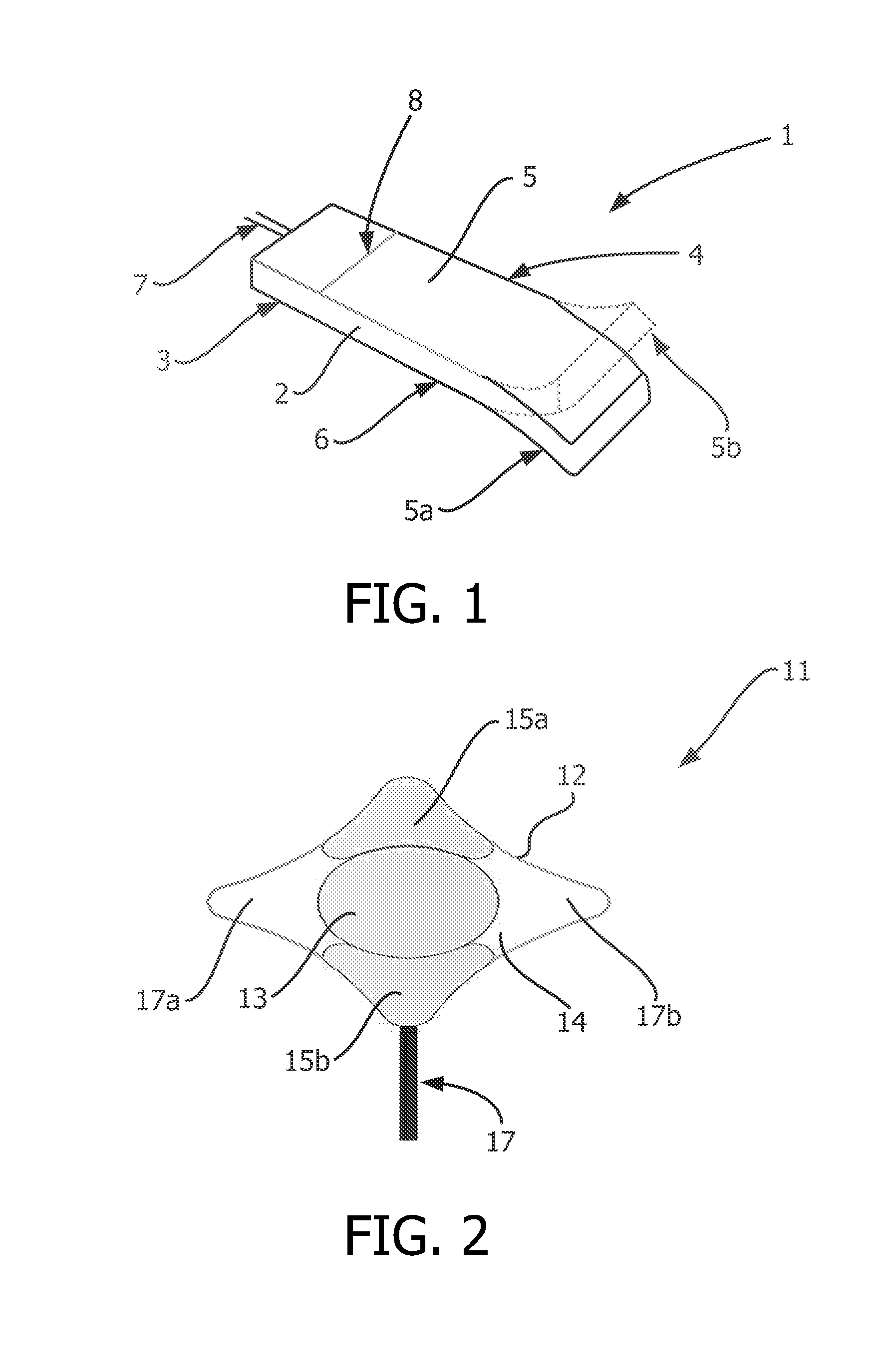
Described and shown is a dielectric antenna (1) having a dielectric feeding section (2), a first transition section (3) comprising a dielectric rod, a dielectric emitting section (5) and, a further, second transition section (4) forming a dielectric horn, wherein the feeding section (2) can be struck with electromagnetic radiation (6), electromagnetic radiation (6) can be guided with the first transition section (3) and the second transition section (4) and the electromagnetic radiation can be emitted from the emitting section (5) as airborne waves.The object of the present invention is to provide a dielectric antenna, which is adaptable as low-loss as possible to different mounting situations, which additionally is as low-reflection as possible and, at the same time is highly bundling.The object of the above-mentioned dielectric antenna is met in that the emitting section (5) is designed as dielectric tube connecting to the second transition section (4).
Owner:KROHNE MESSTECHNICK GMBH & CO KG
Electronic variable stroke devices and system for remote control and interactive play
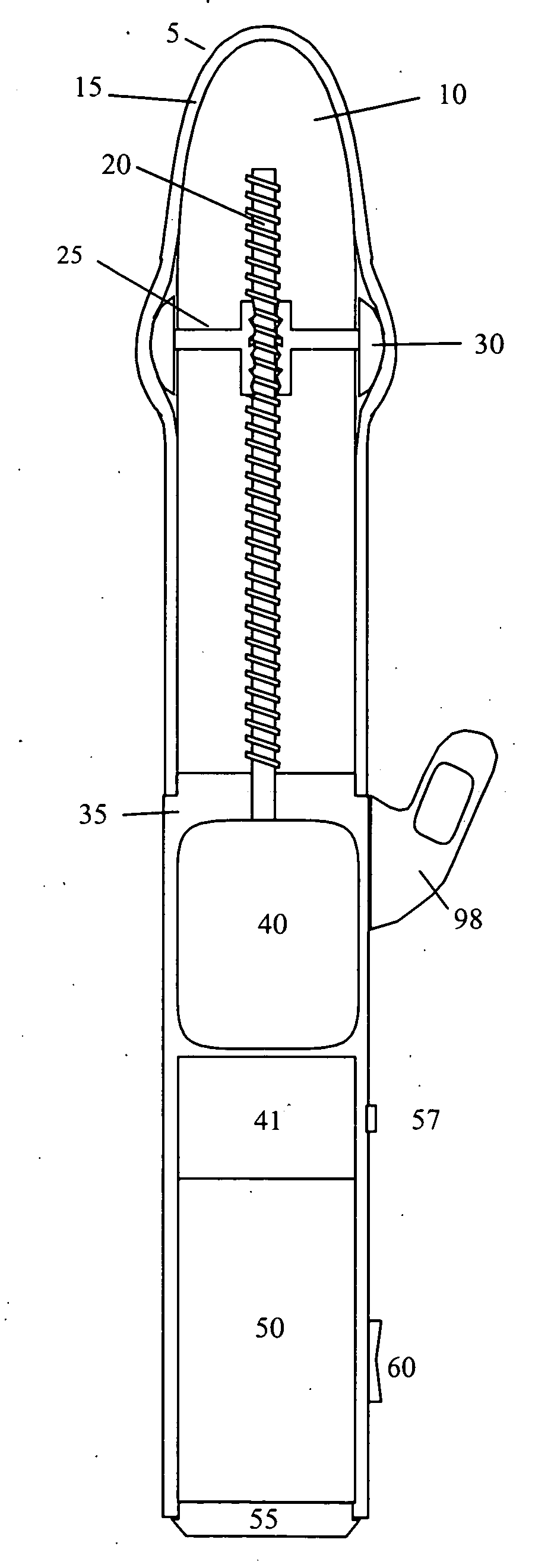
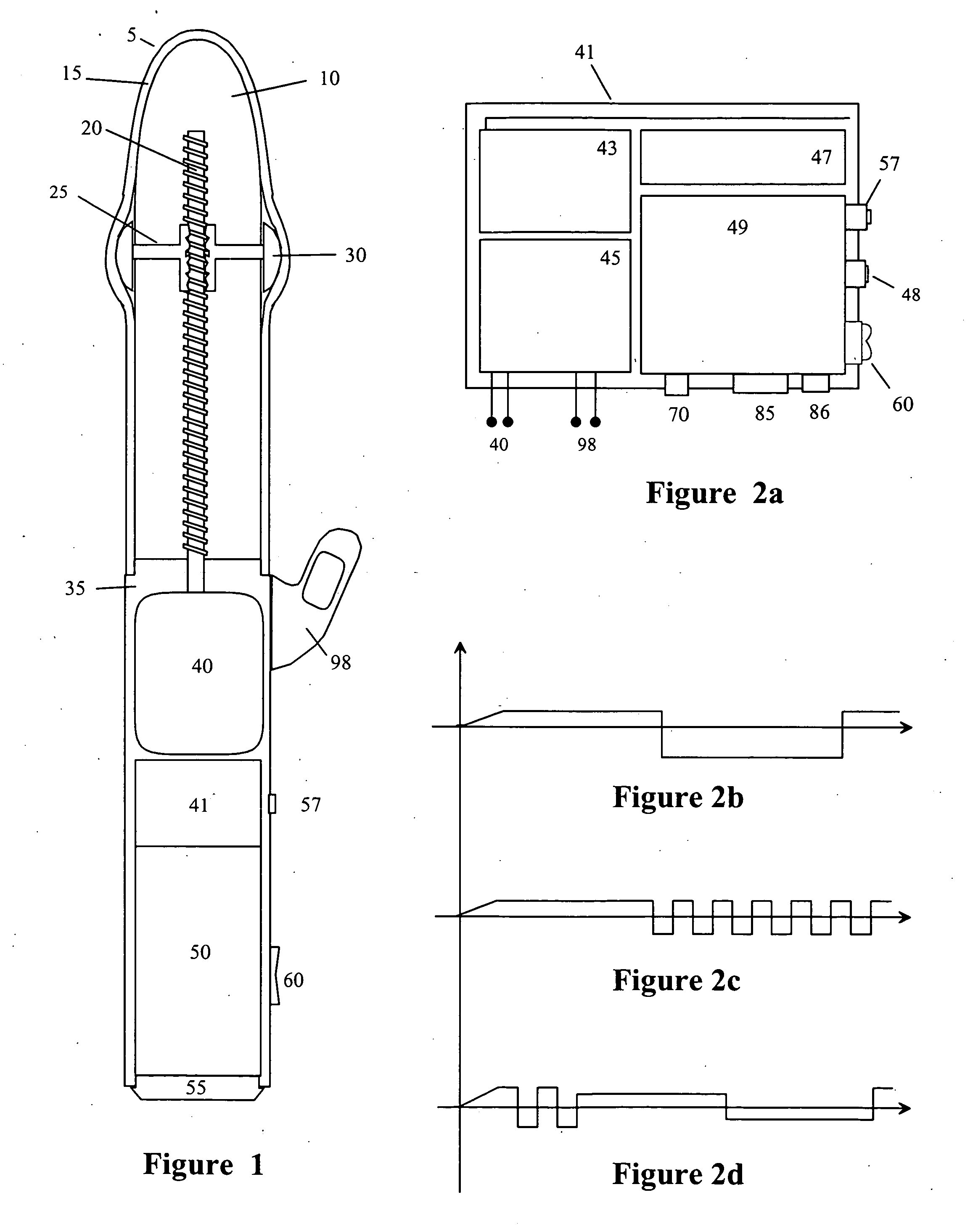
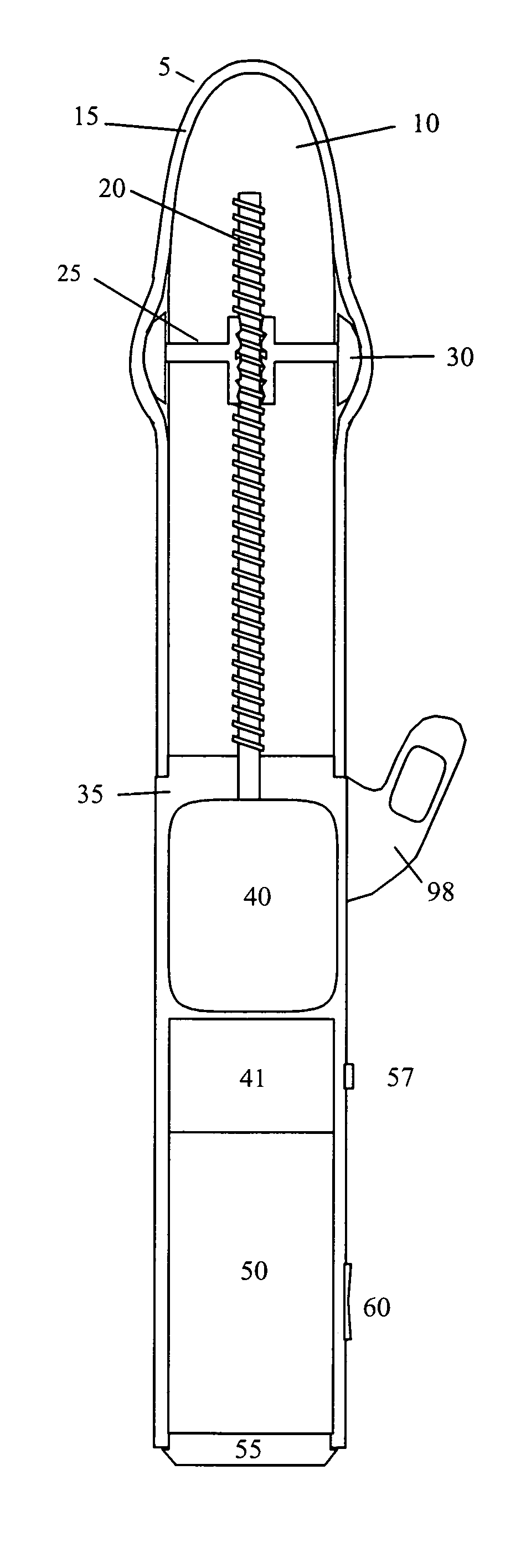
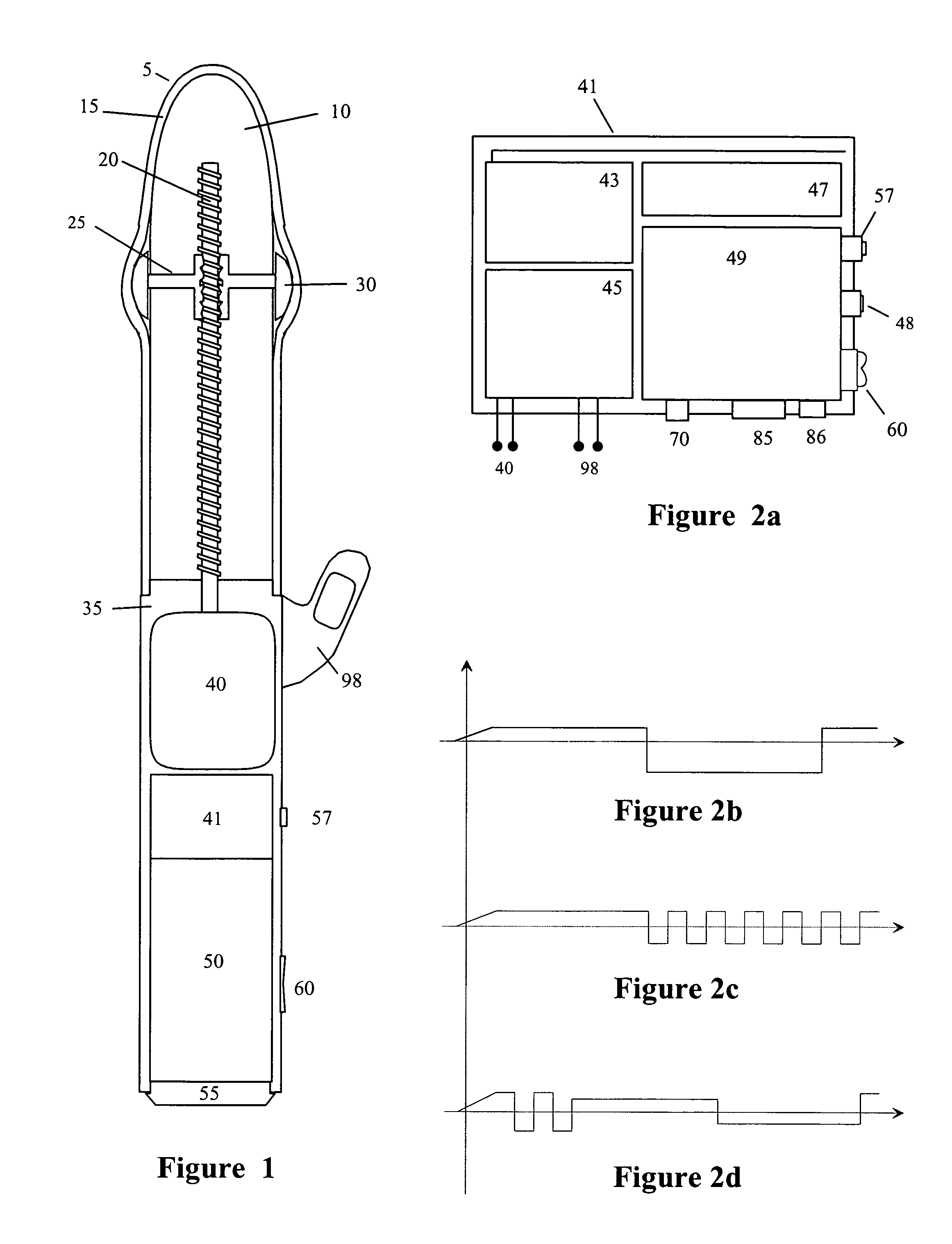
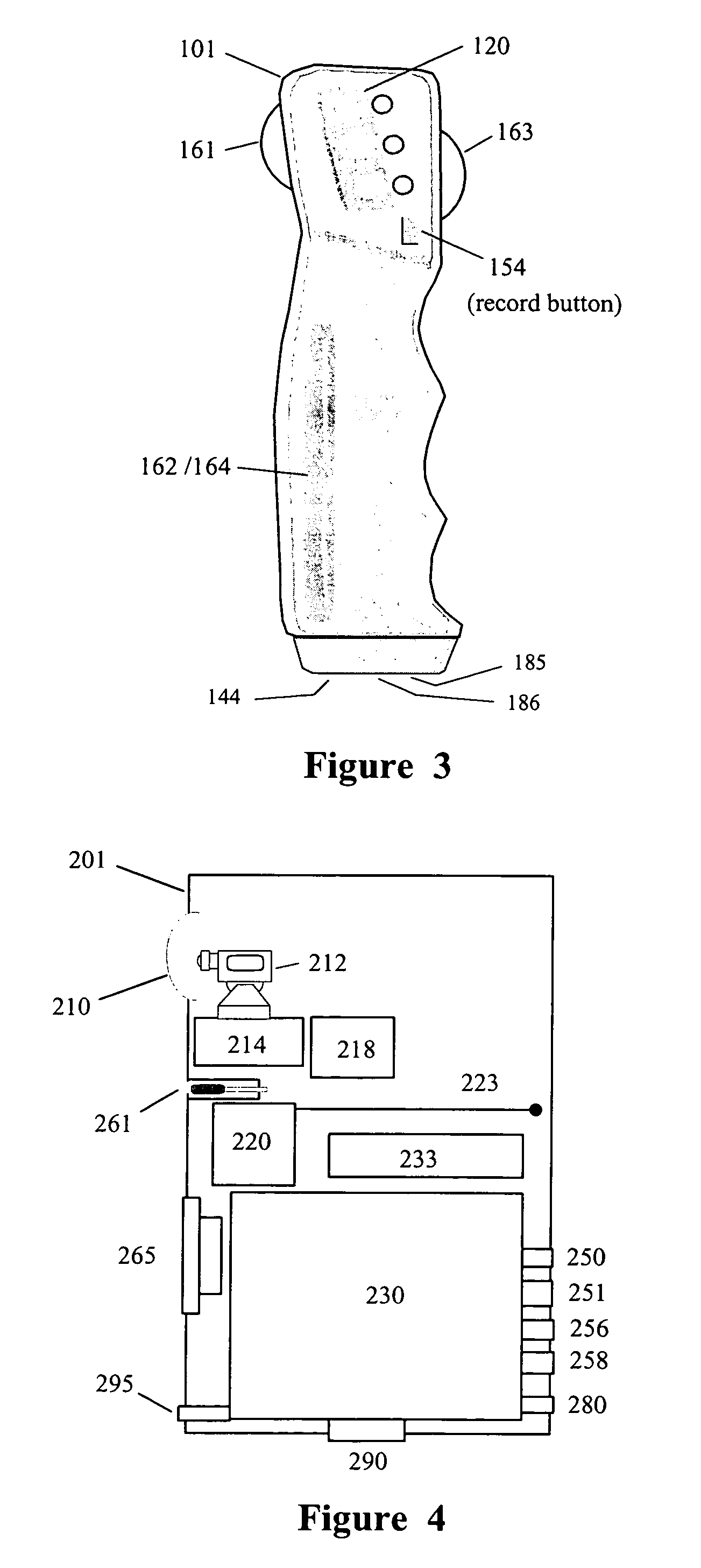
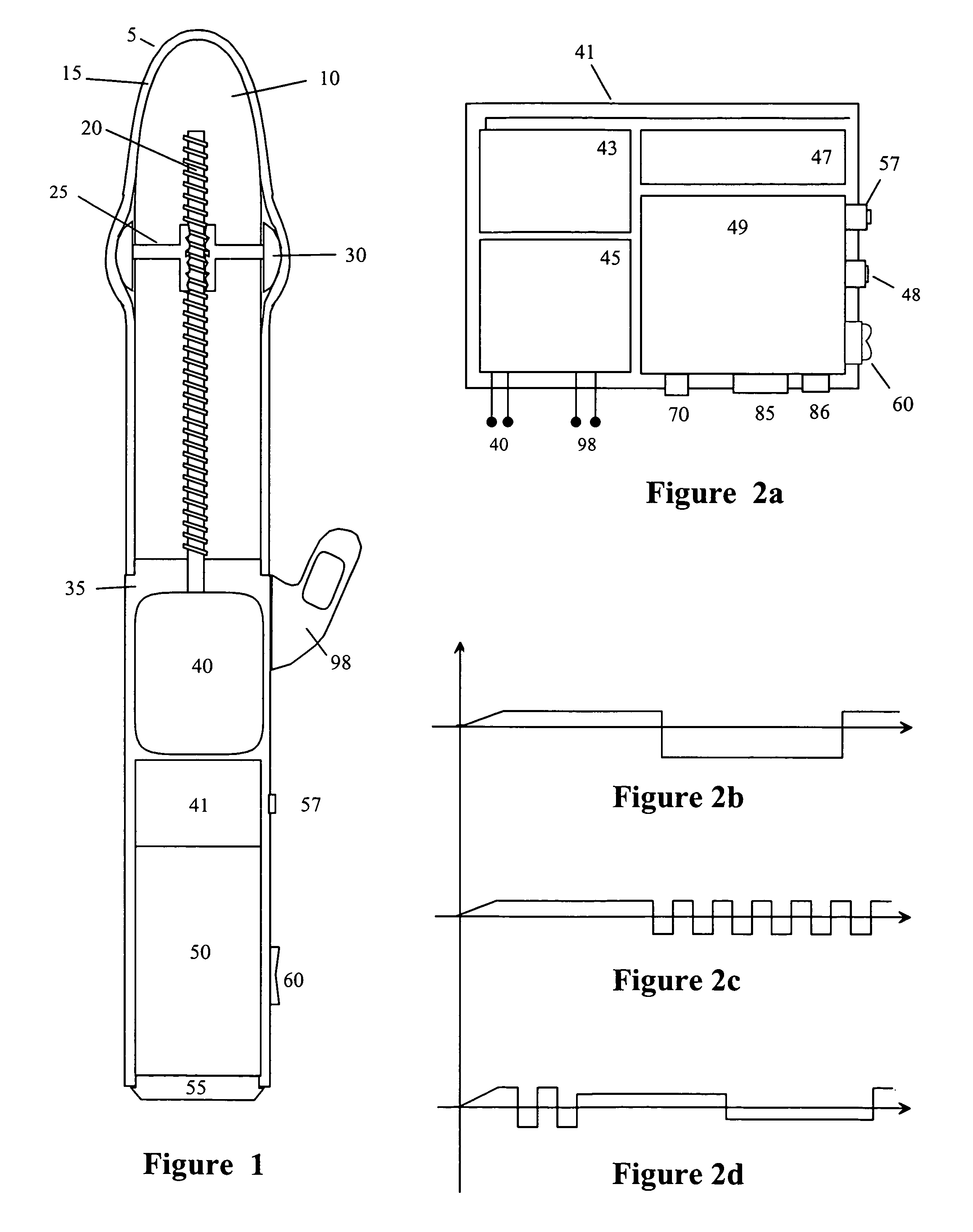
InactiveUS20090099413A1Significant variabilityImprove the environmentVibration massageNon-surgical orthopedic devicesMotor driveControl signal
An electronic variable stroke device comprise a base portion containing a motor-driven screw shaft, an upper portion extending from the base portion having the screw shaft extending longitudinally therein, a traveler engaged with the screw shaft to drive it in reciprocating longitudinal motion. The traveler has an annular shape with an aperture therethrough which is driven in reciprocating longitudinal motions for use as a male sex toy. The device configuration may include a pair of screw threads spaced apart in parallel with the traveler engaged in between them, and / or multiple travelers arranged at different longitudinal positions of the screw shaft(s). A remote controller unit may be provided for ergonomic operation by the user. A network connection unit may be provided to connect the user's device to an external service provider on a network for conducting interactive sessions remotely with another user or users. The device may be adapted as a male toy that can exchange control signals with another user operating a female toy.
Owner:KOBASHIKAWA ALVIN Y +1
Electronic variable stroke device and system for remote control and interactive play
InactiveUS7438681B2Significant variabilityImprove the environmentNon-surgical orthopedic devicesGenitals massageMotor driveReciprocating motion
Owner:KOBASHIKAWA ALVIN Y +1
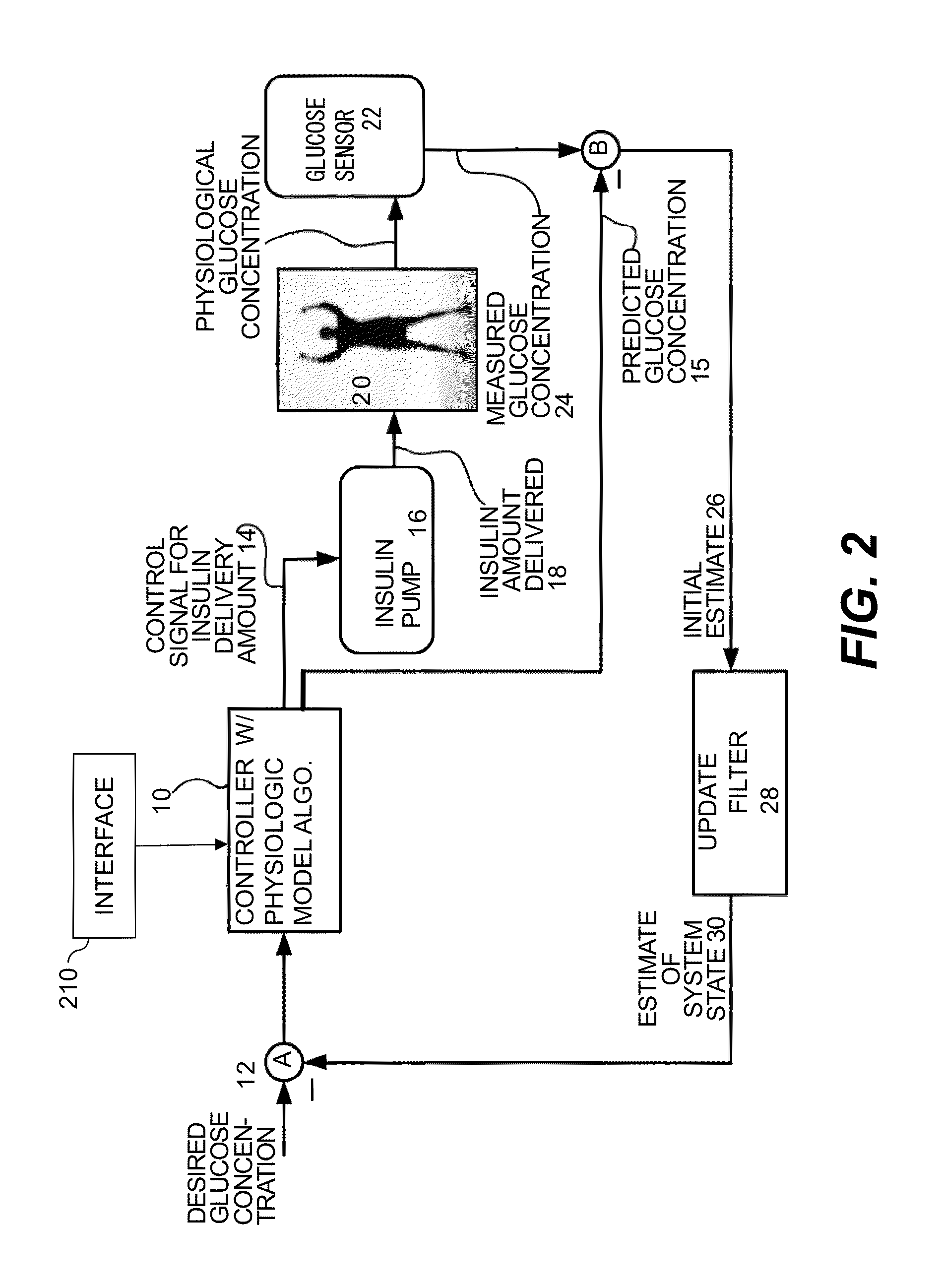
Method and system for closed-loop control of an artificial pancreas
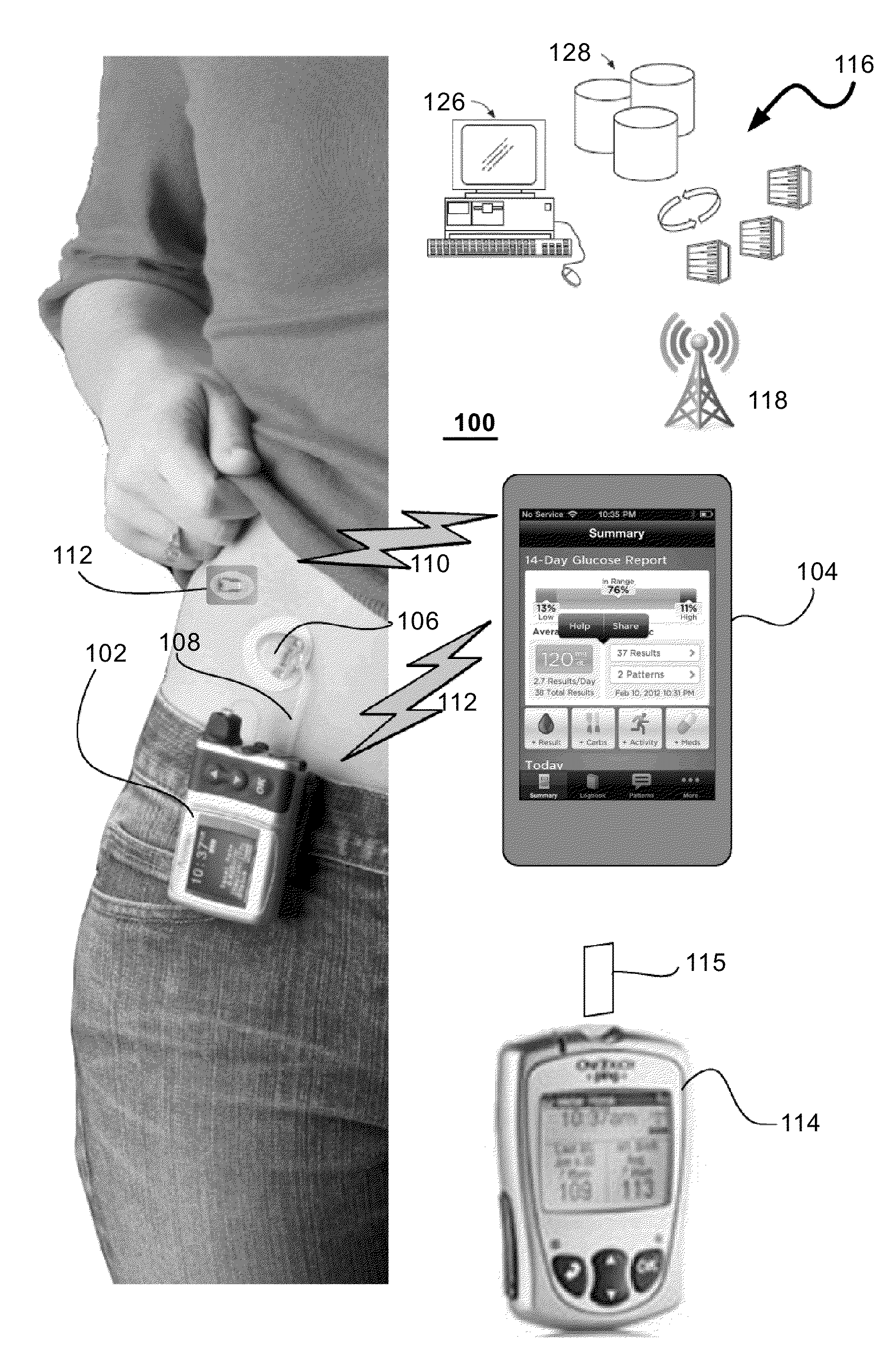
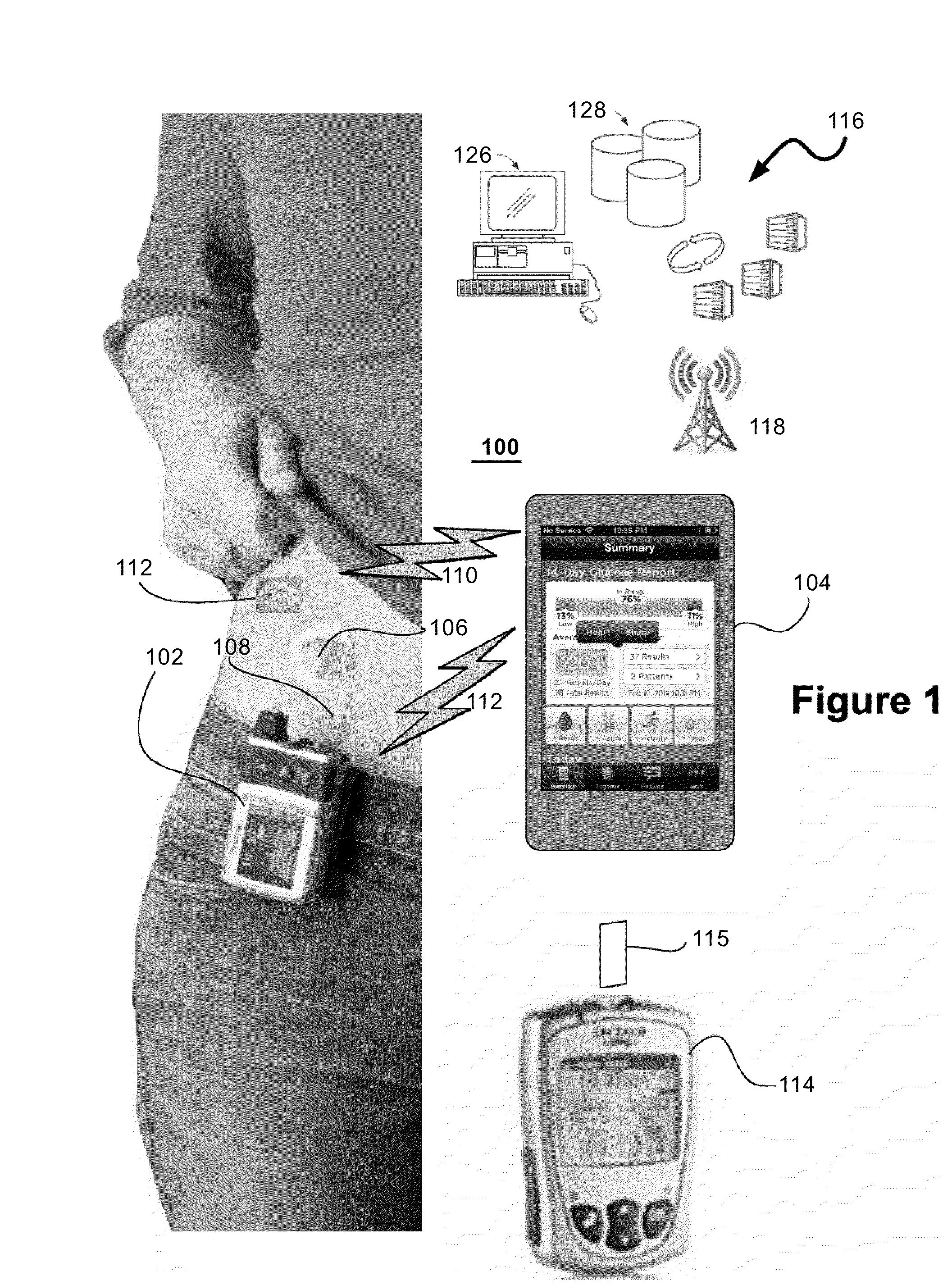
InactiveUS20140276554A1Significant variabilityMaintain blood sugar levelsMedical simulationDrug and medicationsInsulin pumpTime range
Methods and systems for controlling an insulin pump in response to glucose measurements are responsive to a base insulin delivery profile and a temporary insulin delivery profile. These can be used, e.g., to control blood glucose level of a subject using a continuous glucose monitor and an insulin infusion pump. During a selected time range, an insulin amount for the pump to supply is determined using the temporary insulin delivery profile. Outside that time range, the insulin amount is determined using the base insulin delivery profile. The temporary insulin delivery profile can specify an exact amount to be supplied (a “hard” profile), a nominal amount to be supplied if doing so does not drive glucose out of a desired zone (“soft”), or a soft profile with a minimum amount of insulin to be delivered (“semi-soft”).
Owner:JDRF INT +1
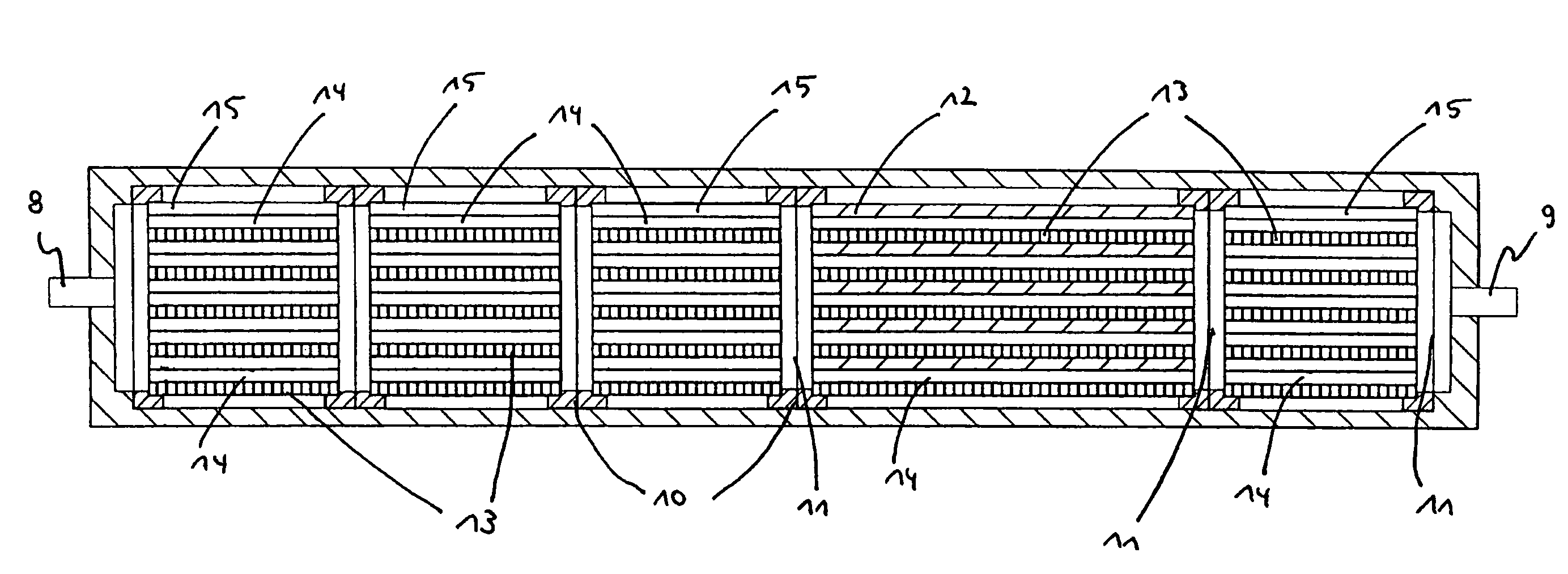
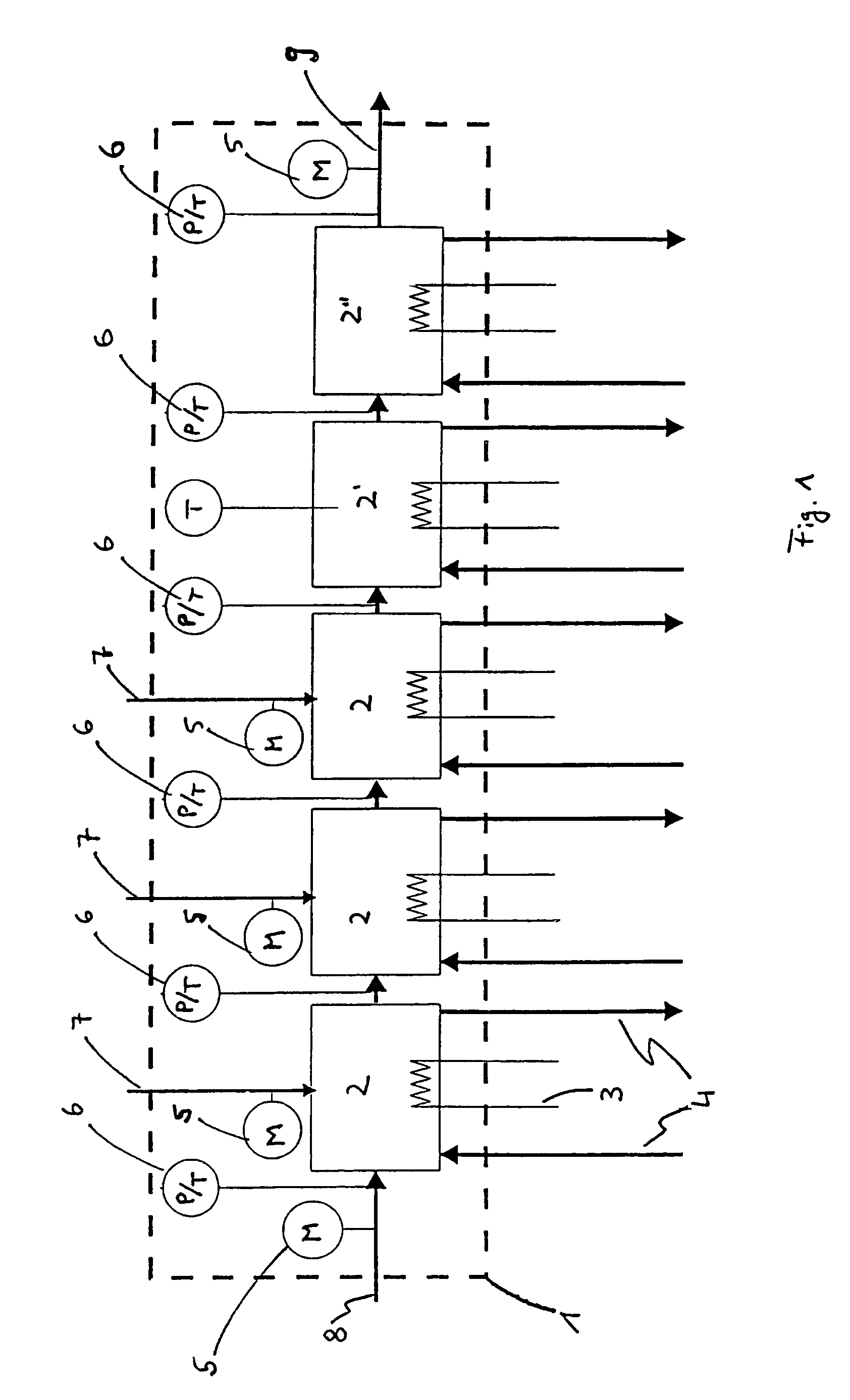
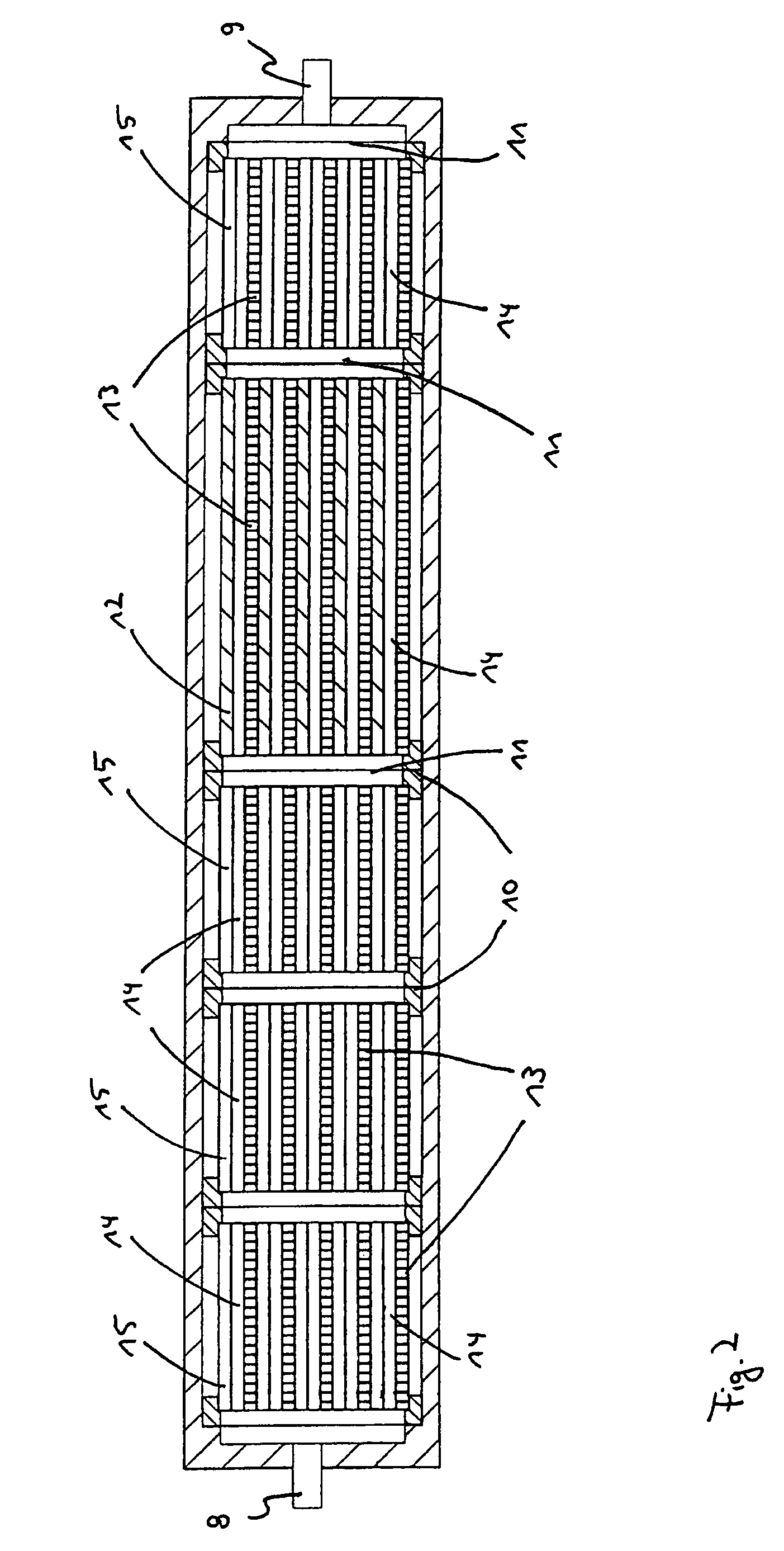
Modular microreaction system
InactiveUS7172735B1Save considerable costSignificant variabilityFlow mixersPiezoelectric/electrostriction/magnetostriction machinesEngineeringHeating element
A modular microreaction system comprising a housing and functional base modules accommodated therein. The housing has at least one fluid inlet and at least one fluid outlet. The base modules are arranged one behind another in a row in the housing and being designed such that fluid can flow successively through them in series. At least some of the base modules are constructed from a plurality of substantially rectangular foils having plate-like surfaces in essentially parallel planes which foils are connected to one another and are arranged in layers one above another, forming a foil stack. At least a first of the foils having at least one of microstructured channels, sensor elements, heating elements and combinations thereof on a plate-like surface of the at least a first of the foils. Each foil stack also has at least one foil which is provided on a plate-like surface of the at least one foil with channels which are constructed such that for one fluid line they lead from one side of the foil stack to another side of the foil stack. The base modules (2, 2′, 2″) each have at least one frame element (10), which is arranged essentially perpendicular to planes of the foils, and is connected to the foil stack in a fluid-tight manner and the foil stacks, together with the frame elements, form base elements that can be inserted into and removed from the housing (1) as a unit.
Owner:IMM INST FUR MIKROTECHNIK GMBH
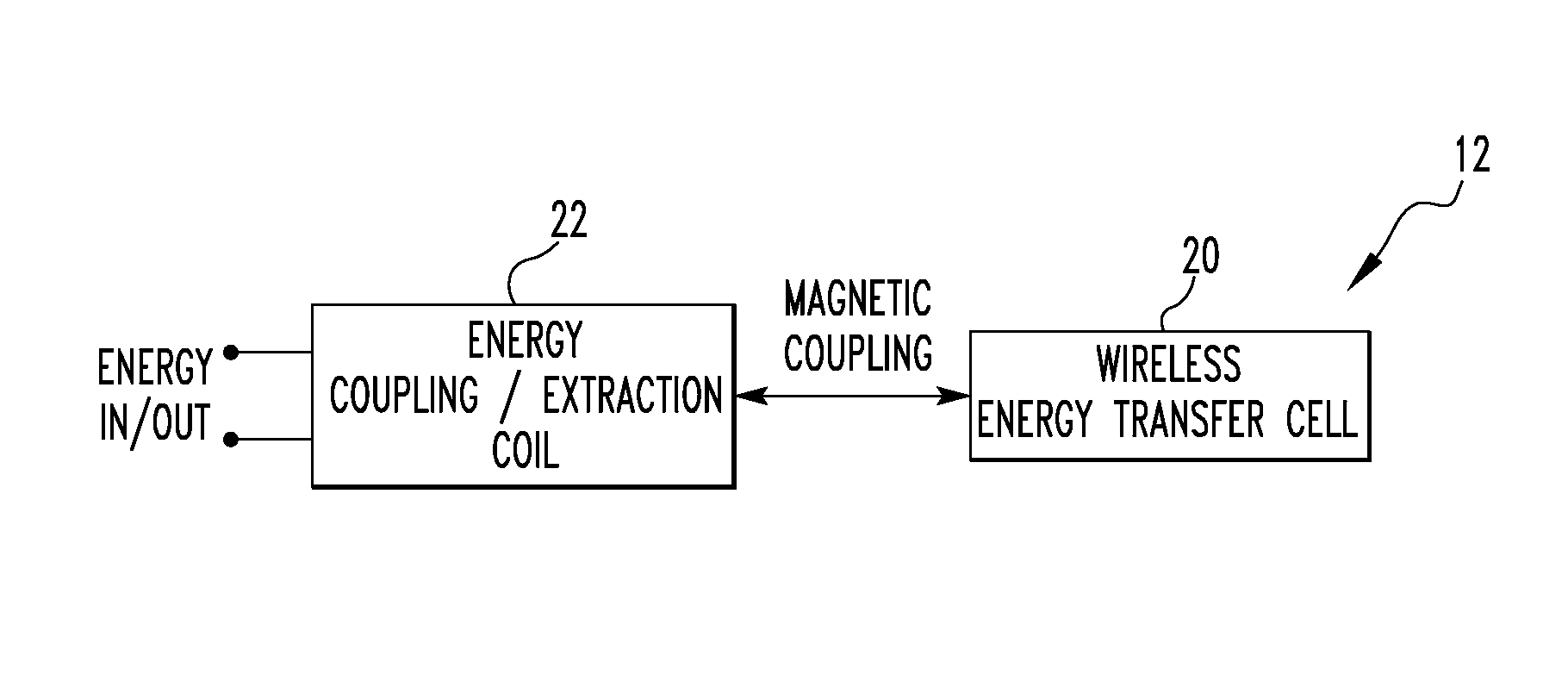
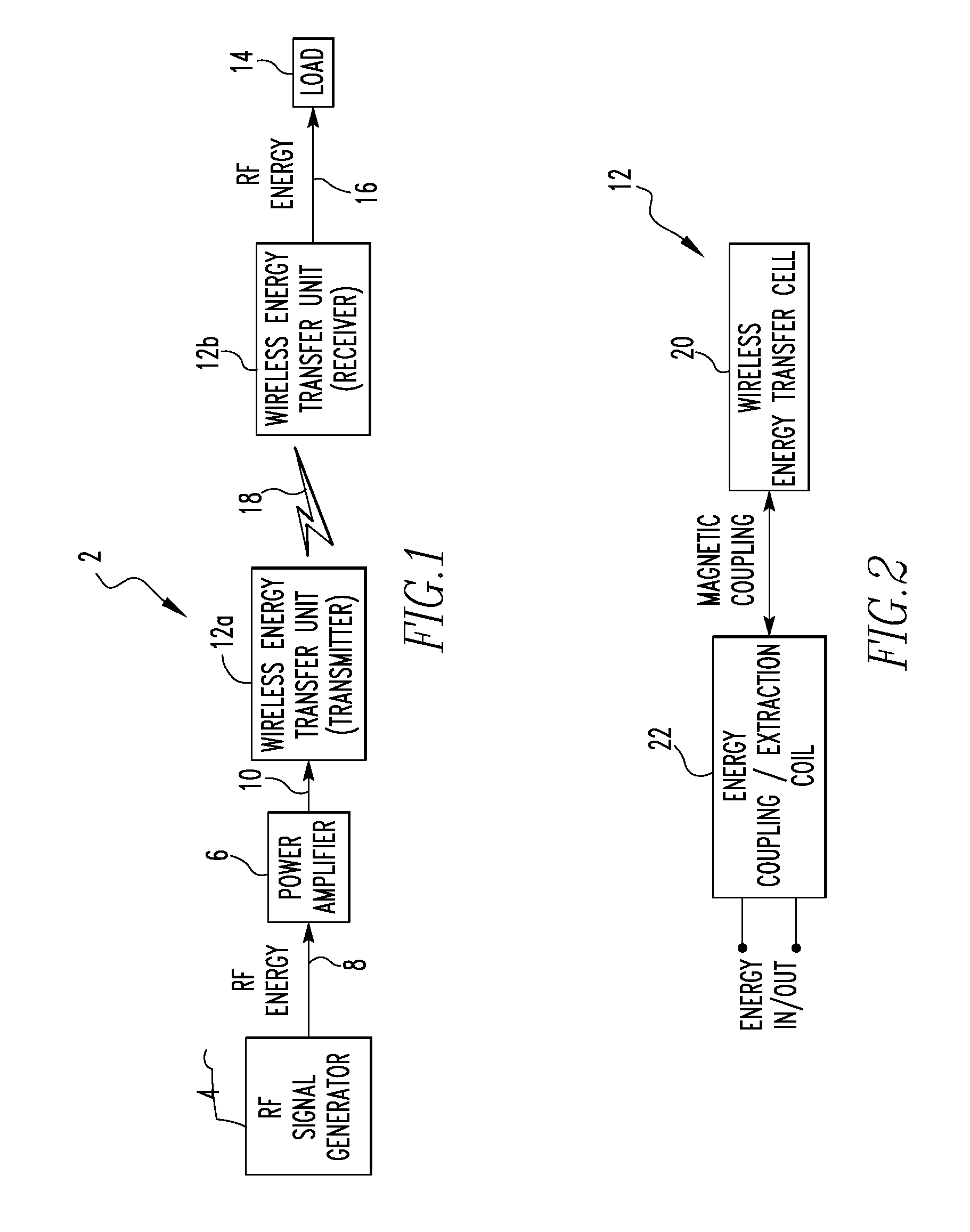
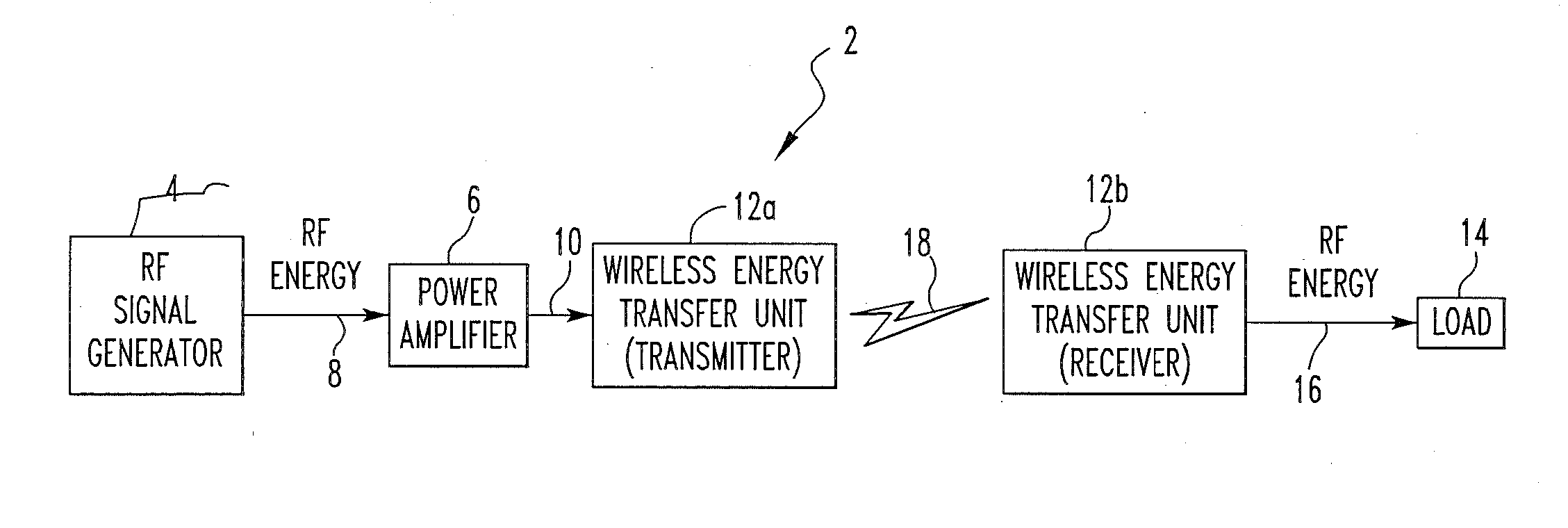
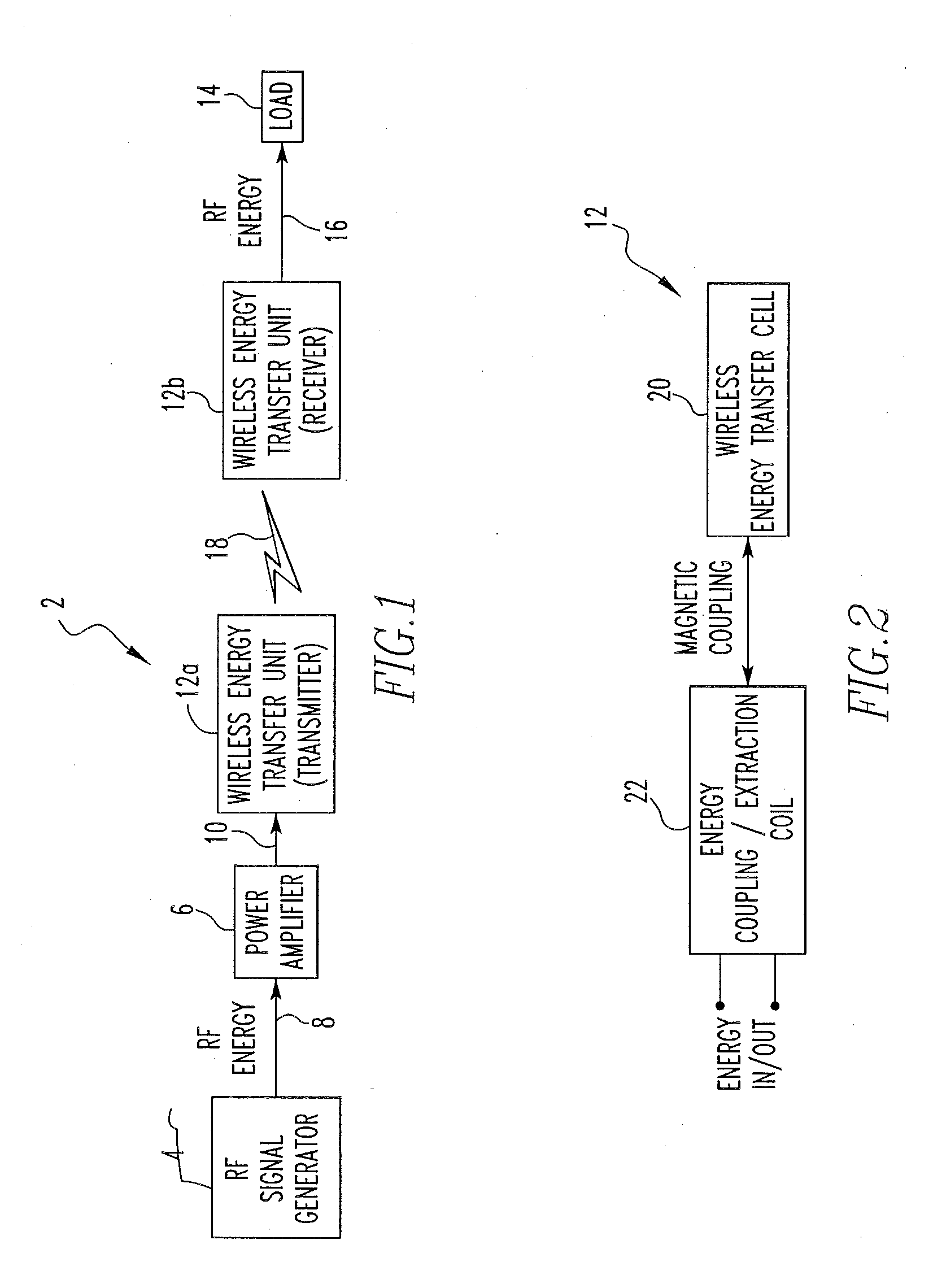
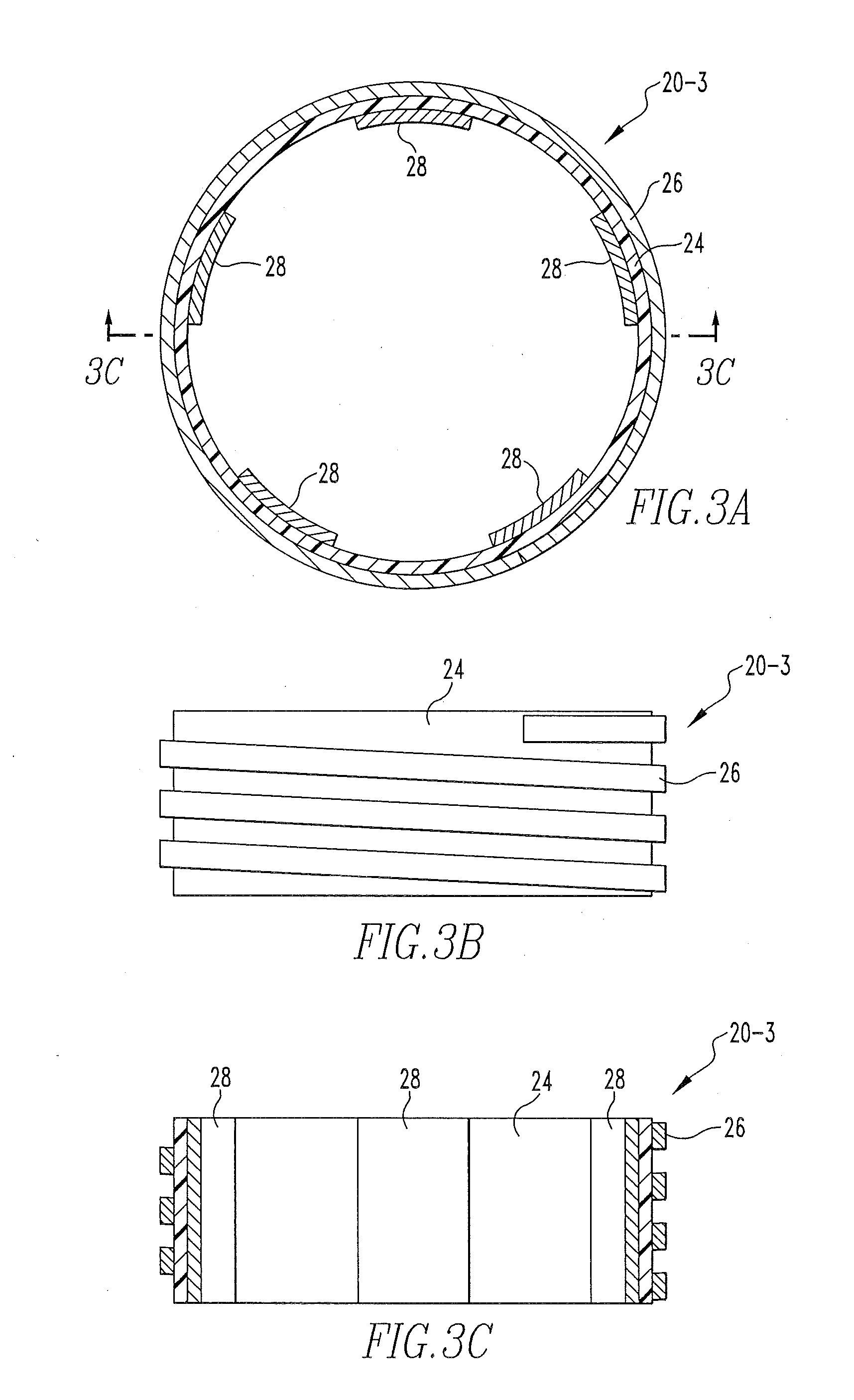
Wireless energy transfer system
ActiveUS8421274B2Significant variabilityCapacitance varyNear-field transmissionTransformersEnergy transferCoupling
A wireless energy transfer system includes a first energy transfer unit having at least one resonant frequency, a second energy transfer unit having the at least one resonant frequency, and a load. The first wireless energy transfer unit includes a first coil magnetically coupled to a first wireless energy transfer cell, and the second wireless energy transfer unit includes a second coil magnetically coupled to a second wireless energy transfer cell. The first coil receives first energy and through the magnetic coupling between the first coil and the first wireless energy transfer cell, the first wireless energy transfer cell is caused to generate second energy, wherein the second wireless energy transfer cell receives the second energy and through the magnetic coupling between the second wireless energy transfer cell and the second coil, the second coil is caused to provide third electromagnetic wave energy to the load.
Owner:UNIVERSITY OF PITTSBURGH
Wireless Energy Transfer System
ActiveUS20140062212A1Significant variabilityCapacitance varyNear-field transmissionTransformersEnergy transferCoupling
A wireless energy transfer system includes a first energy transfer unit having at least one resonant frequency, a second energy transfer unit having the at least one resonant frequency, and a load. The first wireless energy transfer unit includes a first coil magnetically coupled to a first wireless energy transfer cell, and the second wireless energy transfer unit includes a second coil magnetically coupled to a second wireless energy transfer cell. The first coil receives first energy and through the magnetic coupling between the first coil and the first wireless energy transfer cell, the first wireless energy transfer cell is caused to generate second energy, wherein the second wireless energy transfer cell receives the second energy and through the magnetic coupling between the second wireless energy transfer cell and the second coil, the second coil is caused to provide third electromagnetic wave energy to the load.
Owner:UNIVERSITY OF PITTSBURGH
Vehicle headlight system having digital beam-forming optics
InactiveUS7156542B2Improve roadway illuminationImprove driver visibilityNon-electric lightingVehicle headlampsLight irradiationOptoelectronics
A vehicle headlight system including a light source, digital beam forming optics optically coupled to the light source, and a memory storing a plurality of light illumination patterns. The memory is electrically coupled to the digital beam forming optics. The digital beam forming optics is adapted to output light from the light source in the form of at least one of the light illumination patterns in response to at least one vehicle operating condition.
Owner:FORD GLOBAL TECH LLC
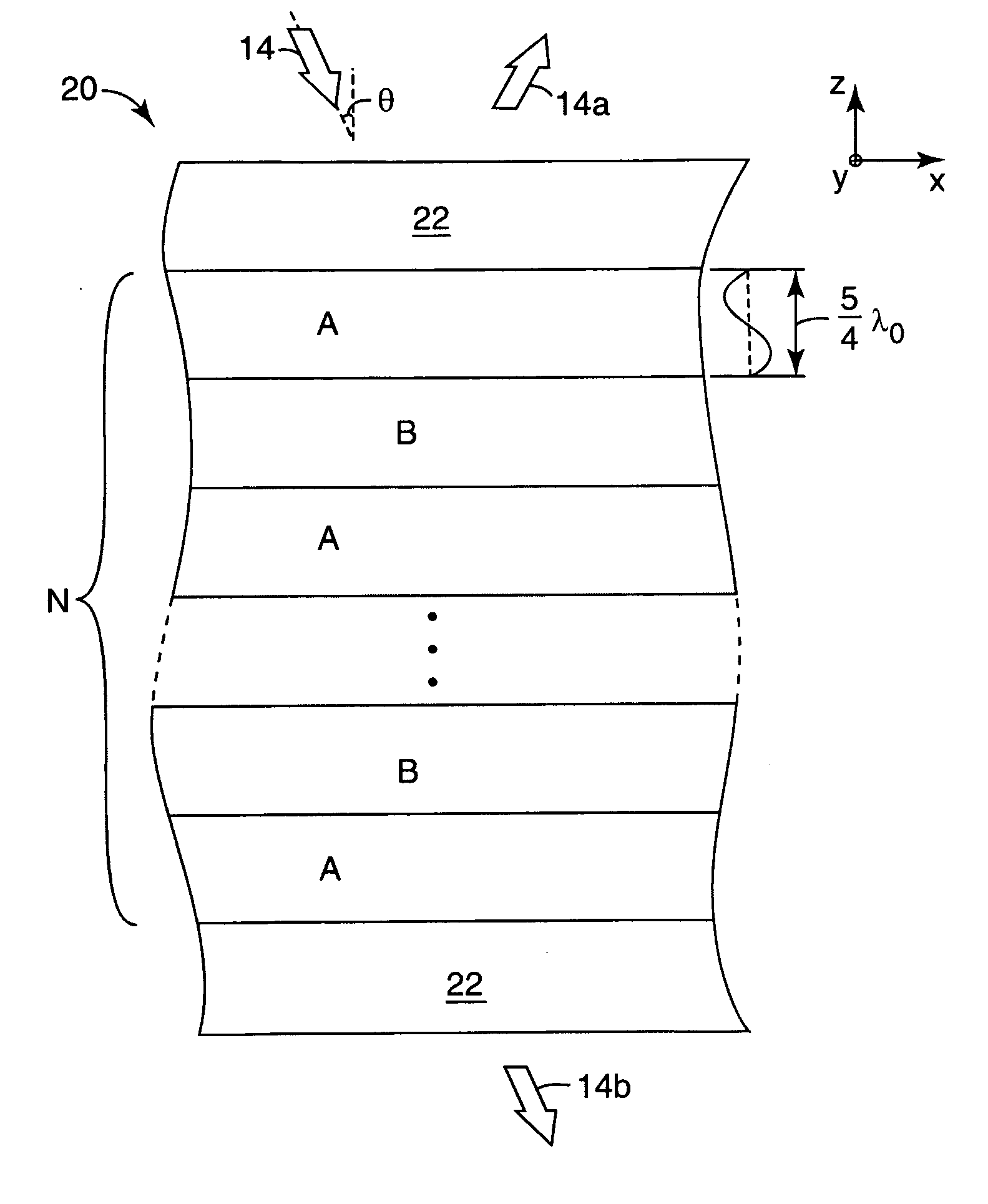
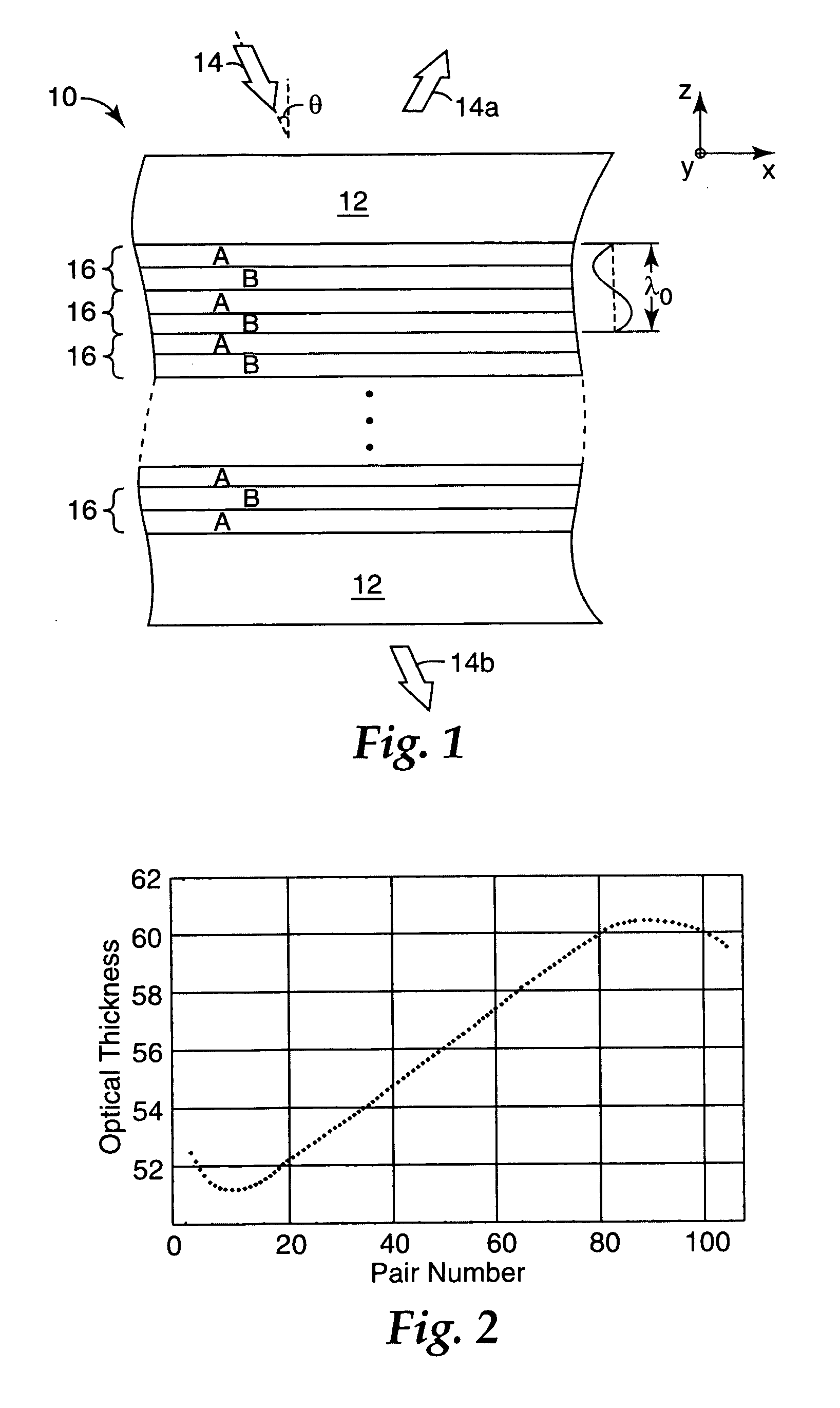
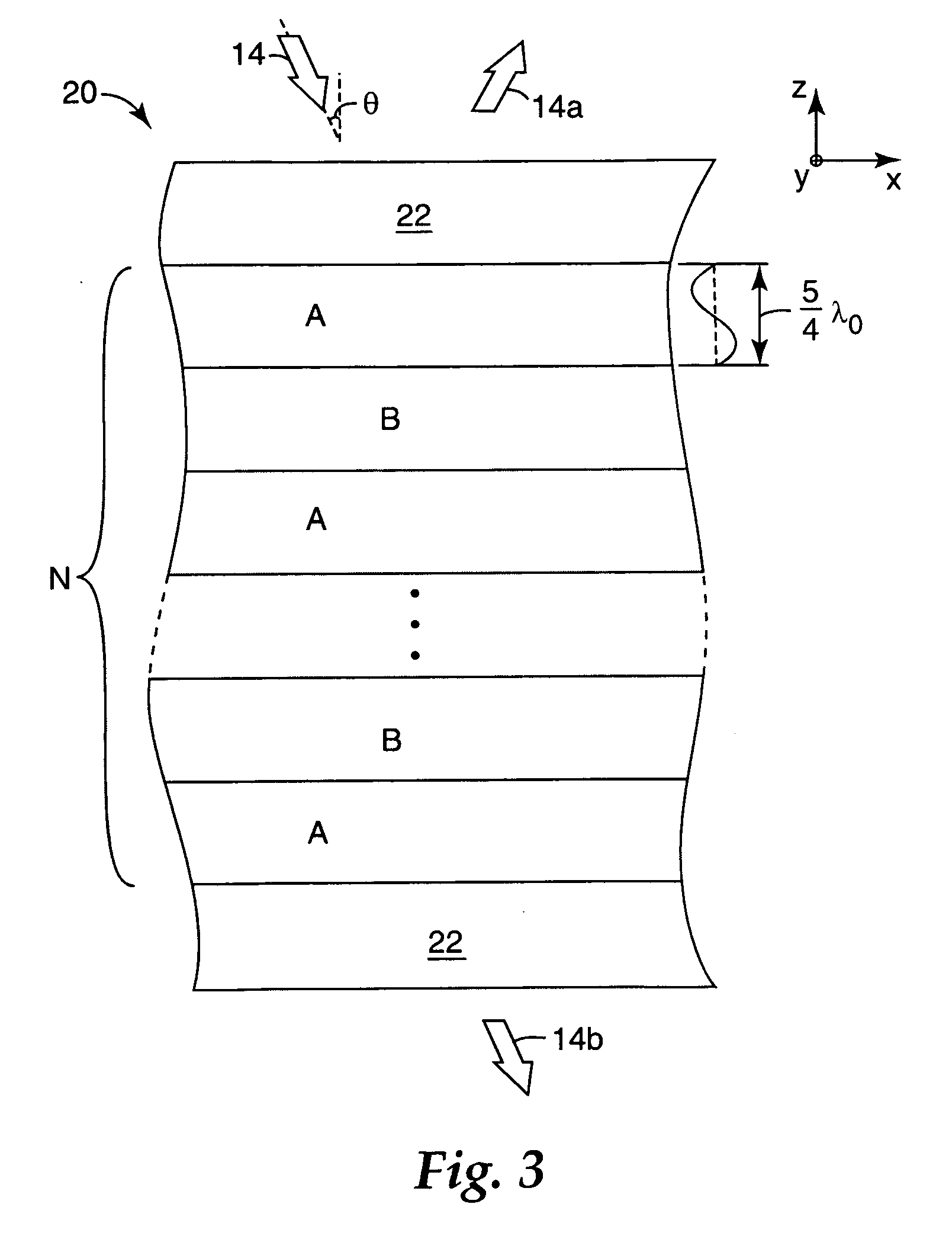
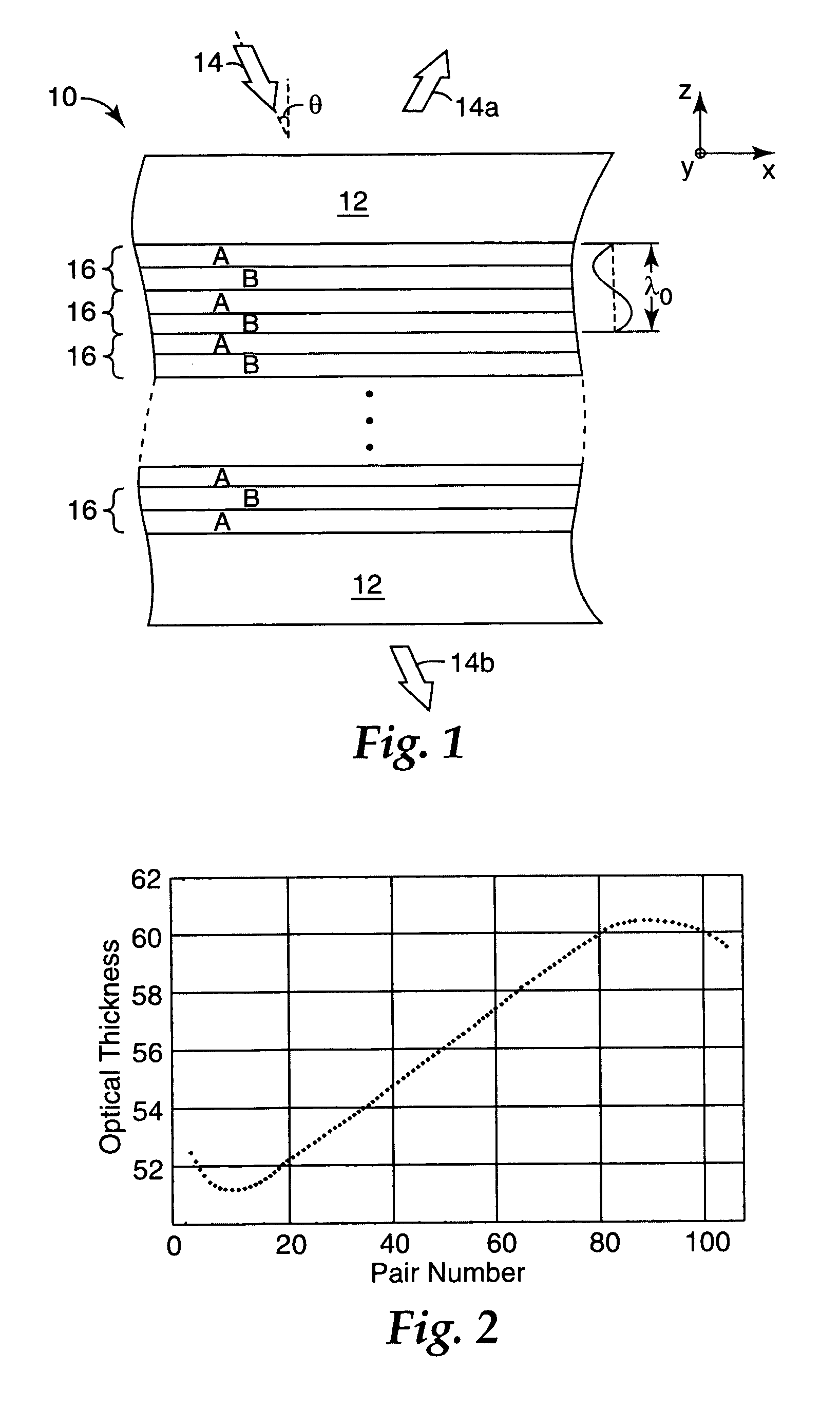
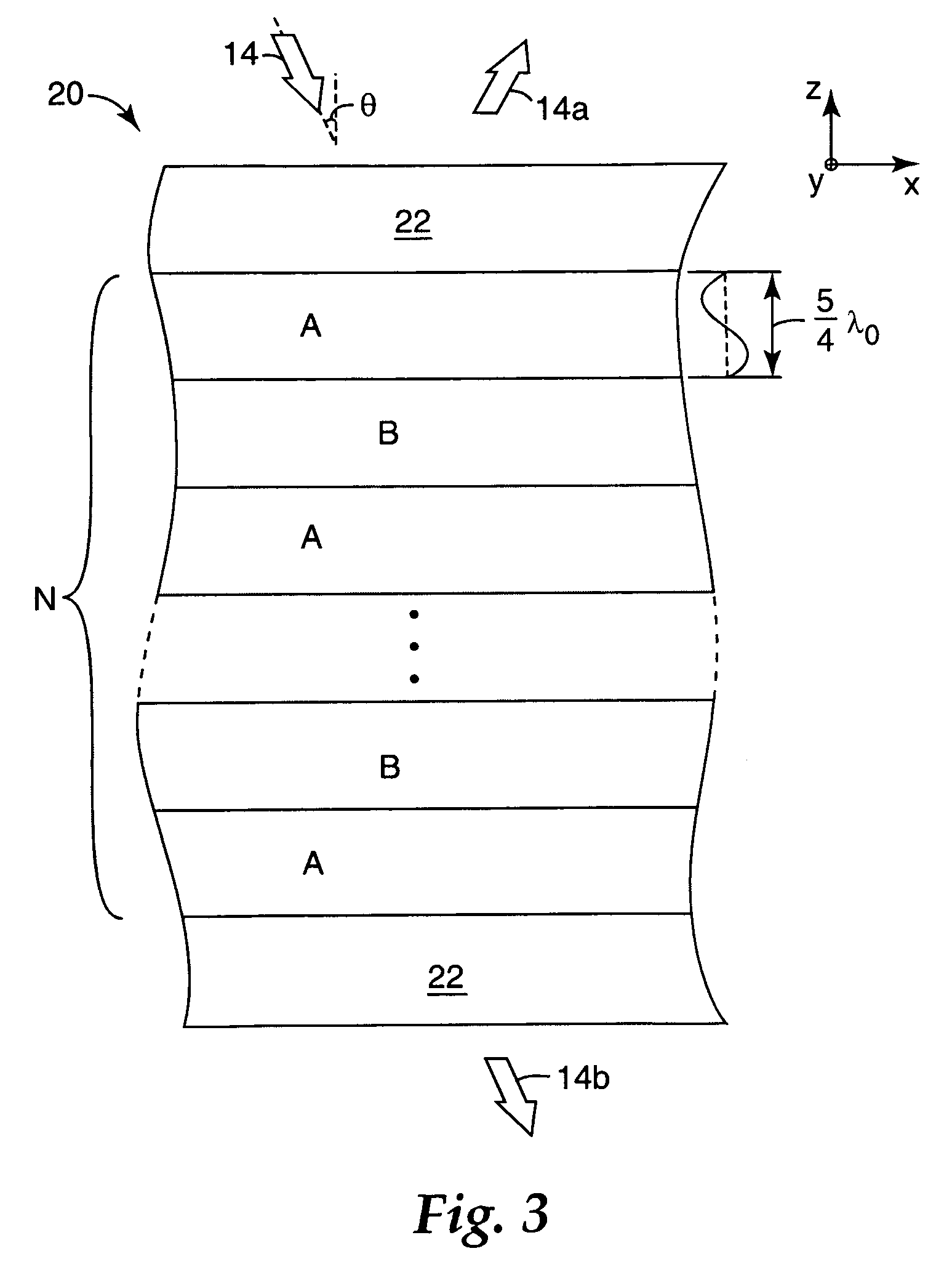
Thick film multilayer reflector with tailored layer thickness profile
ActiveUS20060232863A1Substantial effect on optical performanceSignificant variabilityLayered productsOptical elementsOptoelectronicsPolarizer
A multilayer reflector useable to reflect or transmit light over the visible wavelength range includes optically thick constituent layers. The optical thickness of the constituent layers through the thickness of the reflector defines a layer thickness profile. The layers are arranged so that the thickness profile has a tailored non-uniform distribution, such as a graded distribution or a randomized distribution. The layers desirably have an optical thickness in a range from about one to five or one to ten design wavelengths. The reflector can be a polarizer, reflecting only one normally incident polarization state, or a mirror, reflecting two normally incident orthogonal polarization states.
Owner:3M INNOVATIVE PROPERTIES CO
Electronic variable stroke devices and system for remote control and interactive play
InactiveUS8308631B2Significant variabilityImprove the environmentVibration massageNon-surgical orthopedic devicesMotor driveService provision
An electronic variable stroke device comprise a base portion containing a motor-driven screw shaft, an upper portion extending from the base portion having the screw shaft extending longitudinally therein, a traveler engaged with the screw shaft to drive it in reciprocating longitudinal motion. The traveler has an annular shape with an aperture therethrough which is driven in reciprocating longitudinal motions for use as a male sex toy. The device configuration may include a pair of screw threads spaced apart in parallel with the traveler engaged in between them, and / or multiple travelers arranged at different longitudinal positions of the screw shaft(s). A remote controller unit may be provided for ergonomic operation by the user. A network connection unit may be provided to connect the user's device to an external service provider on a network for conducting interactive sessions remotely with another user or users. The device may be adapted as a male toy that can exchange control signals with another user operating a female toy.
Owner:KOBASHIKAWA ALVIN Y +1
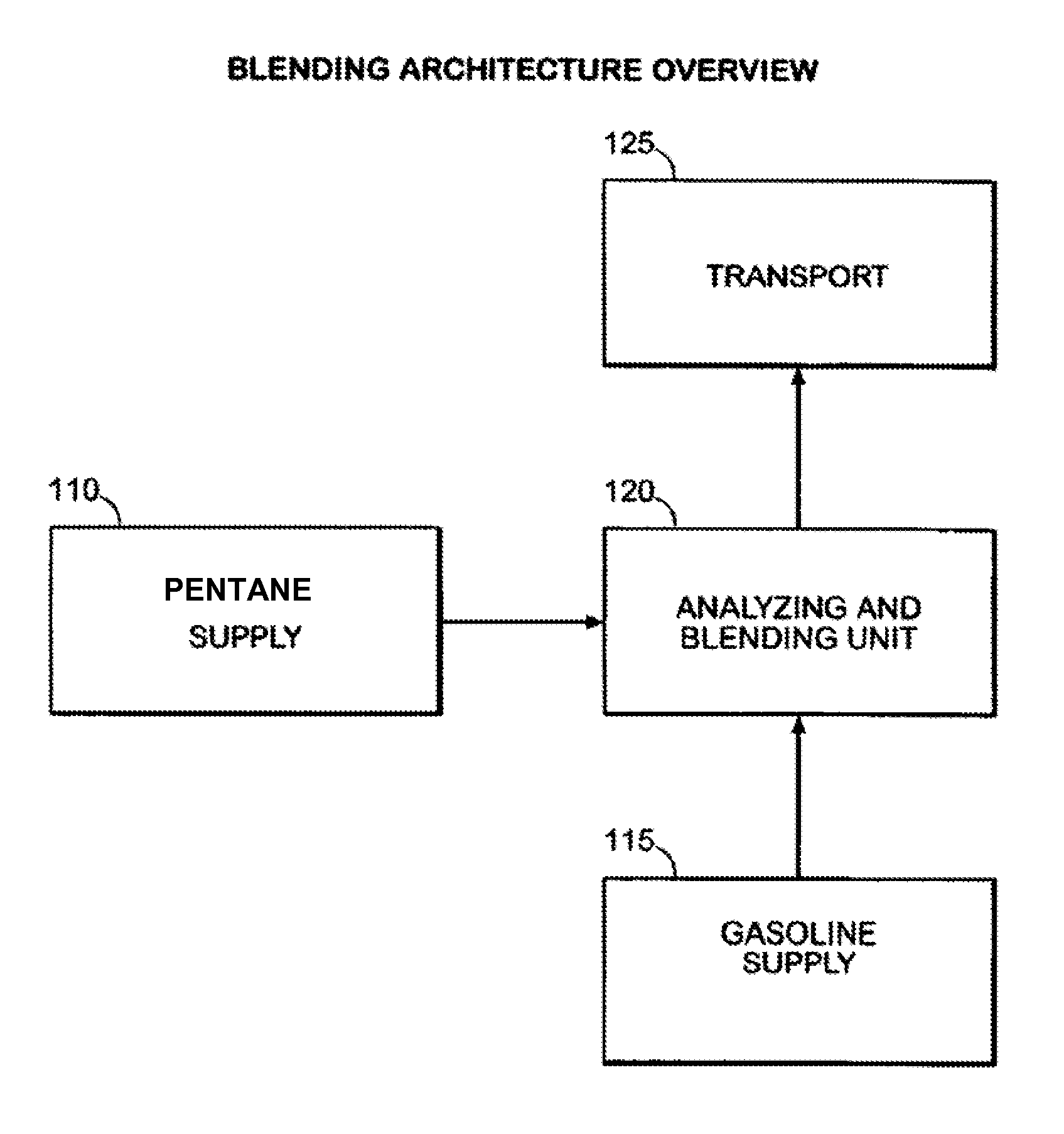
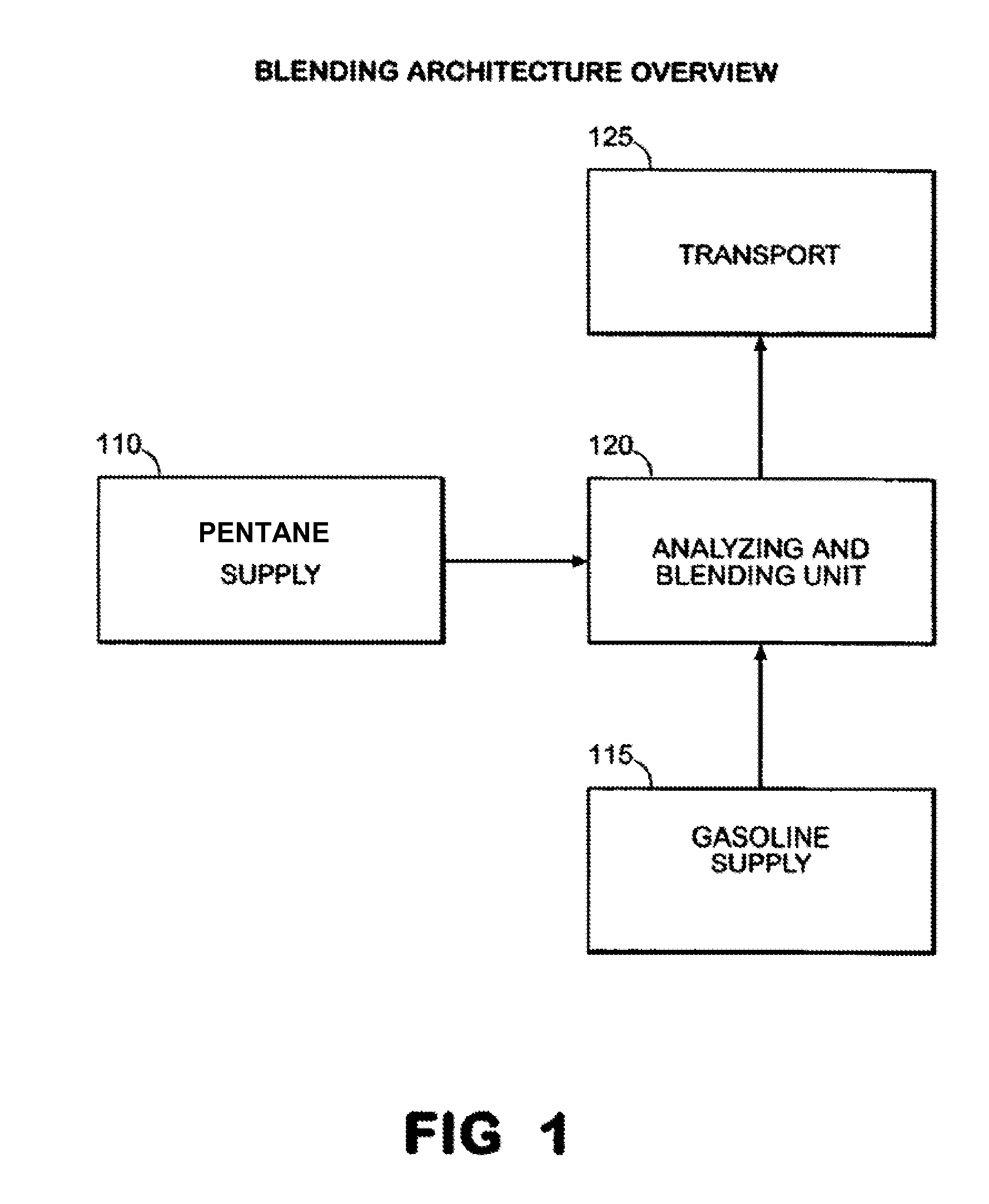
Expansion of fuel streams using mixed hydrocarbons
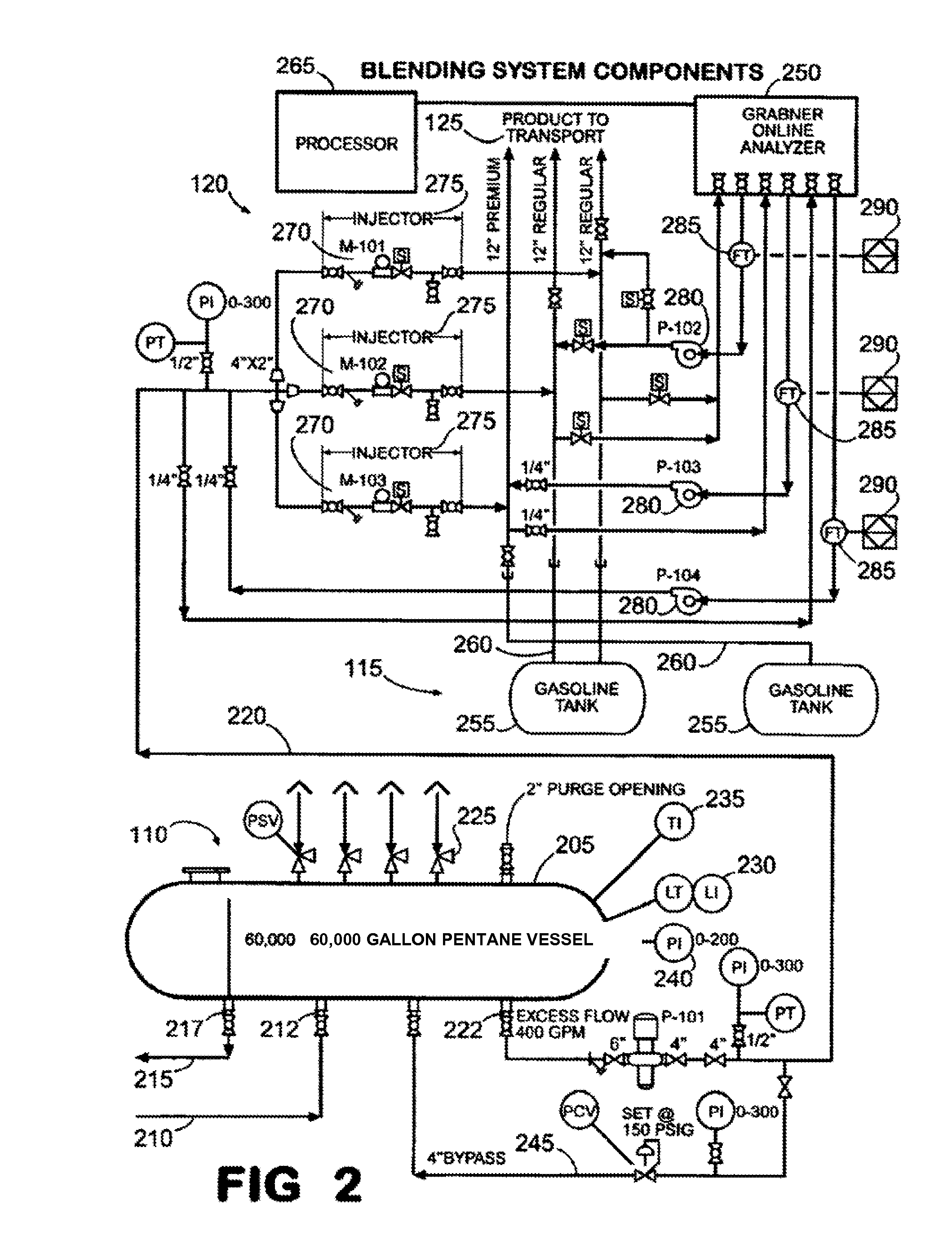
ActiveUS8597380B2Improve octaneReducing octaneLiquid fillingHydrocarbon purification/separationOil refineryHydrocarbon
Owner:SUNOCO PARTNERS MARKETING & TERMINALS LP
Power distribution system
ActiveUS20160190807A1Improve performanceSignificant variabilityBoards/switchyards circuit arrangementsSelective ac load connection arrangementsAutomatic controlTrack lighting
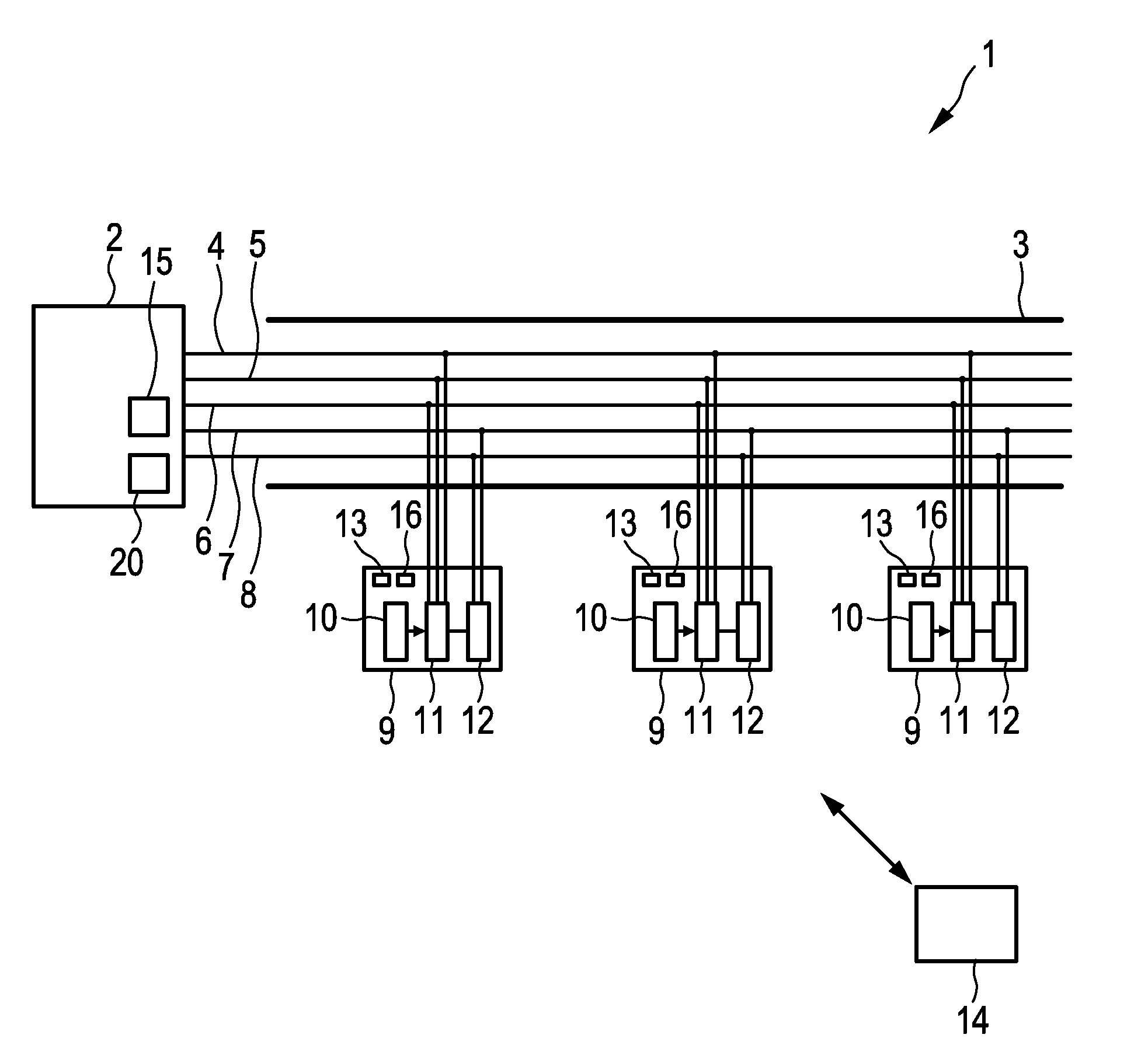
The invention relates a power distribution system (1) like a track lighting system comprising a power supply (2), a power bar (3) comprising several electrical conductors (4, 5, 6) for distributing the power, an electrical load (12) to be powered by the power of the power distribution system, a selector switch (11) connected to the several electrical conductors and to the electrical load, wherein the selector switch is adapted to select via which electrical conductor the power is to be received by the electrical load, and a controlling device (10) for automatically controlling the selector switch. This kind of power distribution system provides a relatively large variability of adapting the power consumption to an actual load situation, which can lead to an improved performance of the power distribution system. In particular, power balancing can be provided in a relatively simple way by automatically controlling the selector switch accordingly.
Owner:SIGNIFY HLDG BV
Beverage sleeve for a container
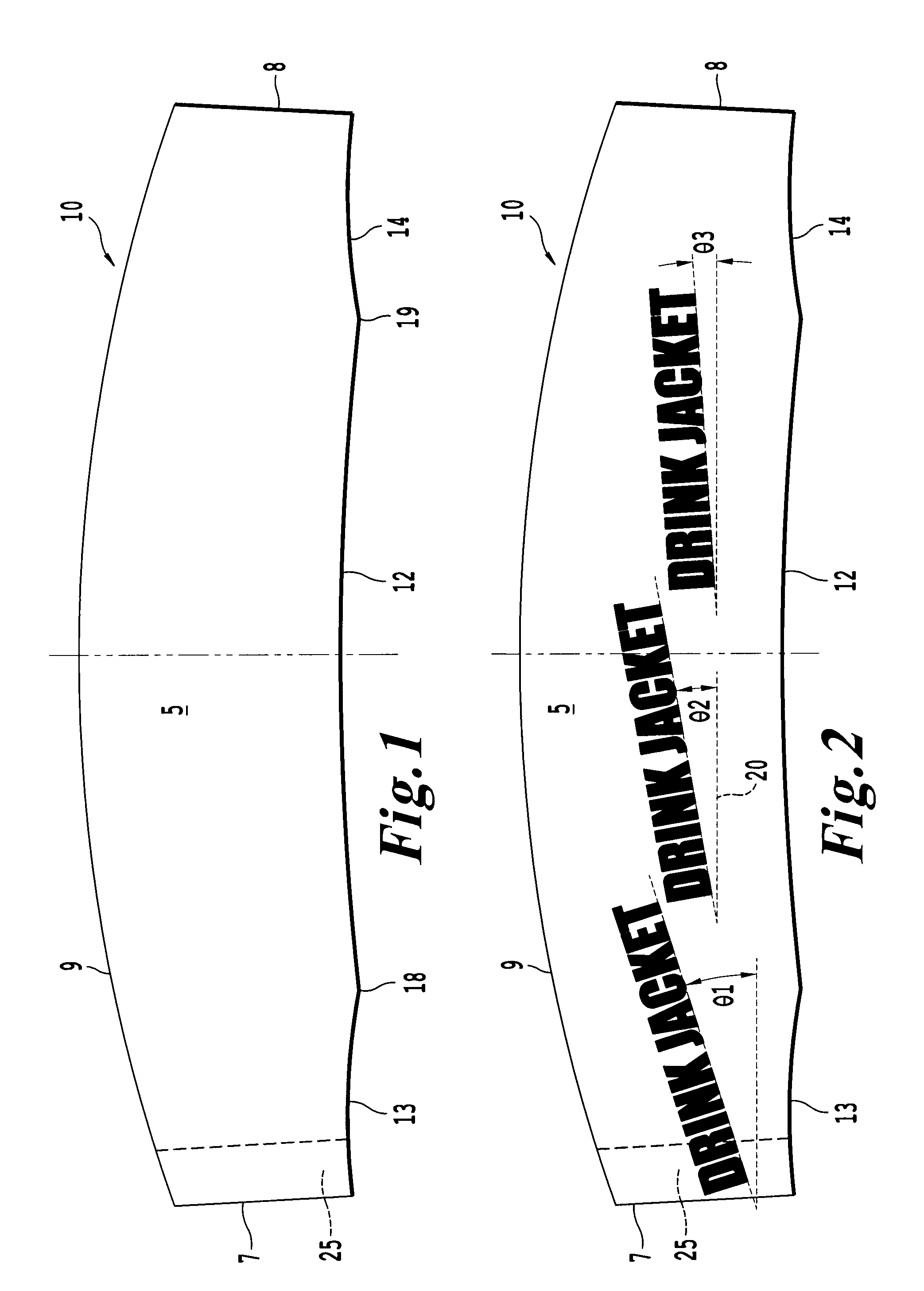
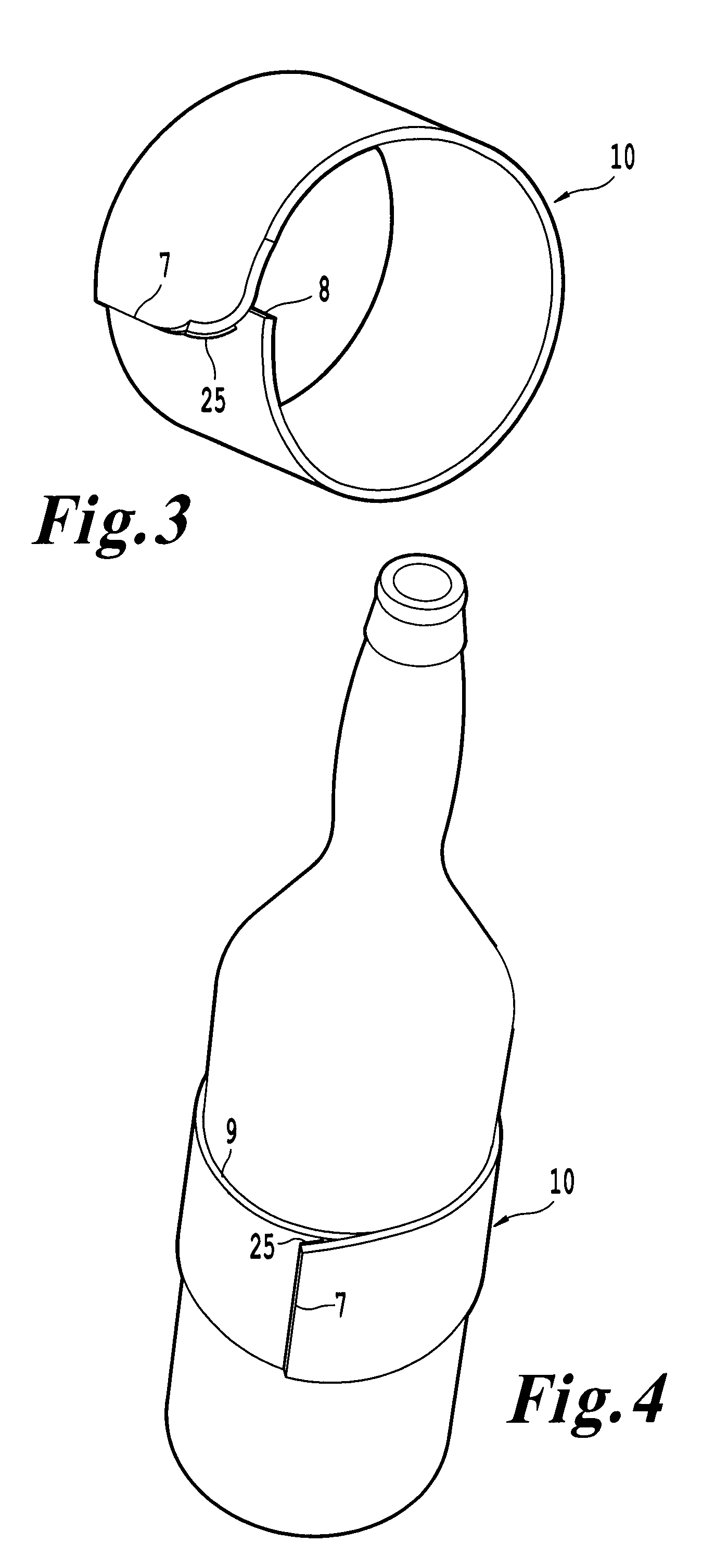
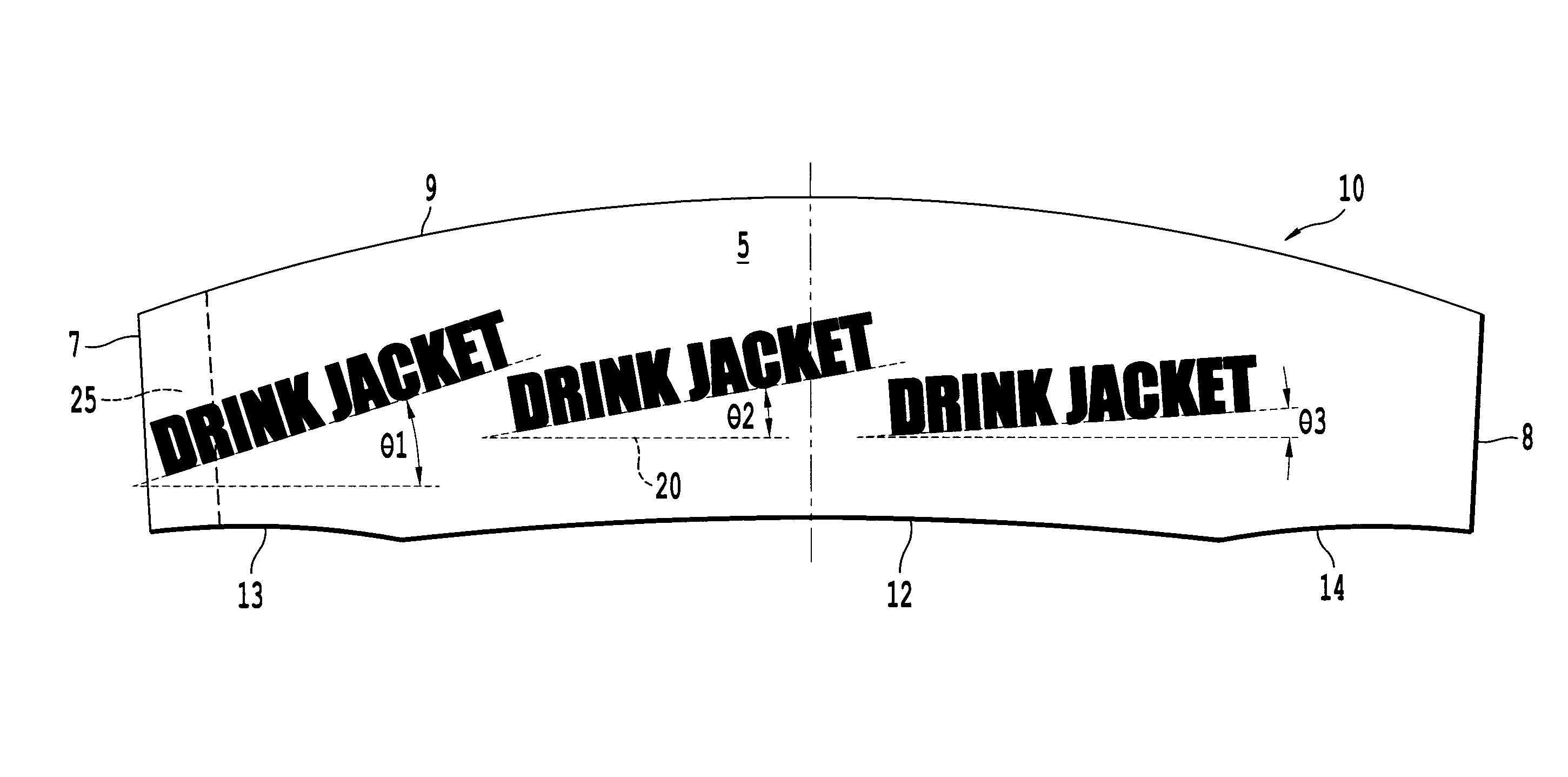
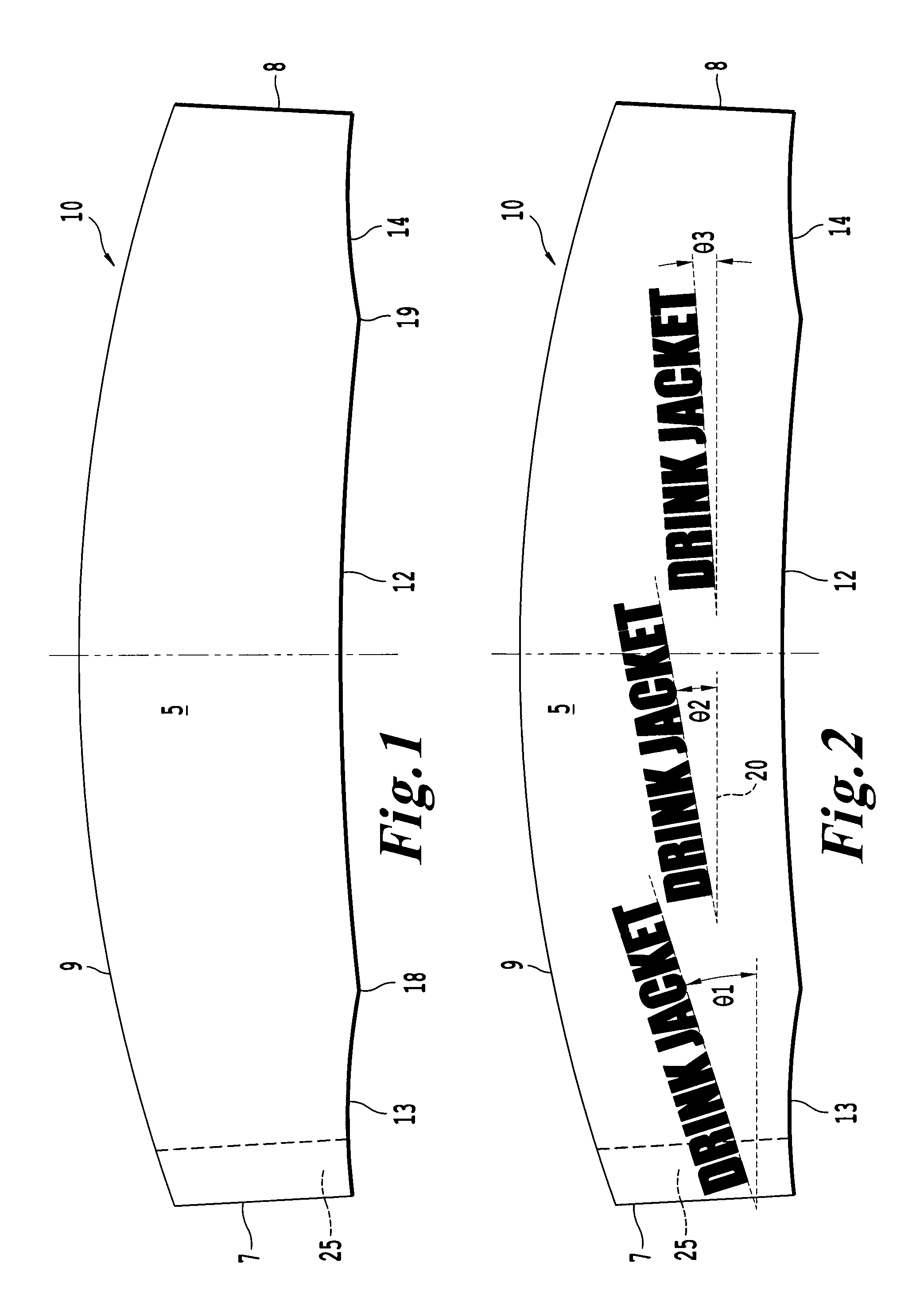
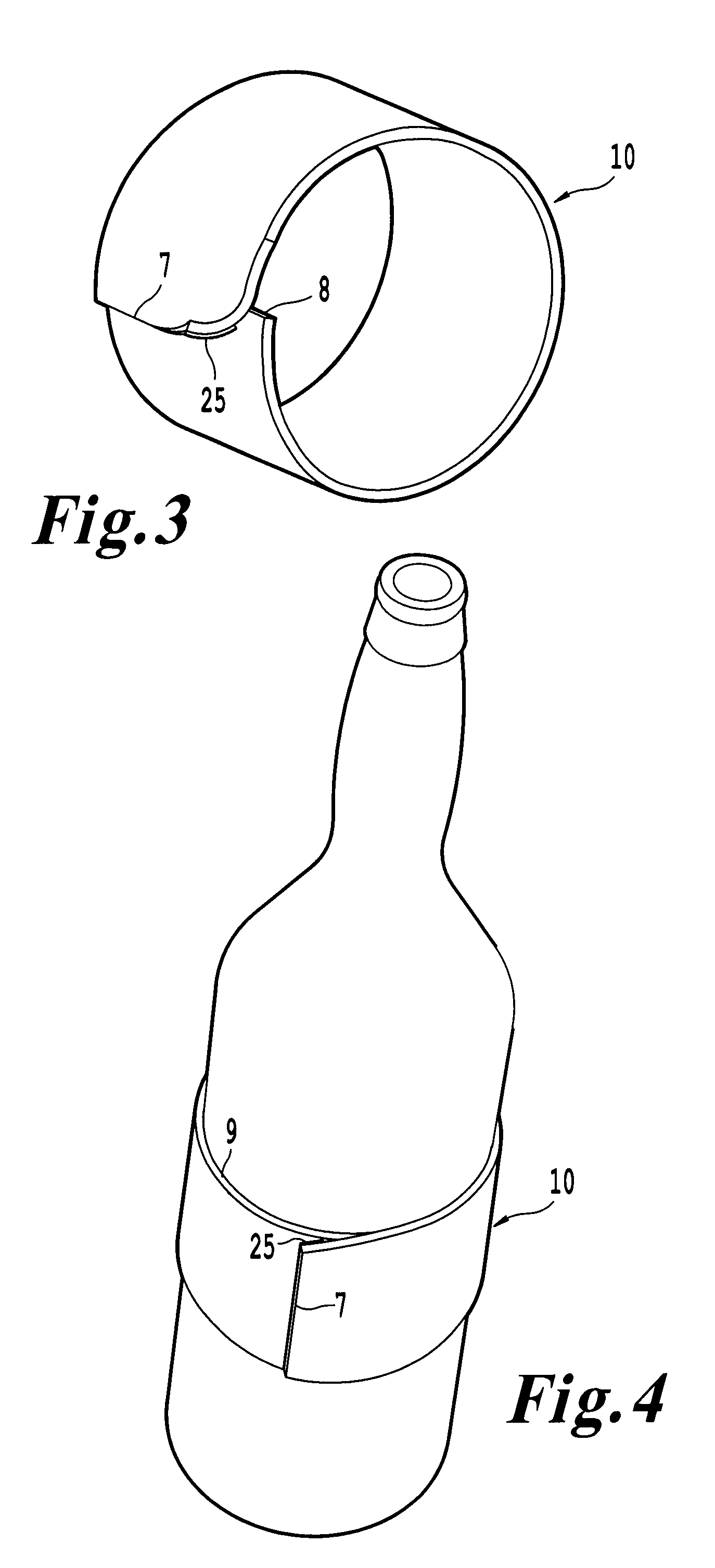
InactiveUS20100018984A1Good insulationEffective positioningStampsContainer decorationsBiomedical engineering
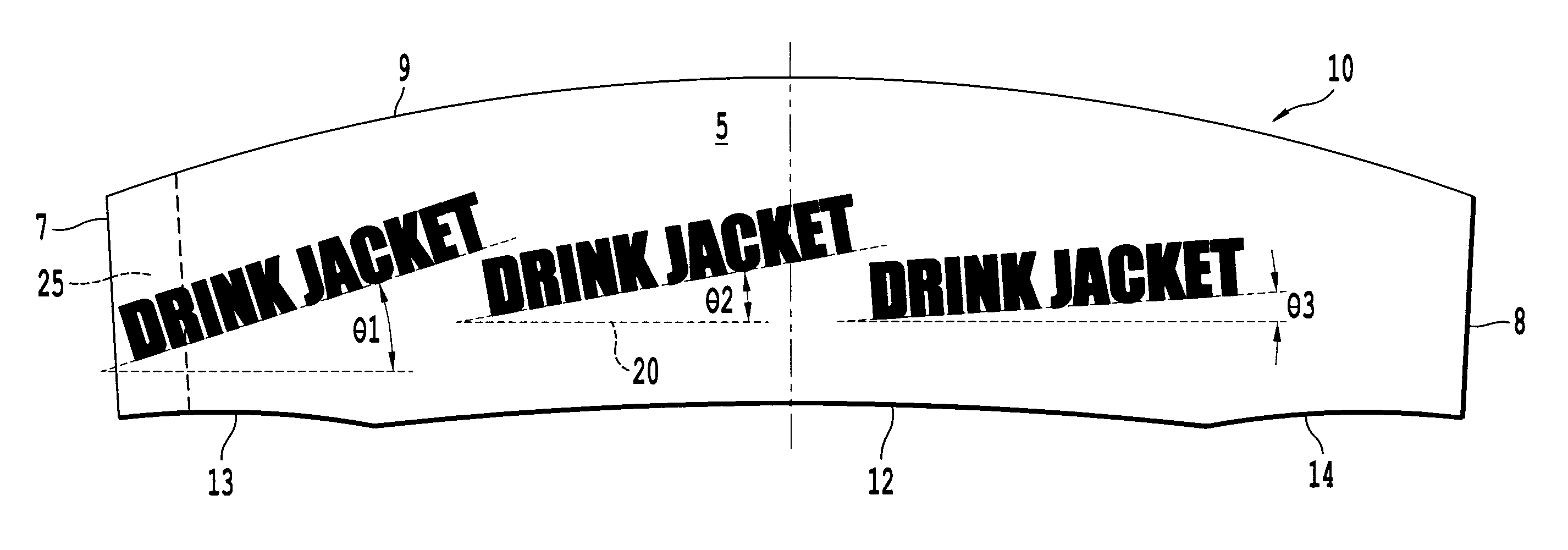
An infinitely variable diameter beverage sleeve includes a first surface configured to contact the container and extend around a circumference of the container, and a second surface configured to extend around the circumference of the container and face away from the container, and a securing device to hold the sleeve to the outside surface of the container. A first portion of the securing device is provided on one end of the sleeve on the first surface, and a second engaging portion of the securing means is provided as the second surface of the sleeve. The top edge of the sleeve is non-planar and the bottom edge of the sleeve is formed of a plurality of segments with at least two segments having a different radius of curvature.
Owner:SOLOMON MERRILL
Dielectric antenna
ActiveUS20100295745A1Highly bundlingSignificant variabilityWaveguide hornsAntenna detailsElectromagnetic radiationDielectric tube
Described and shown is a dielectric antenna (1) having a dielectric feeding section (2), a first transition section (3) comprising a dielectric rod, a dielectric emitting section (5) and, a further, second transition section (4) forming a dielectric horn, wherein the feeding section (2) can be struck with electromagnetic radiation (6), electromagnetic radiation (6) can be guided with the first transition section (3) and the second transition section (4) and the electromagnetic radiation can be emitted from the emitting section (5) as airborne waves.The object of the present invention is to provide a dielectric antenna, which is adaptable as low-loss as possible to different mounting situations, which additionally is as low-reflection as possible and, at the same time is highly bundling.The object of the above-mentioned dielectric antenna is met in that the emitting section (5) is designed as dielectric tube connecting to the second transition section (4).
Owner:KROHNE MESSTECHNICK GMBH & CO KG
Lighting Unit and Light Bar having the Same
InactiveUS20140198494A1Easy to replaceShorten production timeLighting support devicesPoint-like light sourceEffect lightEngineering
Owner:LEXTAR ELECTRONICS CORP
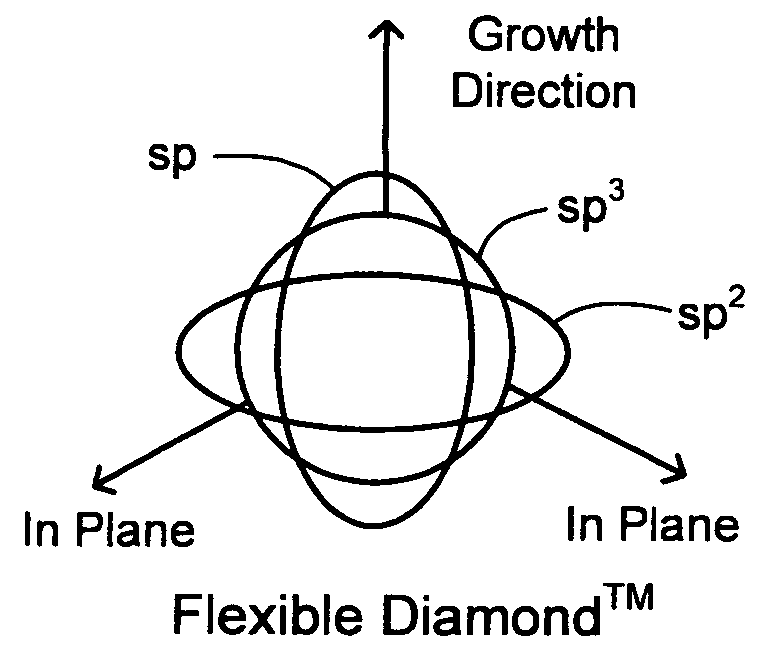
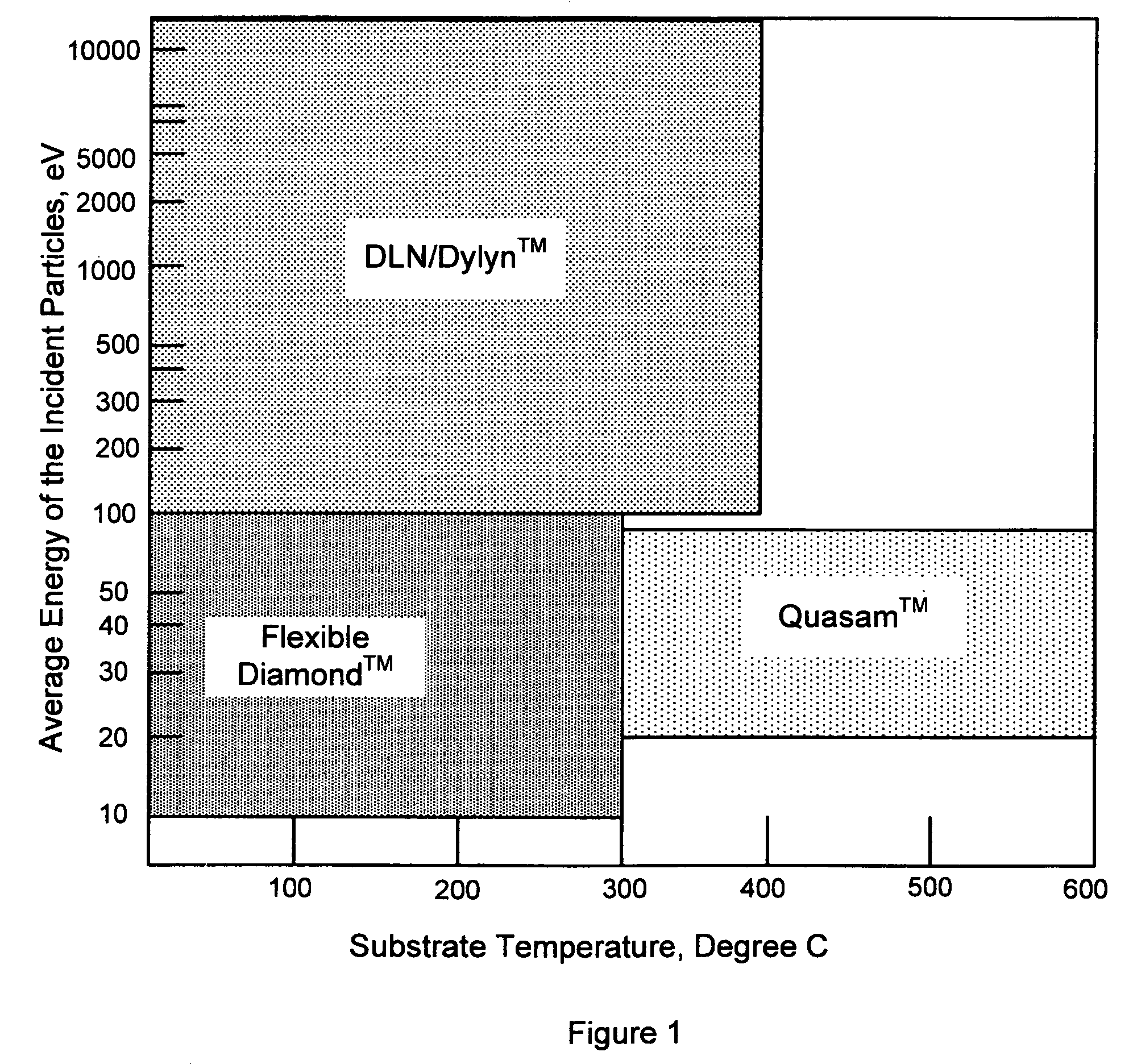
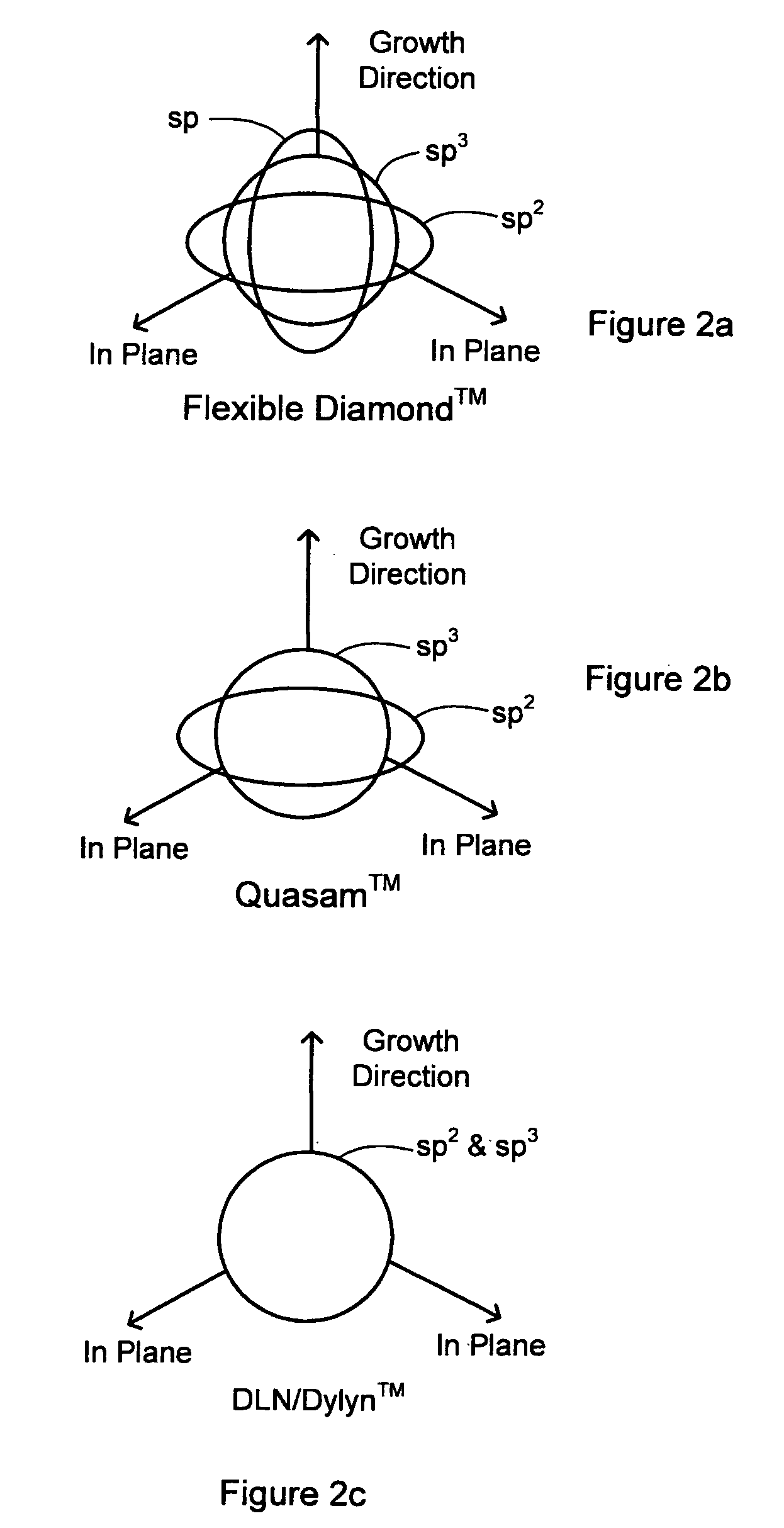
Synergetic SP-SP2-SP3 carbon materials and deposition methods thereof
InactiveUS20050163985A1Simple materialImprove fracture toughnessPolycrystalline material growthUltra-high pressure processesSurface reactionHydrogen
The present invention generally provides carbon materials and methods for producing the carbon materials that include a polymer-like bonded carbon network, a diamond-like bonded carbon network, a graphene-like bonded carbon network, and at least one stabilizing network of at least one alloying element. The material may further include hydrogen, silicone, and oxygen. The carbon materials are generally produced using plasma deposition while accounting for both thermal and incident particle impact activation for surface reactions, which beneficially enables the production of the carbon material at relevantly low incident flux energy and / or relatively low substrate temperatures.
Owner:NANODYNAMICS INC
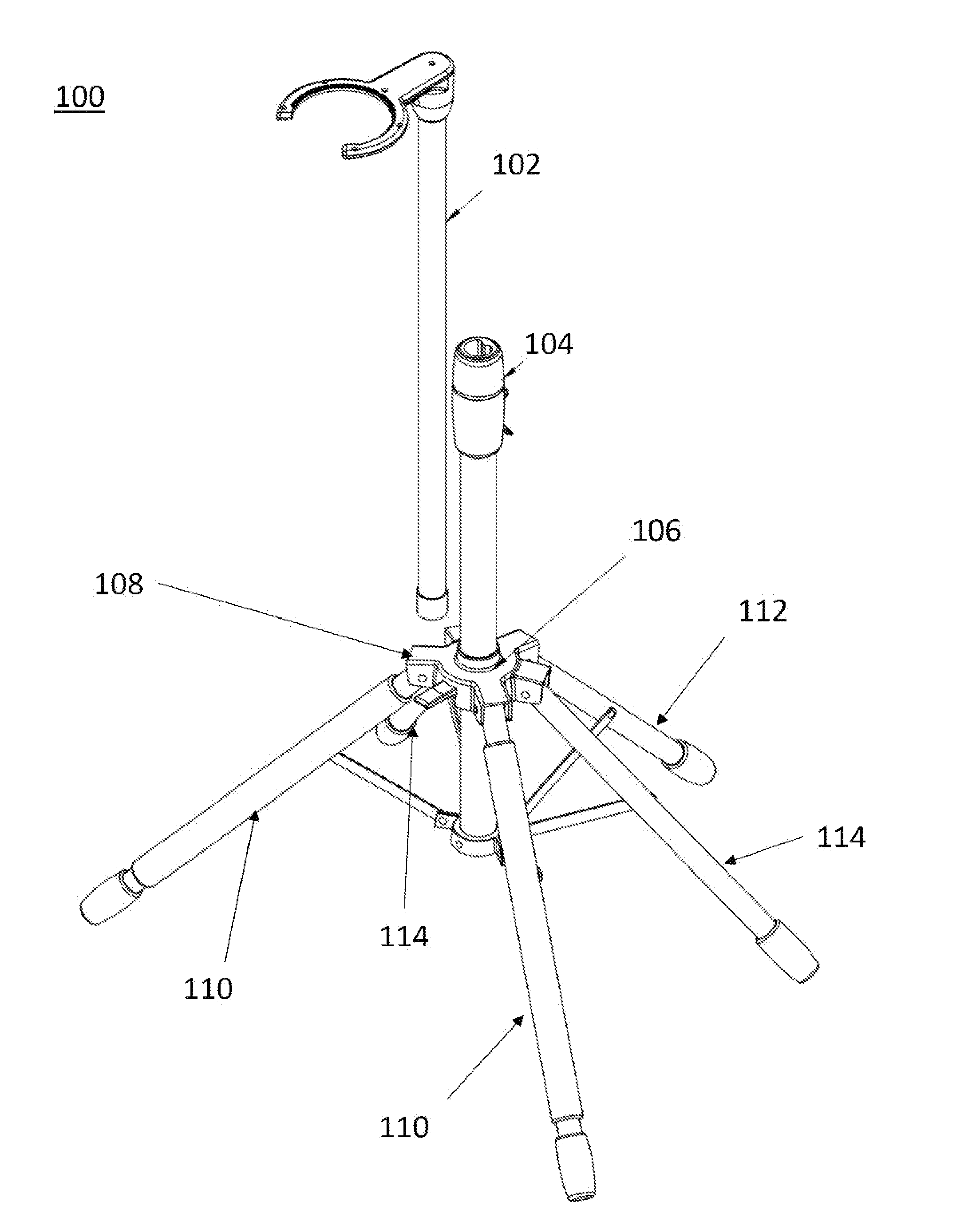
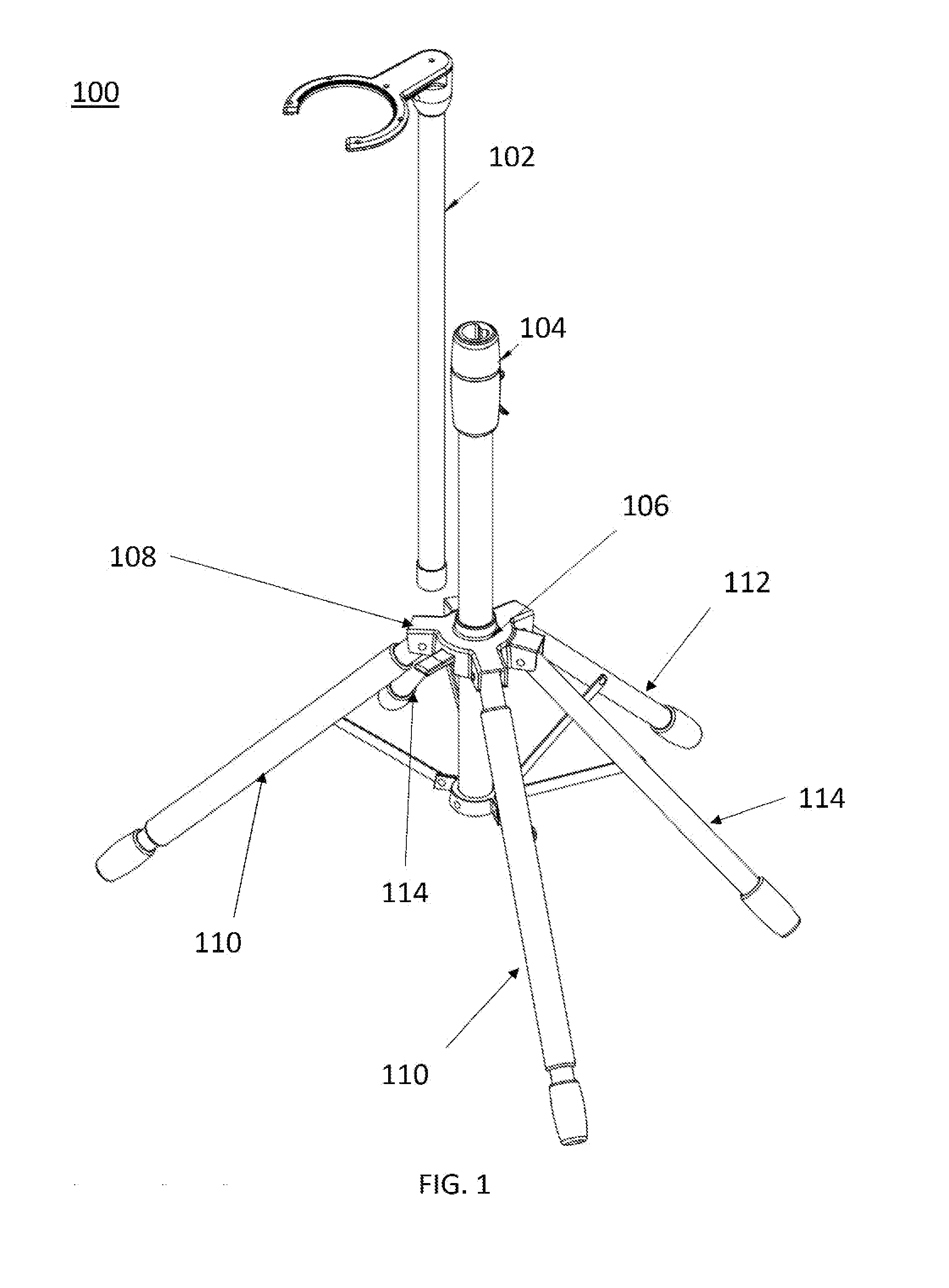
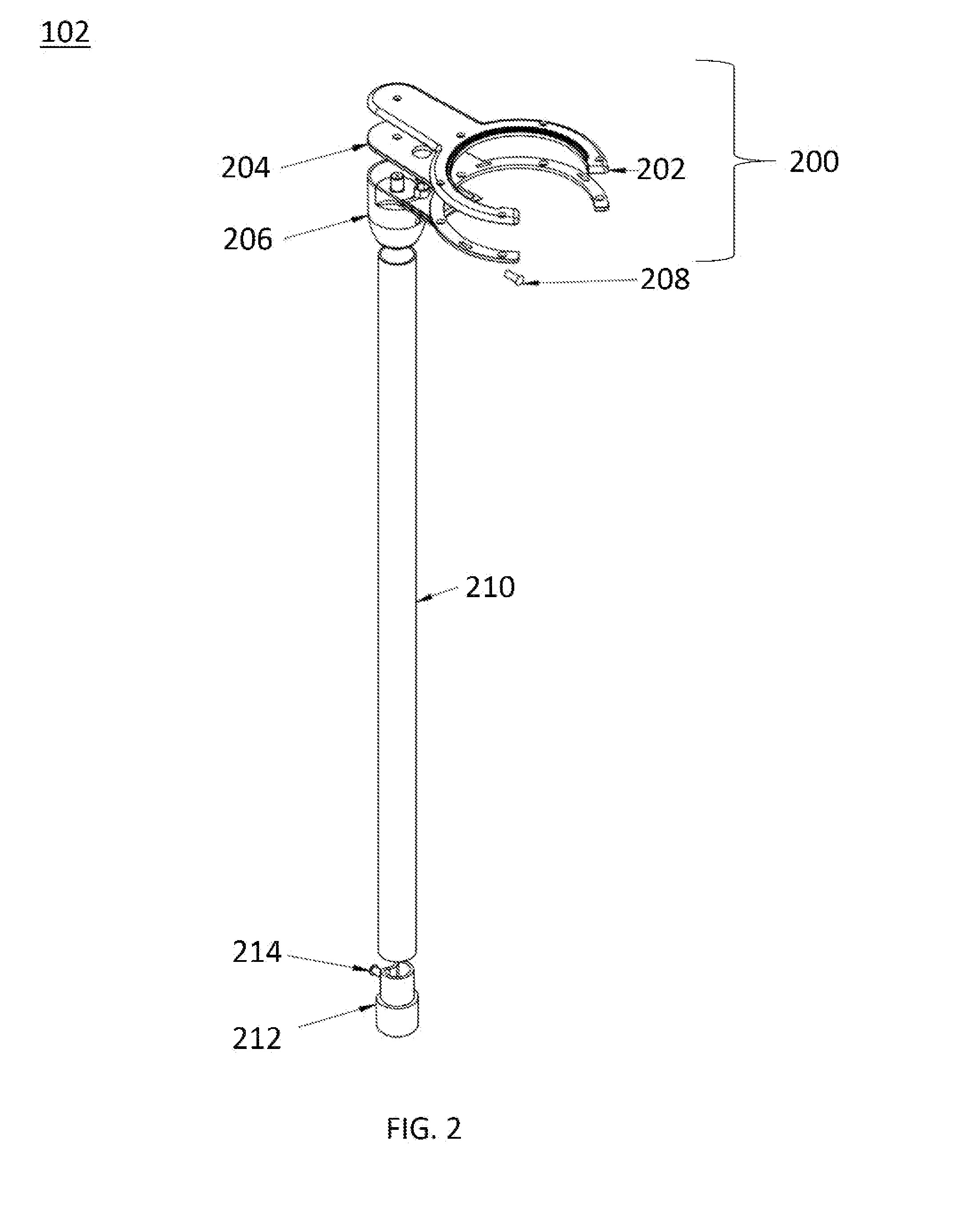
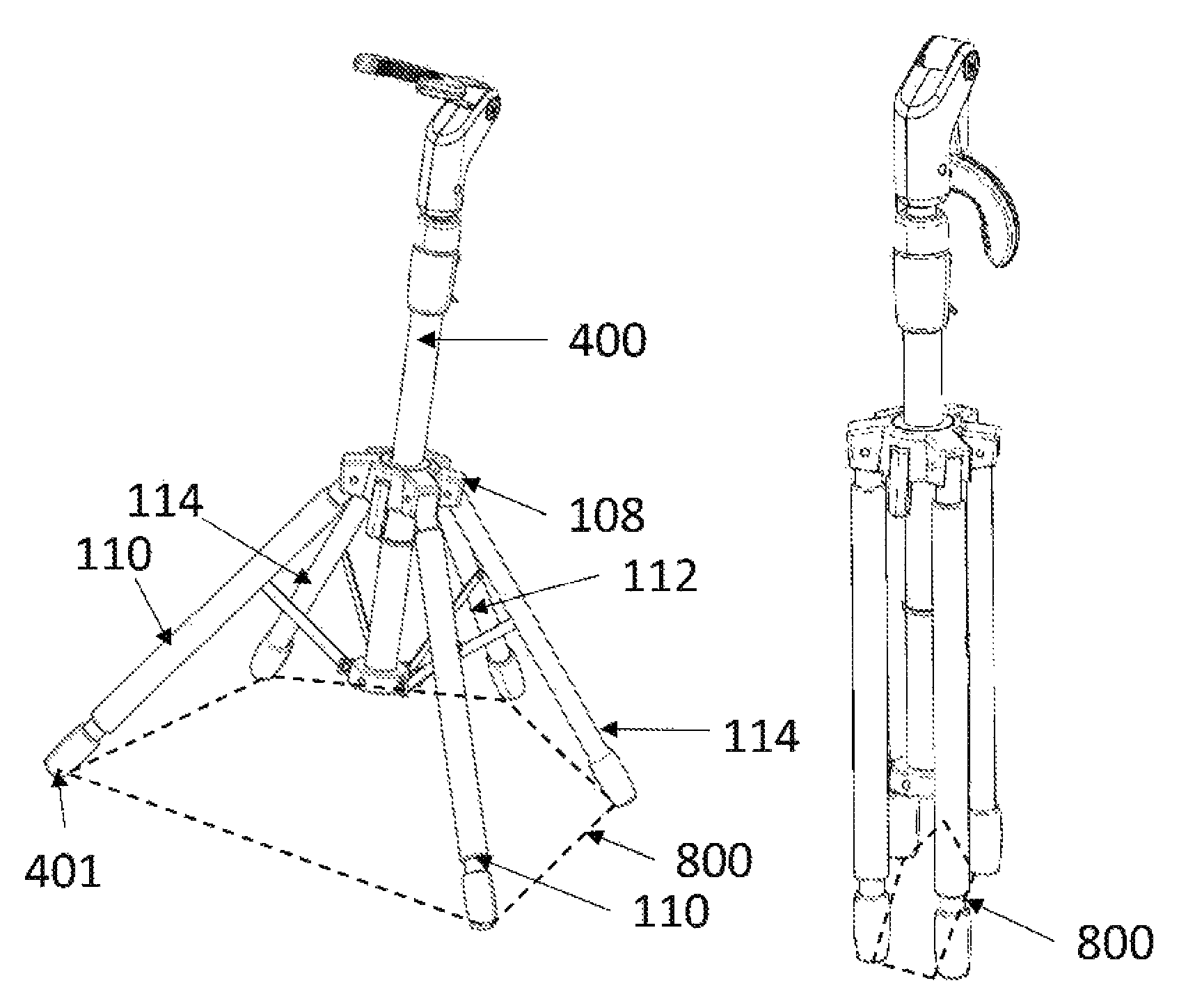
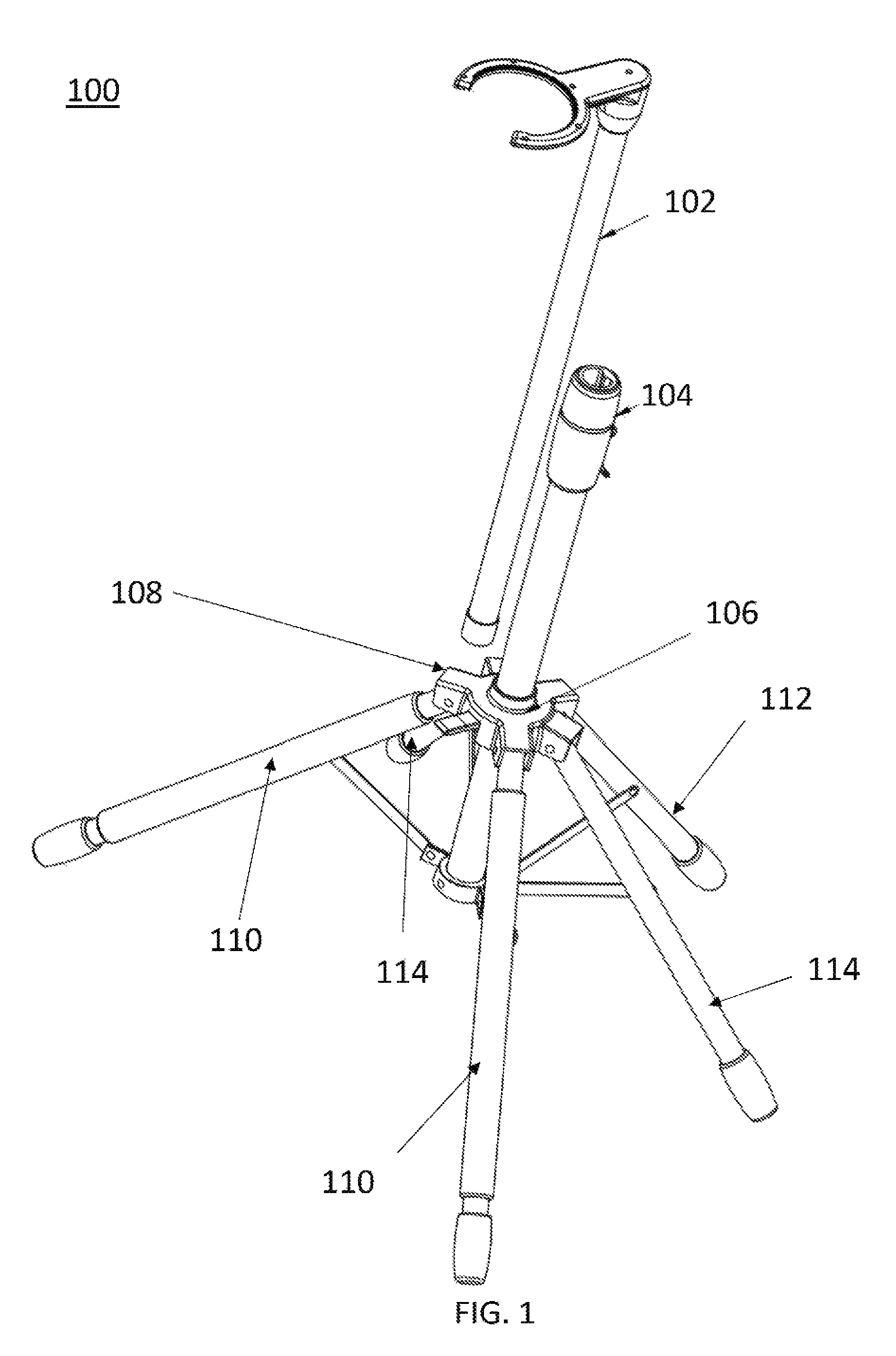
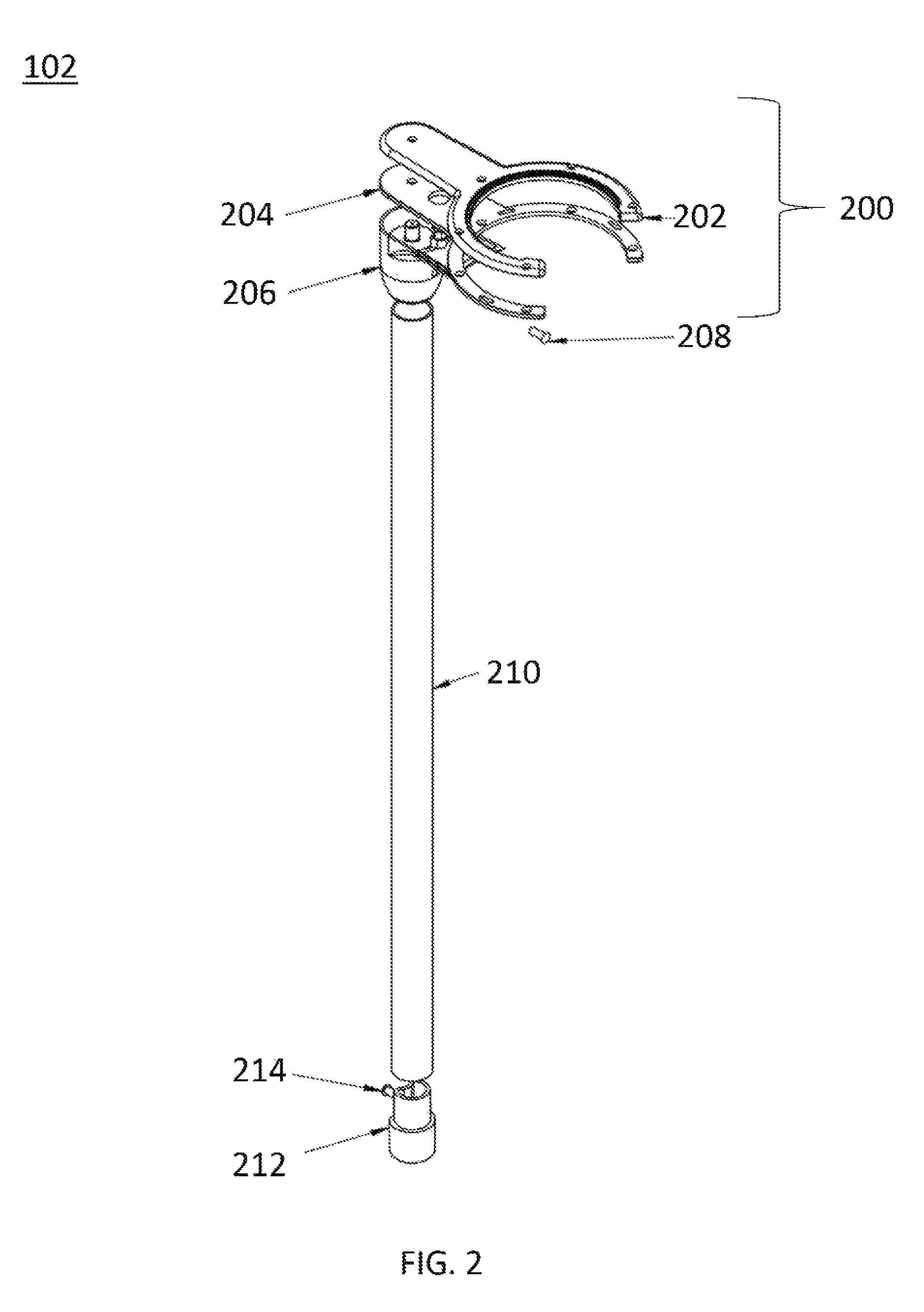
Multi-legged stand with stabilizers
ActiveUS20140151527A1Improve the immunityImprove stabilityPipe supportsStands/trestlesMicrophoneEngineering
Described is a multi-legged instrument stand that is specifically designed to increase stability and resistance to tipping. The stand can be used with any suitable instrument, such as guitars (via a guitar hanger), microphones, etc. The stand includes a base with a body pole. A leg connector is slidably attached with the body pole, with three legs and two stabilizers being pivotally connected with the leg connector. Thus, the legs and stabilizers can collapse for storage or rotate outward to allow the stand to be positioned upon a ground surface with increased stability.
Owner:RKS VENTURES
Method of marking a product, marked product resulting thereof, and method of identifying same
ActiveUS20060228802A1Significant variabilityWork reliablyAnalysis using chemical indicatorsTesting/calibration apparatusIon chromatographyMass Spectrometry-Mass Spectrometry
A method and means for identifying the authenticity and the genuine nature of a solid or liquid bulk material, by incorporating a marking composition containing at least one trace ion into the said bulk material, whereby the total concentration of the incorporated trace ions in the market bulk material is chosen to be lower than the corresponding concentration of the same ions in standard sea water. The authenticity and the genuine nature or the adulteration level of the marked bulk material can be tested in-the-field using electrochemical sensors, and confirmed in the laboratory using a method such as atomic absorption spectroscopy, ion chromatography or mass spectrometry.
Owner:SICPA HLDG SA
Electronic switch for controlling a device in dependency on a sleep stage
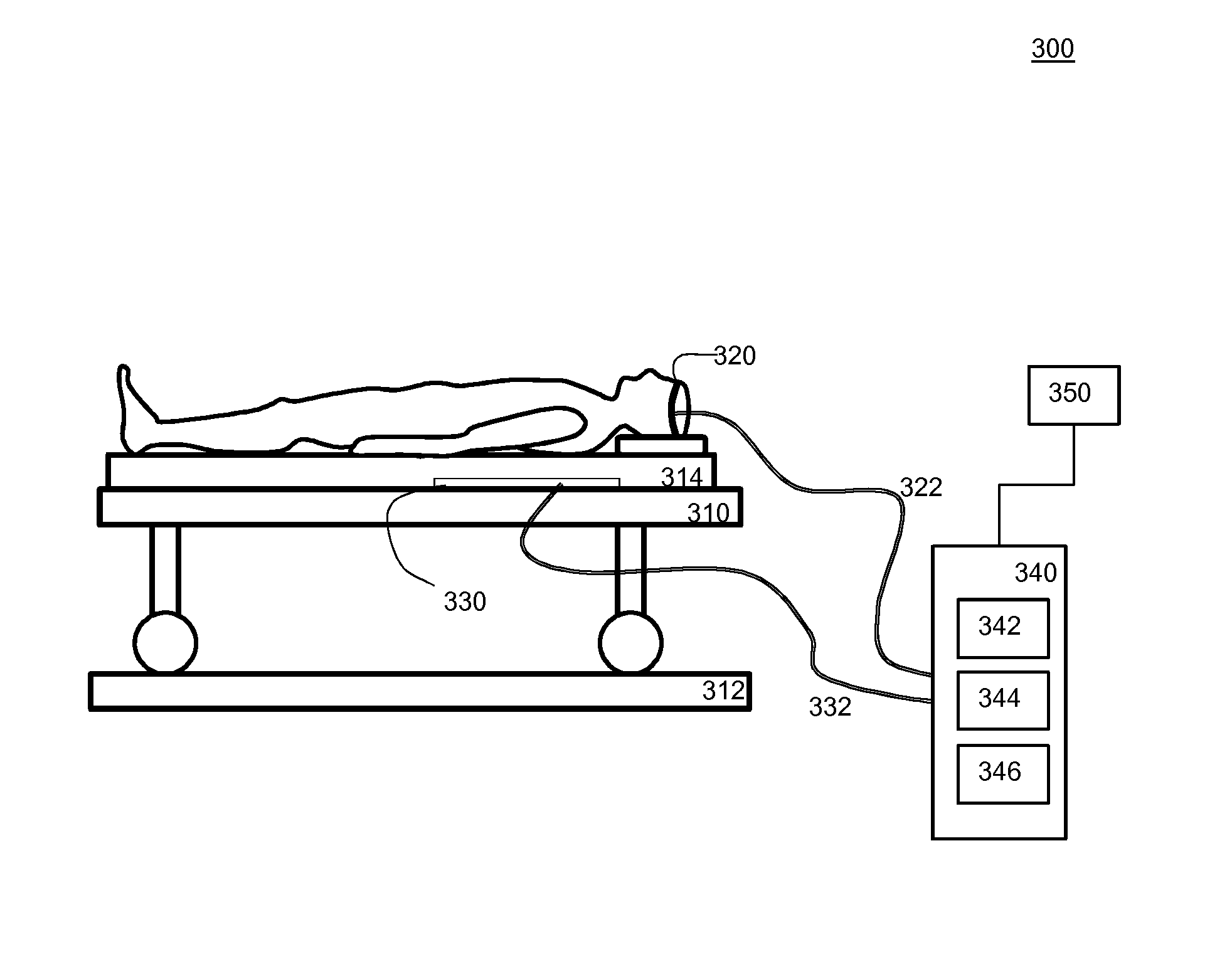
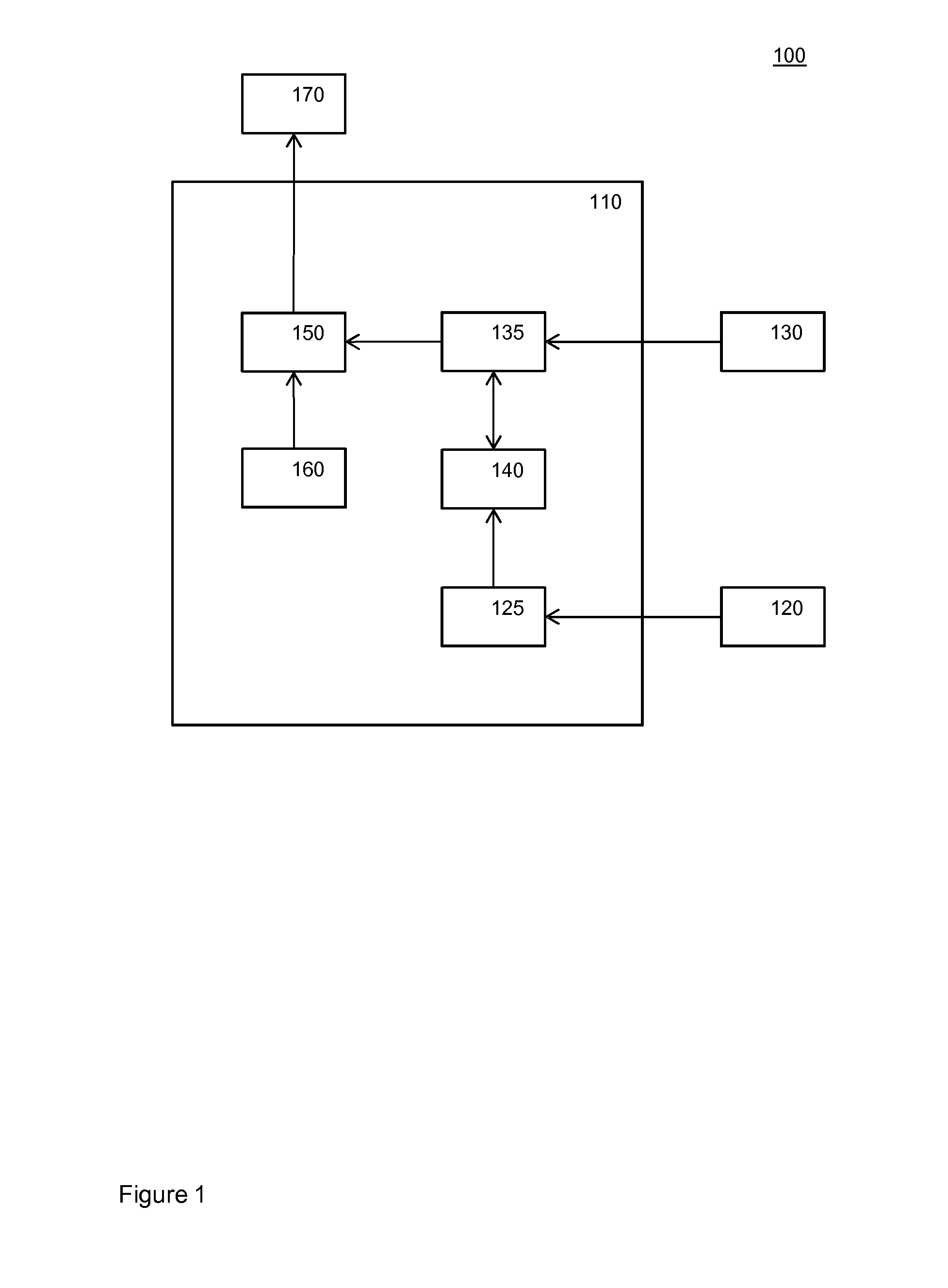
InactiveUS20150302722A1Reduce effectWell linkElectroencephalographyAcoustic time signalsPhases of clinical researchElectricity
An electronic switch for controlling a device (170) by switching a function of the device at least in dependency on a sleep stage of a human, the switch comprising an EEG data interface configured to receive brain activity data from an EEG sensor (120) configured to monitor electrical activity of the brain of the human during a training phase, an EEG sleep classifier (125) configured to classify sleep stages of the human from the received brain activity data, a body data interface configured to receive body activity data from an alternative sensor (30) configured to monitor a bodily function of the human both during the training phase and during a subsequent usage phase, the alternative sensor being different from the EEG sensor, an alternative sleep classifier (135) and a machine learning system (140), the machine learning system being configured to train the alternative sleep classifier (135) to classify a sleep stage of the human from the received body activity data, the learning system using sleep stages classified by the EEG sleep classifier (125) and concurrent body activity data received from the alternative sensor as training data, wherein in the usage phase the device (170) is controlled in dependency on sleep stages of the human classified by the alternative sleep classifier (135), and control logic (150) configured to at least determine that the classified sleep stage is one of a set of particular sleep stages and to switch a function of the device at least in dependency on said determination.
Owner:KONINKLJIJKE PHILIPS NV
Method for retarding agricultural non-point source pollution by applying biological carbon and nitrification inhibitor
InactiveCN105612846AReduce pollutionEasy to operateOther chemical processesOrganic fertilisersAgricultural ecosystemsNitrification inhibitors
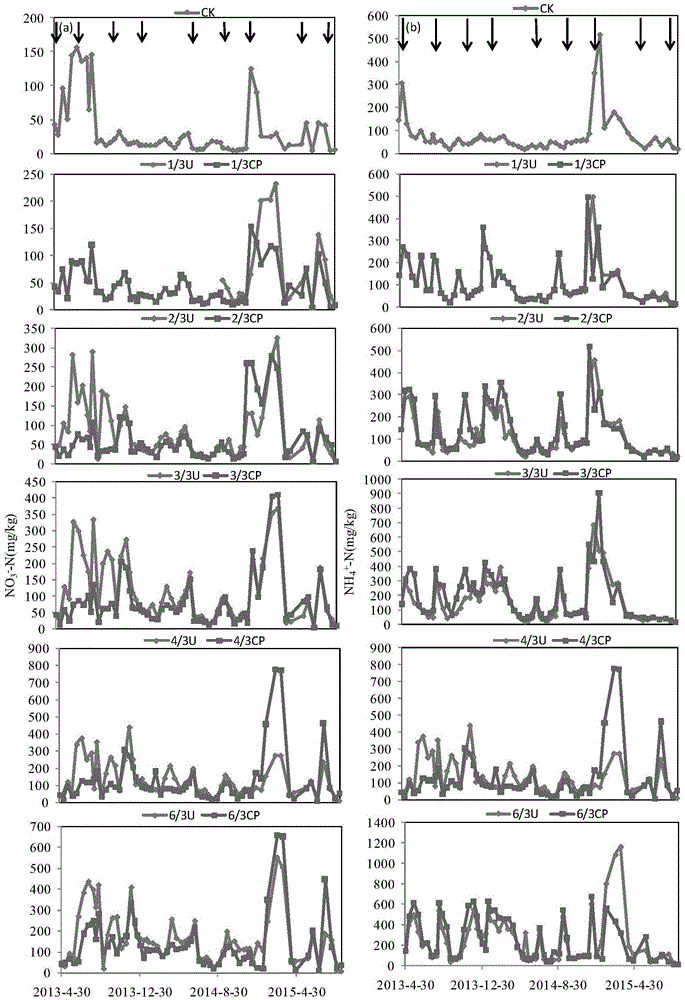
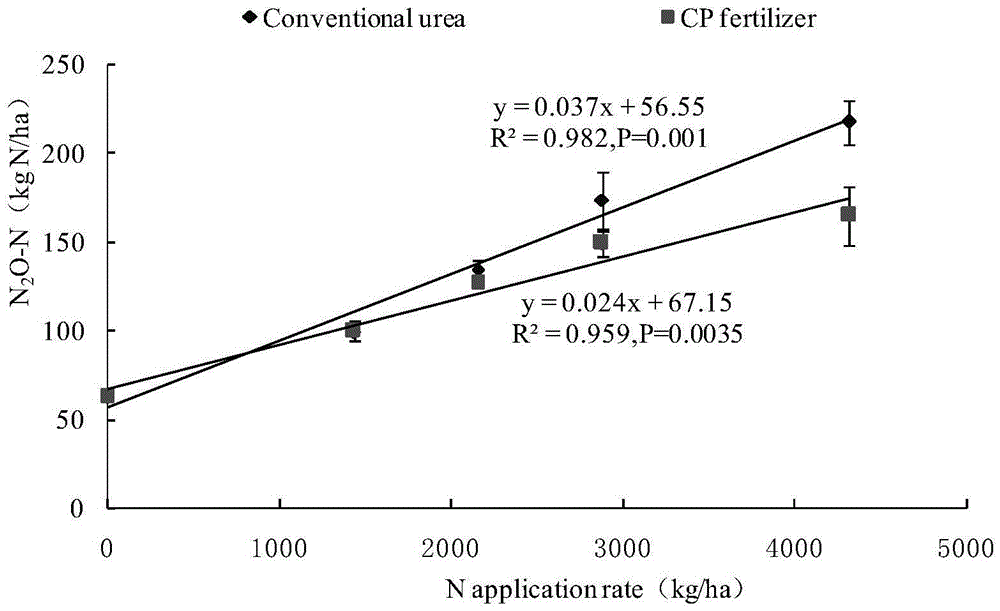
The invention relates to a method for retarding agricultural non-point source pollution by comprehensively applying biological carbon and a nitrification inhibitor, belongs to the field of agricultural non-point source pollution research, and is dedicated to retarding non-point source pollution caused by nitrogen gas losses and subsequent atmospheric deposition in a paddy field and vegetable field ecosystem. According to the method, the biological carbon and the nitrification inhibitor are applied in fields of a rice-wheat crop rotation rice field and intensive vegetable cultivation ecosystem, therefore emission of gaseous nitrogen, such as N2O and NOx, is effectively reduced, and the atmospheric deposition is reduced to retard the non-point source pollution; the utilization rate and the yield of crops are increased, and nitrate nitrogen content of soil and migration of nitrate nitrogen to a water body are reduced to retard the non-point source pollution. The method is a brand-new, rapid, simple and efficient non-point source pollution retarding method, can be used for a wide variety of agricultural ecosystems, is convenient to operate and easily available in material, can be widely applied to prevention and control research on the agricultural non-point source pollution to paddy fields and vegetable fields, and provides an effective means for deeply researching the agricultural non-point source pollution.
Owner:NANJING AGRICULTURAL UNIVERSITY
Method for producing map images of surface sea current velocity vectors and altimetric radar system using the method
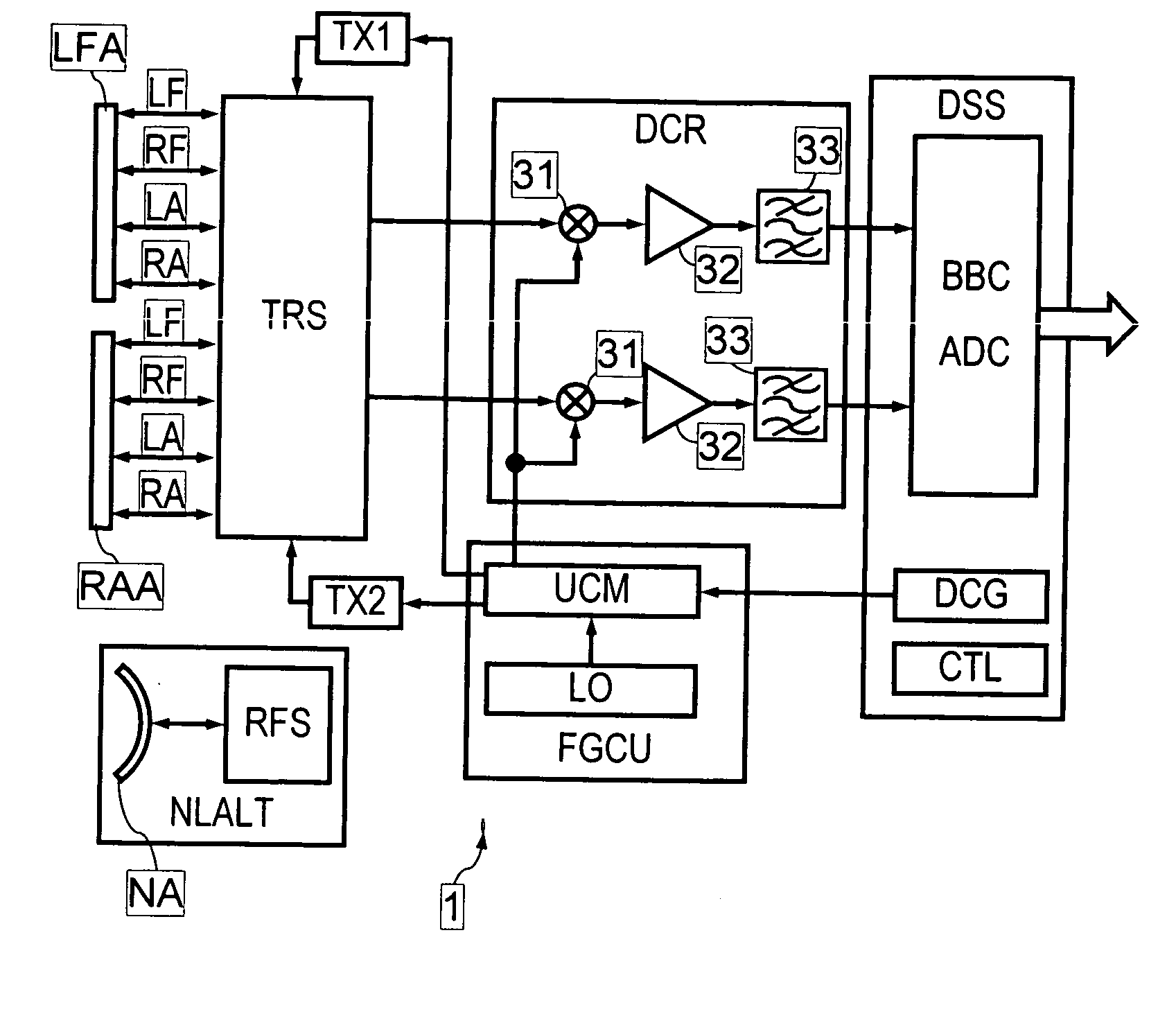
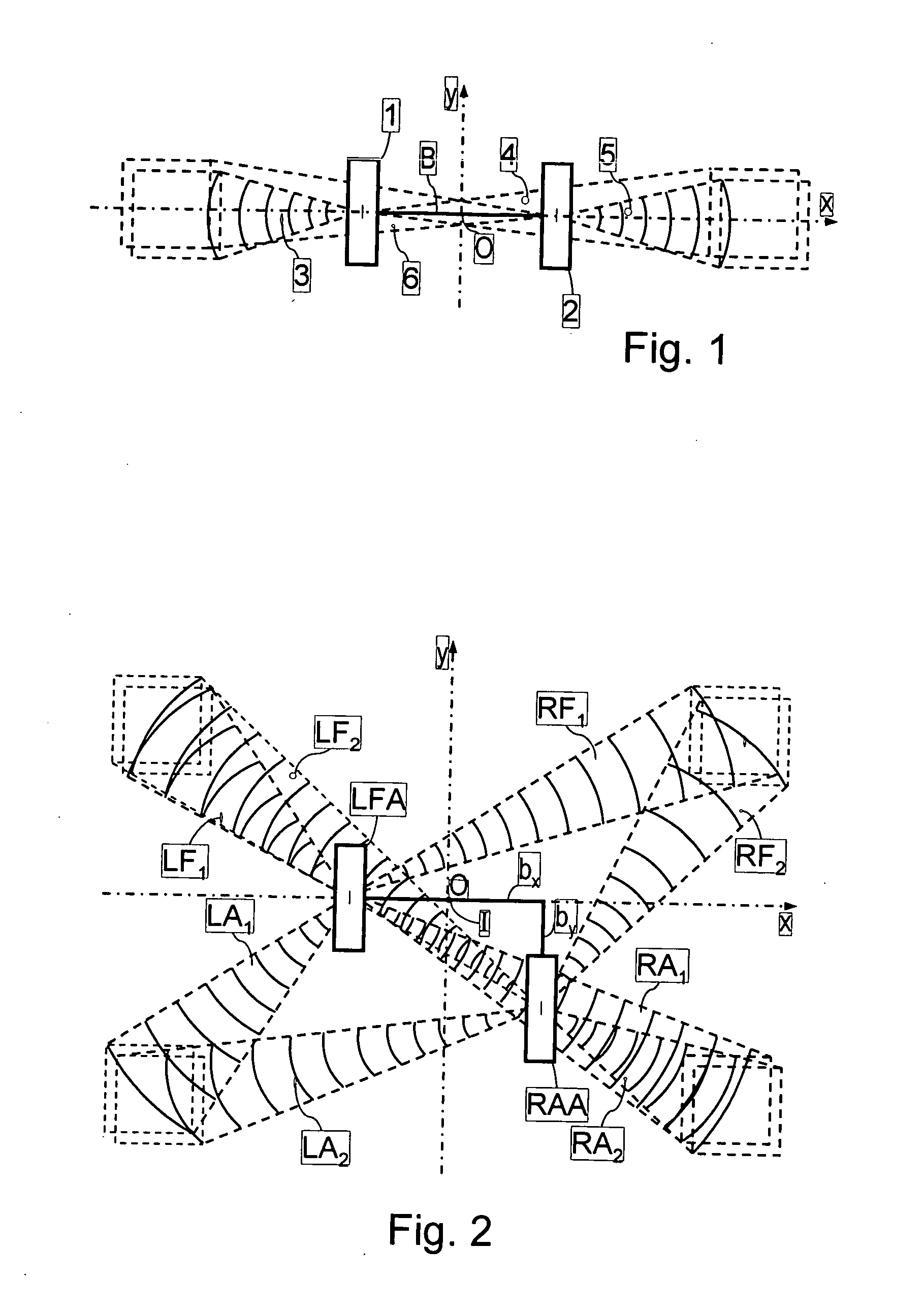
InactiveUS20060262004A1Eliminate disadvantagesSignificant variabilityRadio wave reradiation/reflectionCurrent velocityRadar systems
A method for producing map images of current velocity vectors at the surface sea is described in which beams of electromagnetic waves are emitted towards the surface using a system, towards the left forward and right forward sides of the track of the system, and towards the left aft and right aft sides of the track, from two antennas at a distance from each other along the direction of the track and along a direction perpendicular to the track, and values of roll angle and length of the antenna base connecting the two antennas are determined using differential interferometries applied to the electromagnetic waves reflected by the surface between beams emitted forwards and backwards, the map images being built up using an along-track type differential interferometry, using roll angle and antenna base length values obtained.
Owner:EUROPEAN SPACE AGENCY
Light source
ActiveUS20170241619A1Remove heatLower operating temperatureNon-electric lightingPoint-like light sourcePhosphorAutomotive industry
The light source is based on a high-efficiency solid-state laser source of the excitation coherent radiation and a single crystal phosphor which is machined in a form of an optic element for emitted light parameterisation. The single crystal phosphor is produced from a single crystal material on the basis of garnets of the (Ax, Lu1-x)aAlbO12:Cec general formula or from a single crystal material on the basis of perovskite structure of the B1-qAlO3:Dq general formula. The efficient light source shall be utilized e.g. in the automotive industry.
Owner:CRYTUR SPOL SRO
Multi-legged stand with stabilizers
ActiveUS9330645B2Improve the immunityImprove stabilityStands/trestlesMusical supportsEngineeringMusical instrument
Owner:RKS VENTURES
Thick film multilayer reflector with tailored layer thickness profile
ActiveUS7385763B2Substantial effect on optical performanceSignificant variabilityLayered productsPolarising elementsPolarizerLength wave
A multilayer reflector useable to reflect or transmit light over the visible wavelength range includes optically thick constituent layers. The optical thickness of the constituent layers through the thickness of the reflector defines a layer thickness profile. The layers are arranged so that the thickness profile has a tailored non-uniform distribution, such as a graded distribution or a randomized distribution. The layers desirably have an optical thickness in a range from about one to five or one to ten design wavelengths. The reflector can be a polarizer, reflecting only one normally incident polarization state, or a mirror, reflecting two normally incident orthogonal polarization states.
Owner:3M INNOVATIVE PROPERTIES CO
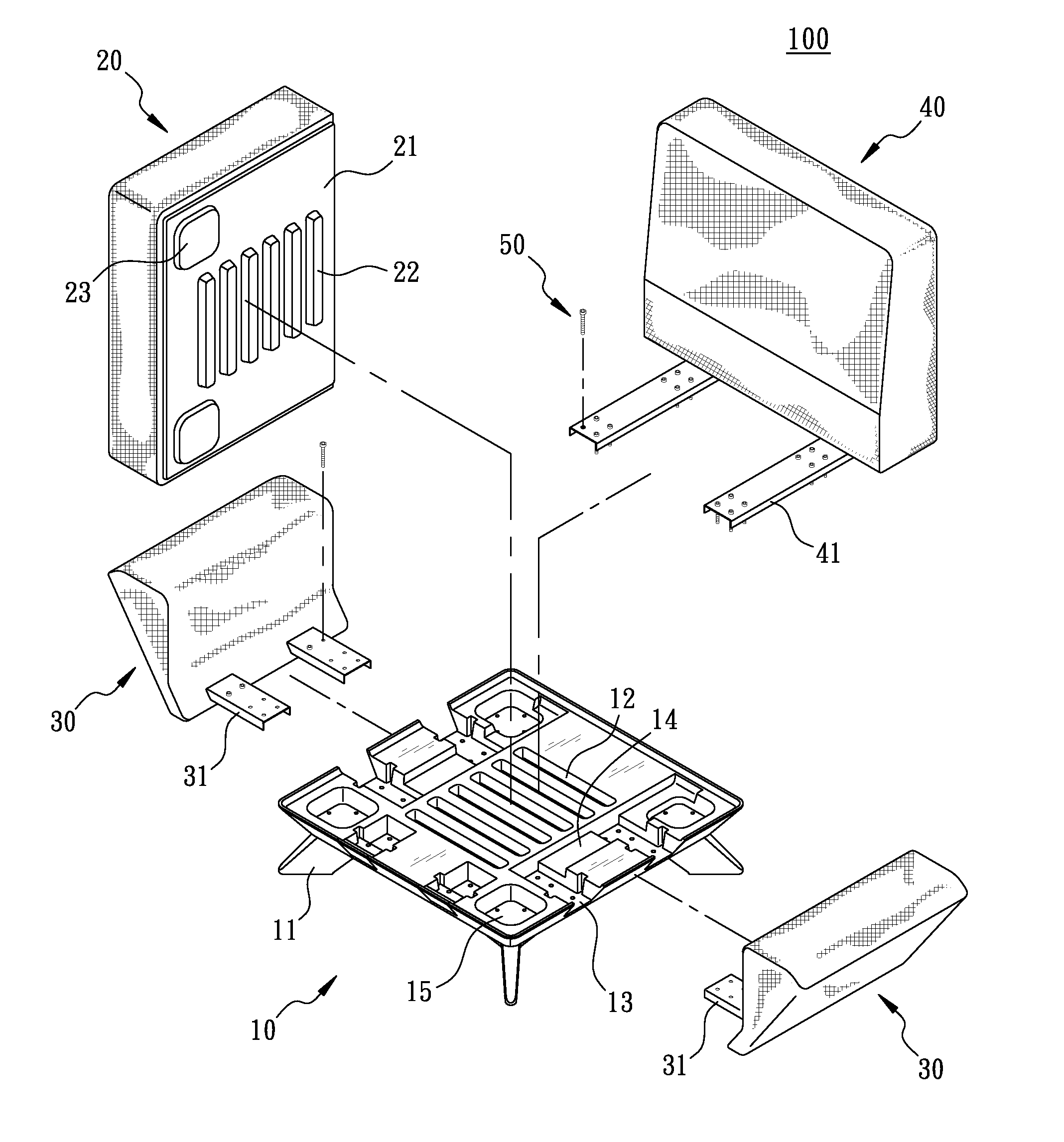
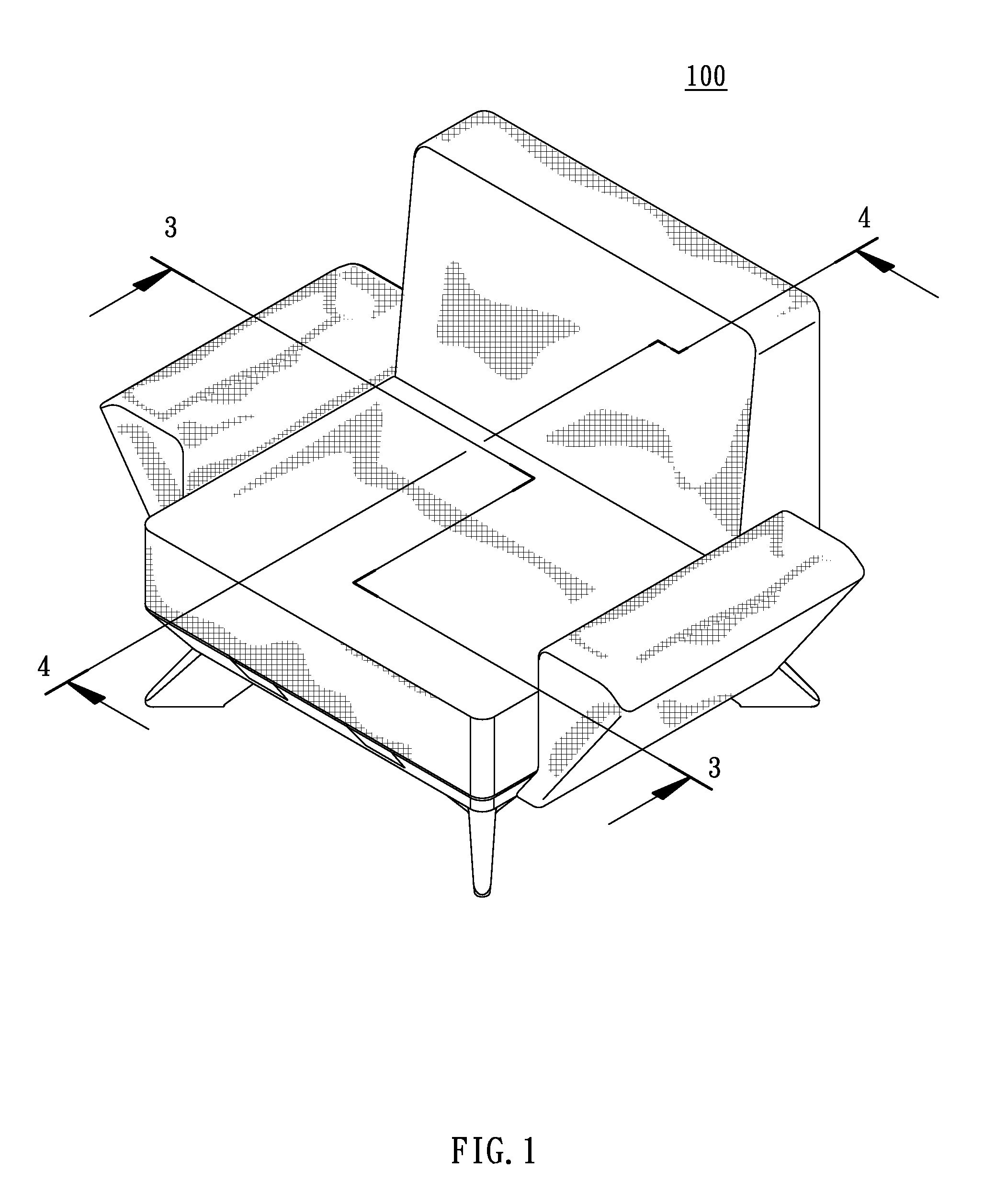
Combination furniture
A combination furniture includes a base, a main cushion, two armrests, a backrest, and a fastener assembly. The top surface of the base is concavely provided with plural strip-shaped coupling grooves. The main cushion has a bottom plate provided with plural coupling strips integrally projecting from the bottom plate for being coupled with the coupling grooves. The two armrests have a pair of combining parts formed in parallel and slidingly and transversely installed on two sides of the base, respectively. The backrest has a pair of fixing parts formed on the backrest in parallel for correspondingly overlapping the two combining parts of the two armrests. The fastener assembly fastens the combining parts and the corresponding fixing parts to the base. Therefore, the combination furniture is easily assembled, manufactured, and transported.
Owner:BUDDHA SHENG INT
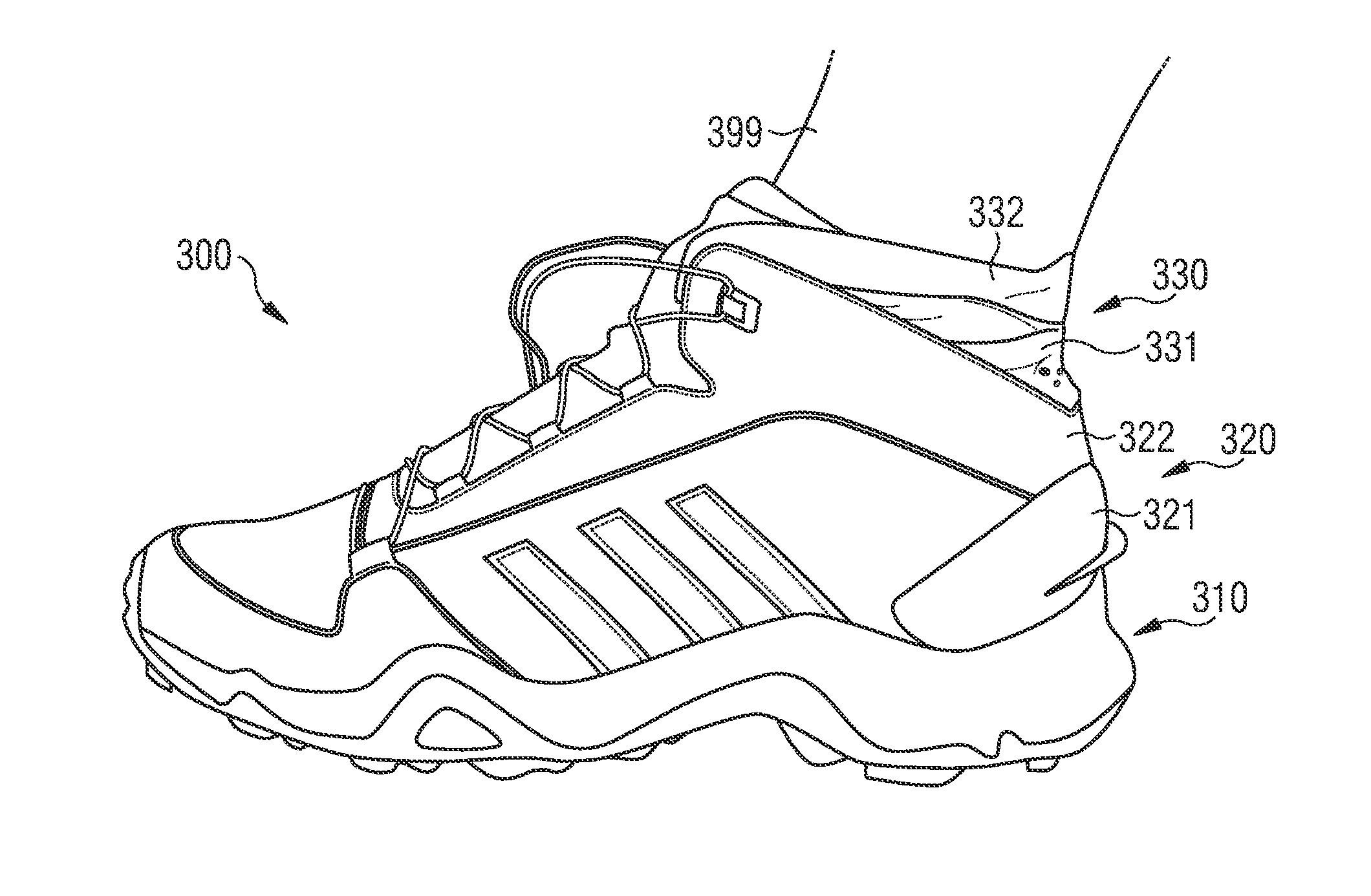
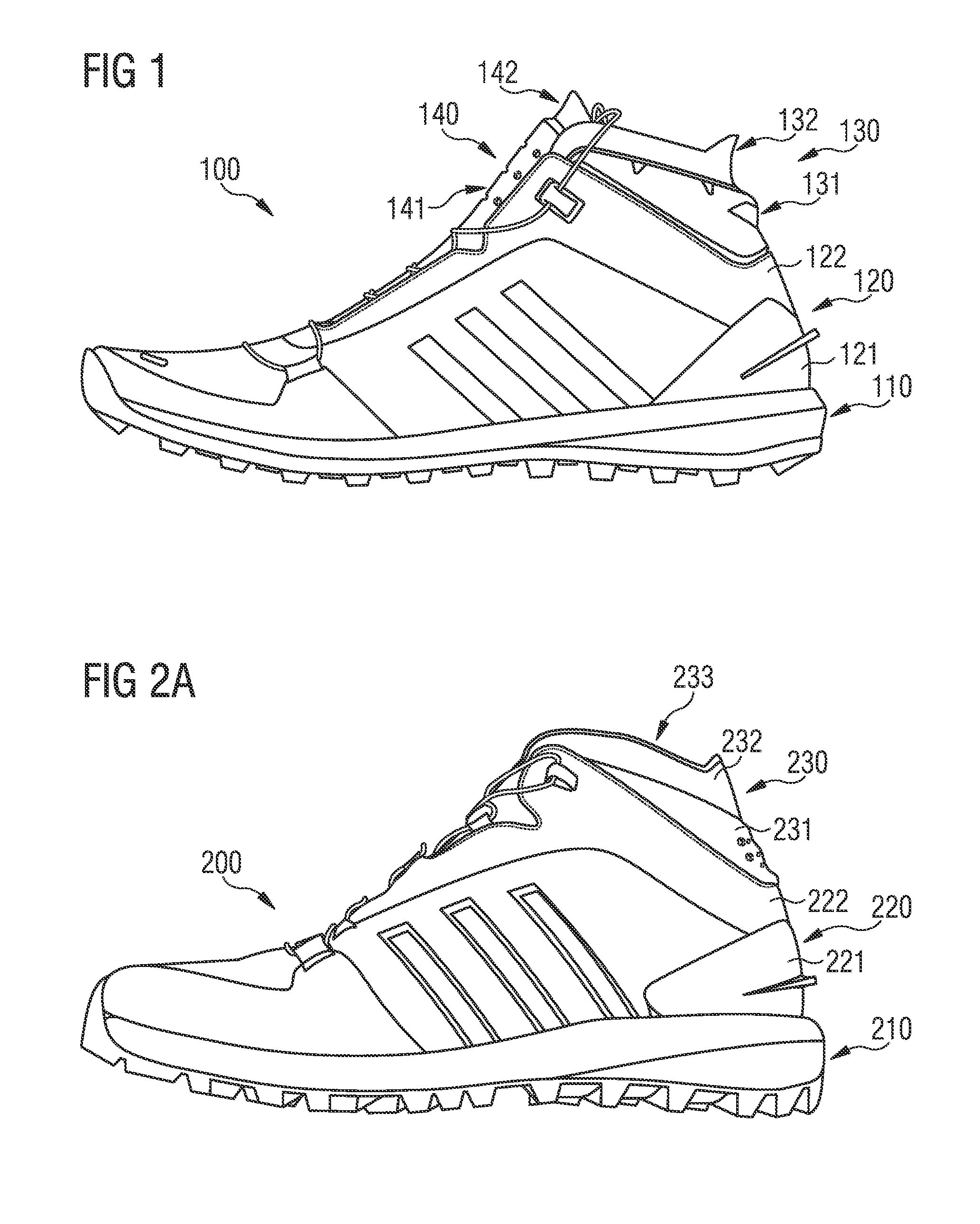
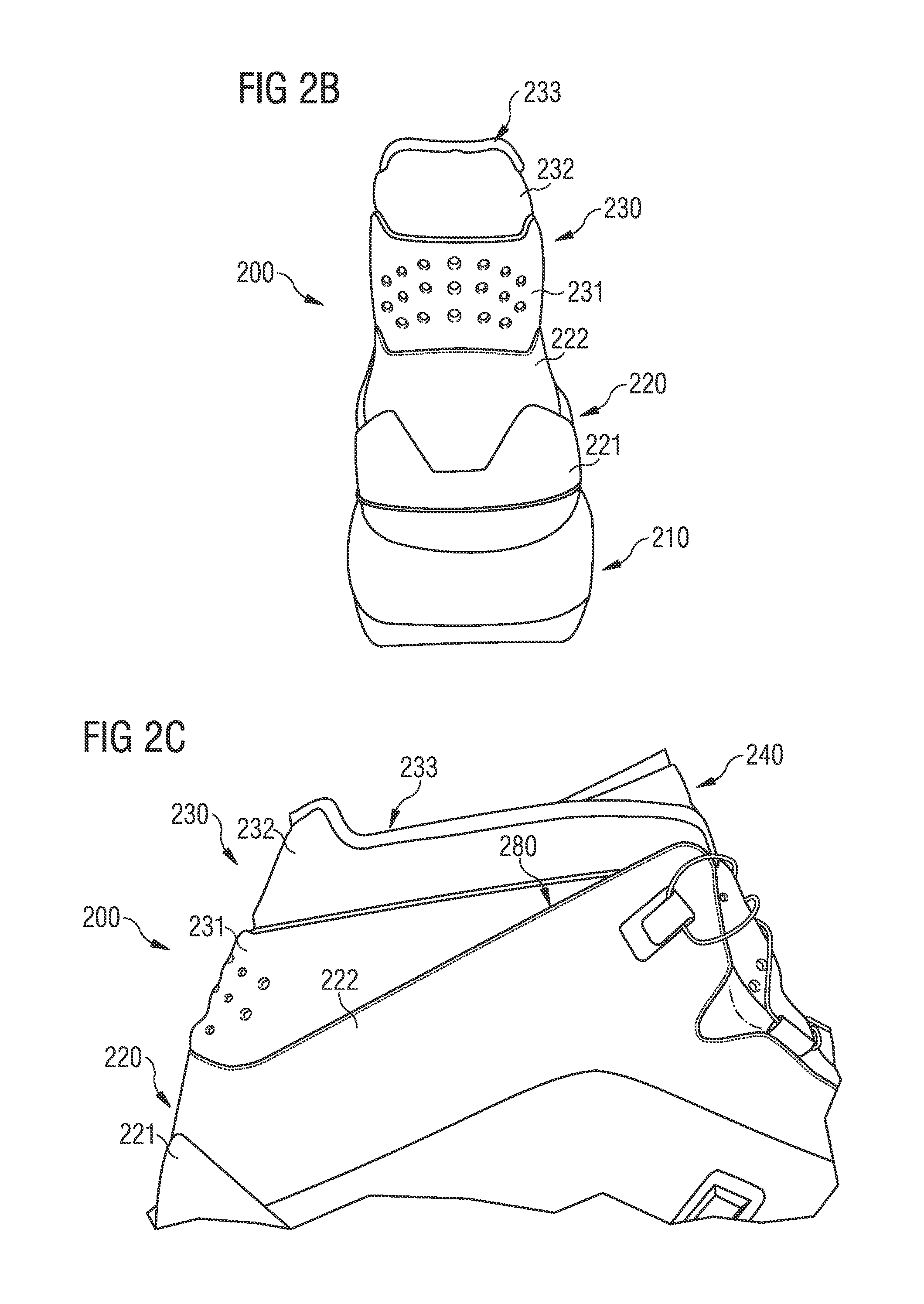
Shoe
Described are shoes with a rigid heel portion, and a collar arranged above the rigid heel portion. The collar includes a first collar portion and a second collar portion, the first collar portion and the second collar portion are configured to partially engage an ankle of a wearer of the shoe on a lateral side, a medial side, and a rear side of the ankle when worn. The first collar portion is more flexible than the rigid heel portion, and the second collar portion is more flexible than the first collar portion.
Owner:ADIDAS
Beverage sleeve for a container
InactiveUS8365947B2Good insulationEffective positioningStampsContainer decorationsBiomedical engineeringEngineering
An infinitely variable diameter beverage sleeve includes a first surface configured to contact the container and extend around a circumference of the container, and a second surface configured to extend around the circumference of the container and face away from the container, and a securing device to hold the sleeve to the outside surface of the container. A first portion of the securing device is provided on one end of the sleeve on the first surface, and a second engaging portion of the securing means is provided as the second surface of the sleeve. The top edge of the sleeve is non-planar and the bottom edge of the sleeve is formed of a plurality of segments with at least two segments having a different radius of curvature.
Owner:SOLOMON MERRILL
User interface device and method
InactiveUS20110241822A1Easy to operateImprove gripInput/output for user-computer interactionComputer controlUser interfaceBiomedical engineering
A user interface device (1) comprises a non-flexible part (3); at least one flexible part (5) coupled to the non-flexible part (3); and at least one sensor, for detecting bending of a respective flexible part (5) with respect to the non-flexible part (3), and for producing a command in response to sensing bending of the respective flexible part (5).
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com