Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
122 results about "Antidepressant medication" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An antidepressant is the name given to a medicine that can help relieve the symptoms of depression, such as low mood, anxiety, and worthlessness. Antidepressants are classified into different types depending on their structure and the way that they work. There are at least seven types of antidepressant: Monoamine oxidase inhibitors (MAOIs)
Method of treating depressive disorders
The invention provides methods of treating depressive disorders, in particular major depression but other depressive orders also, with prodrug stimulants or analogs including amphetamine prodrugs, methylphenidate prodrugs, and methylphenidate analogs, Such methods of treatment may utilize the prodrug stimulant or analog as monotherapy or, more commonly, as an adjunct to antidepressant medication treatment to augment their effect. The invention includes combination methods of treatment in which an amphetamine prodrug, methylphenidate prodrug, or methylphenidate analog is administered to an individual in need with one or more other active agents, either in separate forms or as a single pharmaceutical formulation. Packaged pharmaceutical compositions containing an amphetamine or methylphenidate prodrug, instructions for using the prodrug to treat certain disorders, and optionally one or more other active agents are provided by the invention.
Owner:LUCERNE BIOSCI
Composition and Method for Treatment of Depression and Psychosis in Humans
ActiveUS20140018348A1Eliminate side effectsReduce side effectsBiocideNervous disorderPsychosisAntagonist
Compositions and methods tor the treatment of depression and psychoses in humans are disclosed. More particularly, the invention is directed to formulations containing antipsychotic and / or antidepressant medications and also containing an NMPAR antagonist. The present invention is also directed to methods for the treatment of humans suffering from depression and other psychoses, including, schizophrenia, by administration of the inventive compositions in antidepressant and / or antipsychotic effective amounts.
Owner:GLYTECH
As-needed administration of tricyclic and other non-SRI antidepressant drugs to treat premature ejaculation
InactiveUS6946141B2Slow onsetDelay the onset of ejaculationBiocidePowder deliveryTricyclic anti-depressantsTricyclic antidepressant
A method is provided for treatment of premature ejaculation by administration of an antidepressant drug selected from tricyclic antidepressants, tetracyclic antidepressants, MAO inhibitors, azaspirone antidepressants, and atypical non-SRI antidepressants. In a preferred embodiment, administration is on as “as-needed” basis, i.e., the drug is administered immediately or at most several hours prior to sexual activity. Pharmaceutical formulations and packaged kits are also provided.
Owner:VIVUS
Application of bakuchiol compound
InactiveCN101088498AAchieve therapeuticAchievement or effect of neurological diseaseNervous disorderHydroxy compound active ingredientsDiseaseCytotoxicity
The present invention discloses the application of bakuchiol compound in preparing medicine for treating psychogenic diseases and neurogenic diseases. Extracorporeal experiment and animal experiment show that the bakuchiol compound can suppressing dopamine transport protein, noradrenalin transport protein and 5-hydroxy tryptamine transport protein selectively, and no influence on gamma-aminobutyric acid transport protein and L-glytamic acid transport protein, no cytotoxicity, and antidepressant effect higher than available antidepressant medicine amfebutamoe hydrochloride. Therefore, the bakuchiol compound has excellent marketability in preparing medicine for treating psychogenic diseases and neurogenic diseases.
Owner:上海国联干细胞技术有限公司
Oral contraceptives to prevent pregnancy and diminish premenstrual symptomatology
This invention relates to a method of preventing pregnancy and treating PMS including PMDD. More particularly, the invention relates to a method, which involves administering one of several combination oral contraceptive regimens in combination with an antidepressant and a kit containing the same.
Owner:TEVA WOMENS HEALTH
Assays For Selecting A Treatment Regimen For A Subject With Depression And Methods For Treatment
InactiveCN104053785ANervous disorderMicrobiological testing/measurementTest sampleSerotonin receptor inhibitors
Disclosed herein are novel assays, systems and kits for selecting a treatment regimen for a subject with depression by identifying at least one nucleic acid polymorphism, e.g., but not limited to, at the MTHFR, MTR, or MTRR locus, and / or determining expression levels of peripheral biomarkers (e.g., SAM, SAH, and 4-HNE) in a test sample from a human subject. These biomarkers can be used to determine the effectiveness of treating a depressed subject with a folate-containing compound (alone or as an adjunct to an antidepressant). Additionally, these biomarkers can be used to select an appropriate treatment regimen for subjects with treatment-resistant depression (e.g., resistant to at least one selective serotonin reuptake inhibitor). Methods and compositions for treating a subject with depression and / or determining or improving the effectiveness of an antidepressant drug taken by a subject are also provided herein.
Owner:ALFASIGMA SPA
Preparation method and application of 3-(4-chlorobutyl)-5-cyano-1H-indole
The invention relates to a preparation method and application of 3-(4-chlorobutyl)-5-cyano-1H-indole. The preparation method comprises the following steps: after dissolving 3-(4-chlorobutyryl)-5-cyano-1H-indole in a solvent, adding trifluoroacetic acid, adding sodium borohydride in batches, and treating the reaction liquid to obtain the 3-(4-chlorobutyl)-5-cyano-1H-indole. The method overcomes the defects in the existing preparation method of an important intermediate 3-(4-chlorobutyl)-5-cyano-1H-indole of an antidepressant vilazodone hydrochloride, and has obvious creativity and practical application value. The reaction formula is disclosed in the specification.
Owner:HANGZHOU HEZE PHARMA TECH
Methods to predict the outcome of treatment with antidepressant medication
InactiveUS20080233657A1Sugar derivativesMicrobiological testing/measurementGenetic MaterialsGenotype
The invention provides a method for determining the outcome of treatment with an antidepressant medication in a patient. In particular, the invention provides a method of screening patients to identify those patients with a decreased risk of non-response to treatment with antidepressant medication comprising: (a) obtaining a sample of genetic material from the patients, and (b) assaying the sample for the presence of a genotype in the patients which is associated with a decreased risk of non-response to treatment with antidepressant medication, wherein the genotype is characterized by a polymorphism in a HTR2A, GRIK4, BCL2, or a combination thereof.
Owner:GOVERNMENT OF THE US REPRESENTED BY THE SEC +1
Medicinal composition
The invention relates to a medicinal composition taking a medicinal indianmulberry root oligosaccharide extract and a non-saccharide antidepressant medicament as active ingredients. The medicinal composition is characterized in that: the total content of inulin type oligosaccharide in the medicinal indianmulberry root oligosaccharide extract is 50 to 70 percent, and the content of inulin type penta-polyoligosaccharide is 5 to 15 percent; the non-saccharide antidepressant medicament comprises monoamine oxidase inhibitor type antidepressant medicaments such as moclobemide and the like, norepinephrine reuptake inhibitor type antidepressant medicaments such as imipramine, amitriptyline, maprotiline, doxepin, chlorimipramine or reboxetine and the like, selective 5-serotonin reuptake inhibitor type antidepressant medicaments such as fluoxetine, paroxetine, sertraline or citalopram and the like, or methylepinephrine / selective 5-serotonin dual reuptake inhibitor type non-saccharide antidepressant medicaments such as mirtazapine, duloxetine, venlafaxine or demethyl venlafaxine and the like. The invention also relates to the application of the medicinal composition taking the medicinal indianmulberry root oligosaccharide extract and the non-saccharide antidepressant medicament as the active ingredients in treating nervous and mental diseases such as depression, anxiety disorders, neuropathic pains and the like.
Owner:BEIJING MEIBEITA DRUG RES
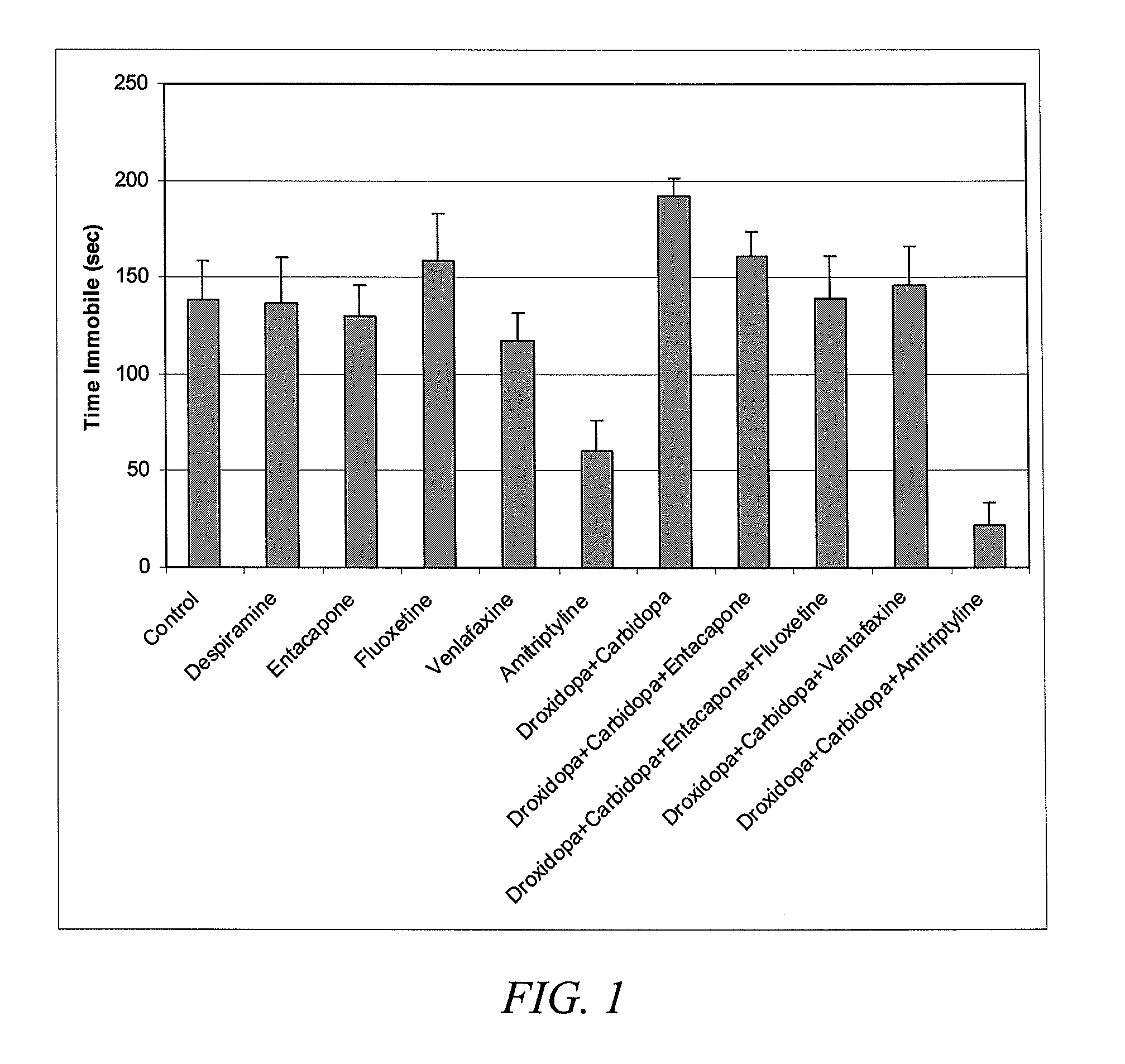
Droxidopa and pharmaceutical composition thereof for the treatment of mood disorders, sleep disorders or attention deficit disorders
ActiveUS20090023705A1Eliminate symptomReduces severity of symptomBiocideNervous disorderAdrenergicSleep disorder
The present invention provides pharmaceutical compositions comprising droxidopa alone, or in combination with one or more further active ingredients, for the treatment of conditions, such as mood disorders, sleep disorders, or attention deficit disorders. In certain embodiments, the compositions useful in the methods of the invention comprise droxidopa and a compound selected from the group consisting of DOPA decarboxylase inhibiting compounds, catechol-O-methyltransferase inhibiting compounds, cholinesterase inhibiting compounds, monoamine oxidase inhibiting compounds, norepinephrine reuptake inhibiting compounds, selective serotonin reuptake inhibiting compounds, tricyclic antidepressant compounds, serotonin norepinephrine reuptake inhibiting compounds, norepinephrine dopamine reuptake inhibiting compound, noradrenergic and specific serotonergic antidepressants, and combinations thereof. The inventive compositions are particularly useful in the treatment of depression, narcolepsy, insomnia, and Attention Deficit / Hyperactivity Disorder (AD / HD).
Owner:CHELSEA THERAPEUTICS
Polymorphism site genotype estimation depression, use and process of medicine effect and kit
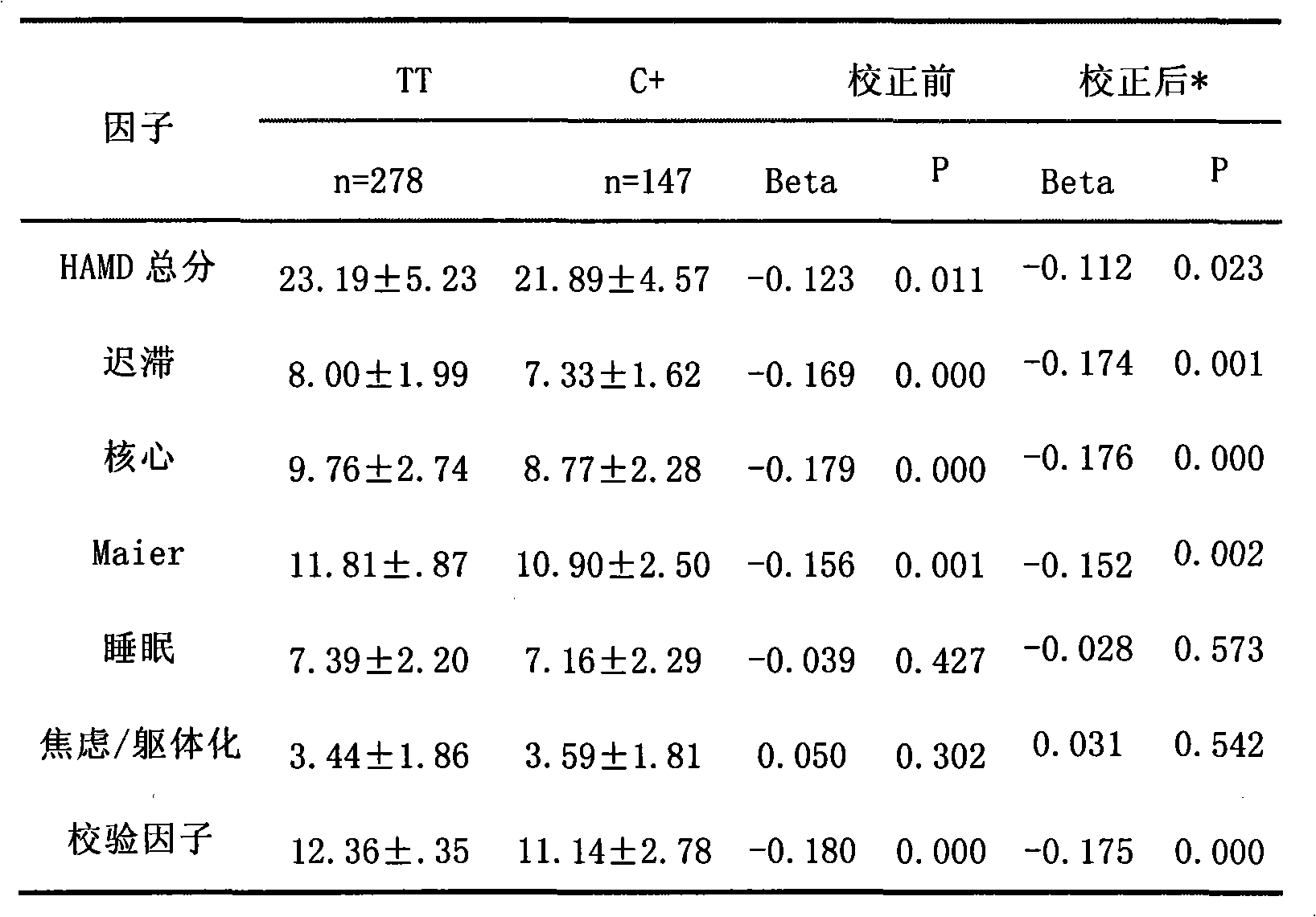
The invention relates to a 5-HTT gene 13C / T polymorphism site genotype used for forecasting an individual depression sickness degree as well as the sickness degree of the core symptom of the depression, forecasting the effect of an antidepressant as well as the usage, the method and the reagent box of the effect of the antidepressant on the core symptom of the depression; wherein, when the 5-HTT gene 13C / T polymorphism site genotype is of 13TT homozygous type, the individual depression sickness degree and the sickness degree of the core symptom of the depression are forecasted to be more serious; the effect after taking the antidepressant is better; the effect of the antidepressant to the core symptom of the depression is better; when the 5-HTT gene 13C / T polymorphism site genotype is of 13CC homozygous type or 13CT heterozygote type, the individual depression sickness degree and the sickness degree of the core symptom of the depression are forecasted to be lighter; the effect after taking the antidepressant is poorer; the effect of the antidepressant to the core symptom of the depression is poorer.
Owner:深圳泰乐德医疗有限公司
Medicine for treating post stroke depression and application thereof
InactiveCN104208583ALow priceEasy to acceptOrganic active ingredientsNervous disorderSalvia miltiorrhizaCannabis
The invention relates to a medicine for treating post stroke depression and an application thereof. The medicine is prepared from the following medicinal raw materials in parts by weight: 25-35 parts of salvia miltiorrhiza, 25-35 parts of rhizoma acori graminei, 10-20 parts of ligusticum wallichii, 10-20 parts of radix paeoniae alba, 10-20 parts of polygala tenuifolia, 5-15 parts of safflower carthamus, 5-15 parts of peach kernel, 7-17 parts of prepared rhizoma cyperi, 5-15 parts of radix bupleuri, 5-15 parts of fructus aurantii, 5-15 parts of radix curcumae, 4-8 parts of pericarpium citri reticulatae viride, 10-20 parts of endothelium corneum gigeriae galli, 25-35 parts of fructus cannabis, 25-35 parts of spina date seed and 25-35 parts of tuber fleeceflower stem. The invention further relates to a composition containing the medicine and fluoxetine hydrochloride. The medicine combined with antidepressant is capable of accelerating the drug effect taking speed, the dose can be controlled, the course of treatment can be shortened, the 'contradictory phenomena' and 'anticholinergic side effect' can be reduced, the effect of the medicine by combining comprehensive rehabilitation therapy is relatively remarkable in the treatment process, and the medicine is capable of gradually replacing the antidepressant in the later period of treatment.
Owner:上海市闸北区中医医院
Oral contraceptives to prevent pregnancy and diminish premenstrual symptomatology
InactiveUS20080064670A1Low ratePreventing pregnancyBiocideOrganic active ingredientsGynecologyObstetrics
This invention relates to a method of preventing pregnancy and treating PMS including PMDD. More particularly, the invention relates to a method, which involves administering one of several combination oral contraceptive regimens in combination with an antidepressant and a kit containing the same.
Owner:TEVA WOMENS HEALTH
Opioid Combination Wafer
InactiveUS20090291123A1Improve complianceEfficient use ofNervous disorderAntipyreticPain therapyHydrophilic polymers
Sheet-like dosage forms for pain therapy, based on hydrophilic polymers, which rapidly dissolve or disintegrate in an aqueous environment and which release active agent combinations when placed into a body orifice or body cavity, and which are preferably orally administrable, with the dosage form containing an active agent combination consisting of an opioid and a second substance The second active agent is a non-steroidal anti-rheumatic (NSAR) or an antidepressant.
Owner:LTS LOHMANN THERAPIE-SYST AG
Marine bacterial novel esterase, as well as preparation method and application thereof
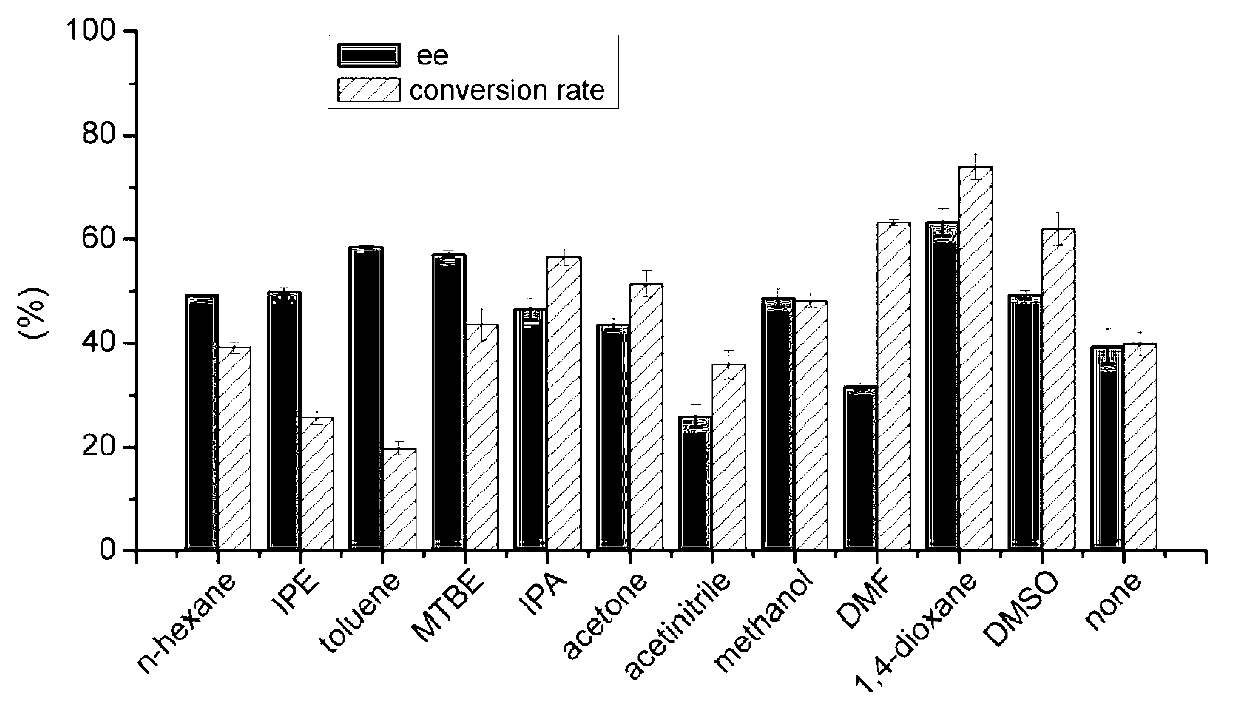
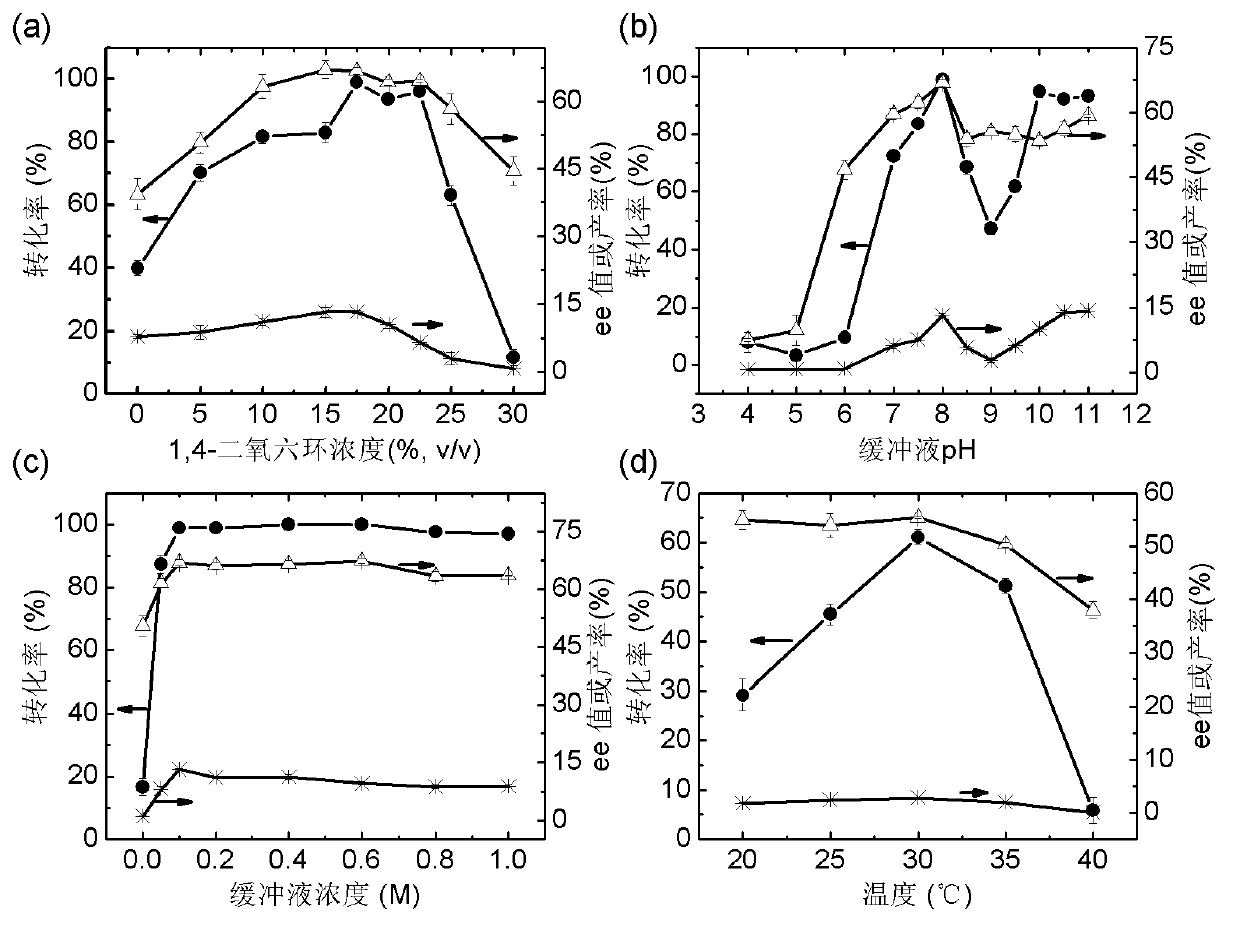
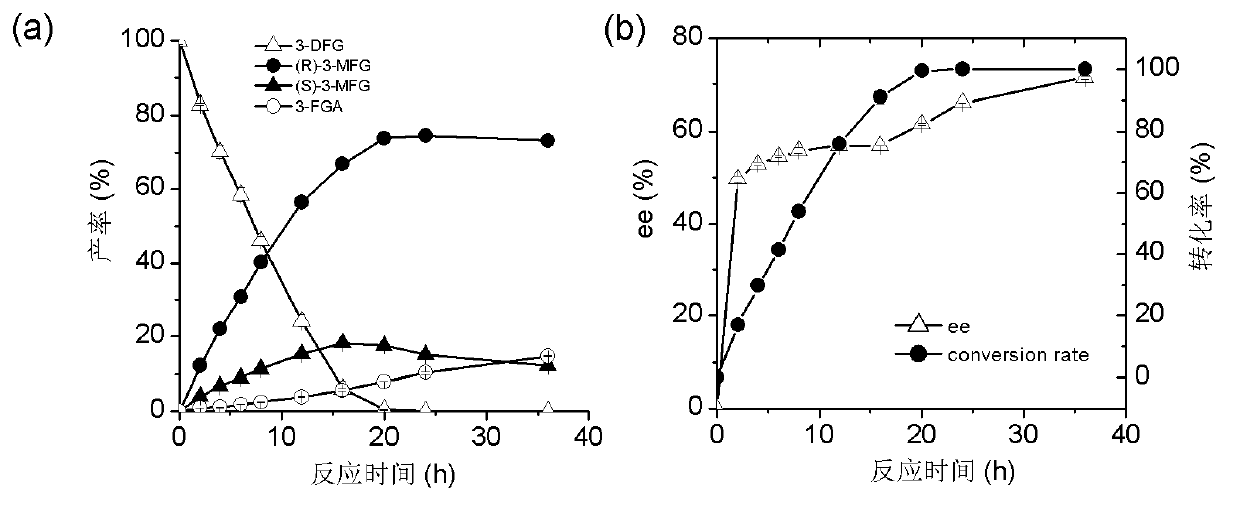
The invention discloses a marine bacterial novel esterase, and a method for producing a drug intermediate (R)-3-(4-fluorophenyl) monomethyl glutarate by chirally catalyzing 3-(4-fluorophenyl) methyl glutarate by using the esterase. The gene of the esterase is cloned to an expression plasmid to transform escherichia coli Rosetta. As the esterase can be highly and solubly expressed in an expression strain, and shows excellent salt resistant, alkali resistance and chiral selectivity, the esterase can be used as potential enzyme for industrial production of the antidepressant drug intermediate (R)-3-(4-fluorophenyl) monomethyl glutarate. As long as the reaction conditions are optimized, the esterase can be used for catalyzing 3-(4-fluorophenyl) methyl glutarate to produce the drug intermediate (R)-3-(4-fluorophenyl) monomethyl glutarate; and as a result, the transformation ratio and chiral selectivity of the esterase are greatly improved.
Owner:SECOND INST OF OCEANOGRAPHY MNR +1
Methods to identify patients at risk of developing adverse events during treatment with antidepressant medication
InactiveUS20080102467A1Increased riskSugar derivativesMicrobiological testing/measurementGRIK2Medicine
The invention provides a method of screening patients to identify those patients more likely to exhibit an increased risk of treatment-emergent suicidal ideation comprising: (a) obtaining a sample of genetic material from the patients, and (b) assaying the sample for the presence of a genotype in the patients which is associated with an increased risk of treatment-emergent suicidal ideation, wherein the genotype is characterized by a polymorphism in a gene selected from the group consisting of glutamine receptor, ionotropic, kainate 2 (GRIK2); glutamate receptor ionotropic AMPA 3 (GRIA3); and combinations thereof.
Owner:GOVERNMENT OF THE US REPRESENTED BY THE SEC +1
Pharmaceutical Dosage Form of an Antidepressant
The present invention relates to pharmaceutical dosage forms of an antidepressant. More particularly, the present invention relates to pharmaceutical dosage forms of Escitalopram oxalate. The present invention also relates to a process for the preparation of pharmaceutical dosage forms of Escitalopram oxalate.
Owner:AUROBINDO PHARMA LTD
Method for preparing high-purity agomelatine
InactiveCN104130154AReduce consumptionReduce pollutionOrganic compound preparationCarboxylic acid amides preparationCyanoacetic acidDehydrogenation
Owner:郭炳华
Anti-depression medicament as well as preparation method and application thereof
InactiveCN101347562ABehaviors that improve depressionLower metabolismNervous disorderPlant ingredientsAlcoholMedicine
The invention discloses an antidepressant drug and is that gardenia (parched) water or alcohol extraction is mixed with pharmaceutically suitable carrier through the mixture of macroporous resin isolation and purification part (effective part I) and cyperus tuber (vinegar bake), Chuanxiong rhizome and Chinese atractylodes (parched) ethanol extraction water-insoluble fraction (effective part II); then mixture is prepared into various preparation. The invention also discloses the preparation method and the medical application of the antidepressant drug.
Owner:SHANGHAI UNIV OF T C M
Application of dendrobium officinale polysaccharides to preparation of antidepressant medicines and antidepressant healthcare products
ActiveCN105963314AImprove securitySignificant antidepressant effectOrganic active ingredientsNervous disorderMedicinePolysaccharide
The invention belongs to the technical field of medicines and provides application of dendrobium officinale polysaccharides to preparation of antidepressant medicines and antidepressant healthcare products. Dendrobium officinale is used as a natural medicine and food for a long time and high in safety when serving as a medicine and a healthcare product. The dendrobium officinale polysaccharides have an excellent antidepressant effect, thereby being used for pharmaceutical production or healthcare product production as antidepressant medicines and antidepressant healthcare products. Further, sources of the antidepressant natural medicines and antidepressant healthcare products are broadened.
Owner:HUAQIAO UNIVERSITY
Anti-depression medicament using salvianolic acid B as raw material and production method thereof
The invention discloses an antidepressant drug which takes salvianolic acid B as a raw material and the dose thereof. The invention further discloses a preparation method of the drug. Experiments prove that the drug can significantly reduce the immobility time of the tail suspension test and the forced swimming test of tested mice, thereby inferring that the drug has the effect of anti-experimental depression.
Owner:张作光
Marker for antidepressant therapy and methods related thereto
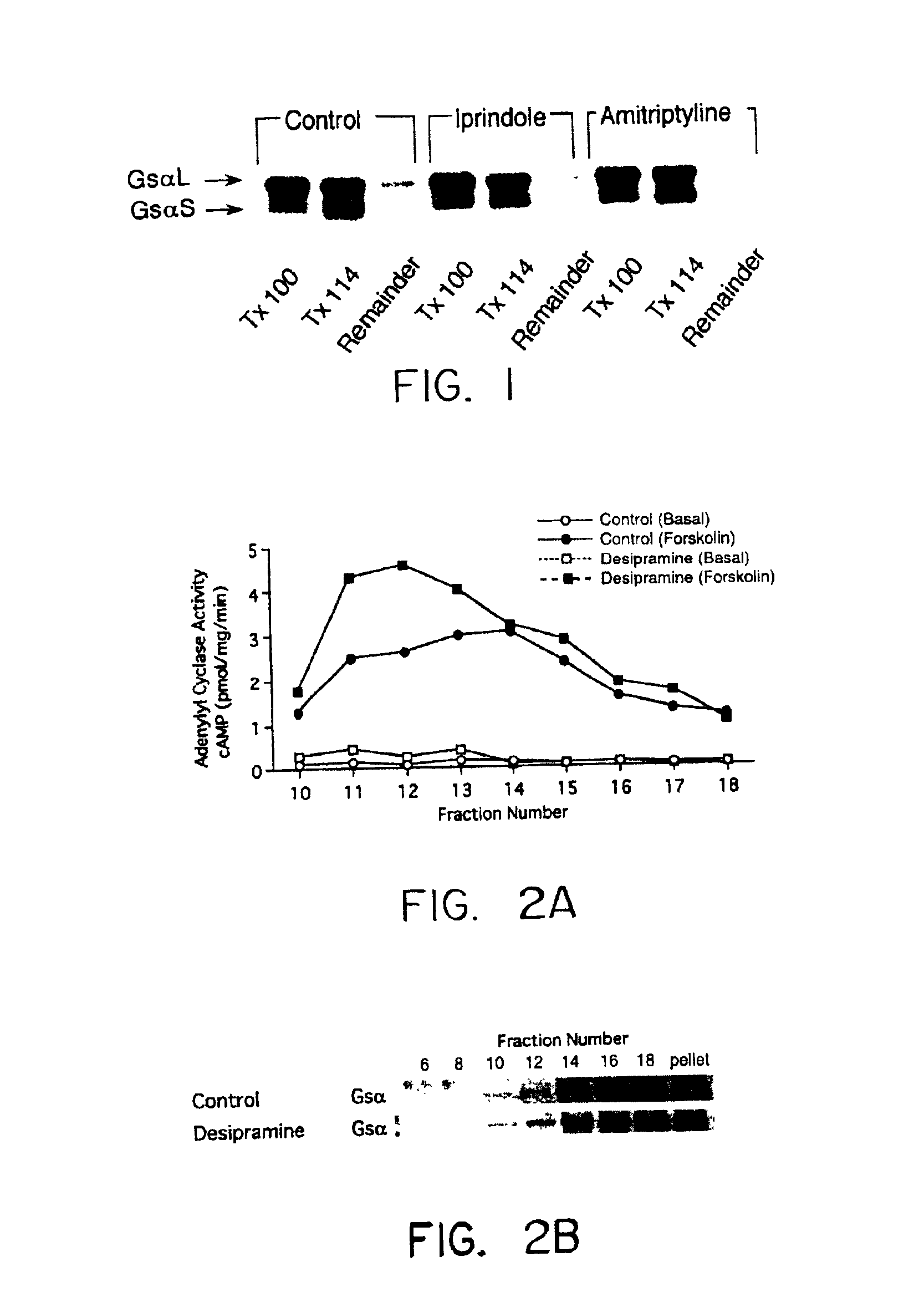
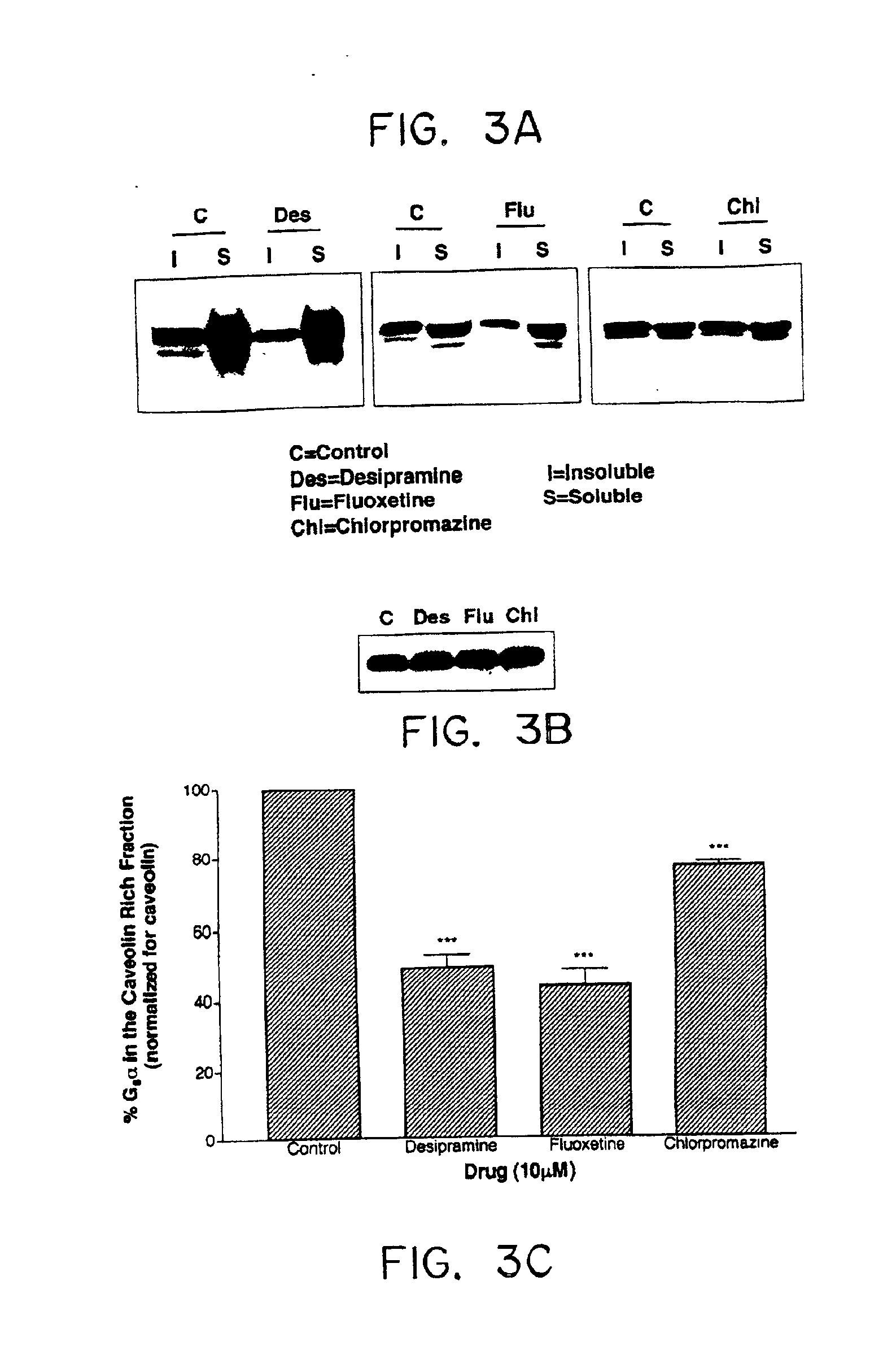
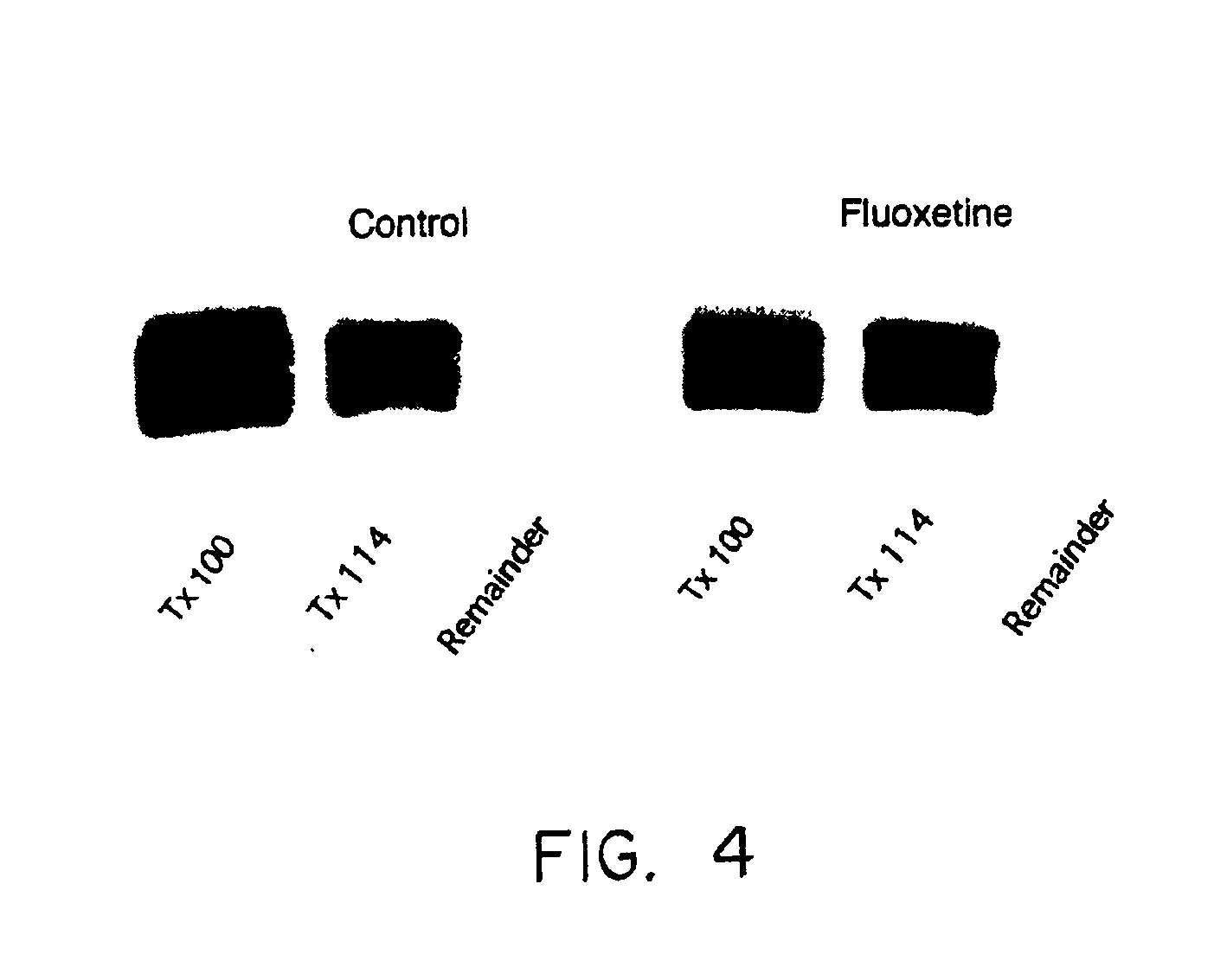
The present invention relates generally to methods for determining the effectiveness of ongoing antidepressant therapy via analysis of the association of Gsα with components of the plasma membrane or cytoskeleton of cells from peripheral tissues of the depressed individual as well as to methods involved in screening for effective antidepressant agents via their ability to cause a difference in the association of Gsα with components of the plasma membrane or cytoskeleton of cells.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Methods to predict the outcome of treatment with antidepressant medication
The invention provides a method for determining the outcome of treatment with an antidepressant medication in a patient. In particular, the invention provides a method of screening patients to identify those patients with a decreased risk of non-response to treatment with antidepressant medication by obtaining a sample of genetic material from the patients, and then assaying the sample for the presence of a genotype which is associated with a decreased risk of non-response to treatment with antidepressant medication. The genotype is characterized by a polymorphism in the genes HTR2A, GRIK4, BCL2, and a combination thereof.
Owner:GOVERNMENT OF THE US REPRESENTED BY THE SEC +1
Long-acting depression relieving microneedle patch and preparation method thereof
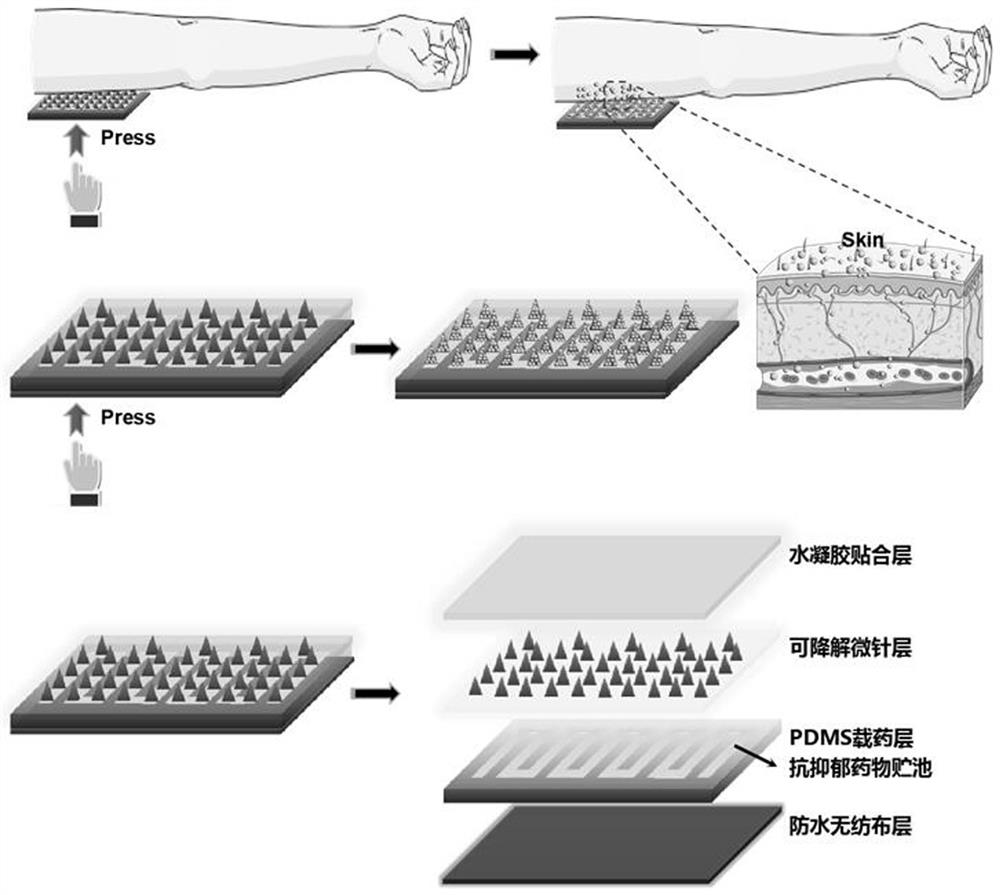
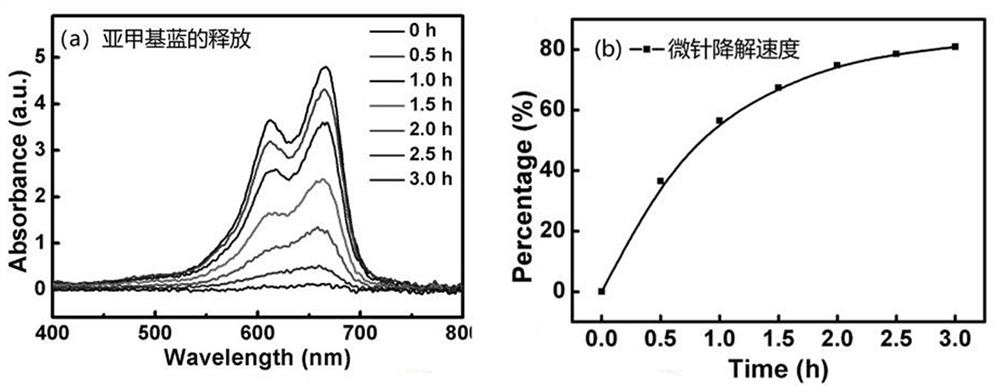
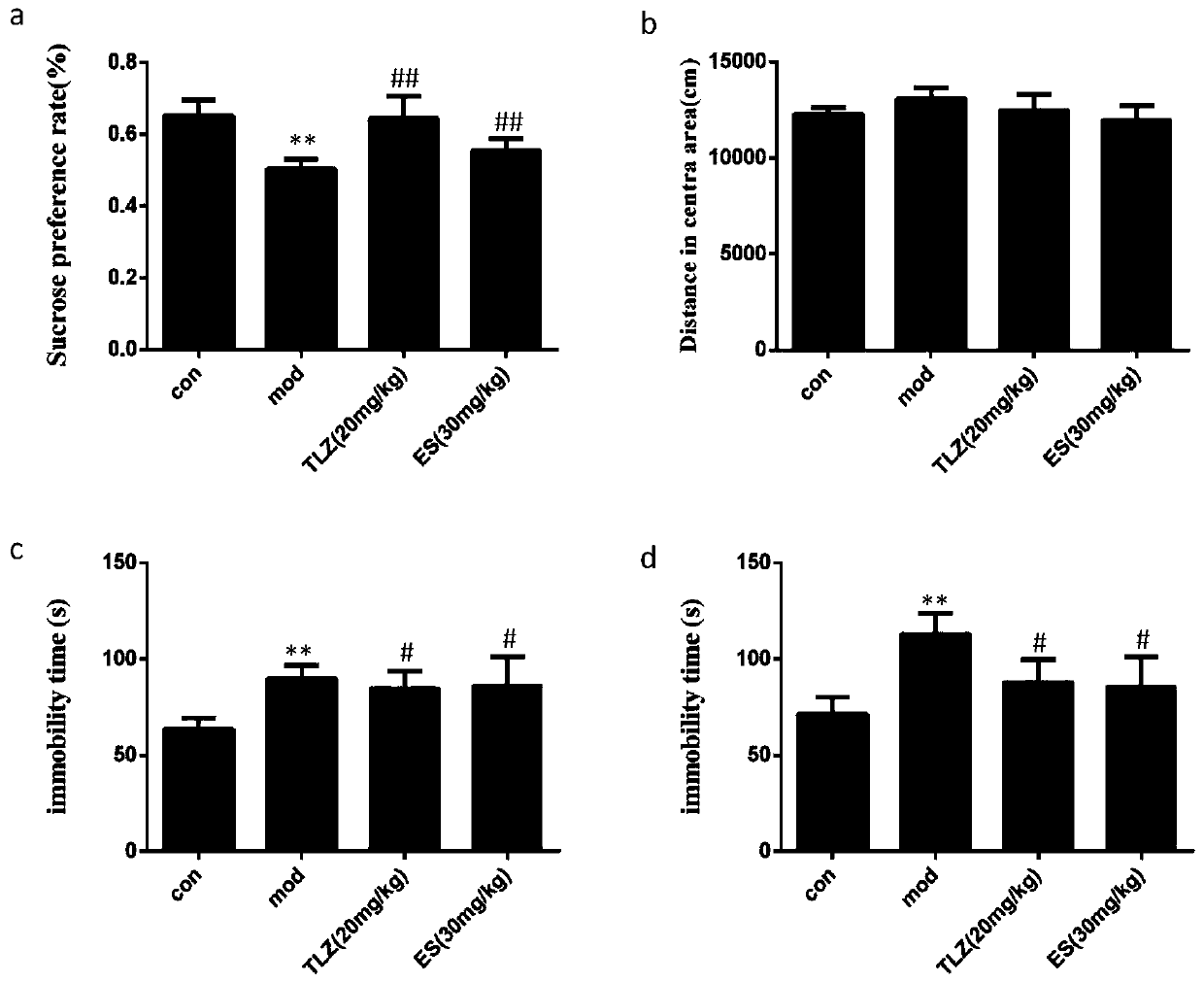
PendingCN113288883AHigh strengthImprove puncture abilityNervous disorderInorganic non-active ingredientsEfficacyNonwoven fabric
The invention relates to a long-acting depression relieving microneedle patch and preparation method thereof, and belongs to the technical field of transdermal or transdermal drug delivery preparations. The Long-acting depression relieving microneedle patch is composed of four layers of effective components, including a hydrogel fitting layer for fitting the skin, a degradable microneedle layer for puncturing the skin, a PDMS drug-loading layer serving as a drug storage tank for loading antidepressant drug components, and a waterproof non-woven fabric layer on the outermost layer. The microneedle is made of a biodegradable high polymer material containing calcium carbonate, the strength of the microneedle is effectively enhanced, the puncture performance of the microneedle is improved, and the microneedle can effectively penetrate through the skin cuticle. Amphipathic tetradecanol is used as a slow-release gate valve of a drug storage tank, on one hand, the amphipathic tetradecanol can wrap drugs with different hydrophilic and hydrophobic properties at the same time, and on the other hand, the slow-release performance is good, so that the microneedle patch can achieve the effect of continuously releasing the drugs for a long time. The manufacturing process is simple, the cost is low, and the safety is high.
Owner:HENAN UNIV OF SCI & TECH
Application of trifolirhizin and antidepressant medicine
InactiveCN111202740AEffective treatmentSignificant antidepressant effectOrganic active ingredientsNervous disorderSide effectXanthonoid
The invention discloses an application of trifolirhizin to preparation of medicines for preventing or treating depression. The trifolirhizin is a flavonoid compound extracted from sophora alopecuroides, is safe and non-toxic in sources, has the effect of resisting depression, can be used for preparing antidepressant medicines, does not produce side effects and is safe and efficient. Besides, the invention also provides an antidepressant medicine. The antidepressant medicine comprises the trifolirhizin and a carrier acceptable for medicines.
Owner:NINGXIA MEDICAL UNIV
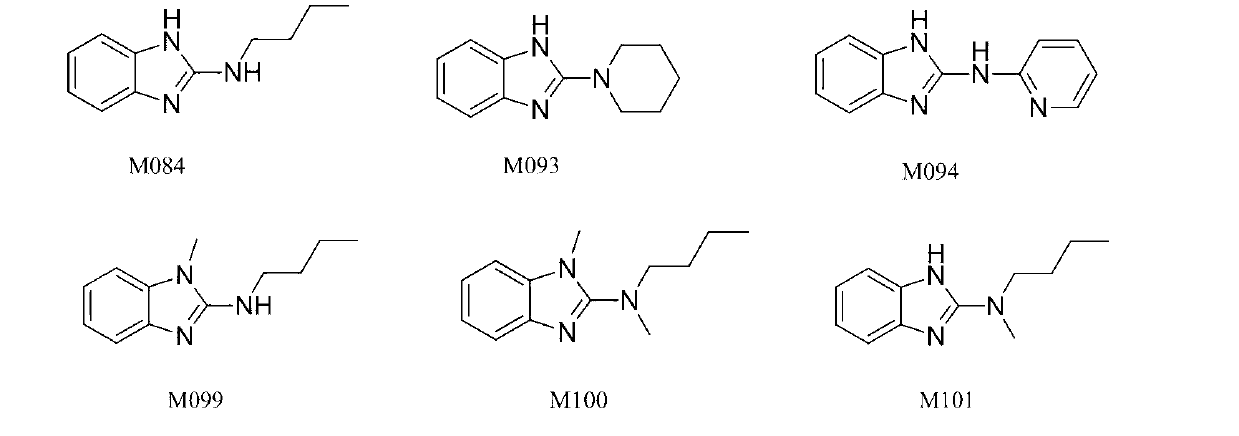
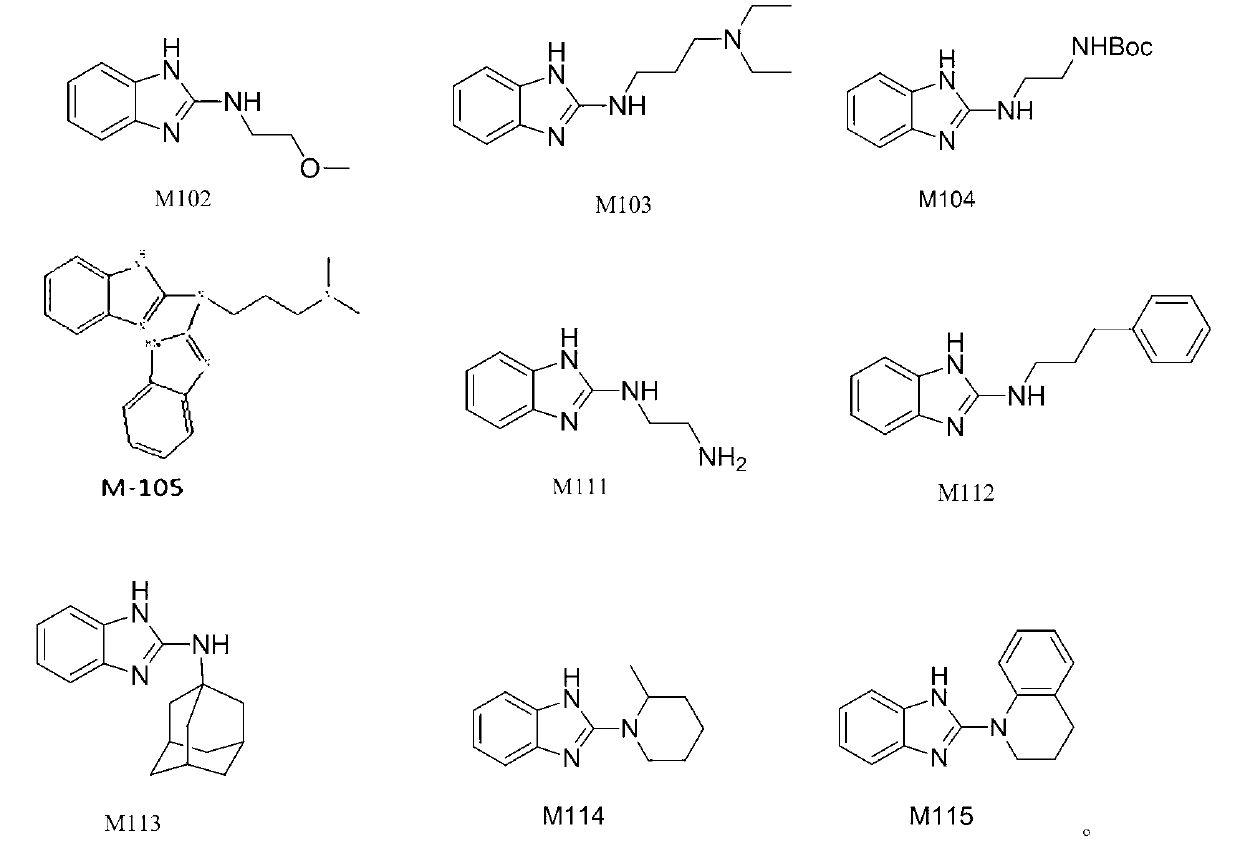
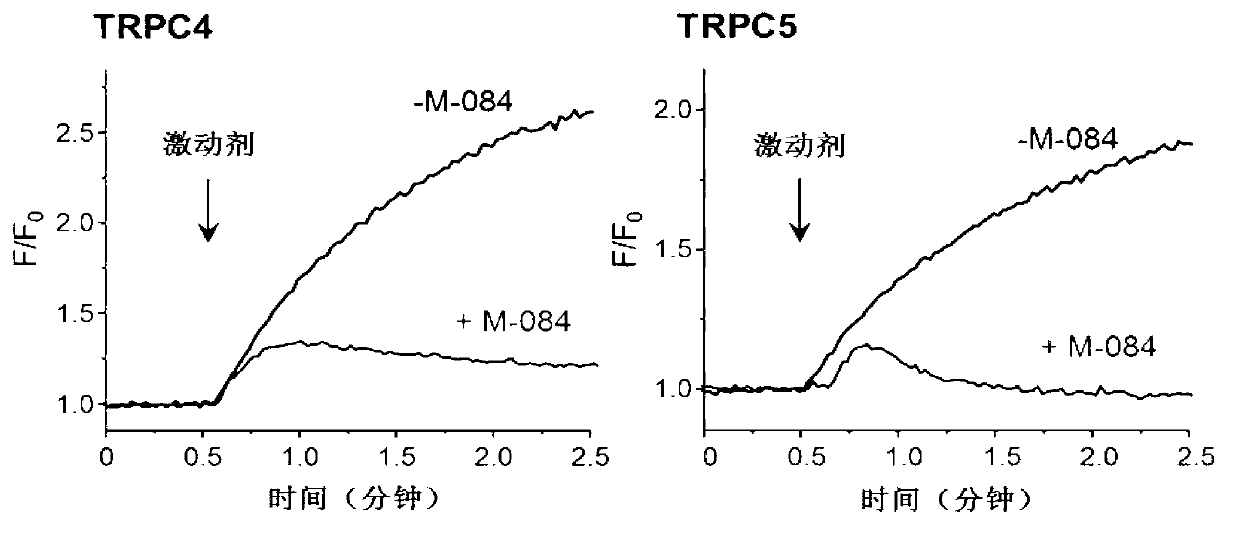
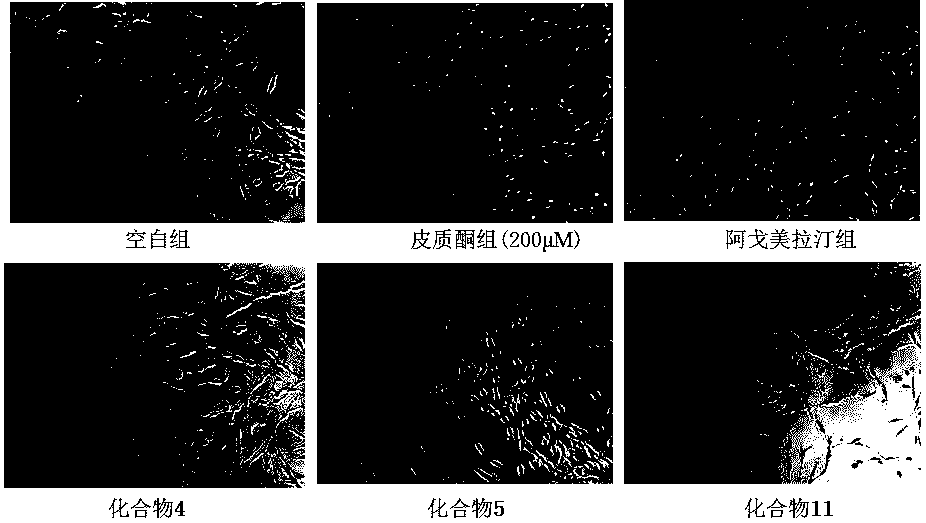
Benzimidazole and derivative thereof, and medicinal composition and application thereof in preparation of antidepressant medicaments
The invention provides benzimidazole and derivative thereof, an antidepressant medicament which contains the benzimidazole and derivative thereof and a pharmaceutical carrier and excipient, and application thereof in preparation of antidepressant medicaments and preparation of functional foods. The benzimidazole and derivative thereof provided by the invention can be prepared into pharmaceutical preparations of various forms, including oral administration, injection, lung inhalation and transdermal preparations, and specifically including injections, oral liquids, tablets, capsules, granules, aerosols, dry powder inhalers, sprays, plasters and the like.
Owner:泸州天演生物医药科技有限公司
Dihydroisoquinoline compounds and their use in the preparation of neuroprotective or antidepressant drugs
InactiveCN104151242BLow toxicityProtective functionOrganic active ingredientsNervous disorderDiseaseNerve cells
The invention belongs to the field of medicines and relates to a dihydro isoquinoline compound and application thereof in treatment of mental disorder, related to emotion, especially depressive disorder. Shown in a structural formula I, the dihydro isoquinoline compound has good protection activity on PC12 cells damaged under the induction action of corticosterone in an in vitro experiment, which suggests that the dihydro isoquinoline compound has a function of protecting nerve cells, further experiments verify that the dihydro isoquinoline compound can effectively improve level of BNDF (brain-derived neurotrophic factor) in nerve cells, the oxidation resistance of the nerve cells can also be effectively enhanced, and growth of the nerve cells is promoted; the dihydro isoquinoline compound has potential of treating mental disorder owning to repairing and protective effect on the nerve cells, is preferable for treating emotion and cognitive mental disorders, such as depressive disorder, senile dementia, anxiety, obsession and schizophrenia and is especially preferable for treatment of depression. In vivo experiments, including forced swimming and open field experiment, the dihydro isoquinoline compound shows obvious effect of alleviating depressive state of experimental animals respectively. The formula (I) is described in the specification.
Owner:SICHUAN UNIV
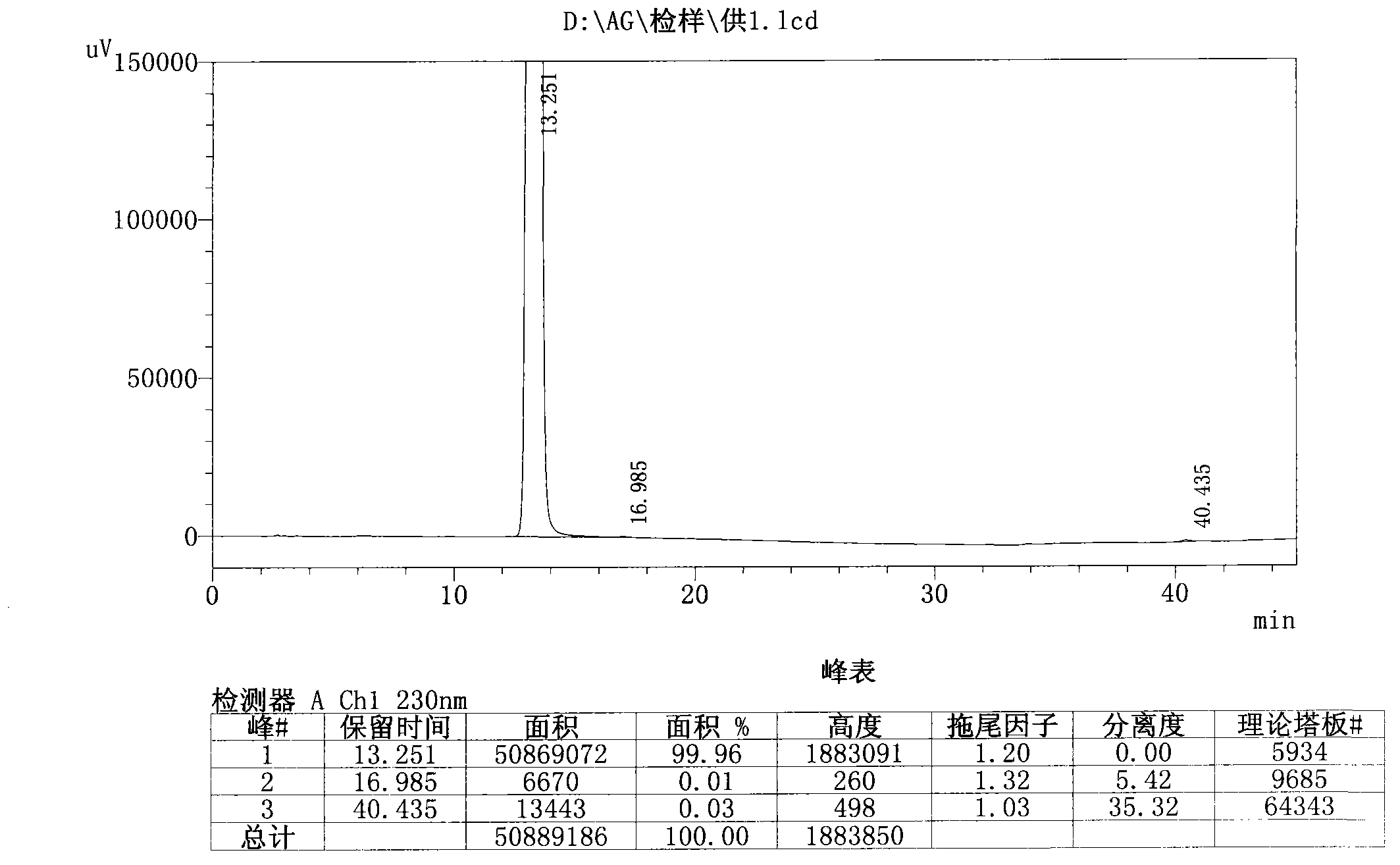
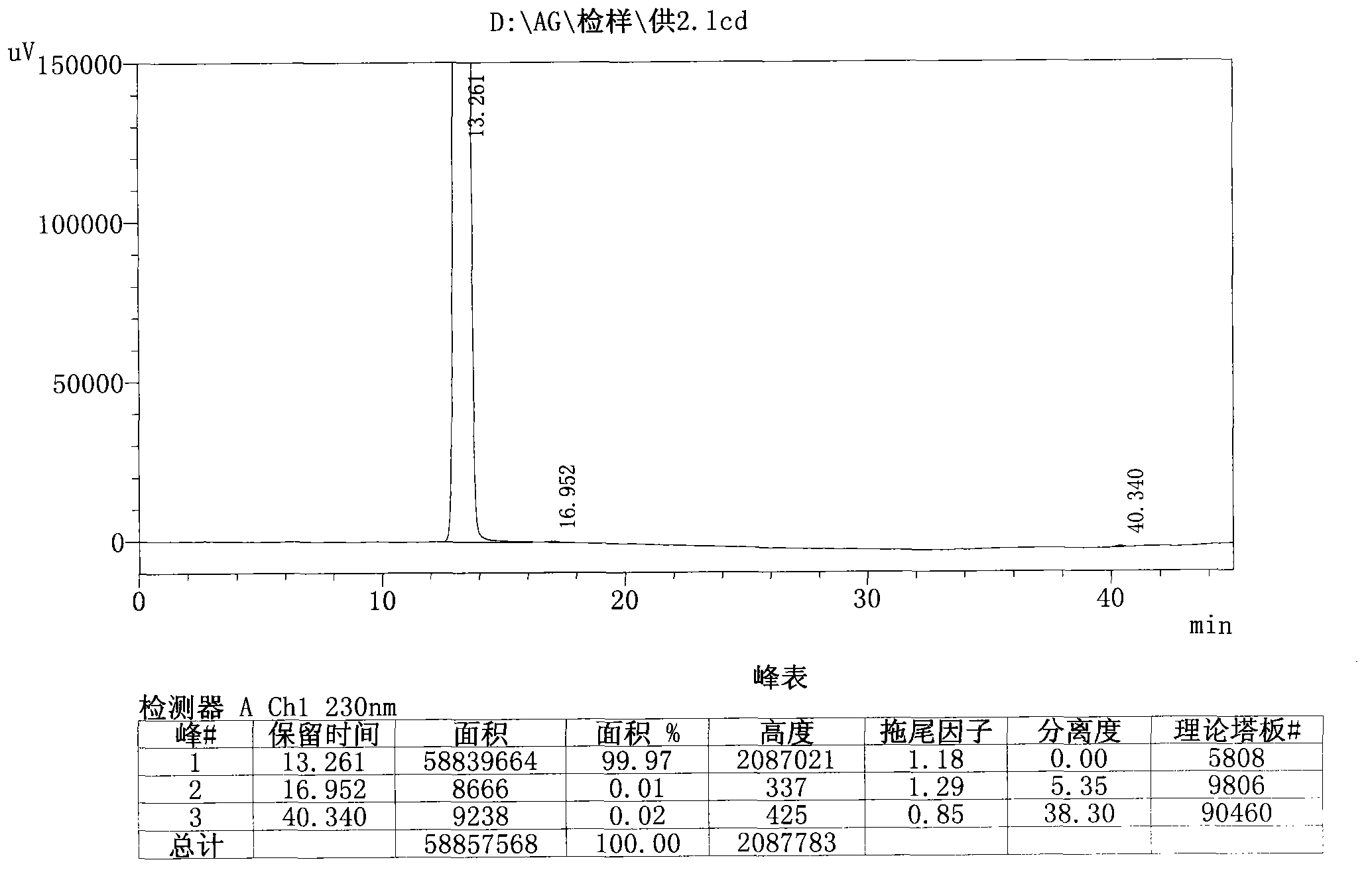
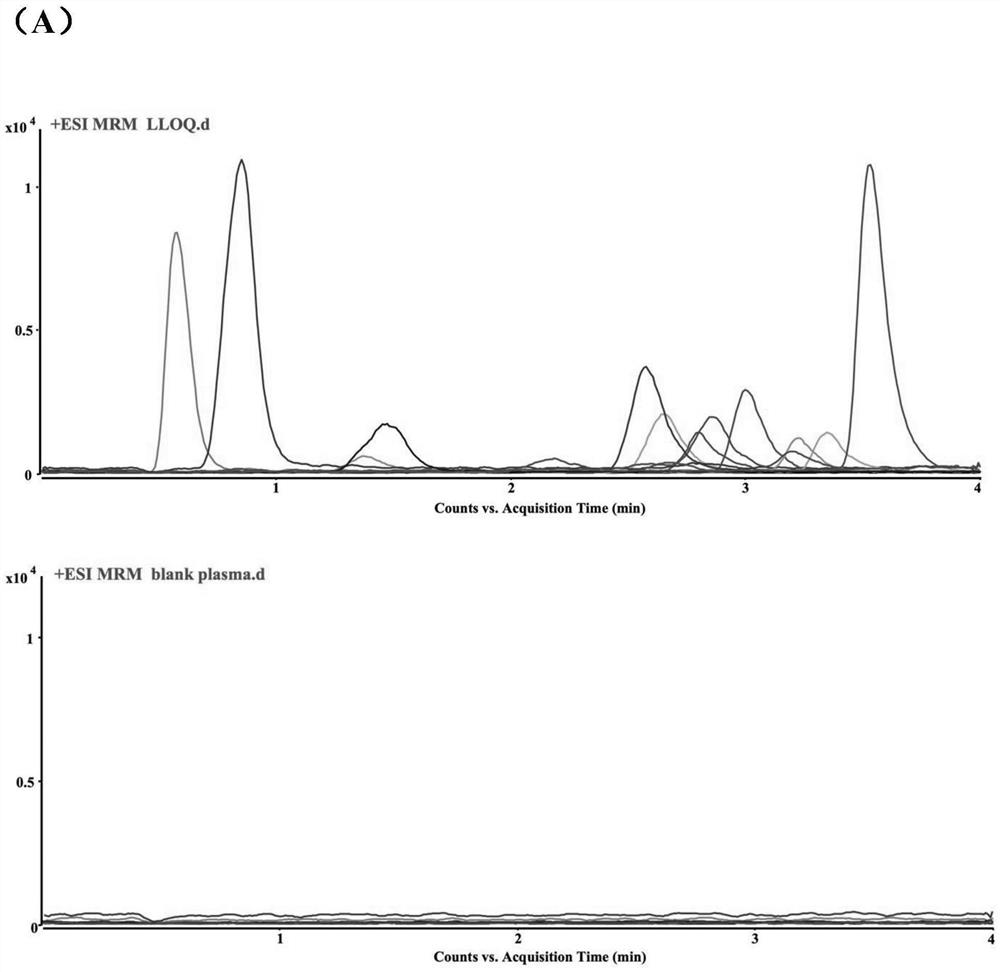
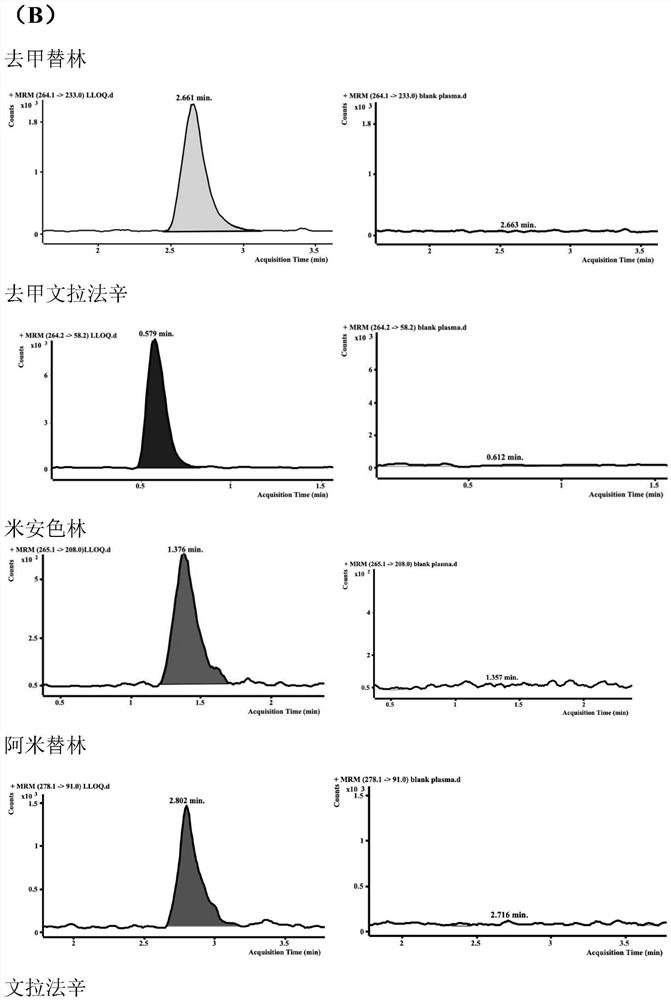
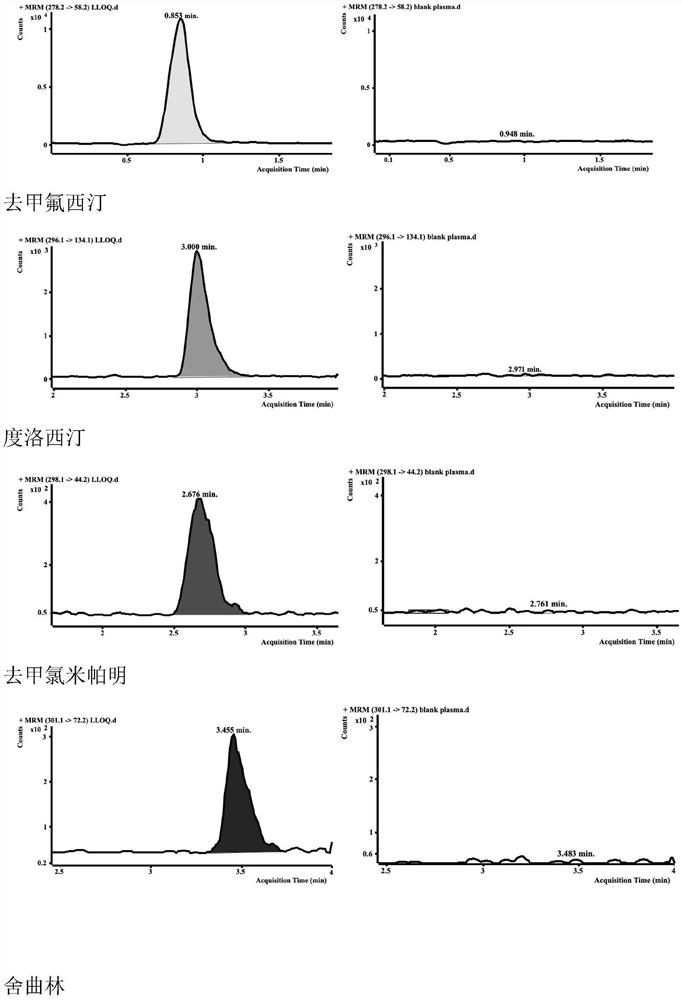
HPLC-MS/MS method for simultaneously determining concentrations of 14 antidepressants in human plasma
PendingCN113820424AMeet the needs of high-throughput detectionLabor savingComponent separationMaterial analysis by electric/magnetic meansChromatographic separationGradient elution
The invention discloses an HPLC-MS / MS (High Performance Liquid Chromatography-Mass Spectrometry / Mass Spectrometry) method for simultaneously determining the concentrations of 14 antidepressants in human plasma, analytes in a plasma sample are extracted by adopting a protein precipitation method, the sample is separated by adopting an Agilent Infinity Lab Poroshell 120EC-C18 chromatographic column, acetonitrile and water are used as mobile phases for gradient elution, and the mass spectrum condition adopts an electrospray ion source positive ion mode and a multi-ion monitoring mode. The total time of chromatographic separation is 4 minutes. According to the present invention, the 14 antidepressants detected by the method have good linearity (R2 > 0.99) in the blank plasma, the intra-batch and inter-batch precision RSD is 0.65-13.18%, the accuracy is 85.21-117.6%, the matrix effect, the recovery rate, the stability, the dilution effect, the residual effect and the like meet the requirements, and the significant clinical data is provided for the optimization of the clinical drug treatment scheme.
Owner:厦门市仙岳医院(厦门市精神卫生中心)
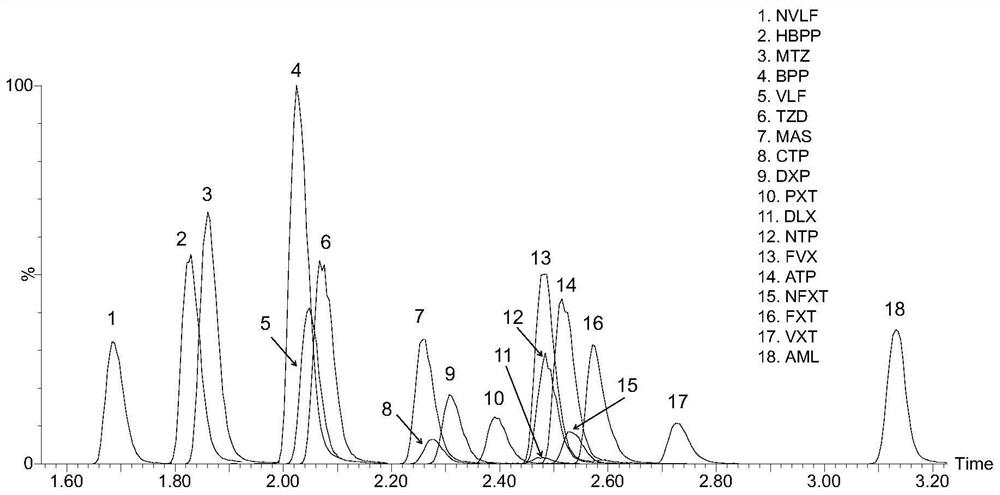
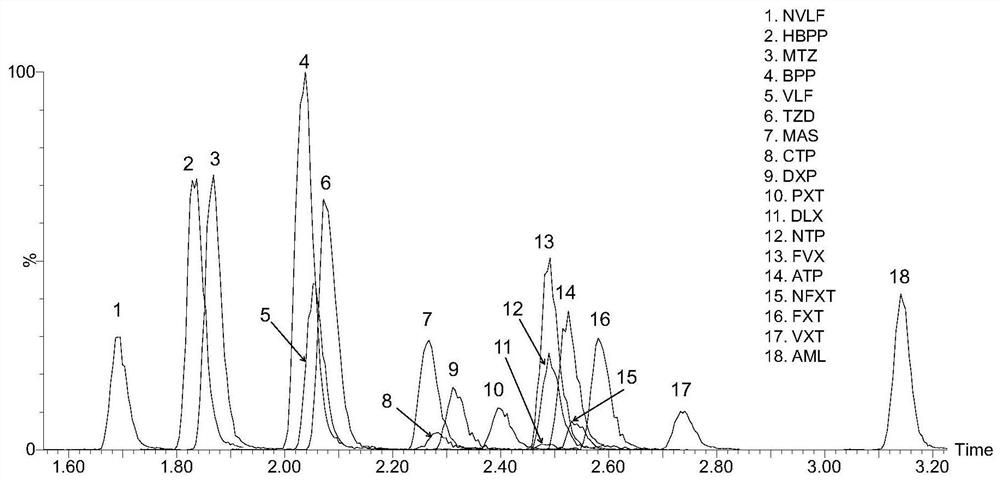
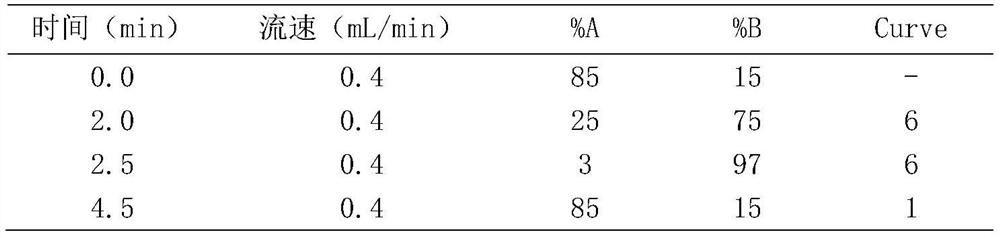
Kit for detecting antidepressant drug in serum through ultra-high performance liquid chromatography-tandem mass spectrometry technology
InactiveCN111665305AShort analysis timeEasy to handleComponent separationFluid phasePharmaceutical drug
The invention relates to a kit for detecting an antidepressant drug in serum through an ultra-high performance liquid chromatography-tandem mass spectrometry technology. The kit is simple in pretreatment process, low in cost, high in sensitivity and high in specificity, separation and detection of the antidepressant drug are completed within 4.5 min, the accuracy and precision basically meet the requirements, the method can be used for quantitative analysis of the clinical antidepressant drug, and a reliable detection method is provided for monitoring the treatment concentration of the clinical antidepressant drug.
Owner:NANJING PINSHENG MEDICAL TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


















![5-alkoxy-tetrazo[1,5-a]qualone derivative and pharmaceutically acceptable salt thereof serving as antidepressants 5-alkoxy-tetrazo[1,5-a]qualone derivative and pharmaceutically acceptable salt thereof serving as antidepressants](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/5124d881-6762-4b8b-9eef-710a4b4b006b/000001.png)
![5-alkoxy-tetrazo[1,5-a]qualone derivative and pharmaceutically acceptable salt thereof serving as antidepressants 5-alkoxy-tetrazo[1,5-a]qualone derivative and pharmaceutically acceptable salt thereof serving as antidepressants](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/5124d881-6762-4b8b-9eef-710a4b4b006b/000002.png)
![5-alkoxy-tetrazo[1,5-a]qualone derivative and pharmaceutically acceptable salt thereof serving as antidepressants 5-alkoxy-tetrazo[1,5-a]qualone derivative and pharmaceutically acceptable salt thereof serving as antidepressants](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/5124d881-6762-4b8b-9eef-710a4b4b006b/000003.png)