Topical Antiviral Formulations
a technology of antiviral and topical formulations, which is applied in the field of formulations with antiviral activity, can solve the problems of not always using safe-sex techniques, not always using them properly, and not always using current safe-sex techniques, so as to increase the probability of composition us
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
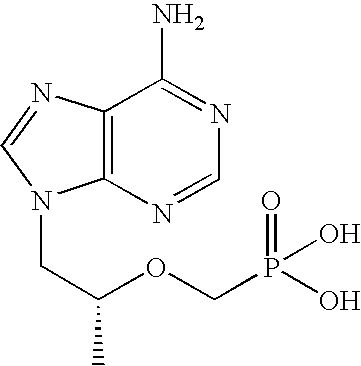
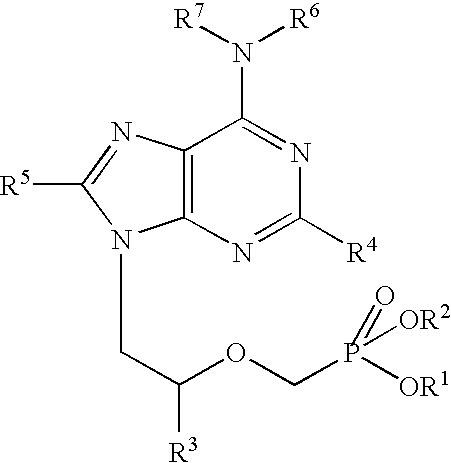
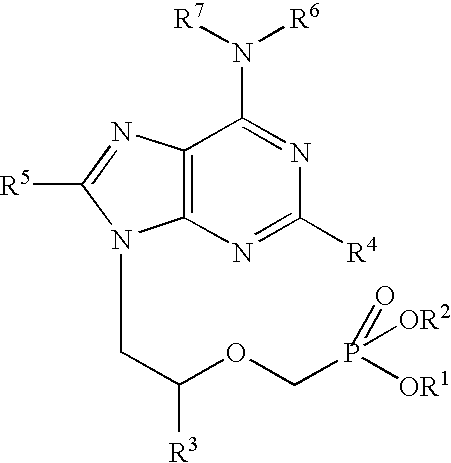
[0056] The following examples further describe and demonstrate particular embodiments within the scope of the present invention. The examples are given solely for illustration and are not to be construed as limitations as many variations are possible without departing from spirit and scope of the Invention. The following examples are intended for illustration only and are not intended to limit the scope of the invention in any way. “Active ingredient” denotes one or more NRTIs, as defined above, preferably tenofovir or a physiologically functional derivative thereof.
[0057] Formulation A (Controlled Release Formulation):
[0058] This formulation is prepared by wet granulation of the ingredients with purified water, followed by the addition of magnesium stearate and compression. The hypromellose can utilize varying viscosity grades.
mg / tabletActive ingredient300Hypromellose112Lactose Monohydrate53Pregelatinized Starch28Magnesium Stearate7Purified Waterq.s.
[0059] Drug release takes pl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight percent | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com