Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30results about How to "Increase and decrease frequency" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
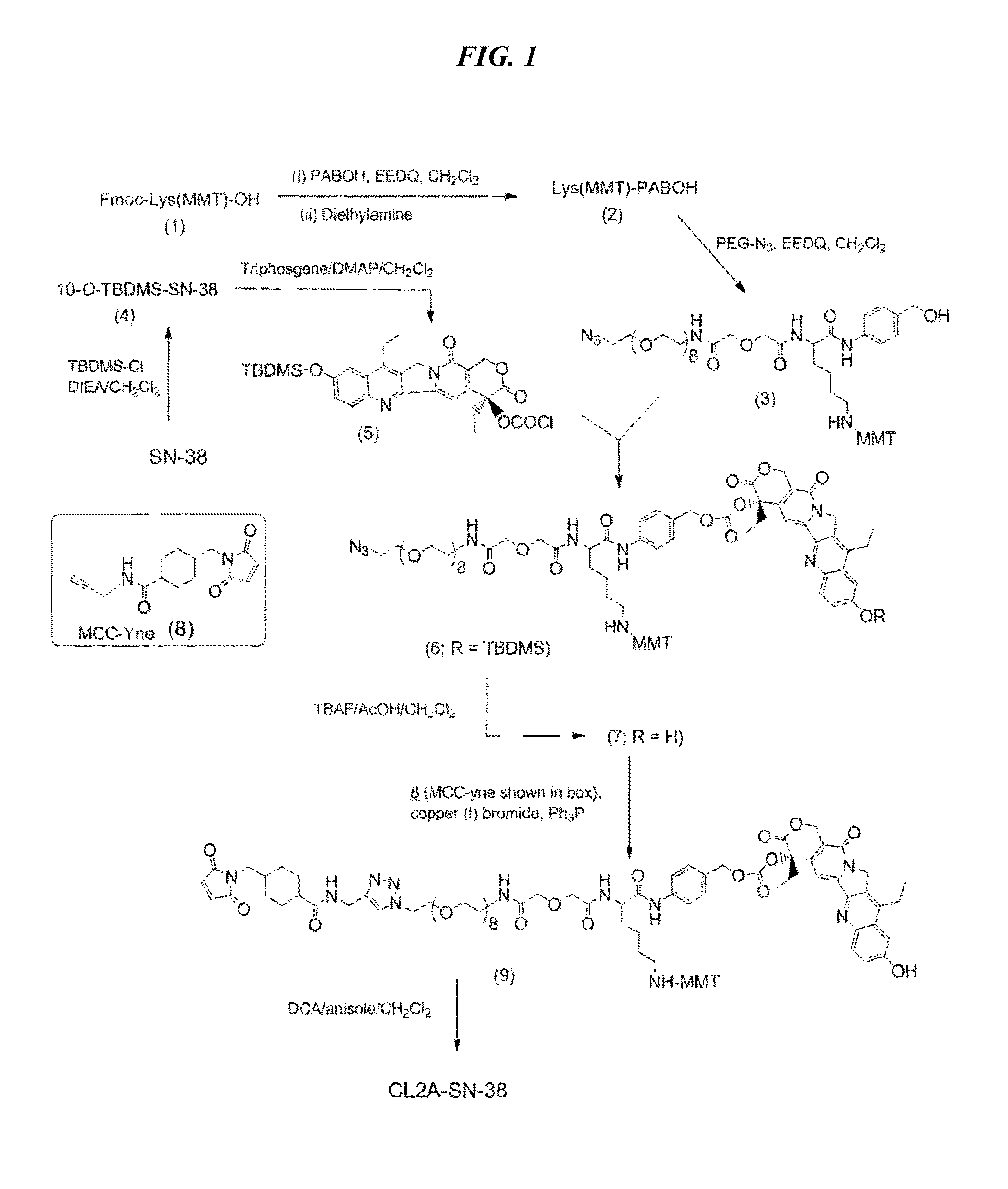
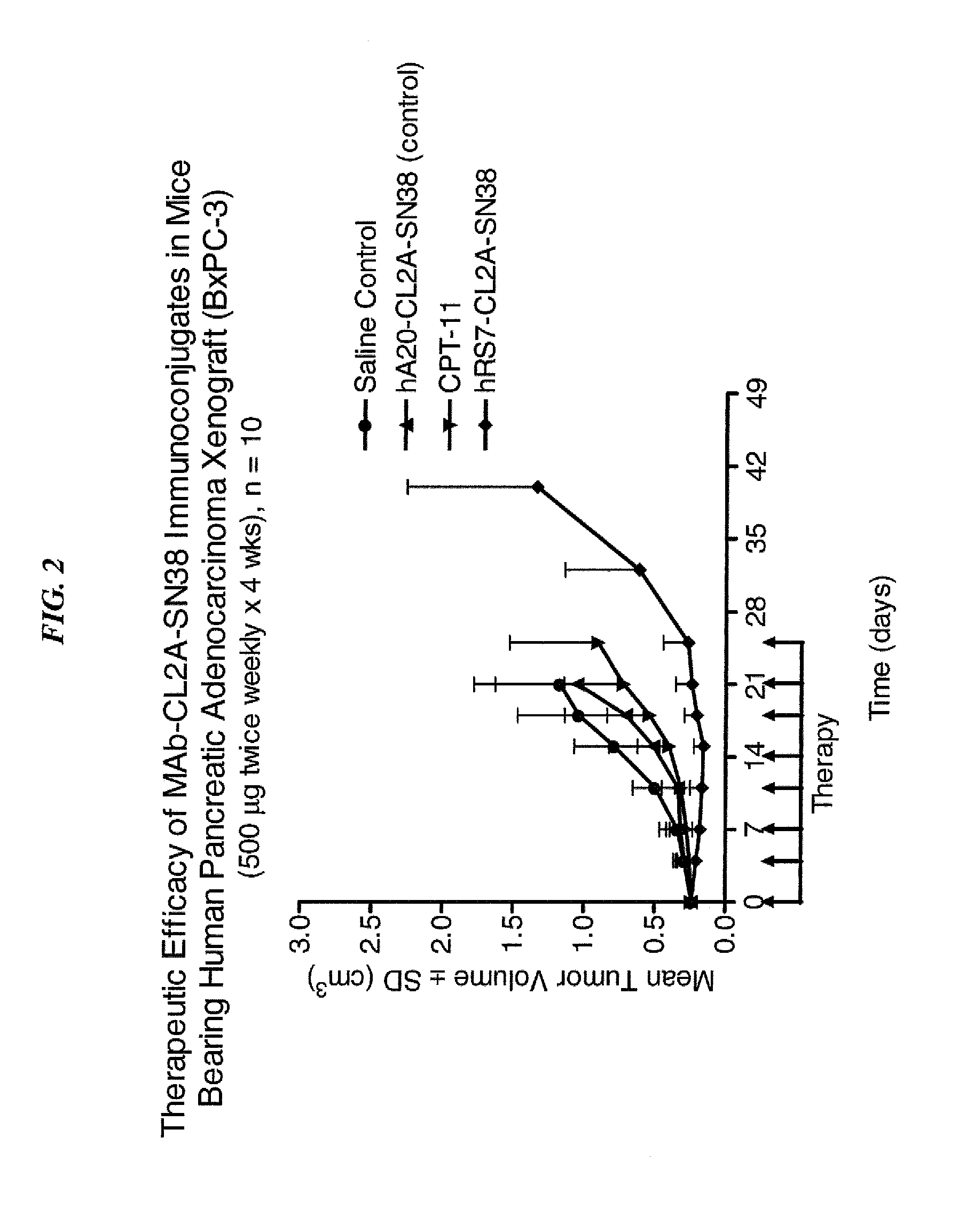
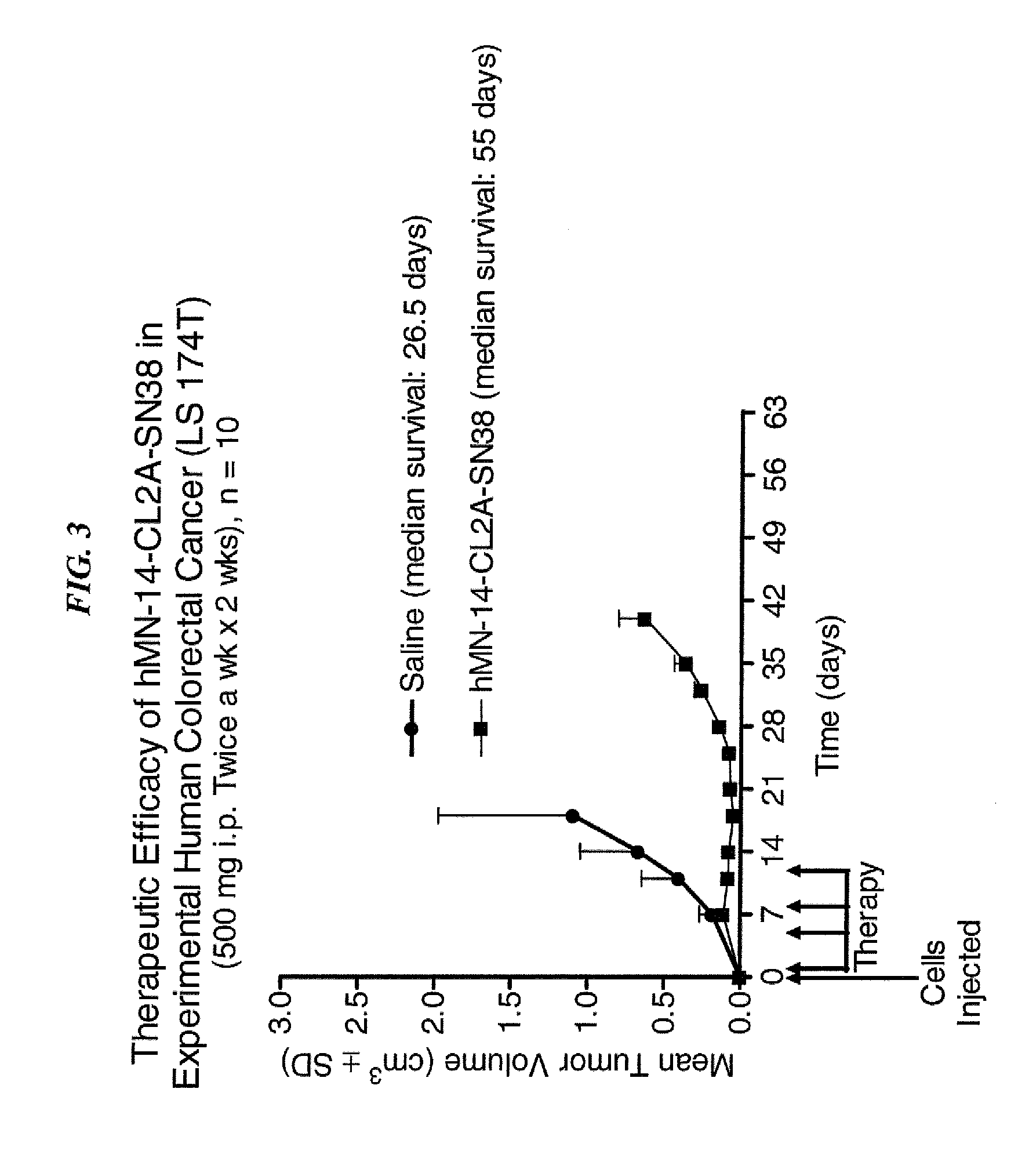
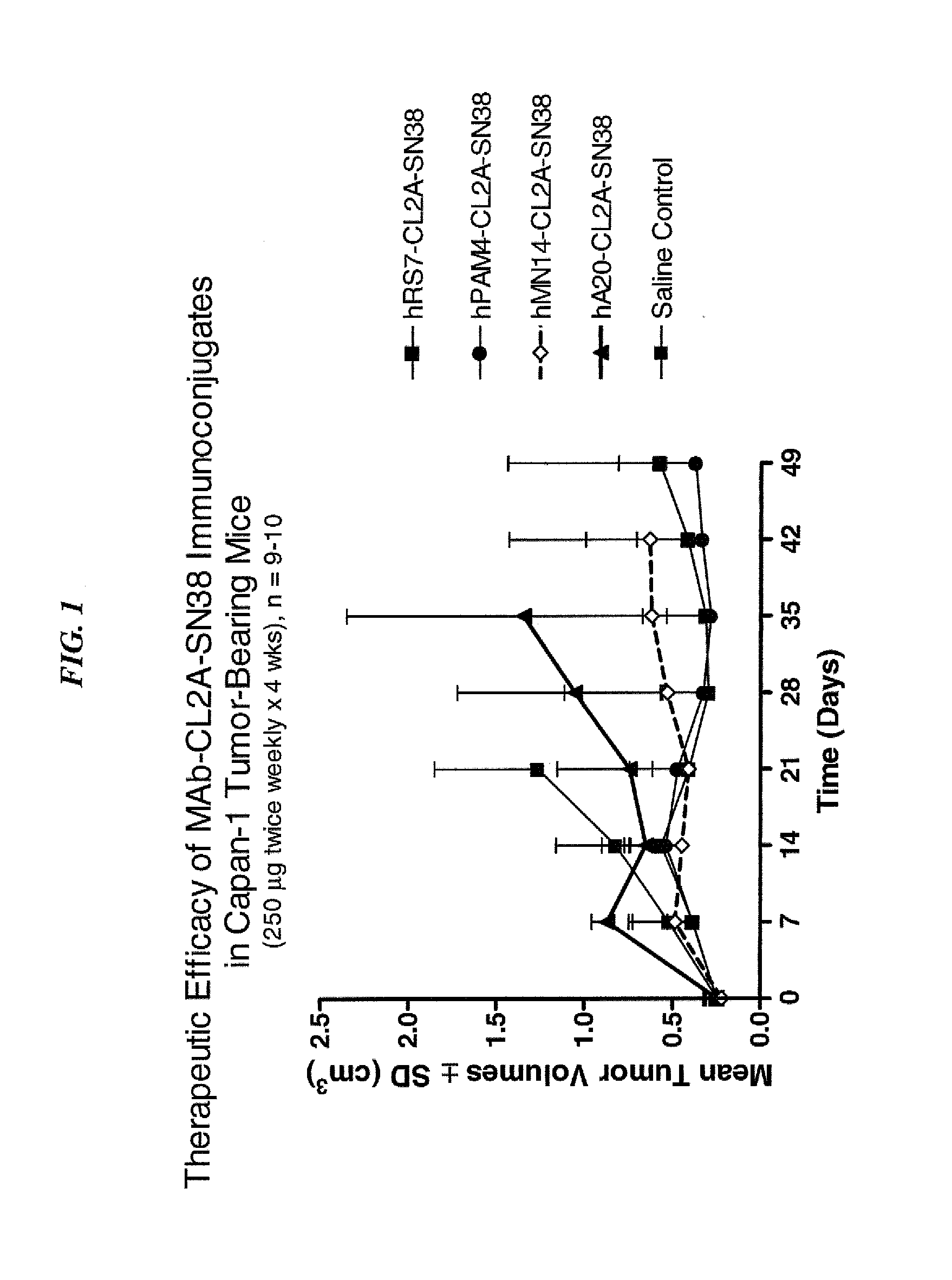
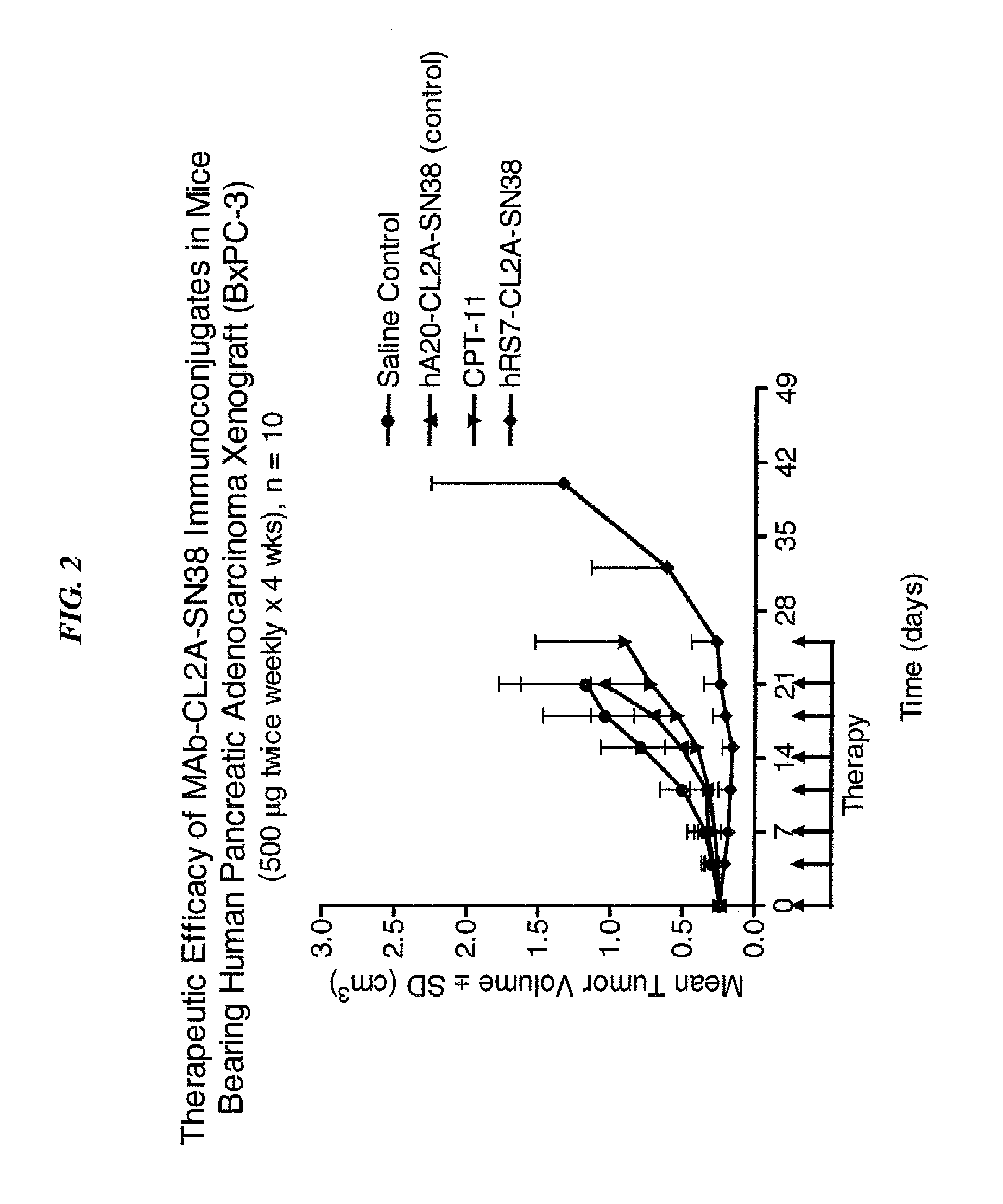
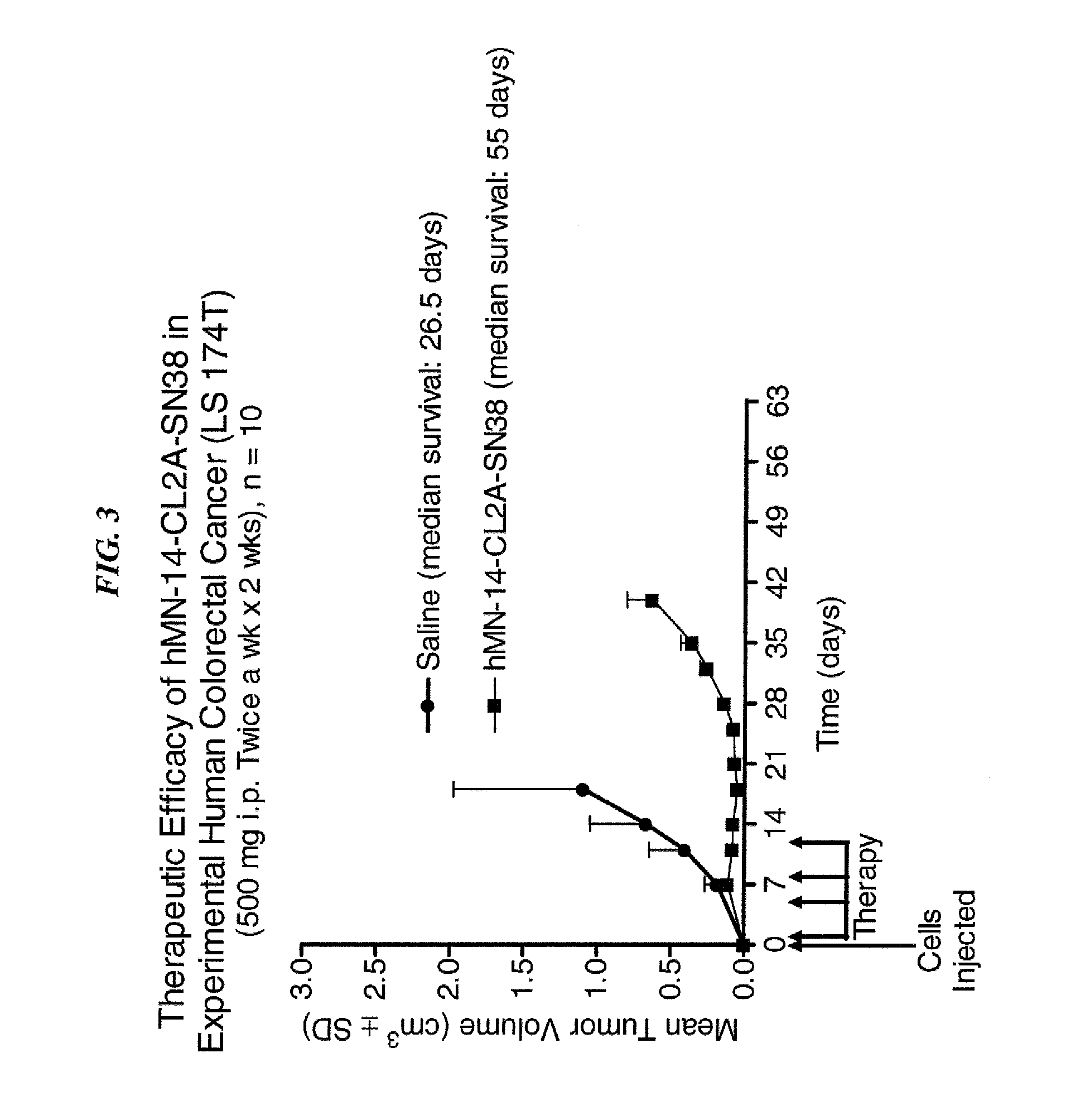
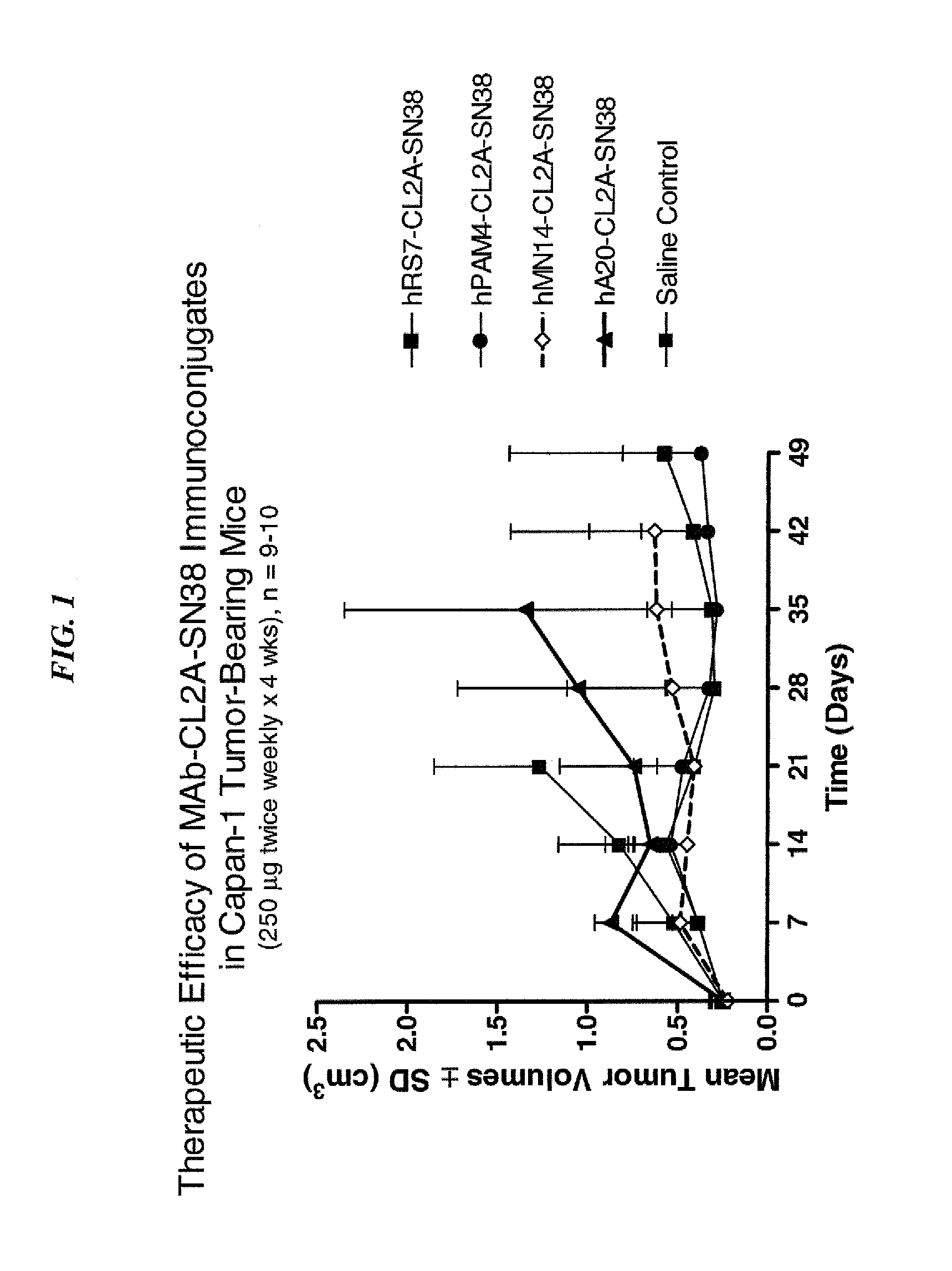
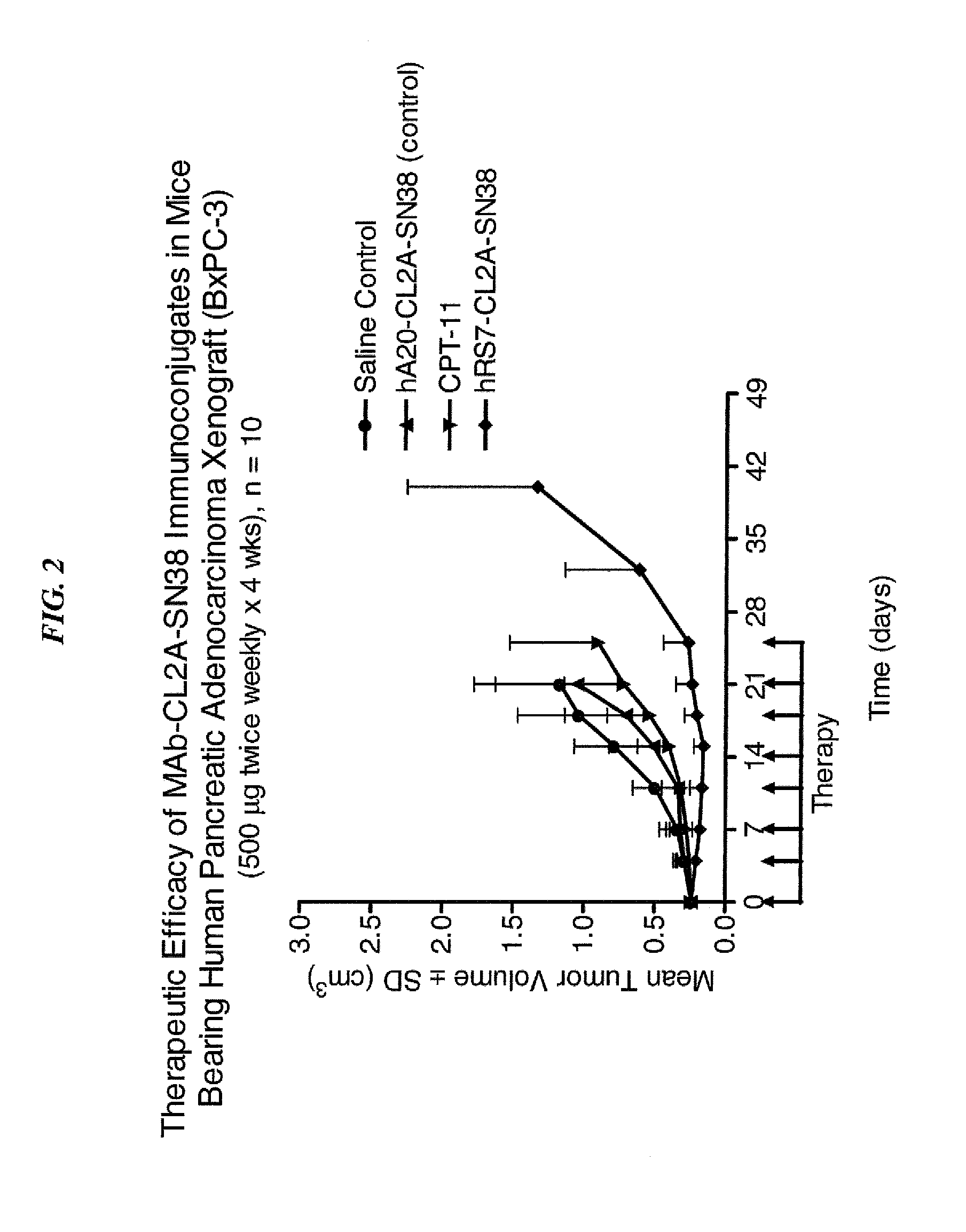
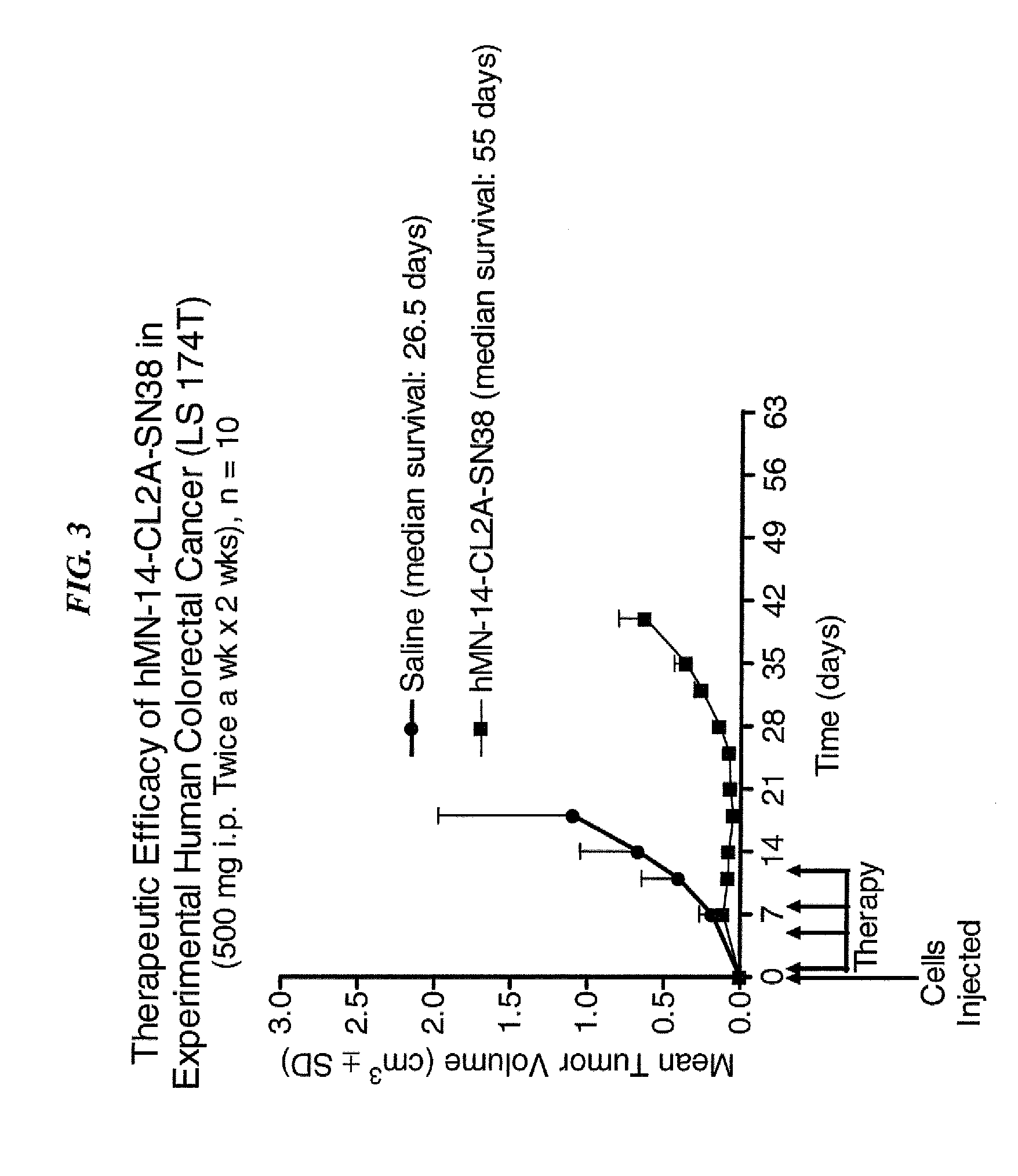
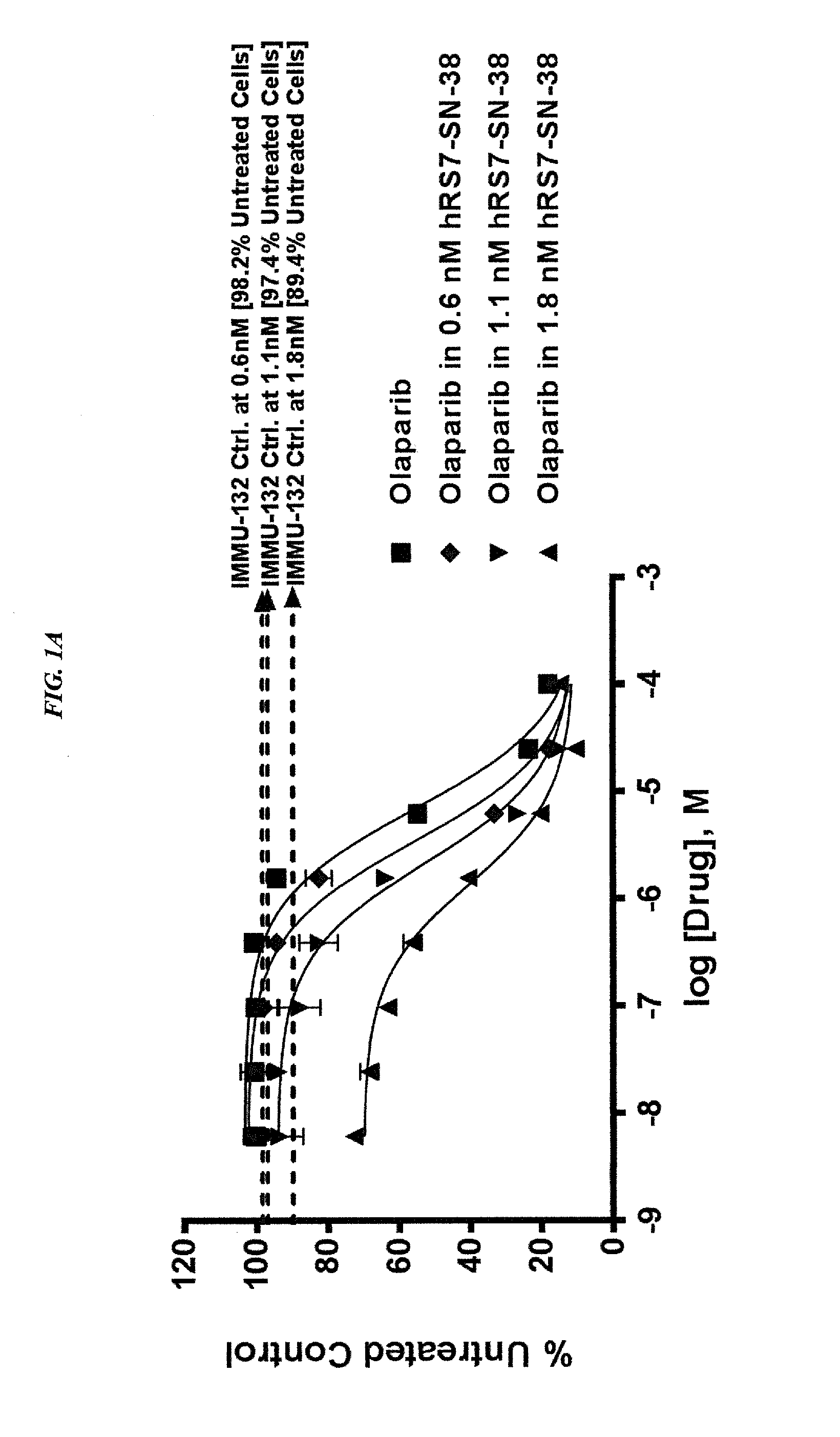
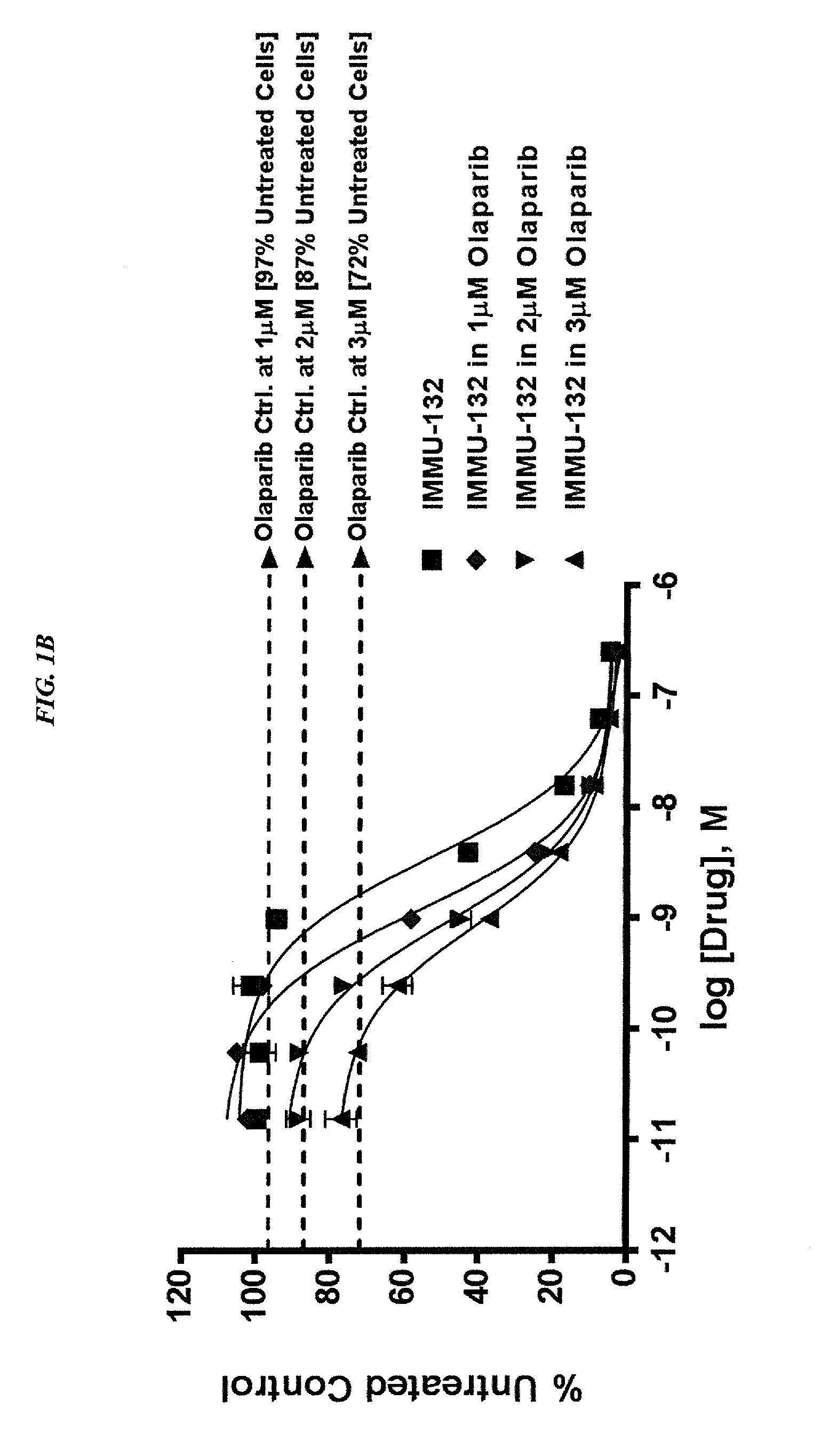
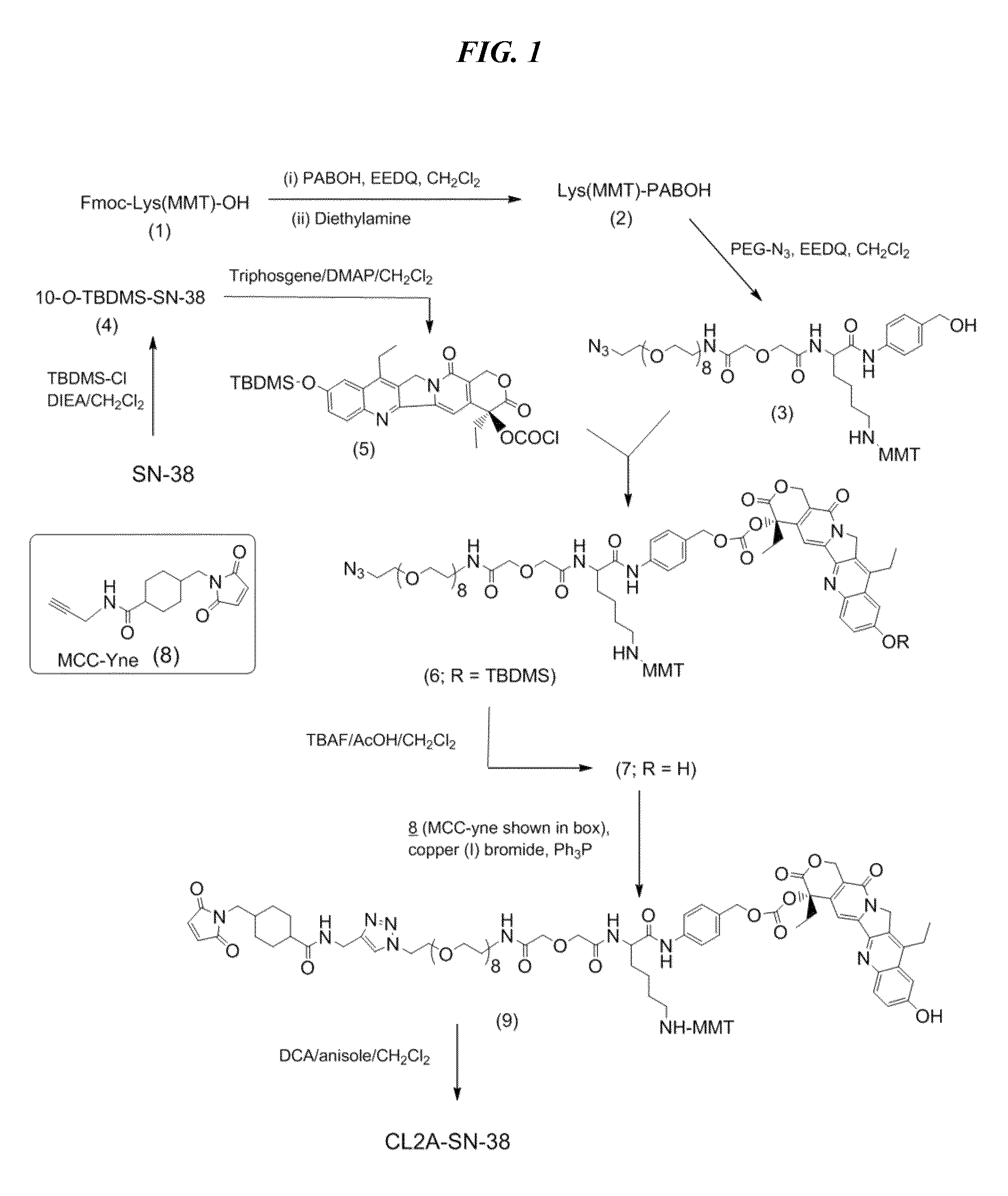
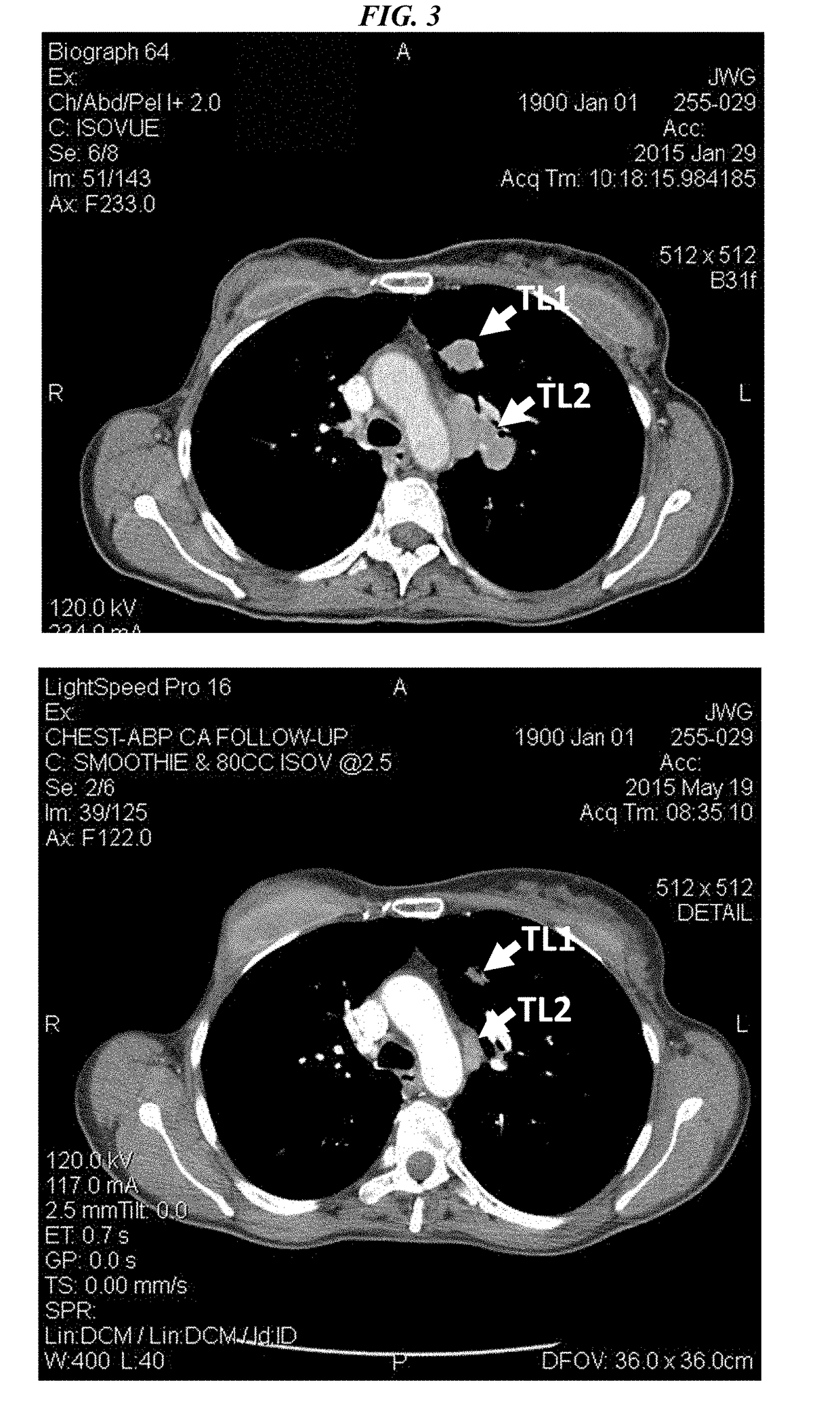
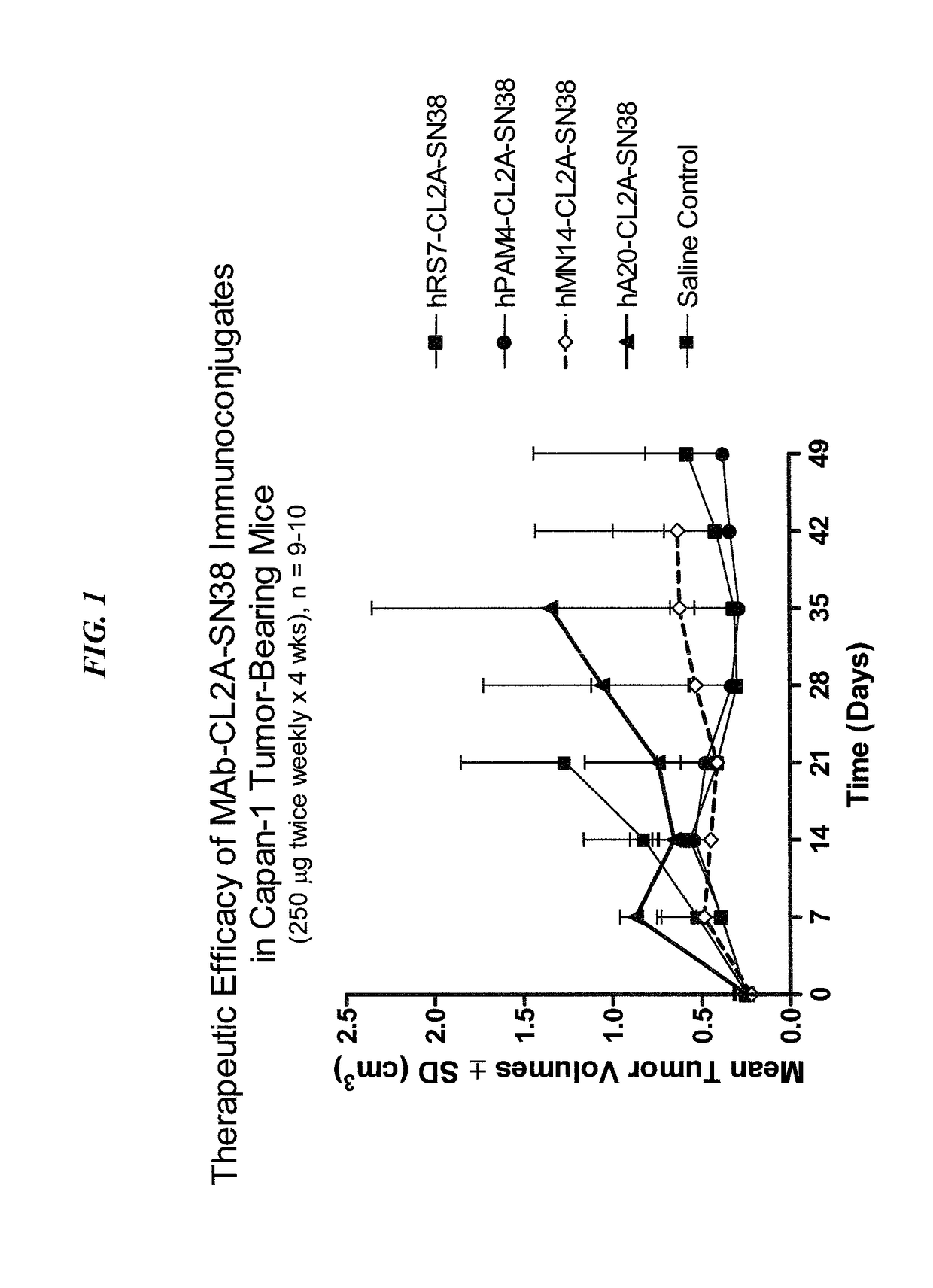
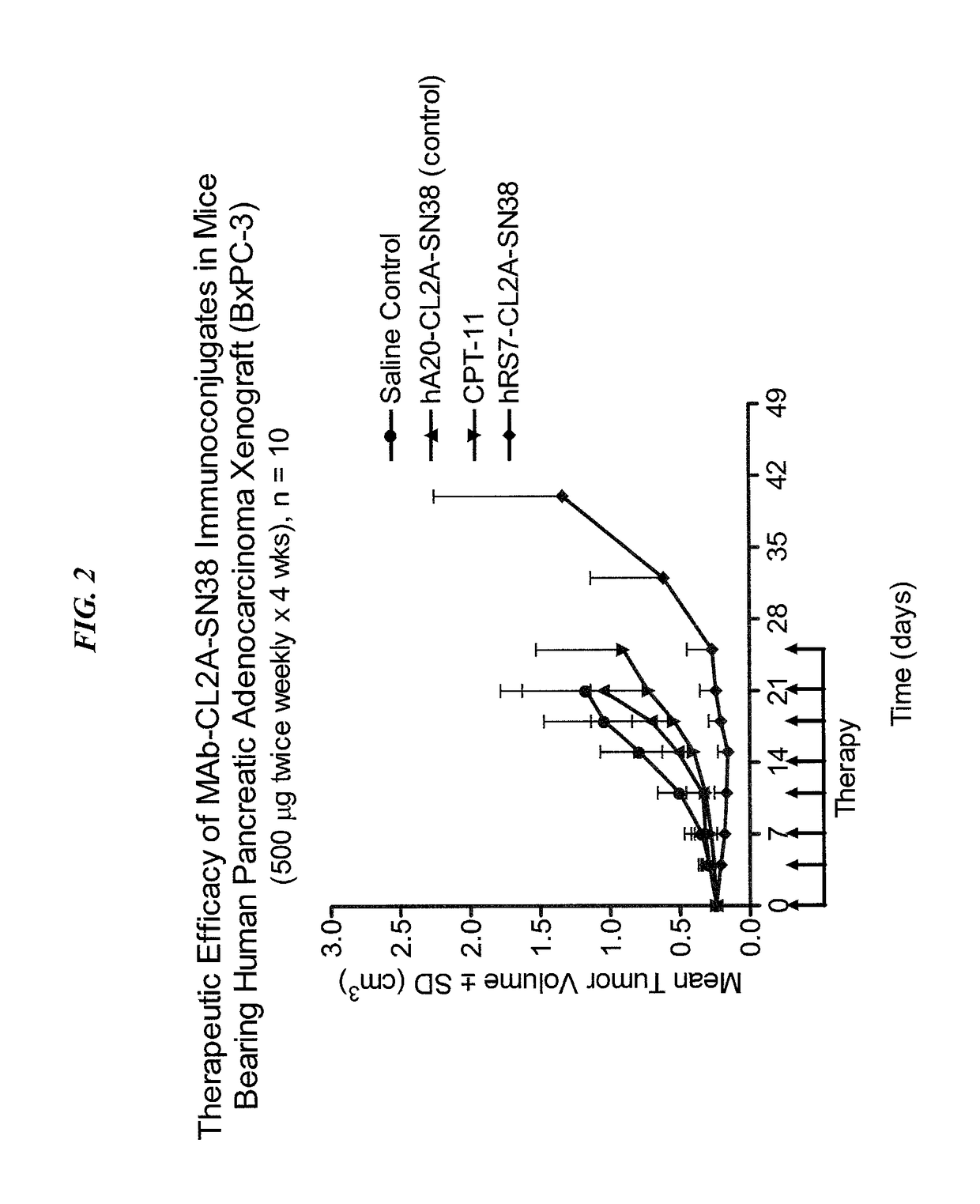
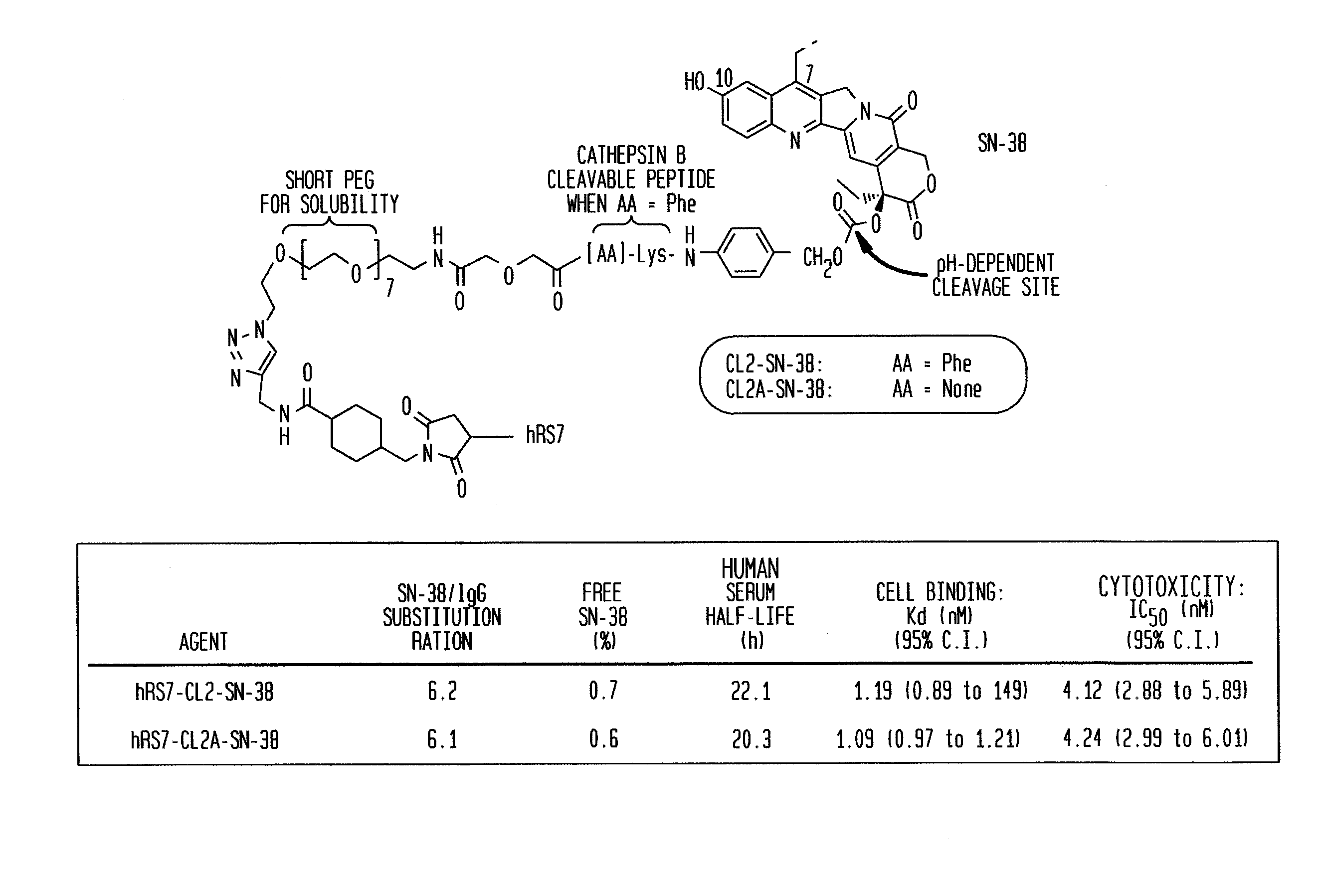
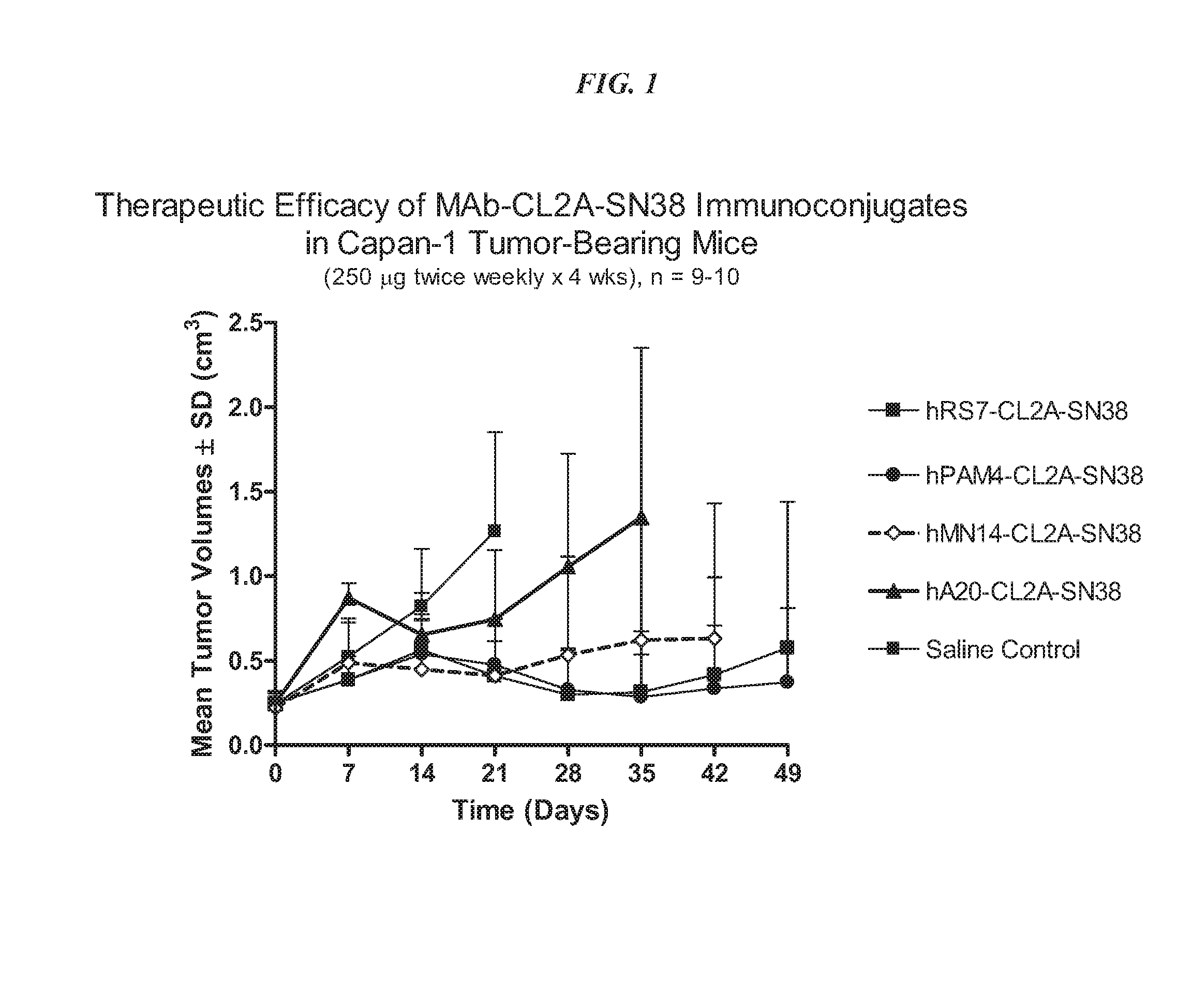
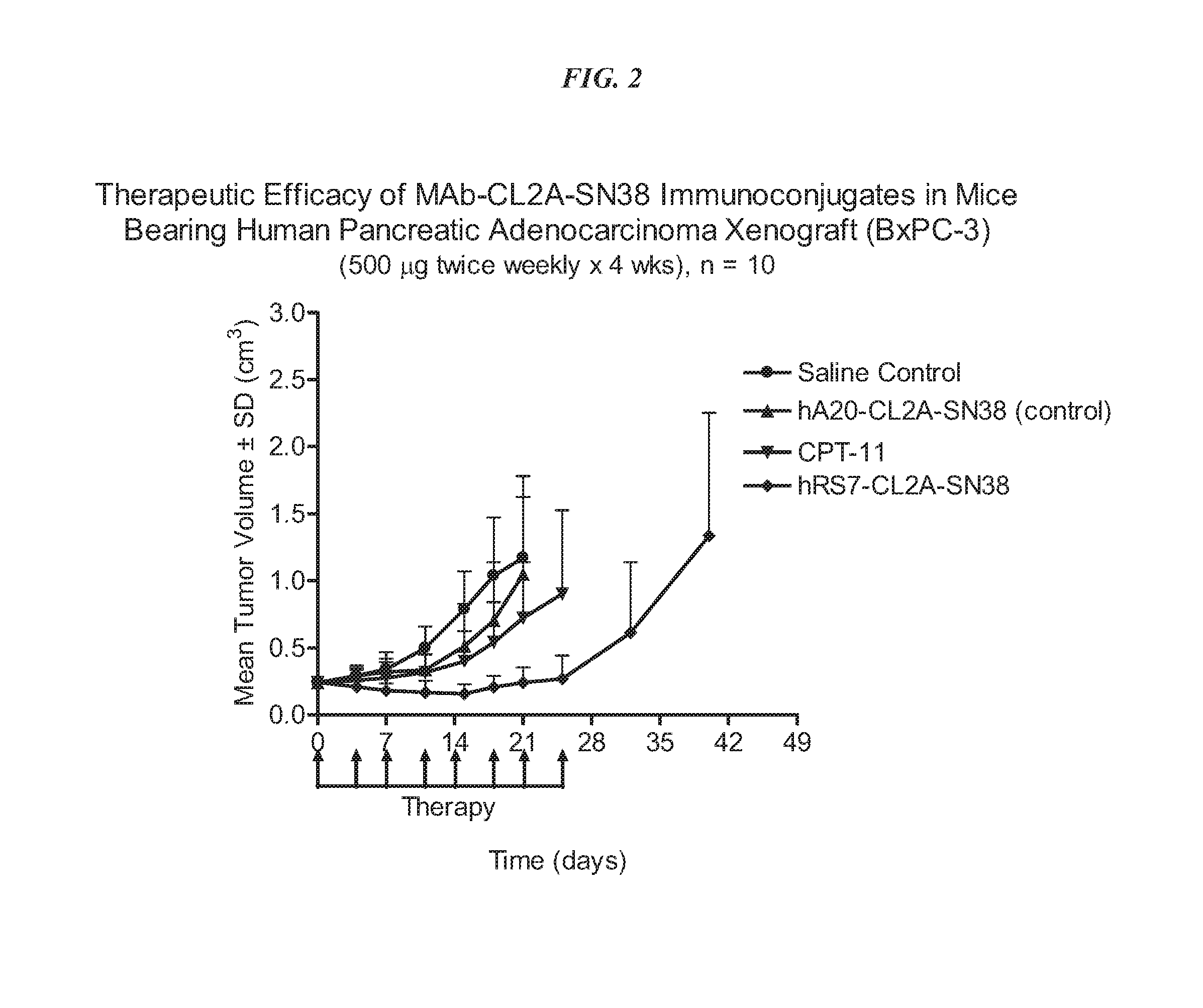
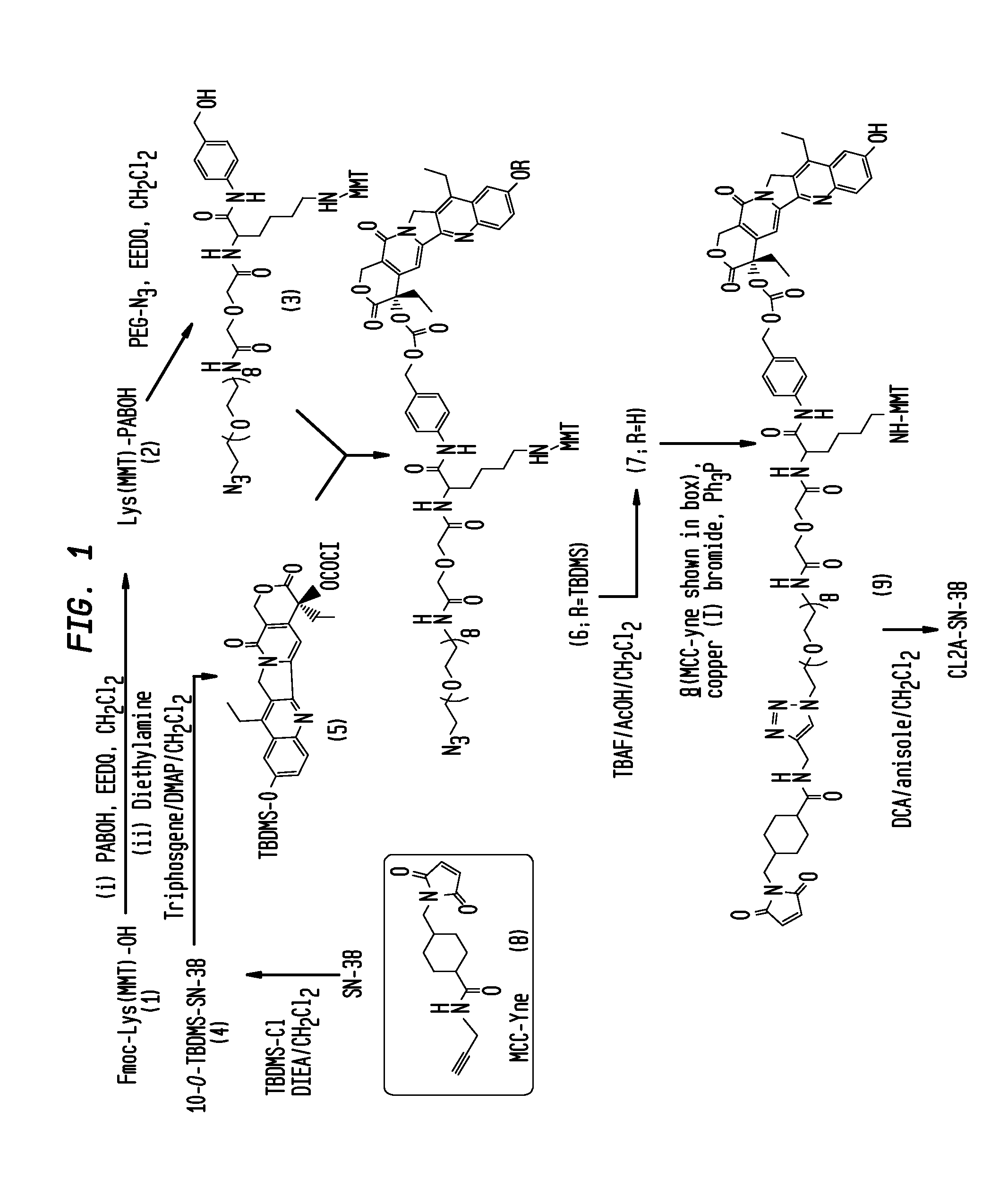
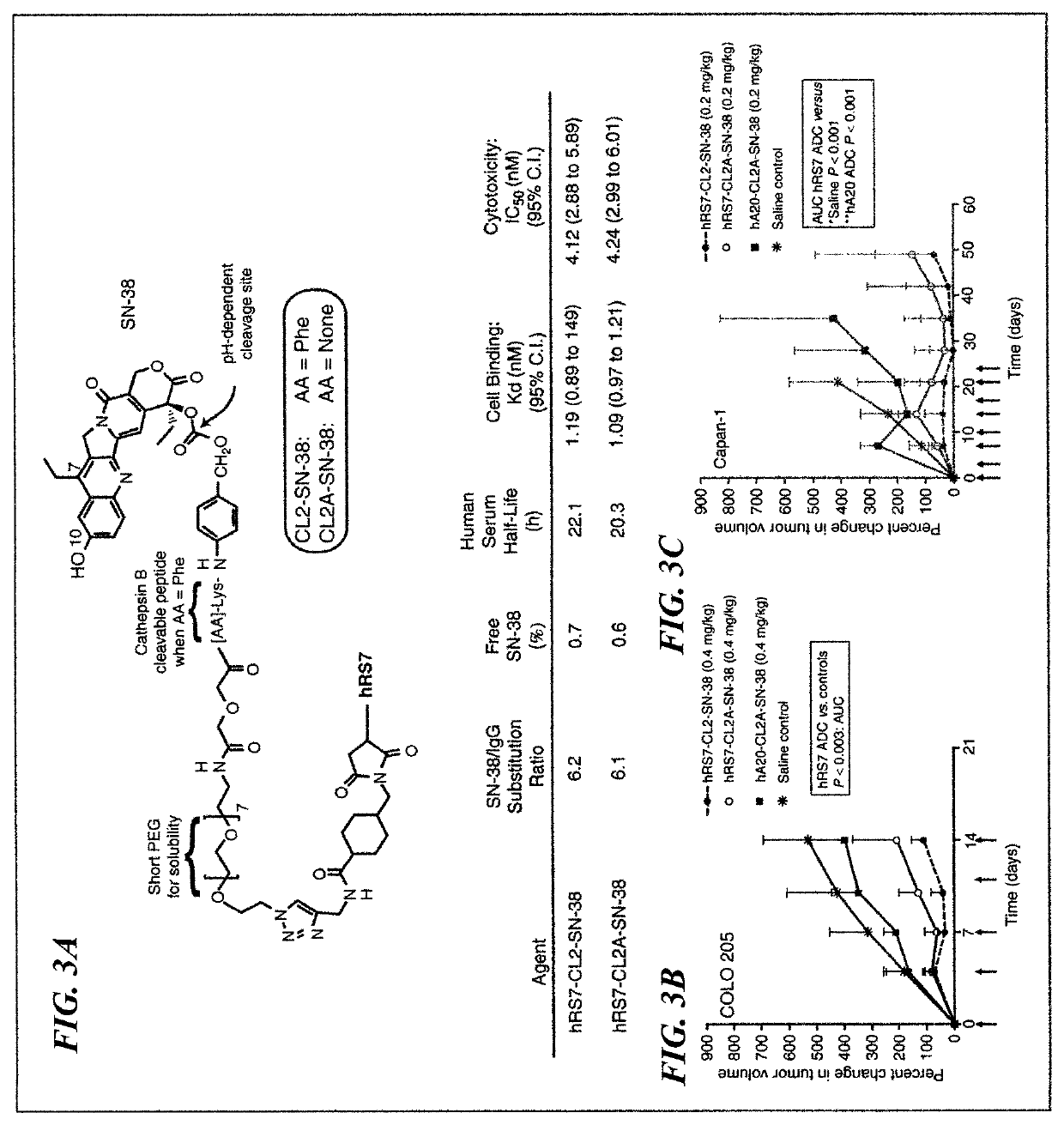
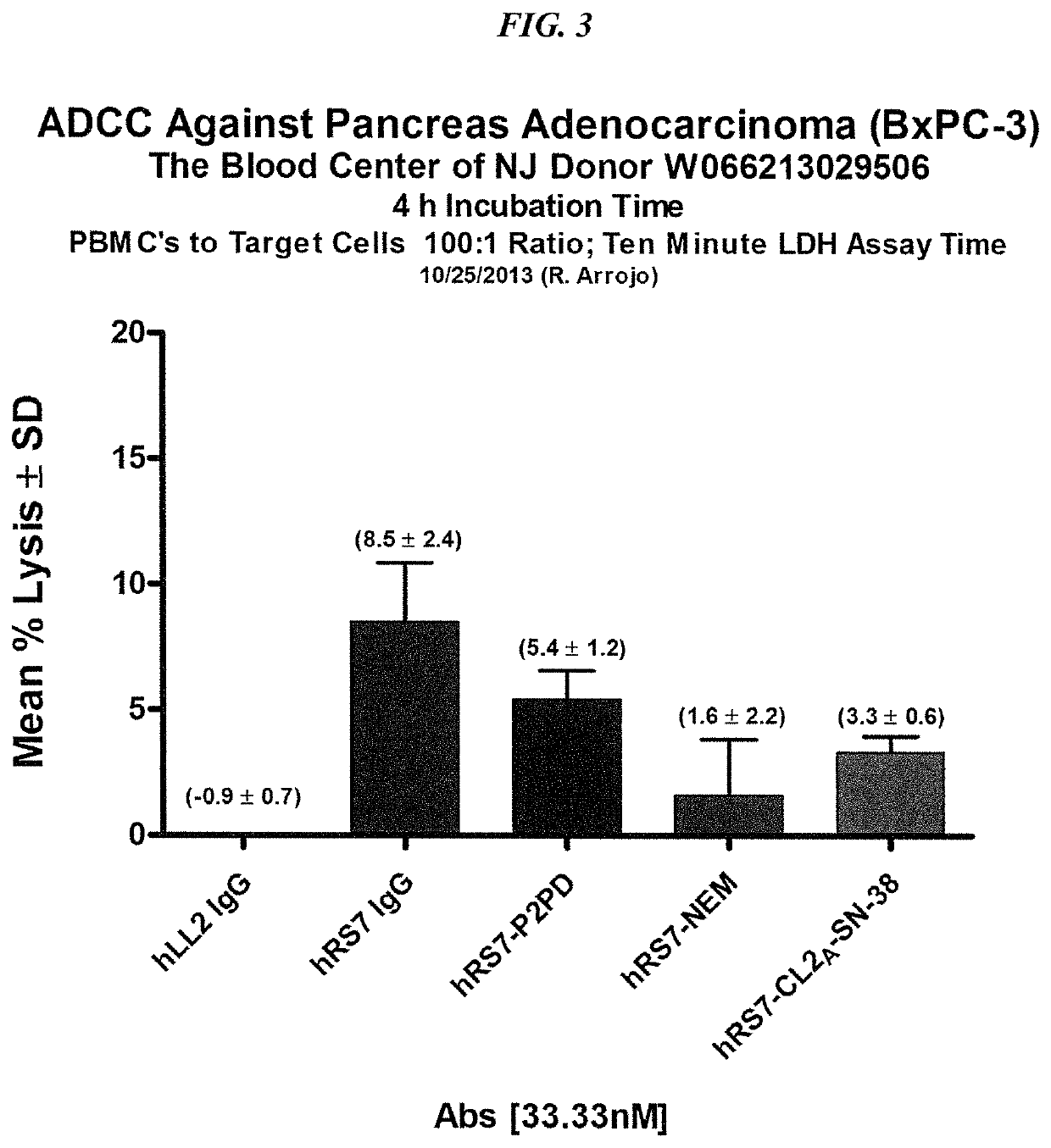
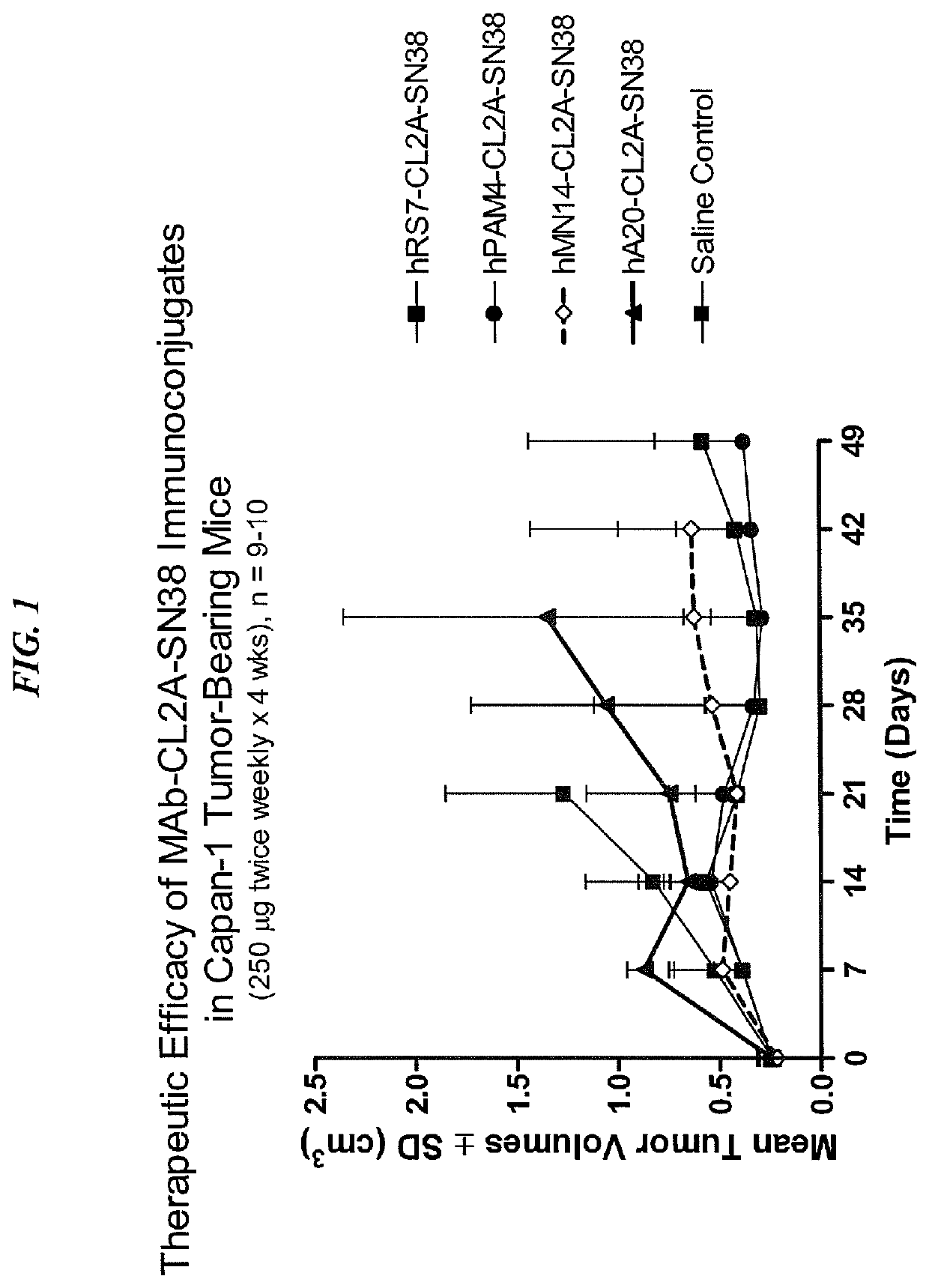
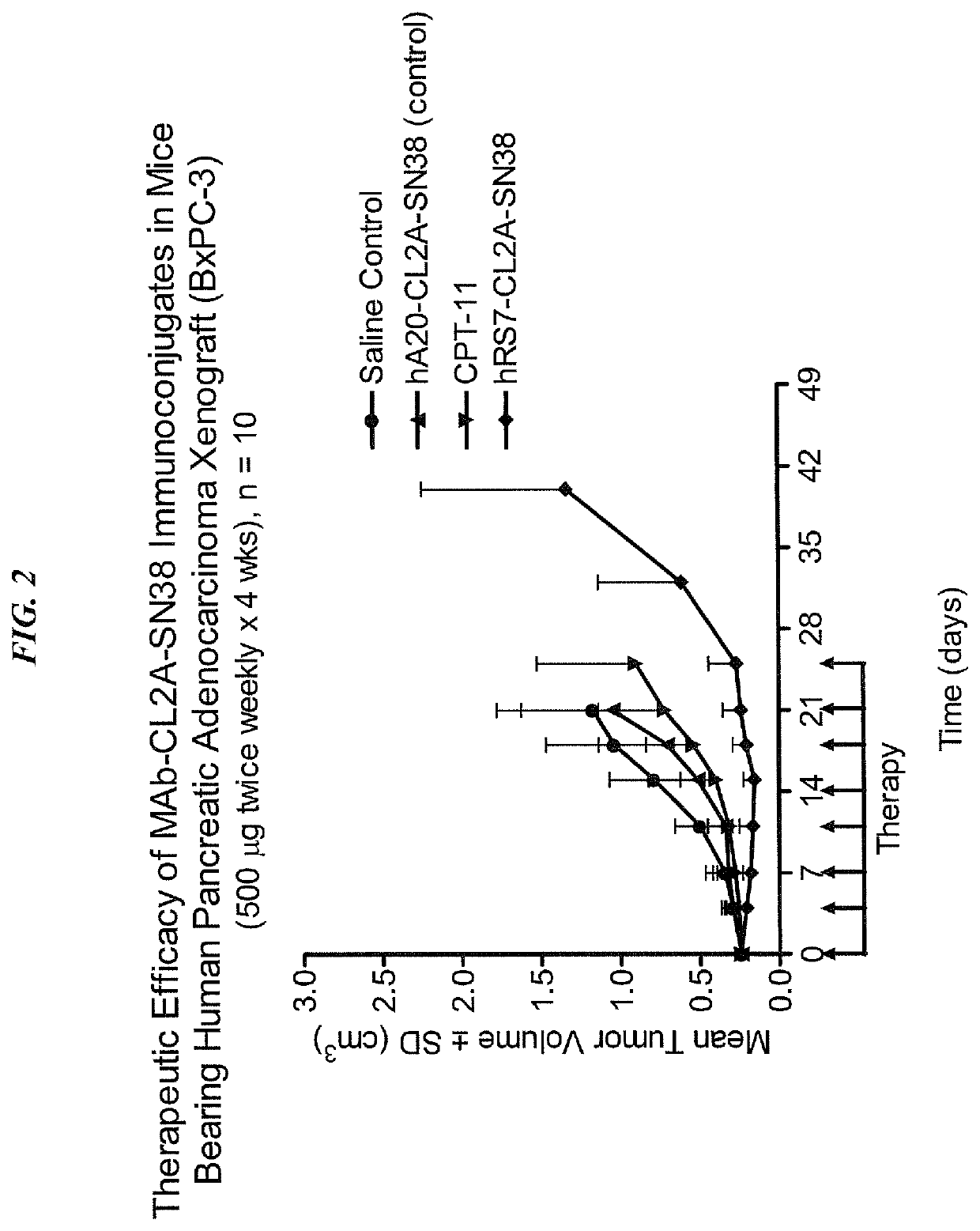
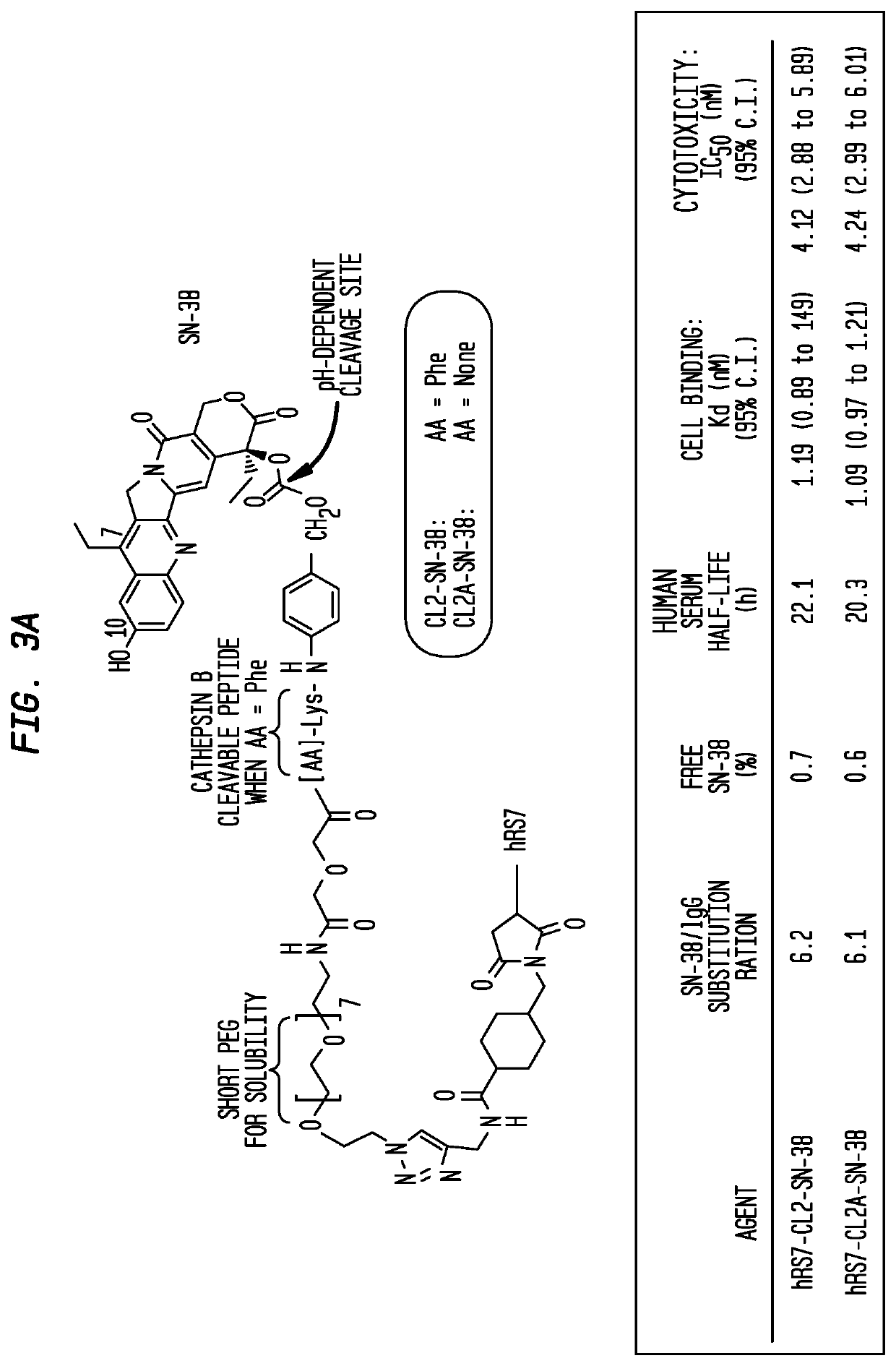
Antibody-sn-38 immunoconjugates with a cl2a linker
ActiveUS20140227180A1Improve drug bioavailabilityGood treatment effectOrganic active ingredientsPeptide/protein ingredientsDiseaseSide effect
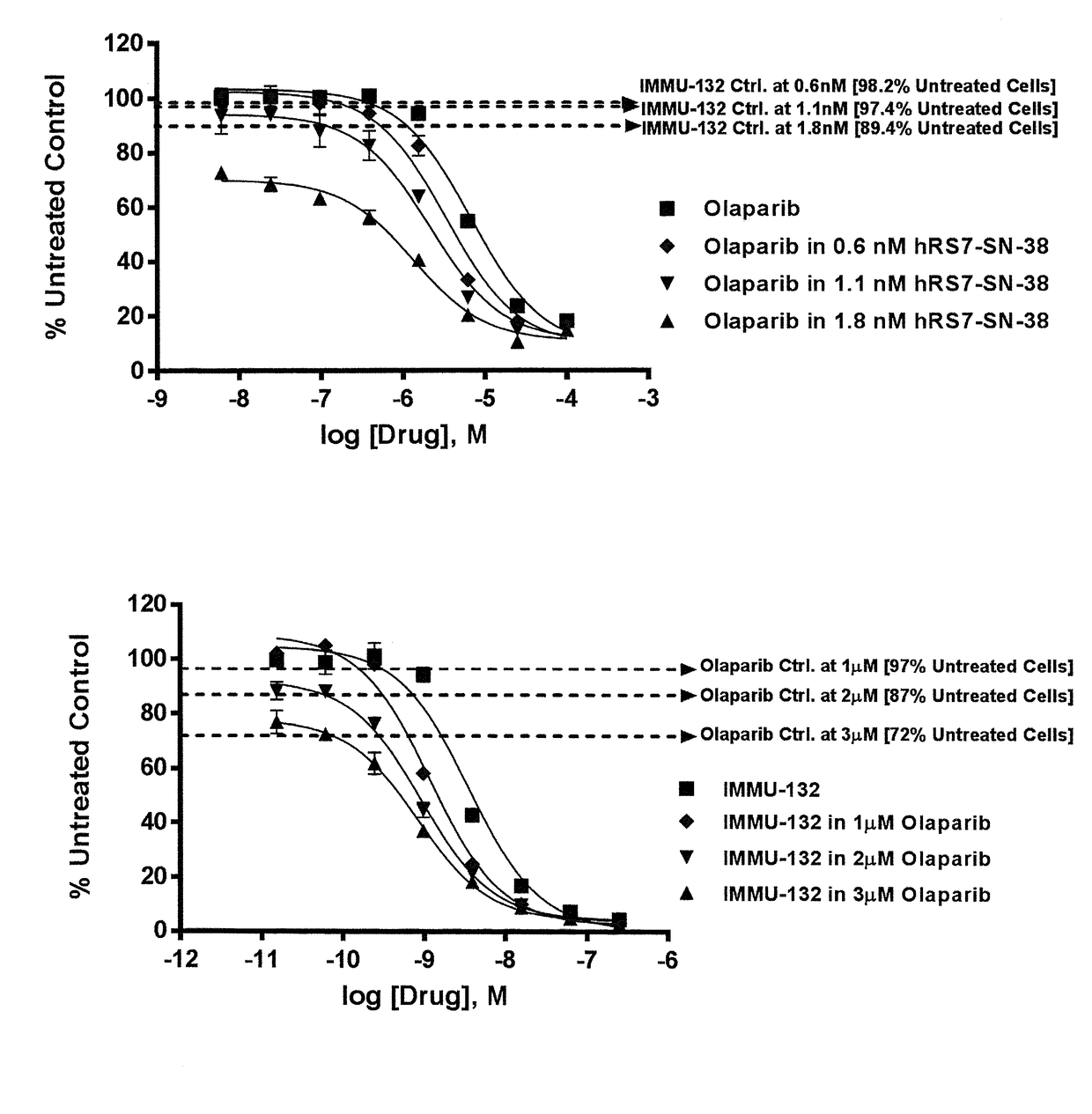
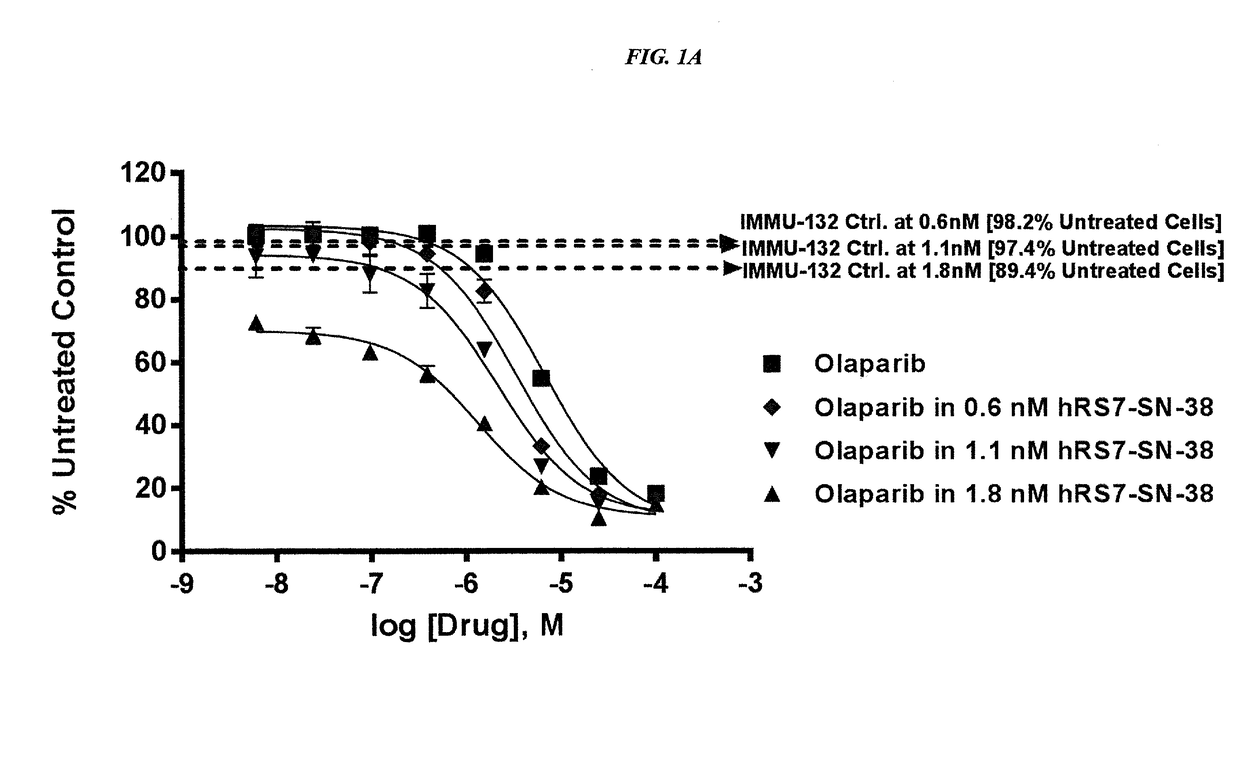
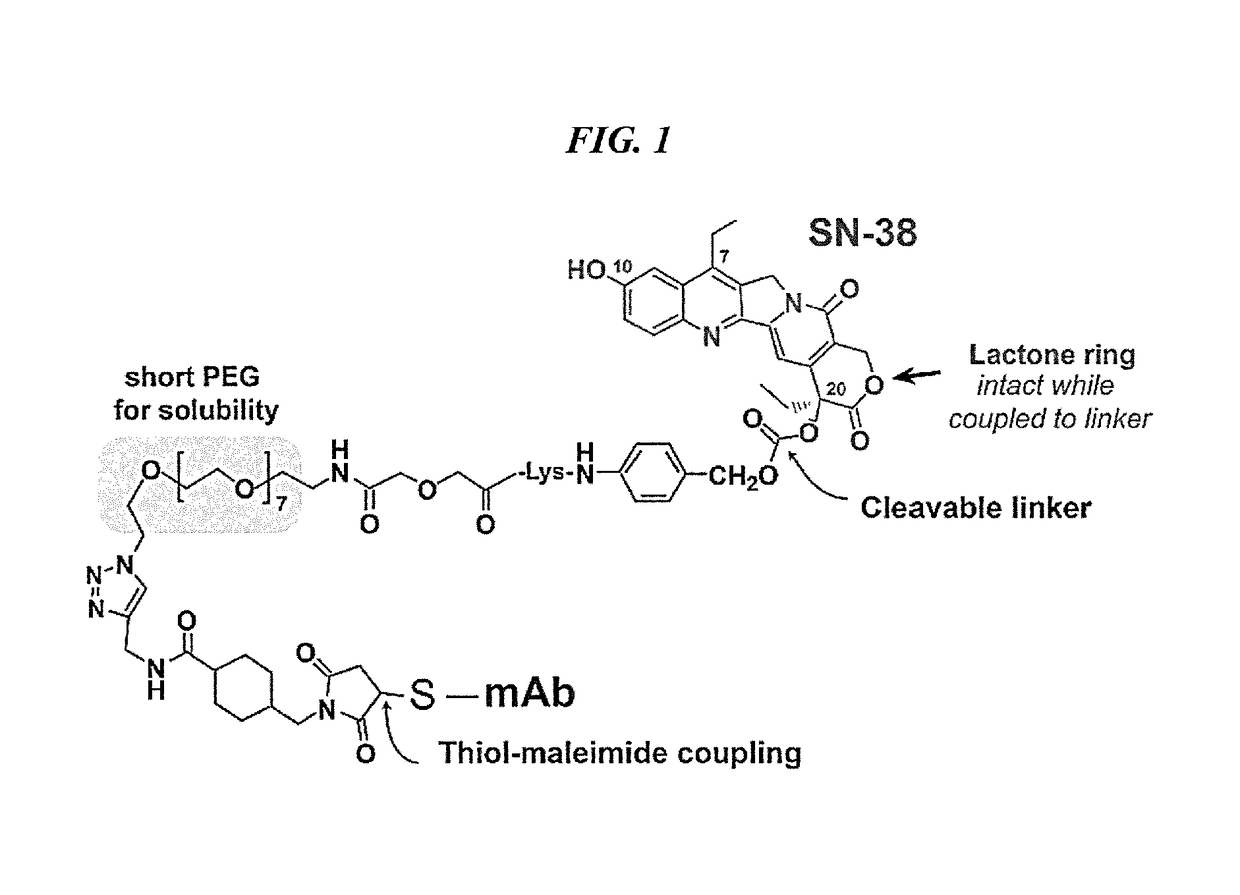
The present invention concerns improved methods and compositions for preparing SN-38 conjugates of proteins or peptides, preferably immunoconjugates of antibodies or antigen-binding antibody fragments. More preferably, the SN-38 is attached to the antibody or antibody fragment using a CL2A linker, with 1-12, more preferably 6 or less, most preferably 1-5 SN-38 moieties per antibody or antibody fragment. Most preferably, the immunoconjugate is prepared in large scale batches, with various modifications to the reaction scheme to optimize yield and recovery in large scale. Other embodiments concern optimized dosages and / or schedules of administration of immunoconjugate to maximize efficacy for disease treatment and minimize side effects of administration.
Owner:IMMUNOMEDICS INC
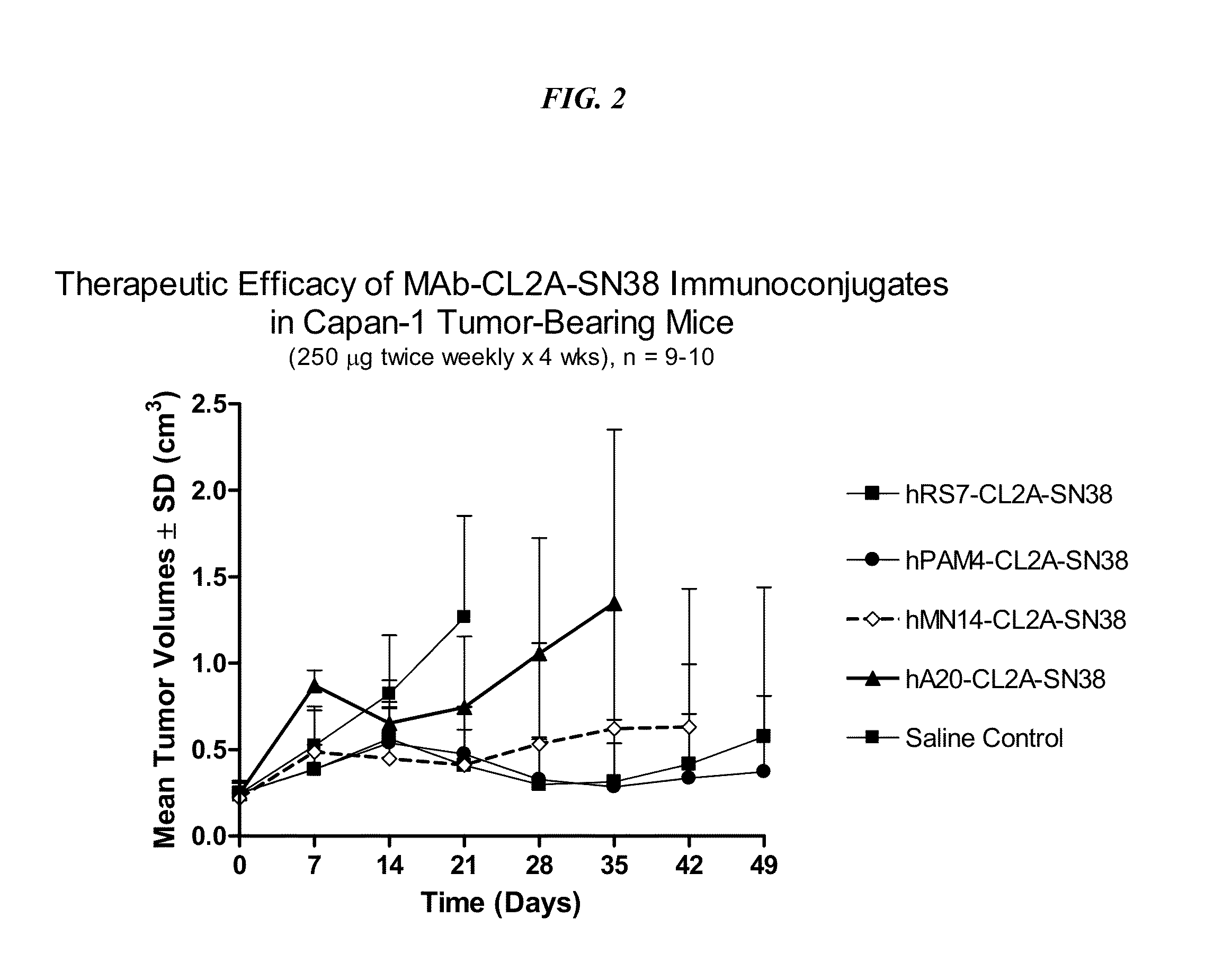
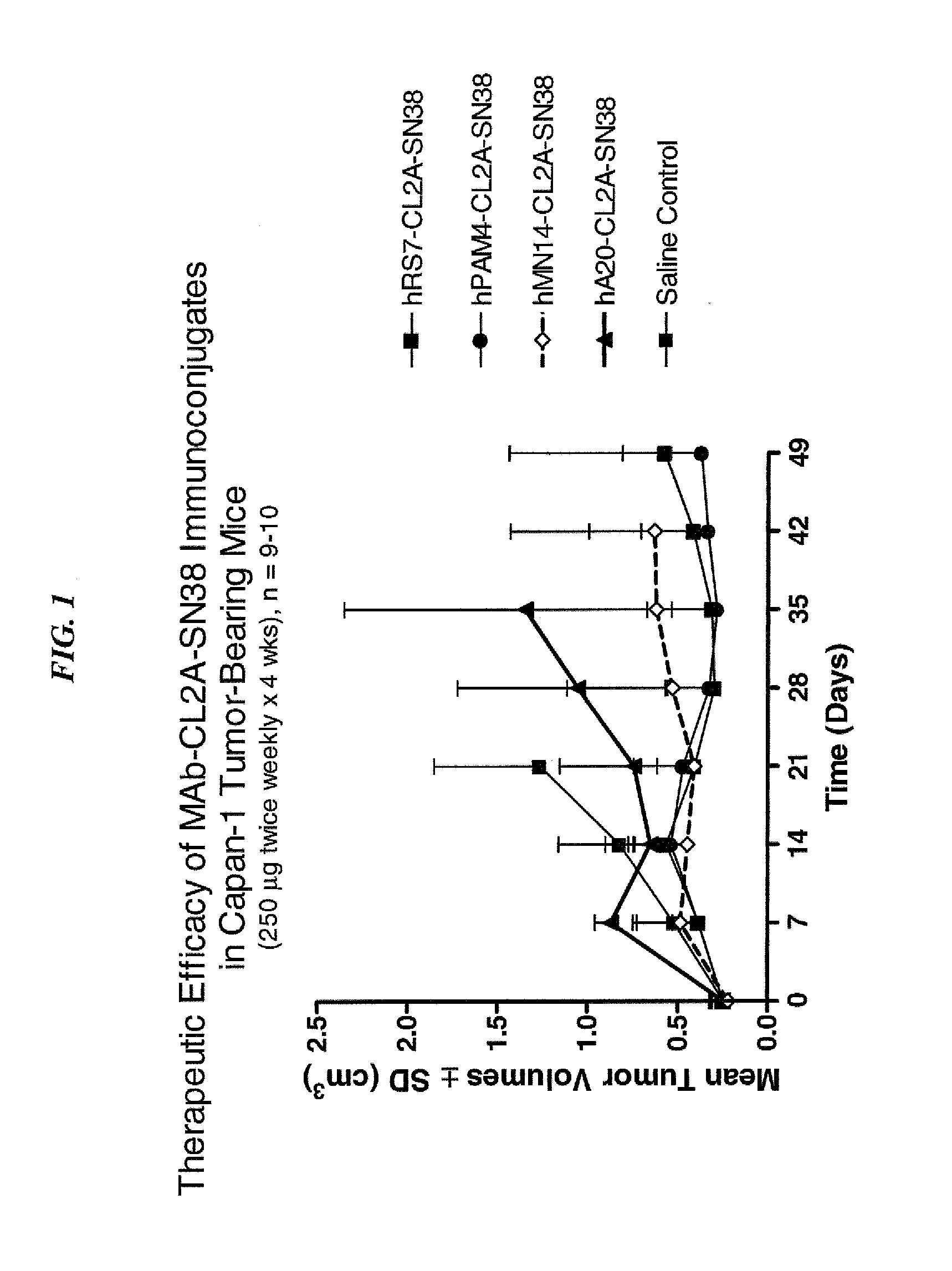
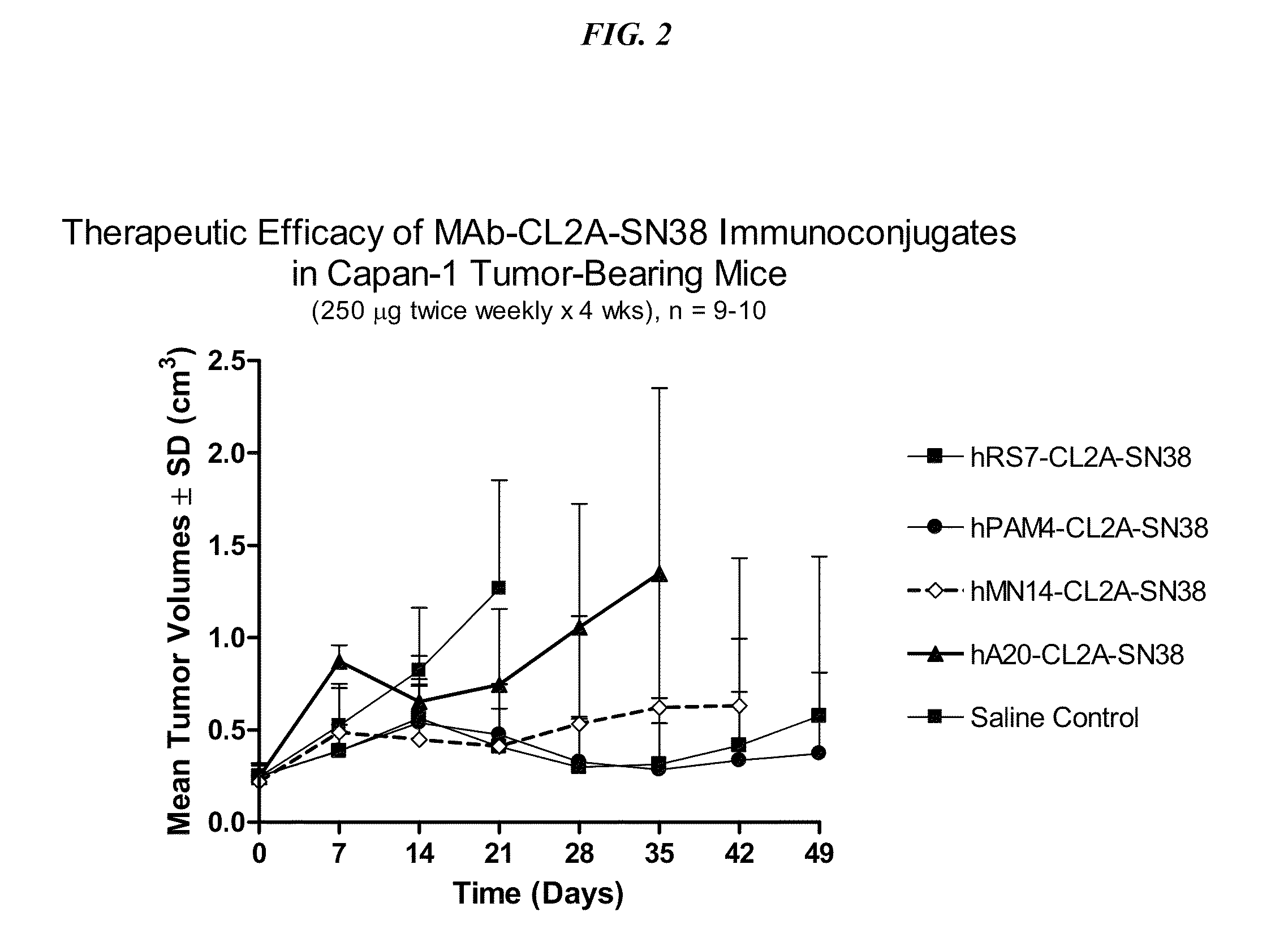
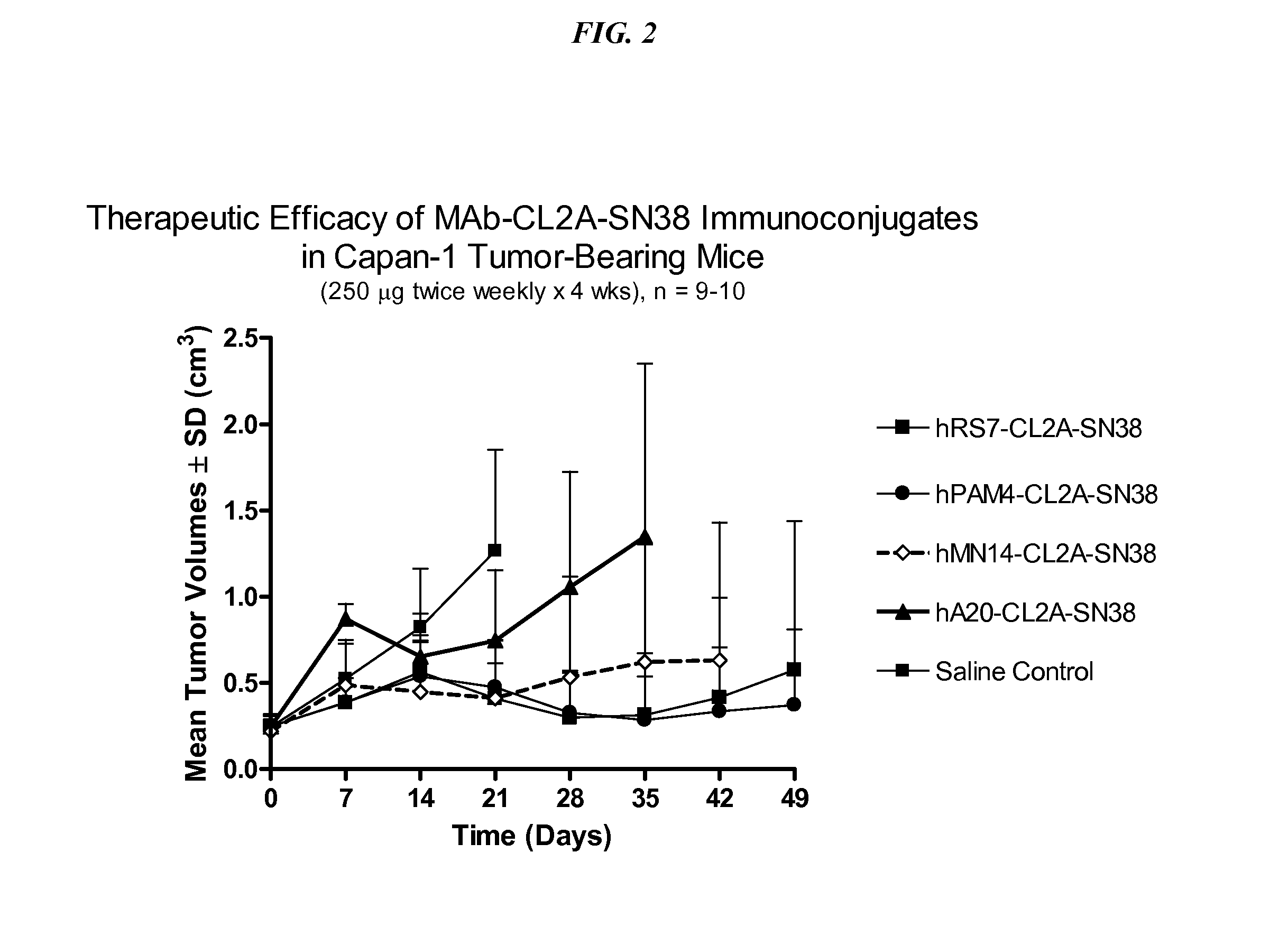
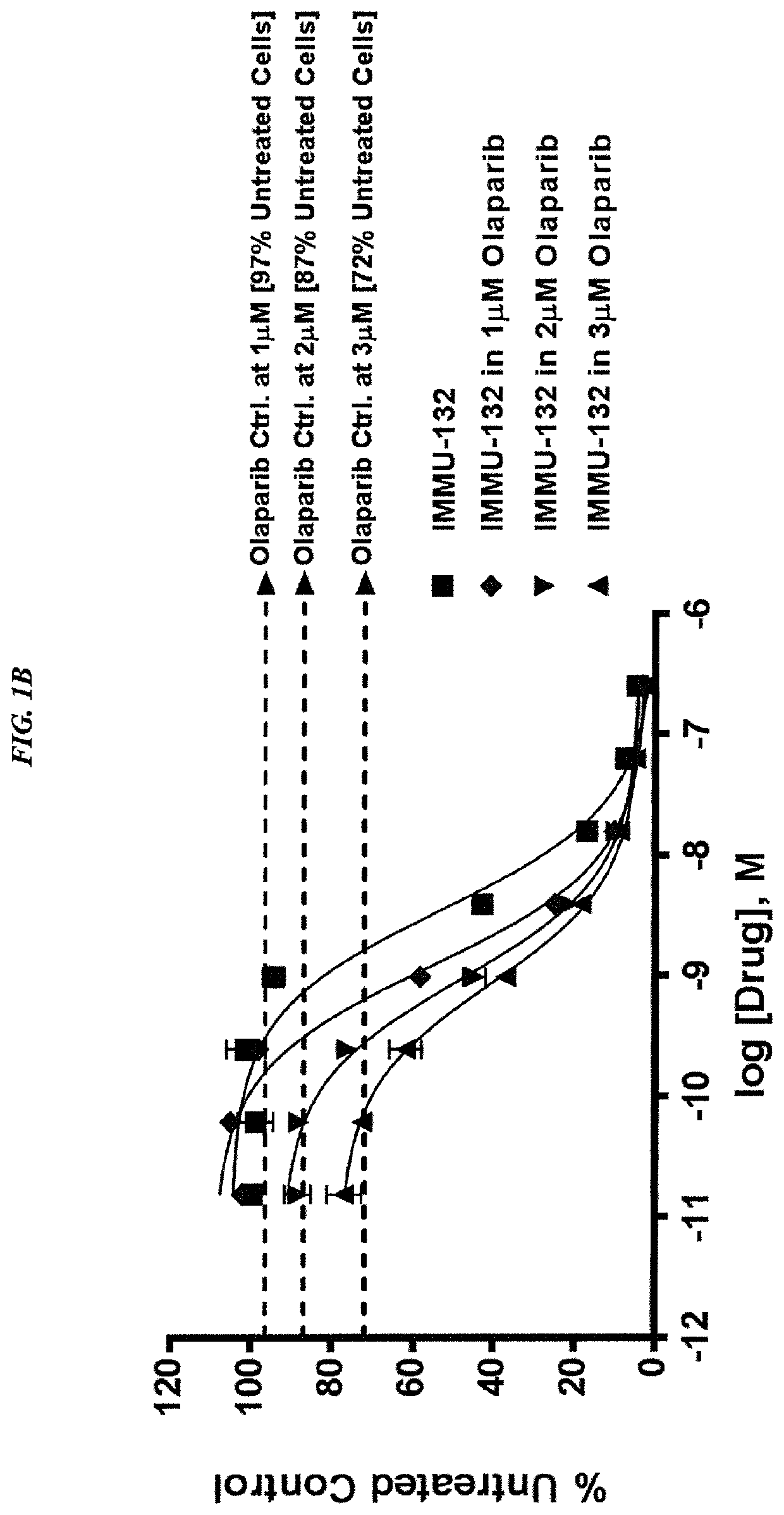
Dosages of Immunoconjugates of Antibodies and SN-38 for Improved Efficacy and Decreased Toxicity
ActiveUS20140170063A1Overcome tumorImprove targetingHeavy metal active ingredientsOrganic active ingredientsCD20Lymphatic Spread
The present invention relates to therapeutic immunoconjugates comprising SN-38 attached to an antibody or antigen-binding antibody fragment. The antibody may bind to EGP-1 ROP-2), CEACAM5, CEACAM6, CD74, CD19, CD20, CD22, CSAp, HLA-DR, AFP or MUC5ac and the immunoconjugate may be administered at a dosage of between 4 mg / kg and 24 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg. When administered at specified dosages and schedules, the immunoconjugate can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy.
Owner:IMMUNOMEDICS INC
Dosages of Immunoconjugates of Antibodies and SN-38 for Improved Efficacy and Decreased Toxicity
ActiveUS20140219914A1Reducing certain severe side effectsReceive treatment wellOrganic active ingredientsRadioactive preparation carriersCD20Lymphatic Spread
The present invention relates to therapeutic immunoconjugates comprising SN-38 attached to an antibody or antigen-binding antibody fragment. The antibody may bind to EGP-1 (TROP-2), CEACAM5, CEACAM6, CD74, CD19, CD20, CD22, CSAp, HLA-DR, AFP or MUC5ac and the immunoconjugate may be administered at a dosage of between 4 mg / kg and 24 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg. When administered at specified dosages and schedules, the immunoconjugate can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy.
Owner:IMMUNOMEDICS INC
Dosages of immunoconjugates of antibodies and SN-38 for improved efficacy and decreased toxicity
ActiveUS9028833B2Reducing certain severe side effectsReceive treatment wellOrganic active ingredientsHeavy metal active ingredientsCD20Lymphatic Spread
The present invention relates to therapeutic immunoconjugates comprising SN-38 attached to an antibody or antigen-binding antibody fragment. The antibody may bind to EGP-1 (TROP-2), CEACAM5, CEACAM6, CD74, CD19, CD20, CD22, CSAp, HLA-DR, AFP or MUC5ac and the immunoconjugate may be administered at a dosage of between 4 mg / kg and 24 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg. When administered at specified dosages and schedules, the immunoconjugate can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy.
Owner:IMMUNOMEDICS INC
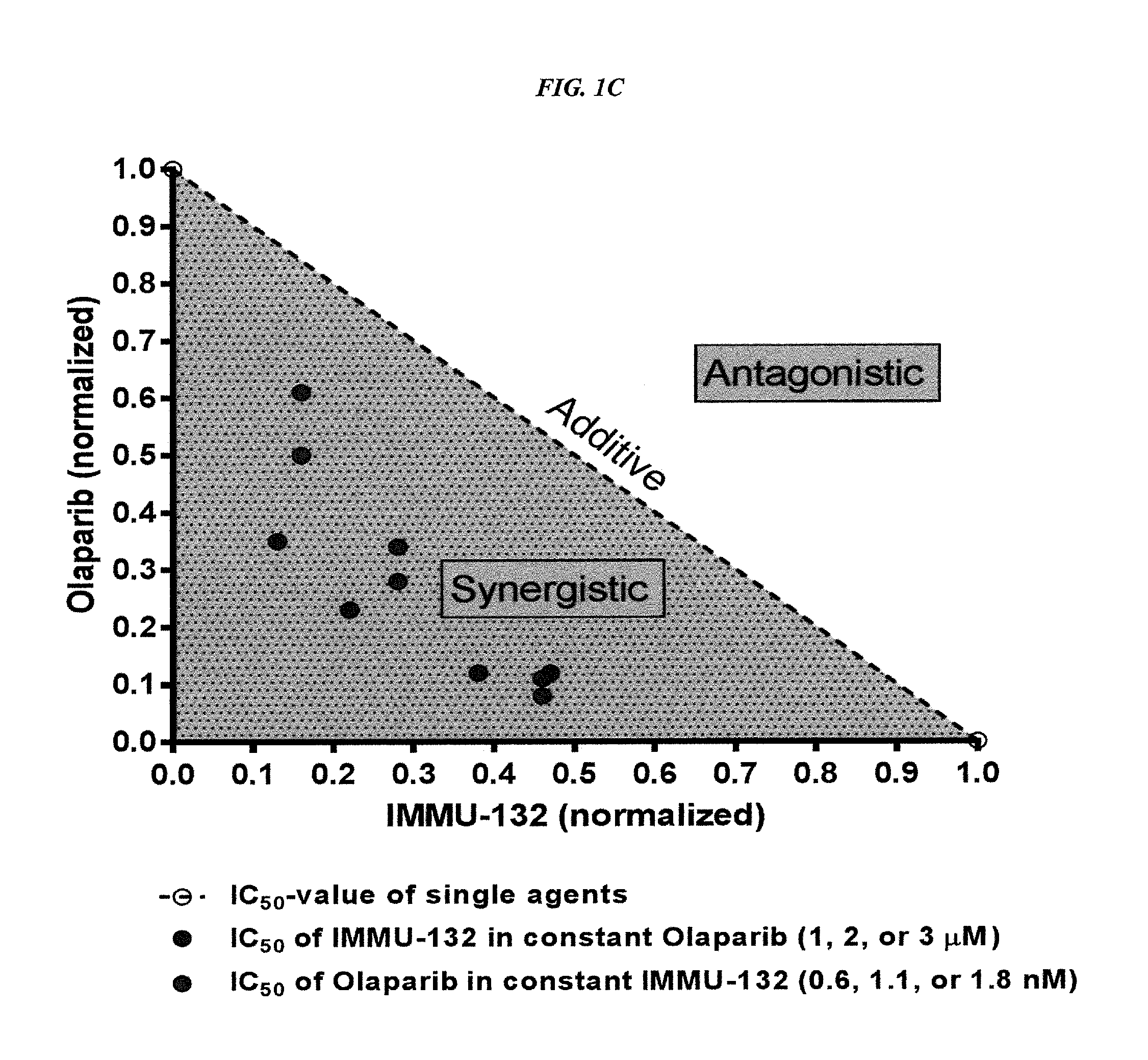
Combining Anti-hla-dr or Anti-trop-2 antibodies with microtubule inhibitors, parp inhibitors, bruton kinase inhibitors or phosphoinositide 3-kinase inhibitors significantly improves therapeutic outcome in cancer
ActiveUS20160296633A1Reducing certain severe side effectsReceive treatment wellHeavy metal active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsLymphatic SpreadTreatment effect
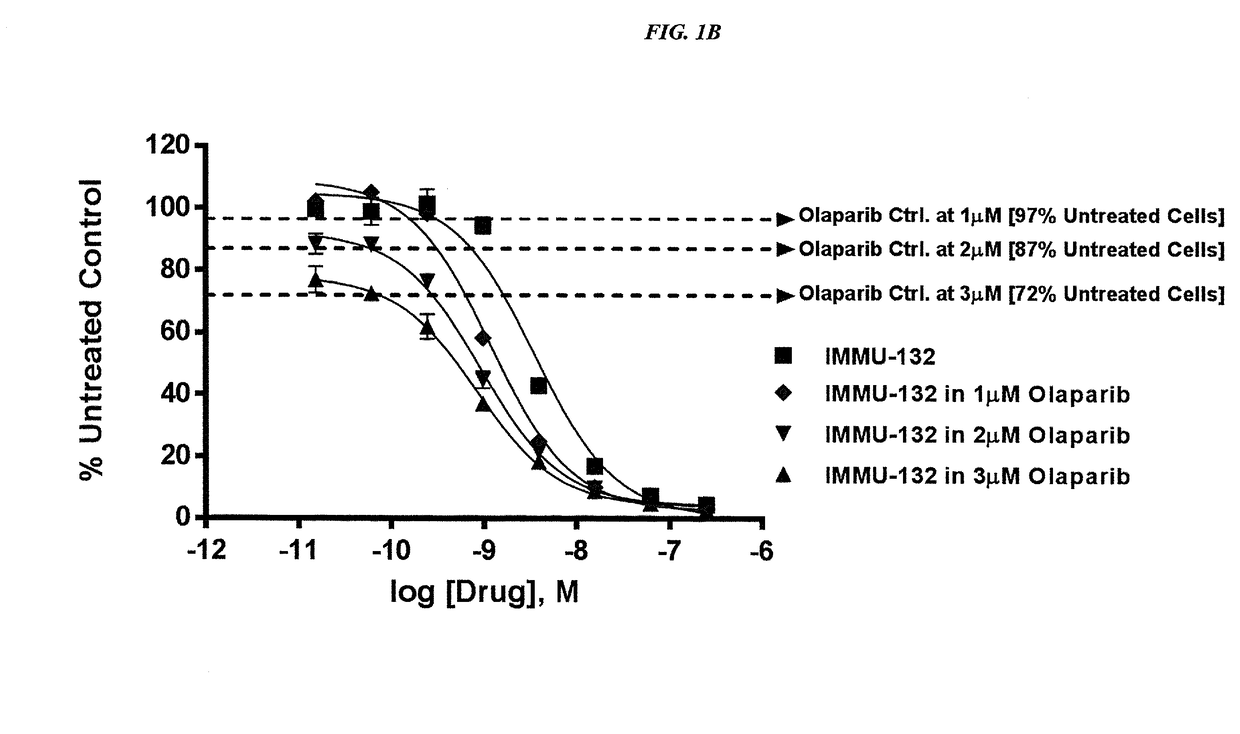
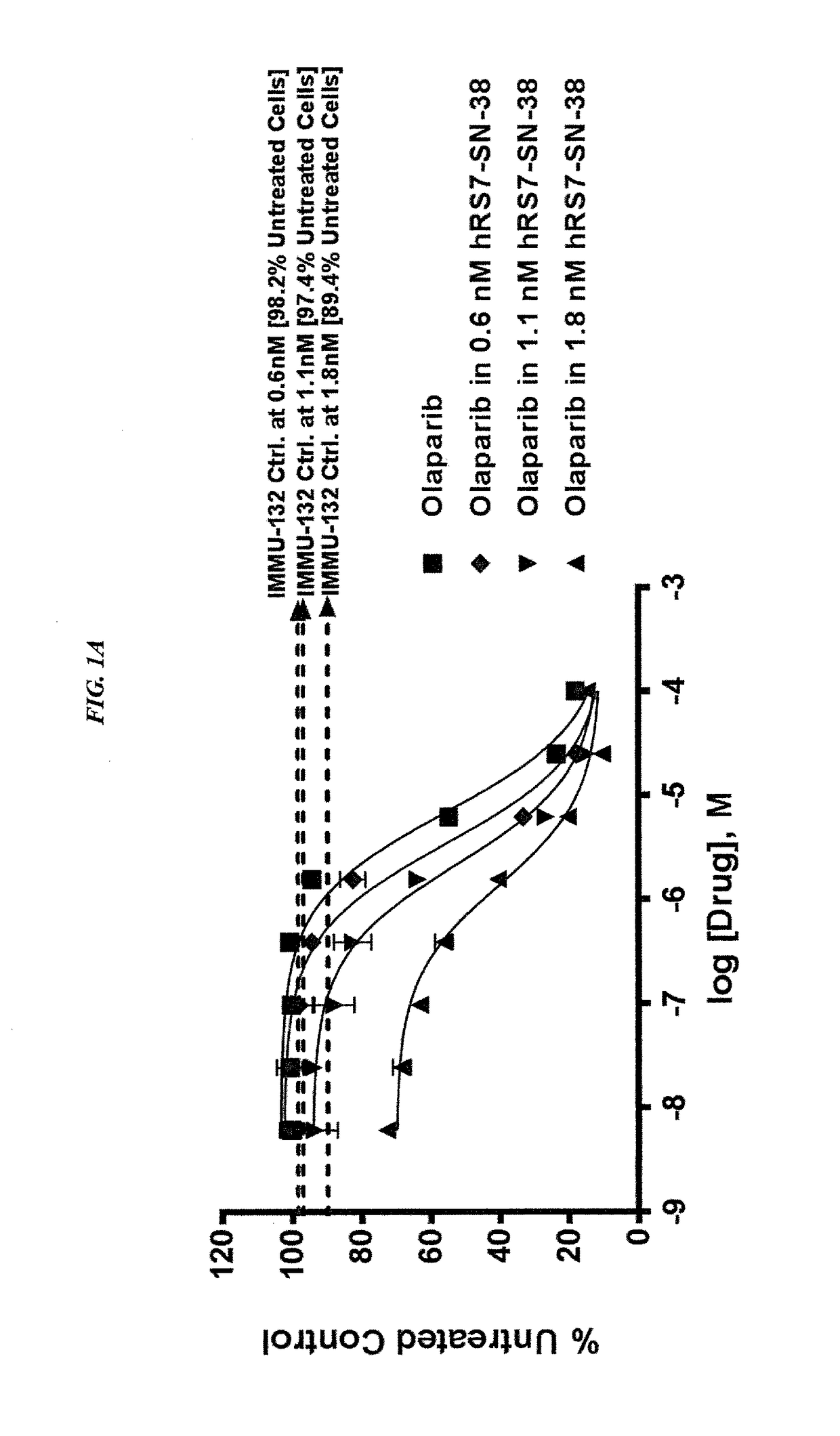
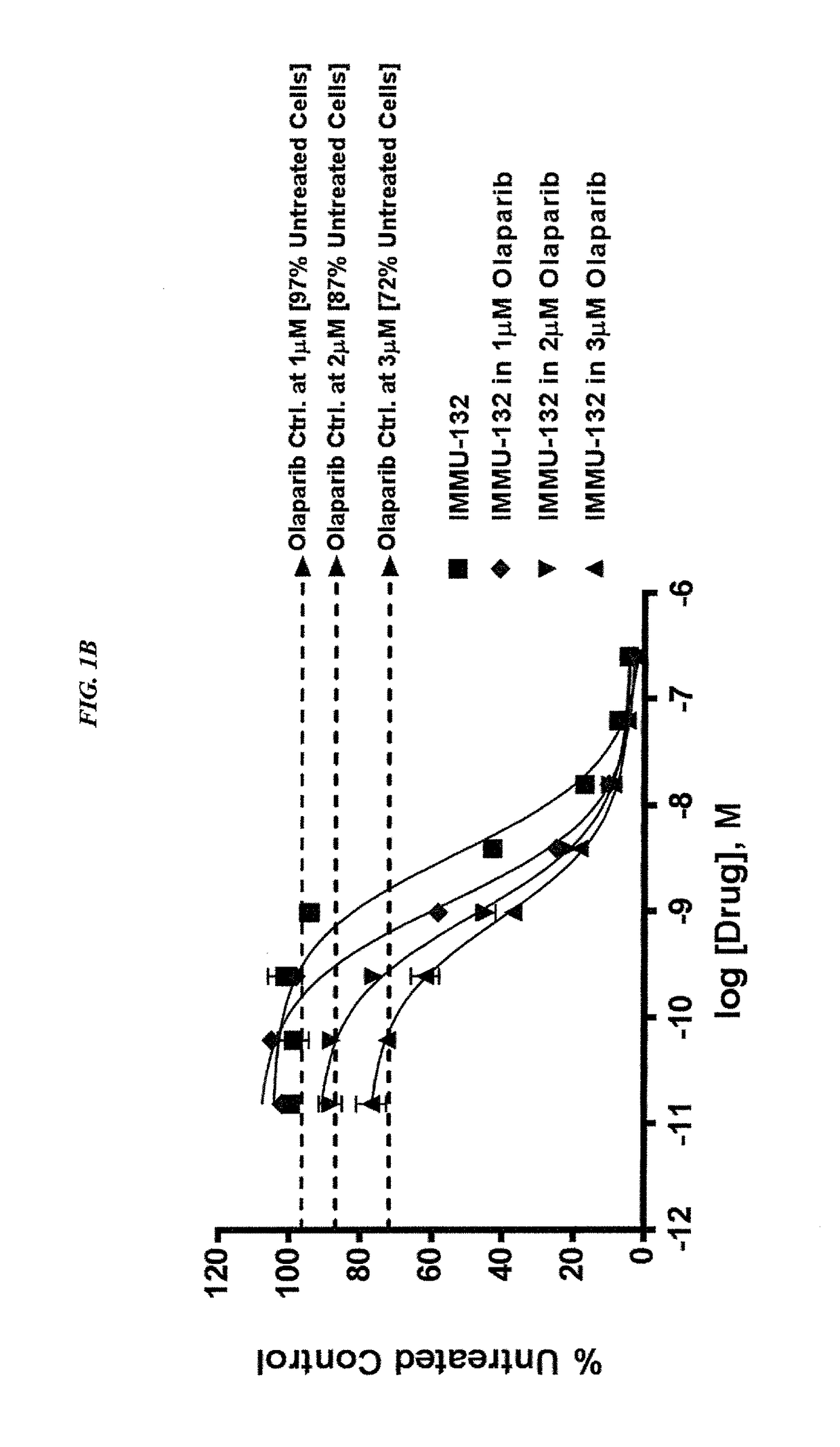
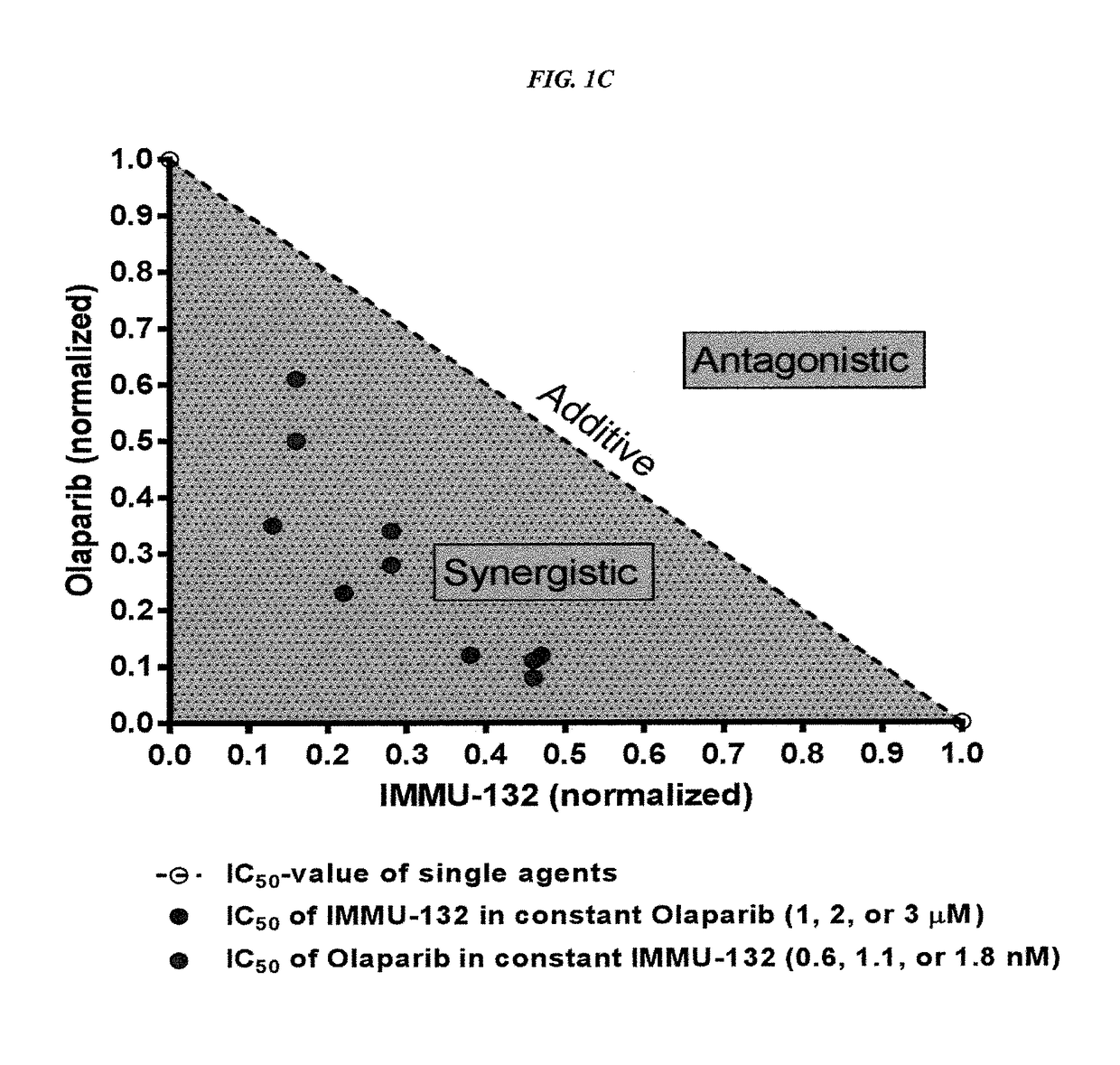
The present invention relates to combination therapy with drugs, such as microtubule inhibitors, PARP inhibitors, Bruton kinase inhibitors or PI3K inhibitors, with antibodies or immunoconjugates against HLA-DR or Trop-2. Where immunoconjugates are used, they preferably incorporate SN-38 or pro-2PDOX. The immunoconjugate may be administered at a dosage of between 1 mg / kg and 18 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg, more preferably 8 or 10 mg / kg. The combination therapy can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. Preferably, the combination therapy has an additive effect on inhibiting tumor growth. Most preferably, the combination therapy has a synergistic effect on inhibiting tumor growth.
Owner:IMMUNOMEDICS INC
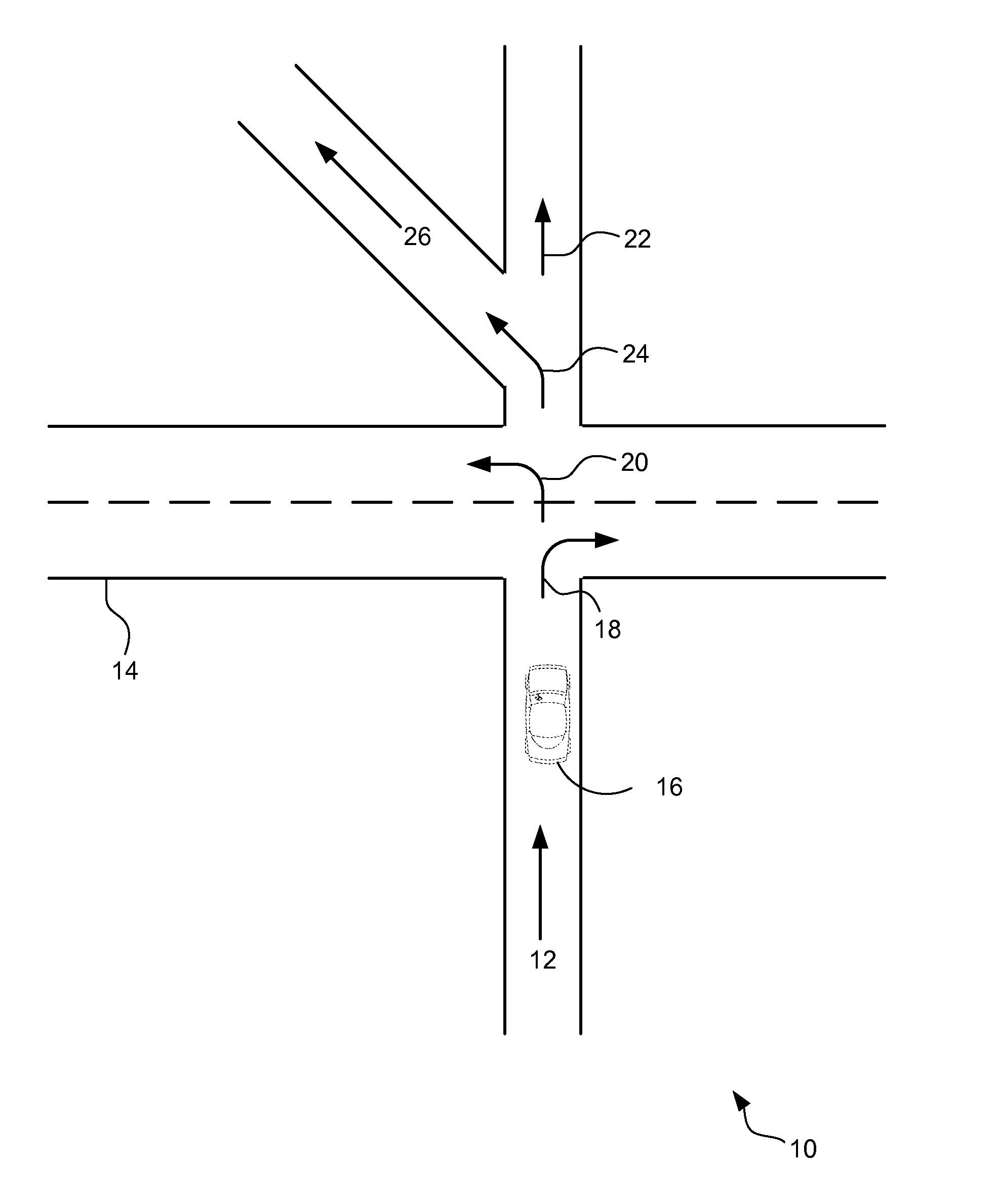
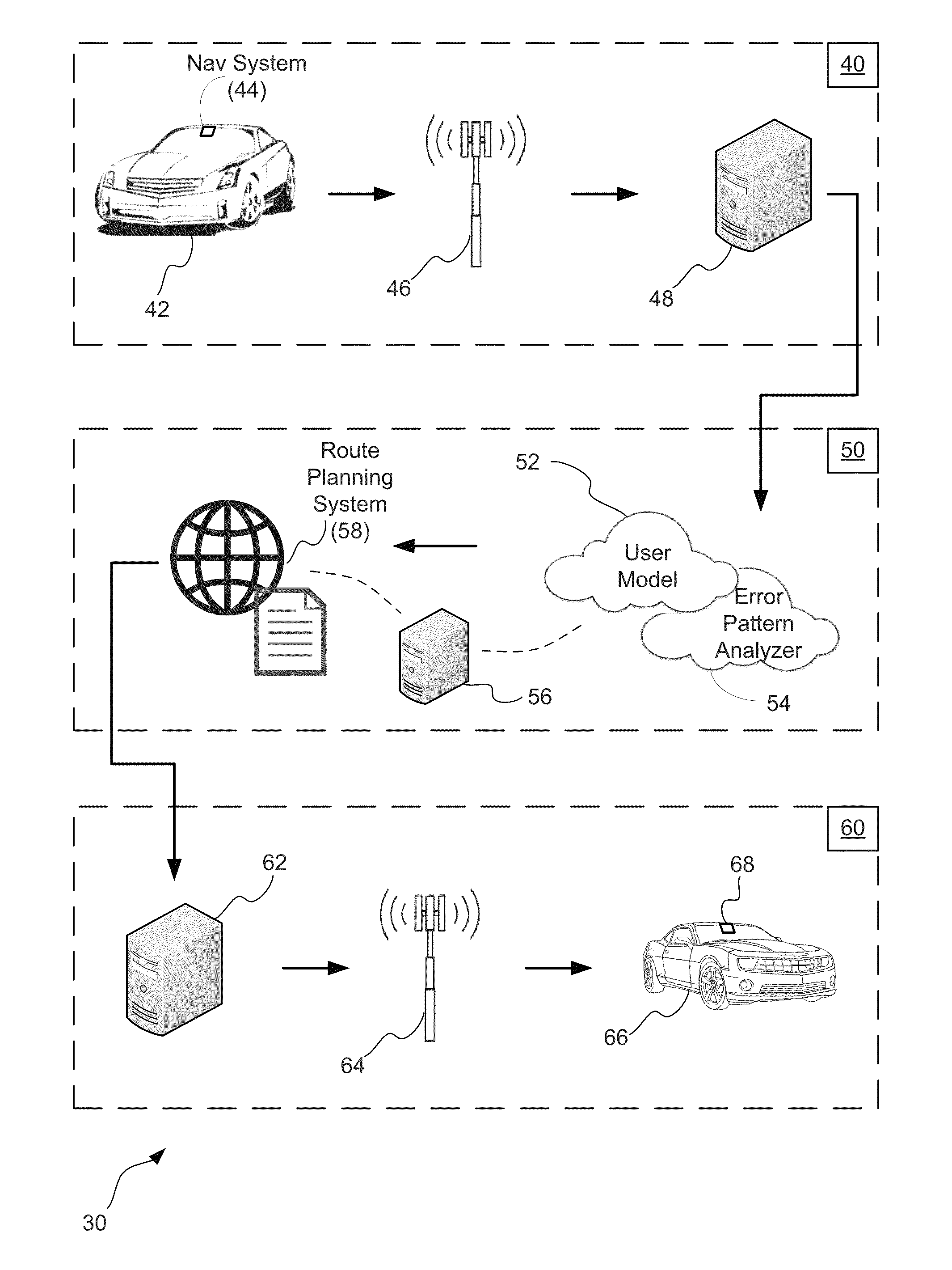
Adaptive user guidance for navigation and location-based services
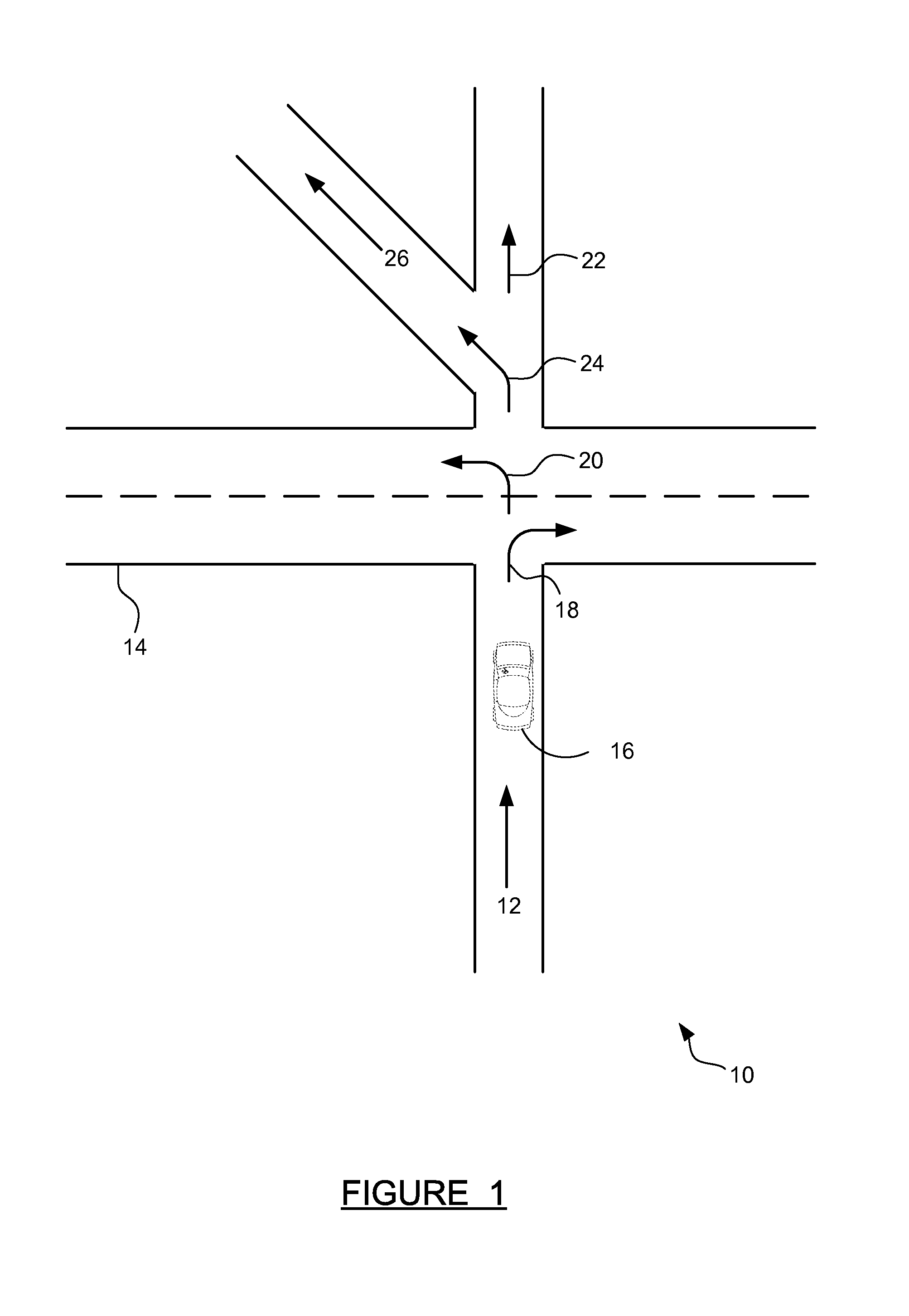
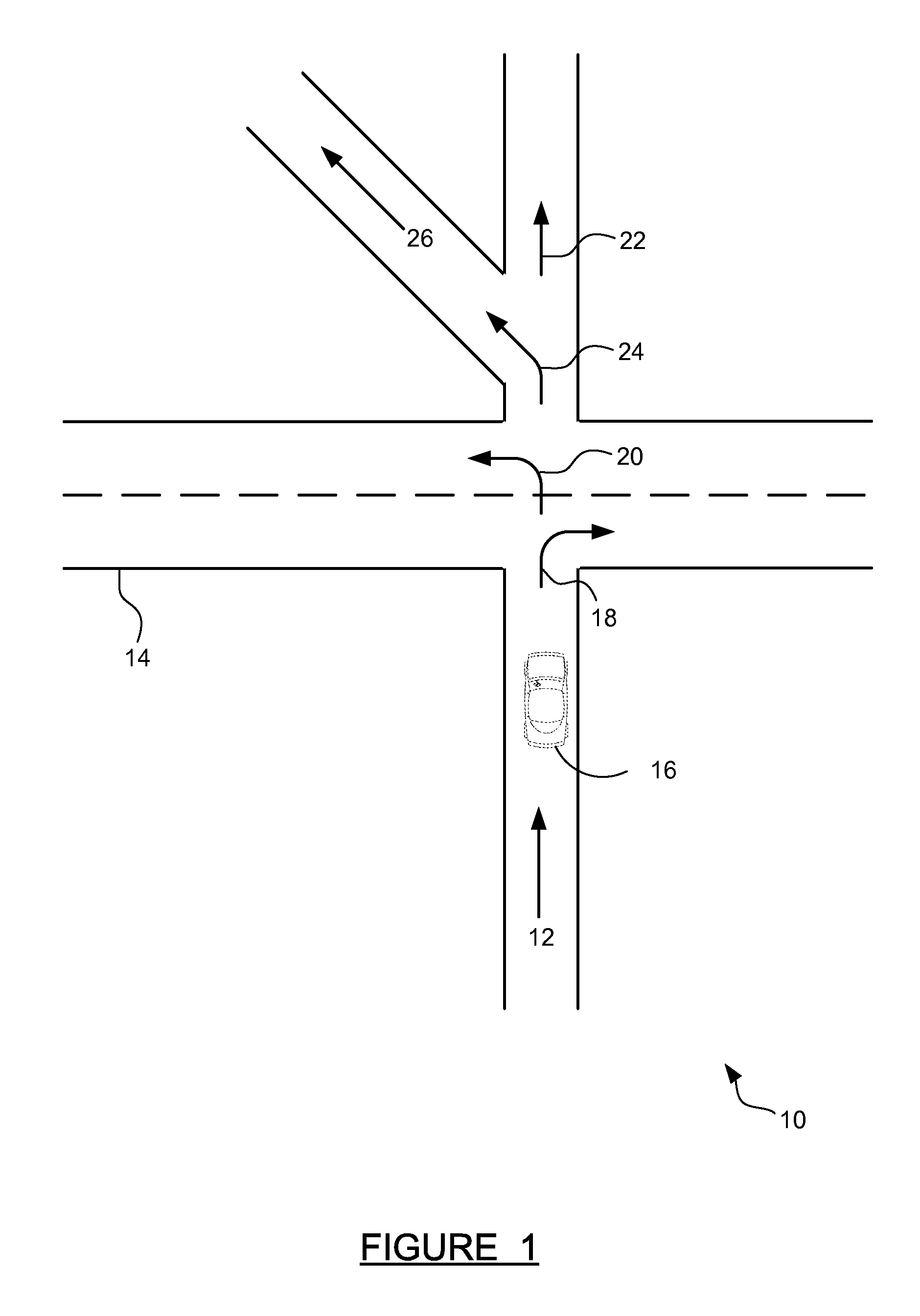
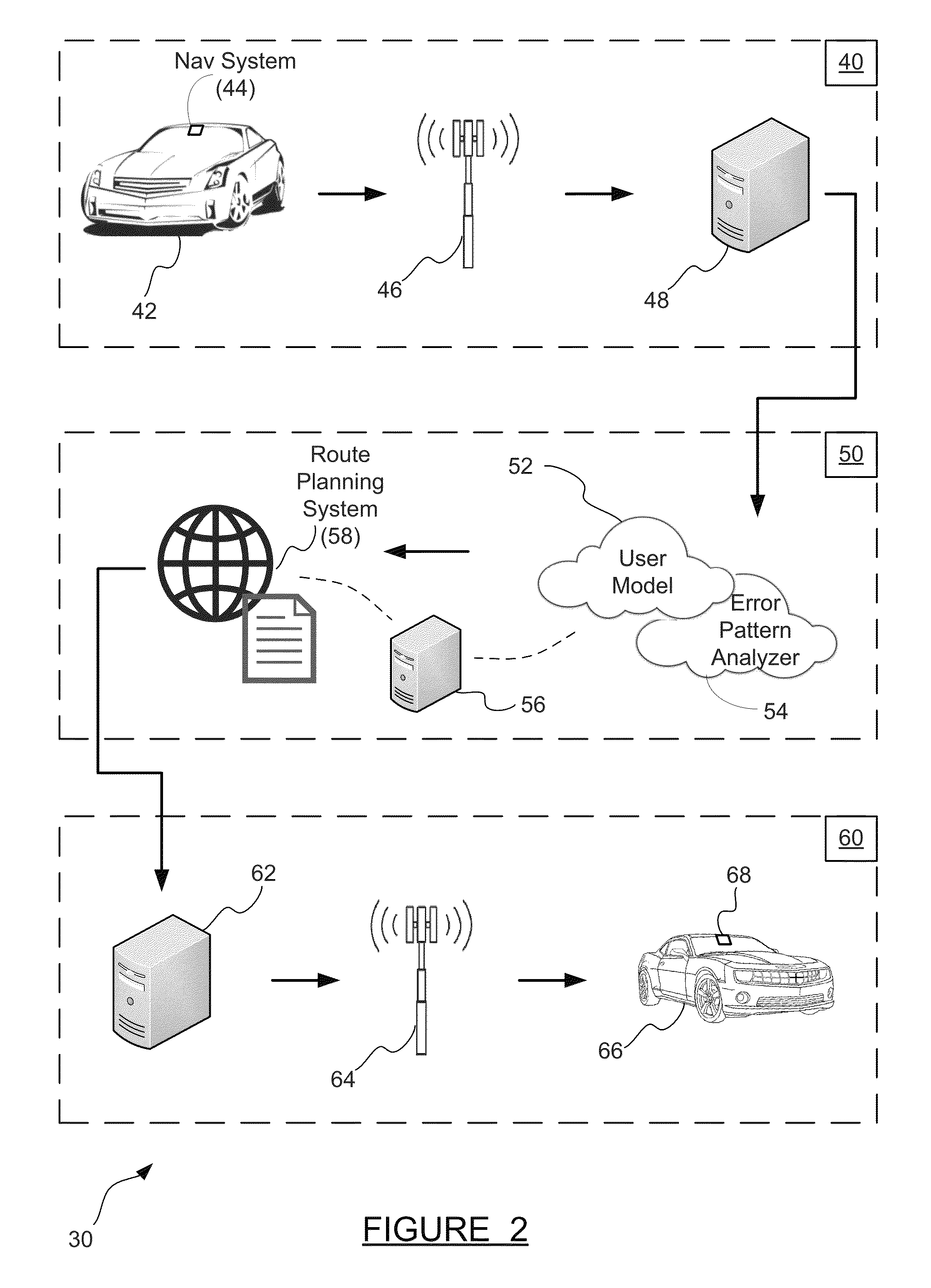
ActiveUS20140214322A1Improve driver performanceIncrease and decrease frequencyInstruments for road network navigationAnalysis dataDriver/operator
A method for adaptive driver guidance for navigation and location-based services. A navigation system onboard a vehicle records errors, including both system errors and user errors, where the errors can be detected by missed turns, re-routing, and similar events. The error data is transmitted to a central server, where the data is analyzed to determine patterns of errors, both for an individual driver and across many drivers. Roadway locations which frequently experience driver navigational errors have the error type integrated as a route feature, and future navigational guidance is adapted to improve driver performance. Adaptations can include increased or decreased frequency, detail, timing and location of navigational instructions. Individual driver guidance can also be adapted based on the driver's tendency to make errors in response to specific guidance instructions. Adaptation of guidance for location-based services is also provided.
Owner:GM GLOBAL TECH OPERATIONS LLC
Combining Anti-hla-dr or Anti-trop-2 antibodies with microtubule inhibitors, parp inhibitors, bruton kinase inhibitors or phosphoinositide 3-kinase inhibitors significantly improves therapeutic outcome in cancer
InactiveUS20170274093A1Reducing certain severe side effectsReceive treatment wellHeavy metal active ingredientsOrganic active ingredientsLymphatic SpreadCombined Modality Therapy
The present invention relates to combination therapy with drugs, such as microtubule inhibitors, PARP inhibitors, Bruton kinase inhibitors or PI3K inhibitors, with antibodies or immunoconjugates against HLA-DR or Trop-2. Where immunoconjugates are used, they preferably incorporate SN-38 or pro-2PDOX. The immunoconjugate may be administered at a dosage of between 1 mg / kg and 18 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg, more preferably 8 or 10 mg / kg. The combination therapy can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. Preferably, the combination therapy has an additive effect on inhibiting tumor growth. Most preferably, the combination therapy has a synergistic effect on inhibiting tumor growth.
Owner:IMMUNOMEDICS INC
Combining anti-HLA-DR or anti-Trop-2 antibodies with microtubule inhibitors, PARP inhibitors, bruton kinase inhibitors or phosphoinositide 3-kinase inhibitors significantly improves therapeutic outcome in cancer
ActiveUS9707302B2Reducing certain severe side effectsReceive treatment wellOrganic active ingredientsHeavy metal active ingredientsLymphatic SpreadCombined Modality Therapy
The present invention relates to combination therapy with drugs, such as microtubule inhibitors, PARP inhibitors, Bruton kinase inhibitors or PI3K inhibitors, with antibodies or immunoconjugates against HLA-DR or Trop-2. Where immunoconjugates are used, they preferably incorporate SN-38 or pro-2PDOX. The immunoconjugate may be administered at a dosage of between 1 mg / kg and 18 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg, more preferably 8 or 10 mg / kg. The combination therapy can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. Preferably, the combination therapy has an additive effect on inhibiting tumor growth. Most preferably, the combination therapy has a synergistic effect on inhibiting tumor growth.
Owner:IMMUNOMEDICS INC
Antibody-SN-38 immunoconjugates with a CL2A linker
ActiveUS9107960B2Improve drug bioavailabilityGood treatment effectOrganic active ingredientsHeavy metal active ingredientsDiseaseSide effect
The present invention concerns improved methods and compositions for preparing SN-38 conjugates of proteins or peptides, preferably immunoconjugates of antibodies or antigen-binding antibody fragments. More preferably, the SN-38 is attached to the antibody or antibody fragment using a CL2A linker, with 1-12, more preferably 6 or less, most preferably 1-5 SN-38 moieties per antibody or antibody fragment. Most preferably, the immunoconjugate is prepared in large scale batches, with various modifications to the reaction scheme to optimize yield and recovery in large scale. Other embodiments concern optimized dosages and / or schedules of administration of immunoconjugate to maximize efficacy for disease treatment and minimize side effects of administration.
Owner:IMMUNOMEDICS INC
Biomarkers for antibody-drug conjugate monotherapy or combination therapy
PendingUS20210093730A1Predict resistancePredict sensitivityMicrobiological testing/measurementOrganic non-active ingredientsDiseaseAnticarcinogen
The present invention relates to biomarkers of use in cancer therapy, wherein the therapy comprises treatment with anti-Trop-2, anti-CEACAM5 or anti-HLA-DR ADCs (antibody-drug conjugates), alone or in combination with and one or more anti-cancer agents, such as a DDR inhibitor, an ABCG2 inhibitor, a microtubule inhibitor, a checkpoint inhibitor, a PI3K inhibitor, an AKT inhibitor, a CDK 4 inhibitor, a CDK 5 inhibior, a tyrosine kinase inhibitor or a platinum-based chemotherapeutic agent. Preferably, the combination therapy has a synergistic effect on inhibiting tumor growth. The biomarkers are of use to predict efficacy and / or toxicity of ADC therapy, determine tumor response to treatment, identify minimal residual disease or relapse, determine prognosis, stratify patients for initial therapy or to optimize treatment for the patient, based on the specific biomarkers detected.
Owner:IMMUNOMEDICS INC
Efficacy of Anti-trop-2-sn-38 antibody drug conjugates for therapy of tumors relapsed/refractory to checkpoint inhibitors
ActiveUS20170313781A1Improve targetingLow toxicityOrganic active ingredientsDigestive systemAntiendomysial antibodiesEfficacy
The present invention relates to therapeutic ADCs comprising SN-38 attached to an anti-Trop-2 antibody or antigen-binding antibody fragment, more particularly sacituzumab govitecan. The ADC is administered to a subject with a Trop-2 positive cancer that is resistant to or relapsed from prior treatment with a checkpoint inhibitor. The therapy is effective to treat cancers that are resistant to checkpoint inhibitors.
Owner:IMMUNOMEDICS INC
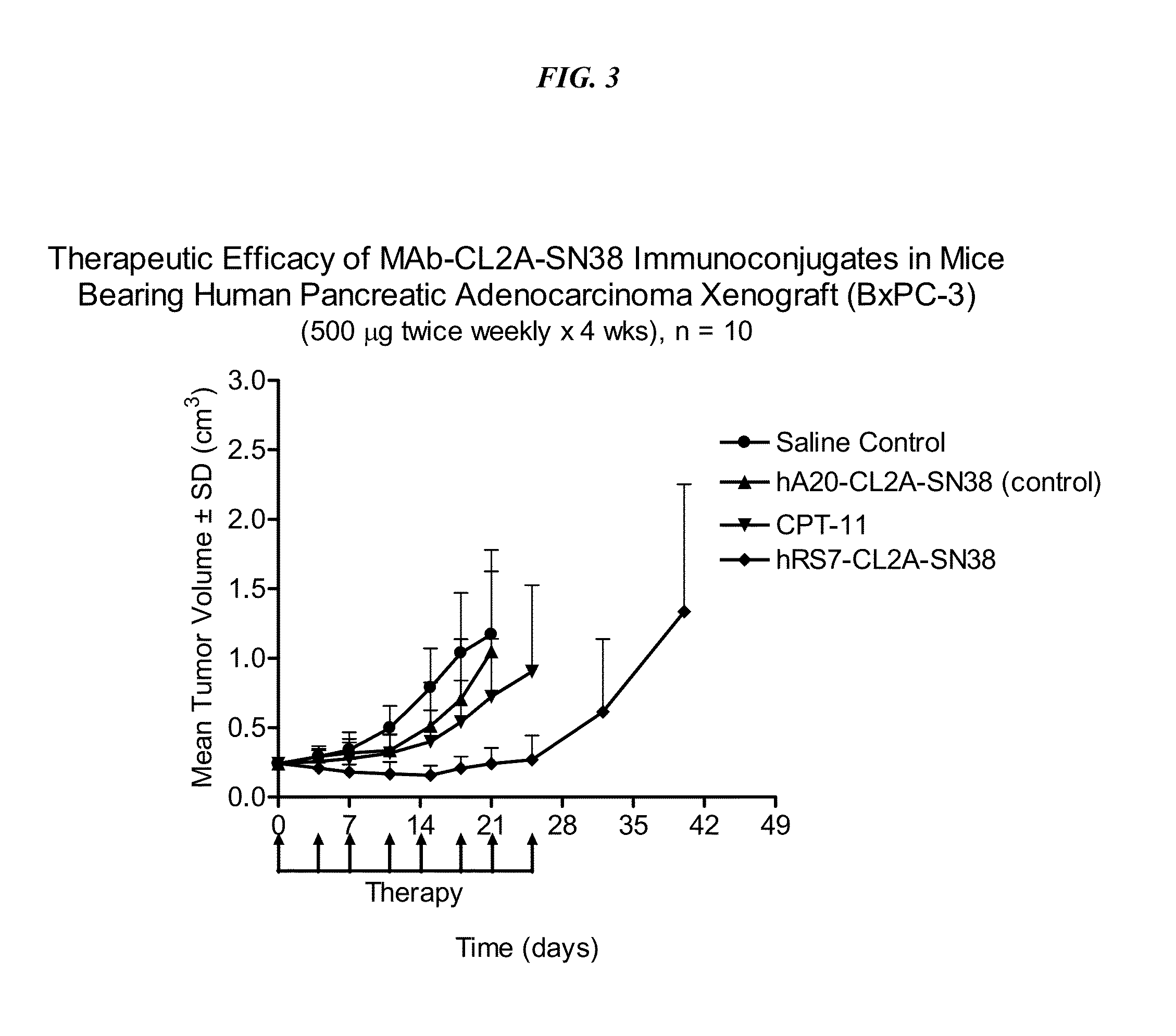
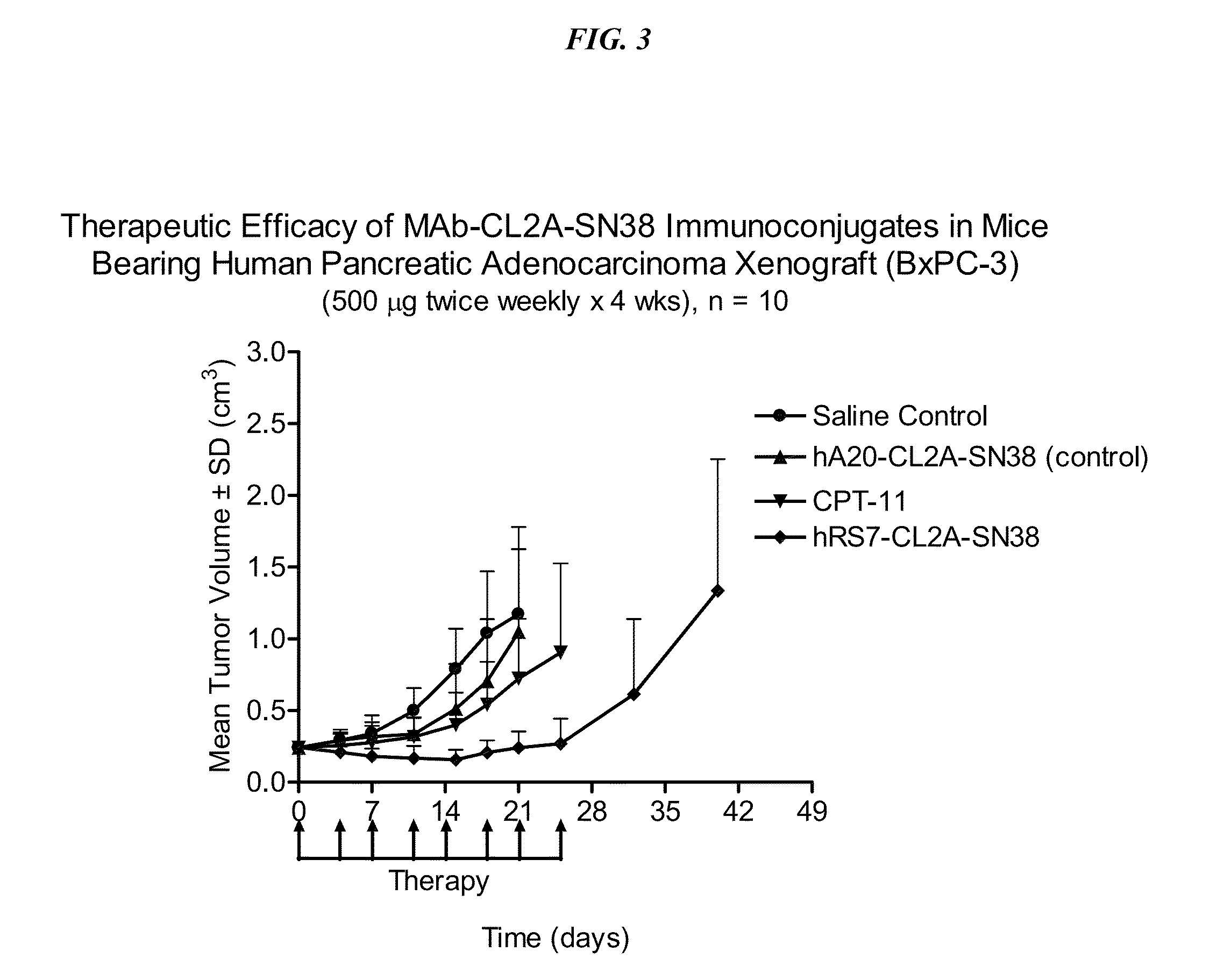
Therapy of small-cell lung cancer (SCLC) with a topoisomerase-i inhibiting antibody-drug conjugate (ADC) targeting trop-2
ActiveUS20180185351A1Good effectLow toxicityOrganic active ingredientsHeavy metal active ingredientsCarboplatinLymphatic Spread
The present invention relates to treatment of SCLC with therapeutic ADCs comprising a drug attached to an anti-Trop-2 antibody or antigen-binding antibody fragment. Preferably, the drug is SN-38. More preferably, the antibody is an hRS7 antibody and the ADC is sacituzumab govitecan. The ADC may be administered at a dosage of between 4 mg / kg and 16 mg / kg, preferably 4, 6, 8, 9, 10, 12, or 16 mg / kg, mostly preferably 8 to 10 mg / kg. When administered at specified dosages and schedules, the ADC can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. Surprisingly, the ADC is effective to treat cancers that are refractory to or relapsed from irinotecan or topotecan. Preferably, the ADC is administered as a combination therapy with one or more other anti-cancer treatments, such as carboplatin or cisplatinum.
Owner:IMMUNOMEDICS INC
Adaptive user guidance for navigation and location-based services
ActiveUS9127955B2Increase and decrease frequencyImprove performanceInstruments for road network navigationRoad vehicles traffic controlDriver/operatorAnalysis data
Owner:GM GLOBAL TECH OPERATIONS LLC
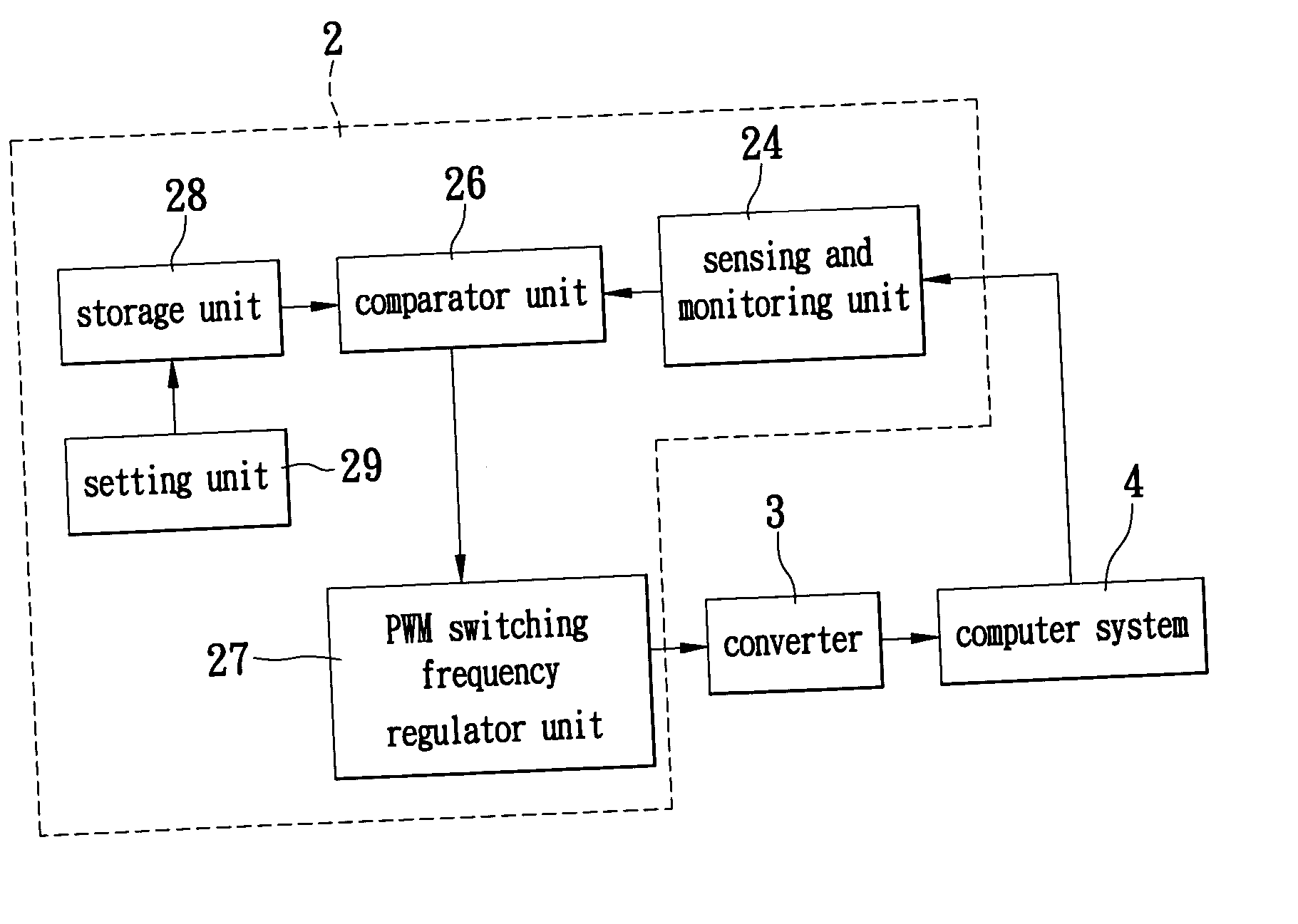
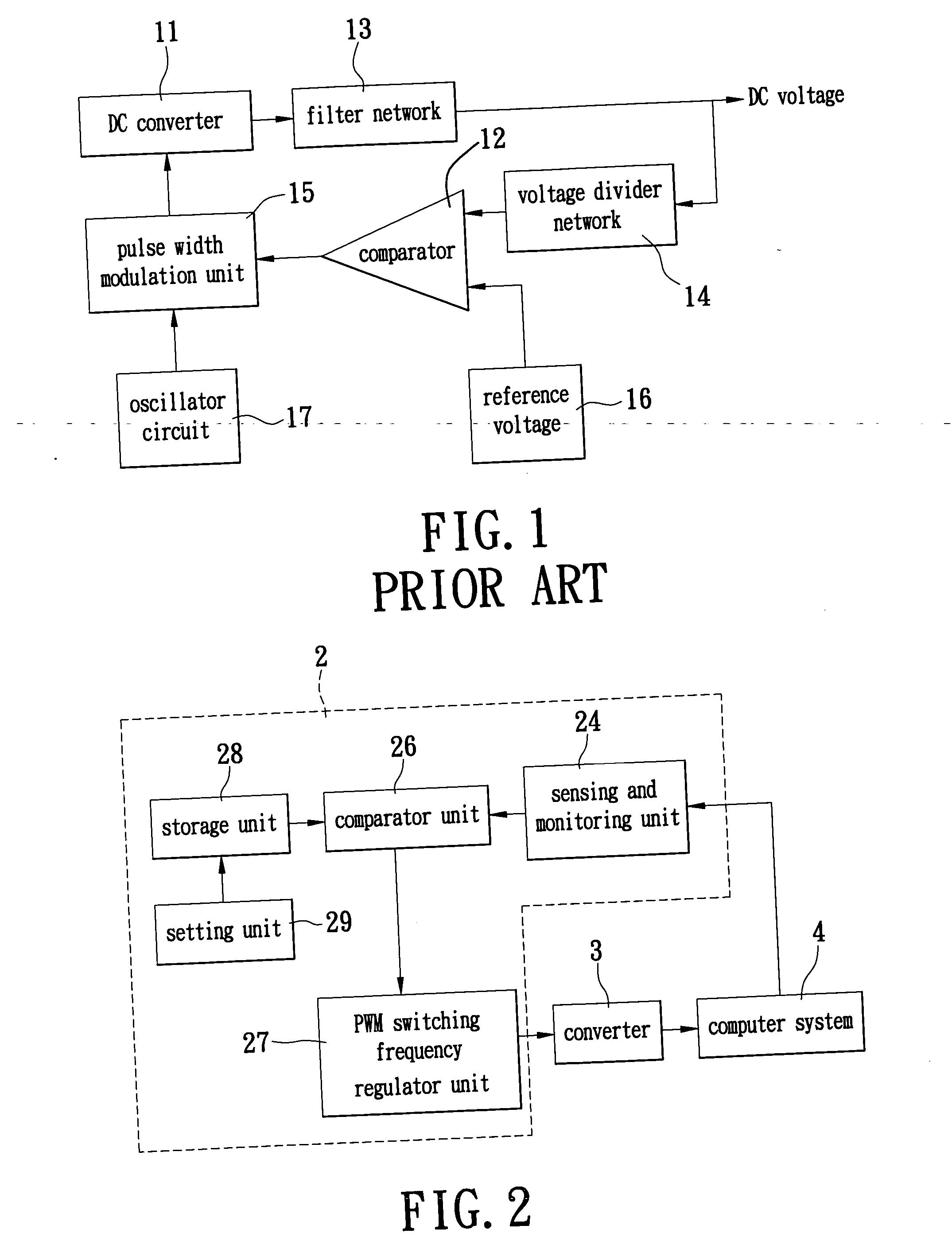
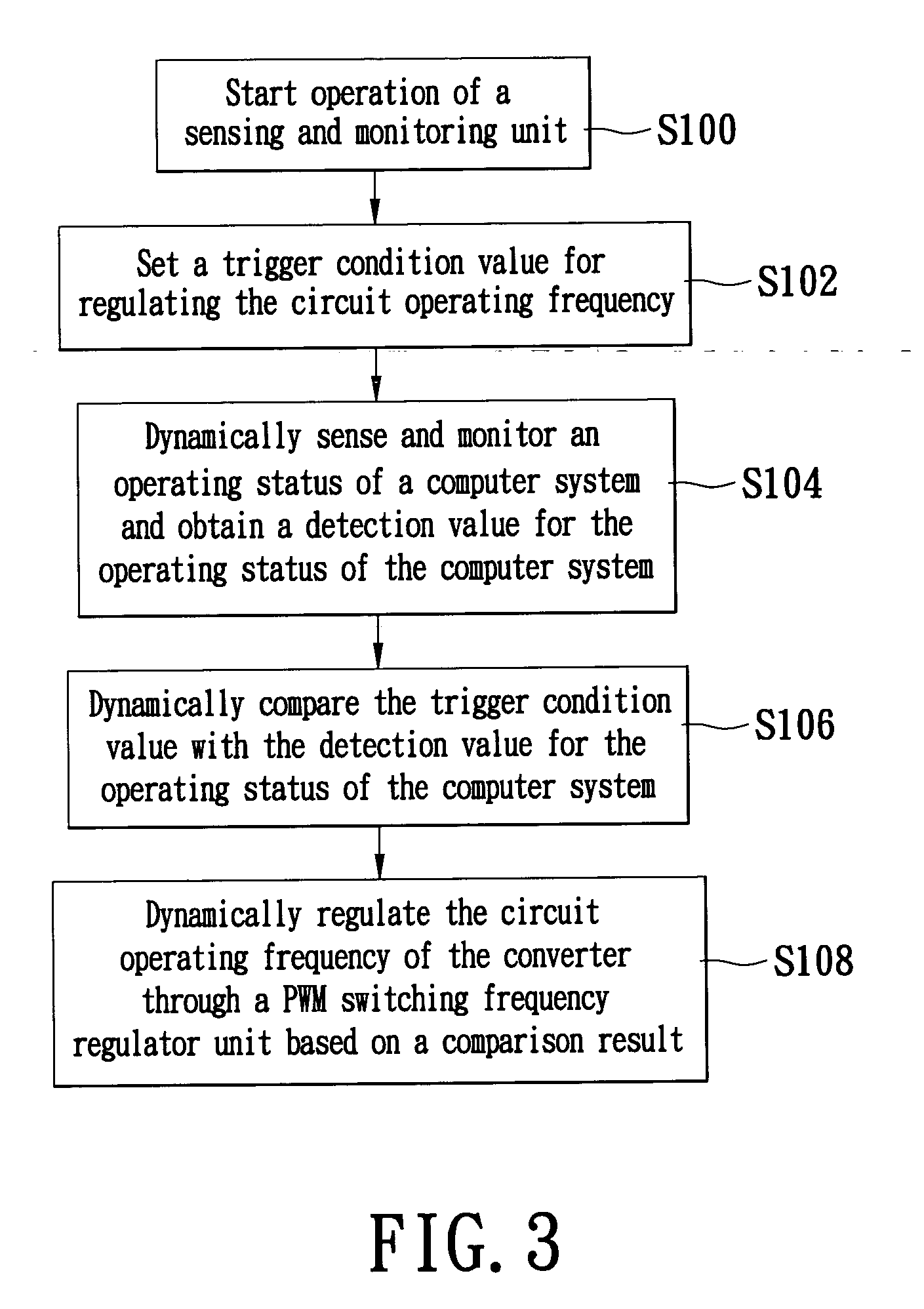
Switching power supply system for automatically regulating circuit operating frequency and method thereof
ActiveUS6992470B1Increase and decrease frequencyEasy to operateEfficient power electronics conversionDc-dc conversionAuto regulationOutput compare
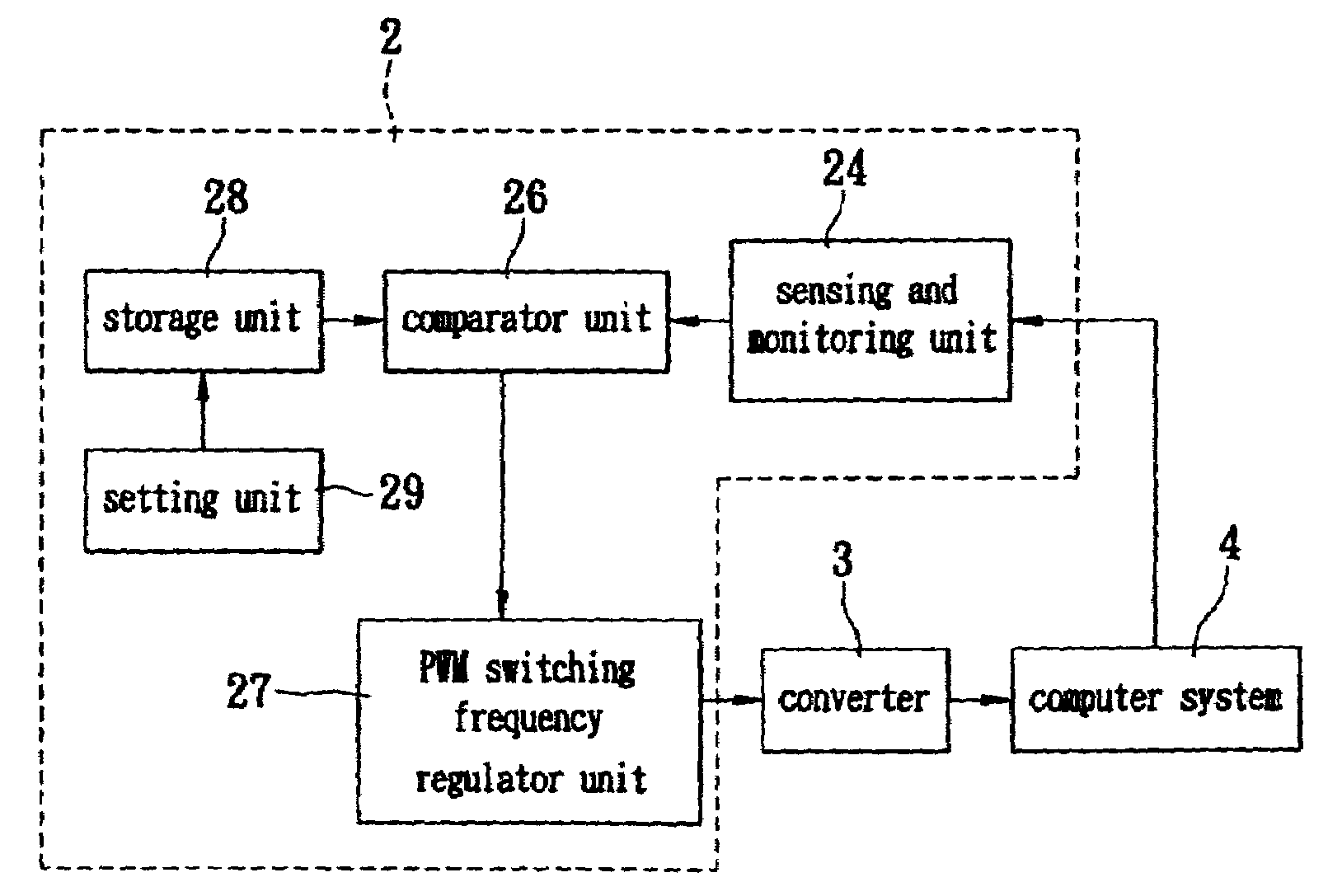
The invention describes a switching power supply system for automatically regulating an operating frequency and the method. The switching power supply system has a sensing and monitoring unit connected to the computer system for detecting the operating status of the computer system and outputting a detection value, a setting unit for setting a trigger condition value, a storage unit connected to the setting unit for storing the trigger condition value, a comparator unit connected to the storage unit and the sensing and monitoring unit for comparing the detection value with the trigger condition value and outputting a comparison result signal, and a PWM switching frequency regulator unit connected to the comparator unit and the converter for receiving the comparison result signal and regulating the operating frequency of the pulse width modulation so that a converter can supply power to the computer system more efficiently.
Owner:GIGA BYTE TECH CO LTD
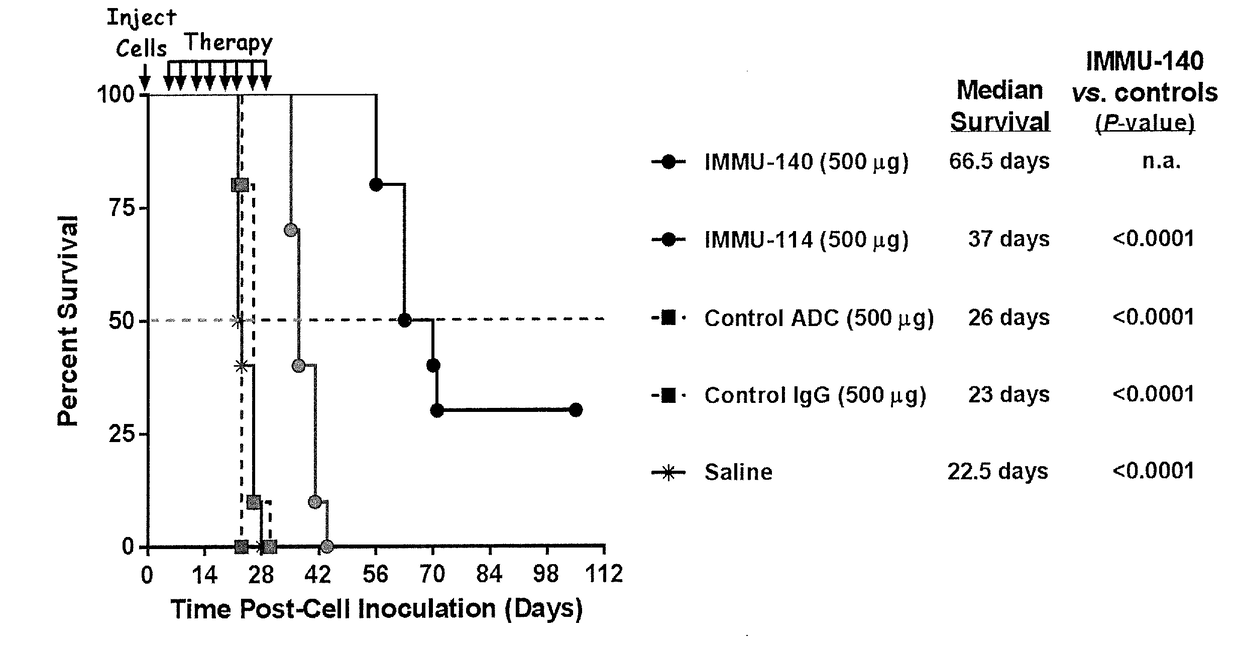
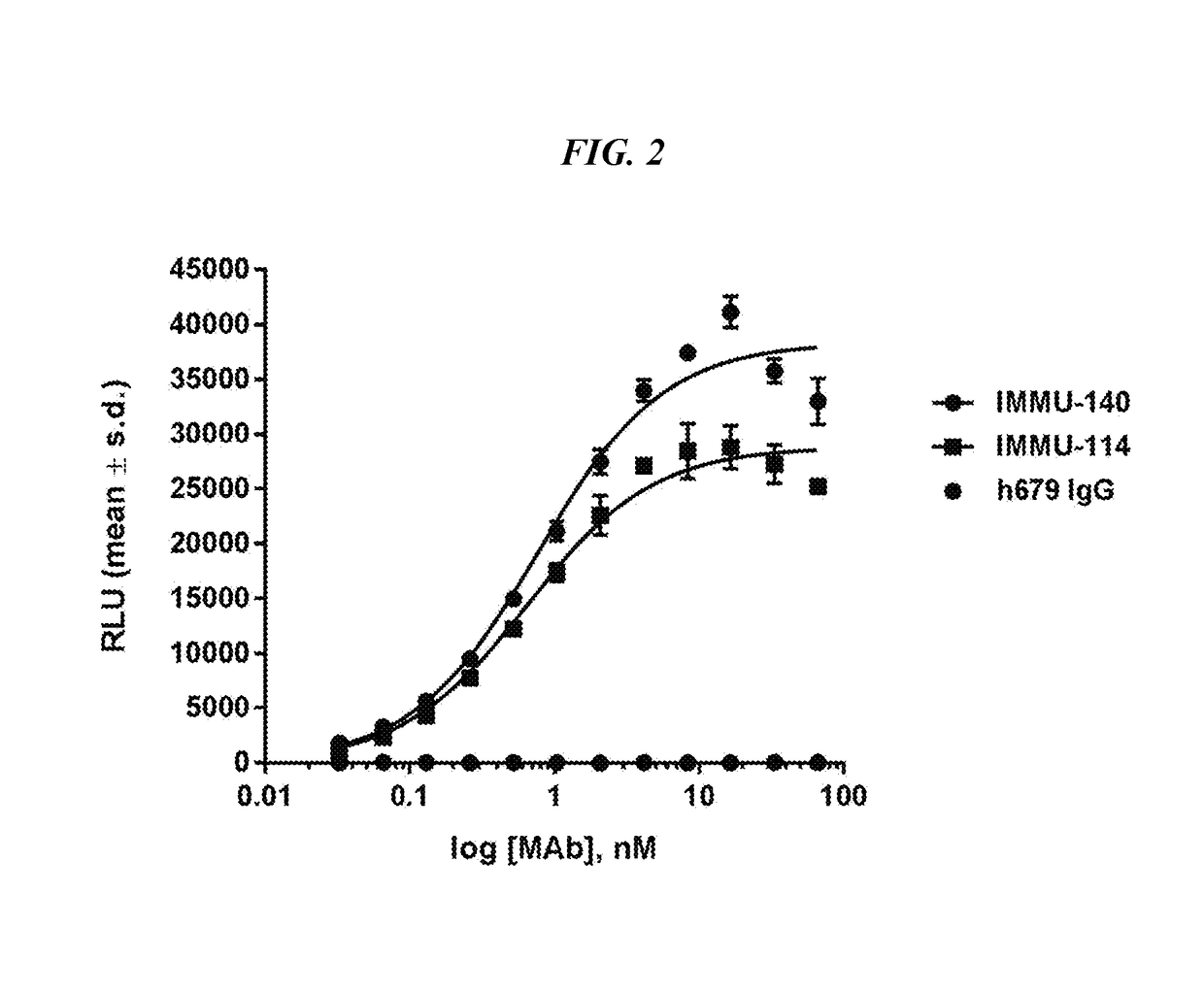
Efficacy of anti-HLA-DR antiboddy drug conjugate IMMU-140 (hL243-CL2A-SN-38) in HLA-DR positive cancers
ActiveUS10206918B2Reducing certain severe side effectsReceive treatment wellOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsLymphatic SpreadAntibody fragments
The present invention relates to therapeutic immunoconjugates comprising SN-38 attached to an anti-HLA-DR antibody or antigen-binding antibody fragment. The immunoconjugate may be administered at a dosage of between 3 mg / kg and 18 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg, more preferably 8, 10 or 12 mg / kg. When administered at specified dosages and schedules, the immunoconjugate can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. The methods and compositions are particularly useful for treating AML, ALL or multiple myeloma.
Owner:IMMUNOMEDICS INC
Efficacy of anti-trop-2-SN-38 antibody drug conjugates for therapy of tumors relapsed/refractory to checkpoint inhibitors
ActiveUS10266605B2Reducing certain severe side effectsReceive treatment wellOrganic active ingredientsDigestive systemAntibody fragmentsRefractory
Owner:IMMUNOMEDICS INC
Antibody-drug conjugates and uses thereof
ActiveUS20170014527A1Increase and decrease frequencyGood effectOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody fragmentsAntigen binding
The present invention relates to therapeutic immunoconjugates comprising SN-38 attached to an antibody or antigen-binding antibody fragment. The antibody may bind to Trop-2 or CEACAM5 and the immunoconjugate may be administered at a dosage of between 4 mg / kg and 16 mg / kg, preferably 4, 6, 8, 9, 10, 12, or 16 mg / kg. When administered at specified dosages and schedules, the immunoconjugate can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. Surprisingly, the immunoconjugate is effective to treat cancers that are refractory to or relapsed from irinotecan.
Owner:IMMUNOMEDICS INC
Switching power supply system for automatically regulating circuit operating frequency and method thereof
ActiveUS20060012359A1Efficient supplyEfficient power supplyEfficient power electronics conversionDc-dc conversionAuto regulationOutput compare
The invention describes a switching power supply system for automatically regulating an operating frequency and the method. The switching power supply system has a sensing and monitoring unit connected to the computer system for detecting the operating status of the computer system and outputting a detection value, a setting unit for setting a trigger condition value, a storage unit connected to the setting unit for storing the trigger condition value, a comparator unit connected to the storage unit and the sensing and monitoring unit for comparing the detection value with the trigger condition value and outputting a comparison result signal, and a PWM switching frequency regulator unit connected to the comparator unit and the converter for receiving the comparison result signal and regulating the operating frequency of the pulse width modulation so that a converter can supply power to the computer system more efficiently.
Owner:GIGA BYTE TECH CO LTD
Antibody-sn-38 immunoconjugates with a cl2a linker
InactiveUS20150306243A1Improve drug bioavailabilityGood treatment effectOrganic active ingredientsPeptide/protein ingredientsDiseaseSide effect
The present invention concerns improved methods and compositions for preparing SN-38 conjugates of proteins or peptides, preferably immunoconjugates of antibodies or antigen-binding antibody fragments. More preferably, the SN-38 is attached to the antibody or antibody fragment using a CL2A linker, with 1-12, more preferably 6 or less, most preferably 1-5 SN-38 moieties per antibody or antibody fragment. Most preferably, the immunoconjugate is prepared in large scale batches, with various modifications to the reaction scheme to optimize yield and recovery in large scale. Other embodiments concern optimized dosages and / or schedules of administration of immunoconjugate to maximize efficacy for disease treatment and minimize side effects of administration.
Owner:IMMUNOMEDICS INC
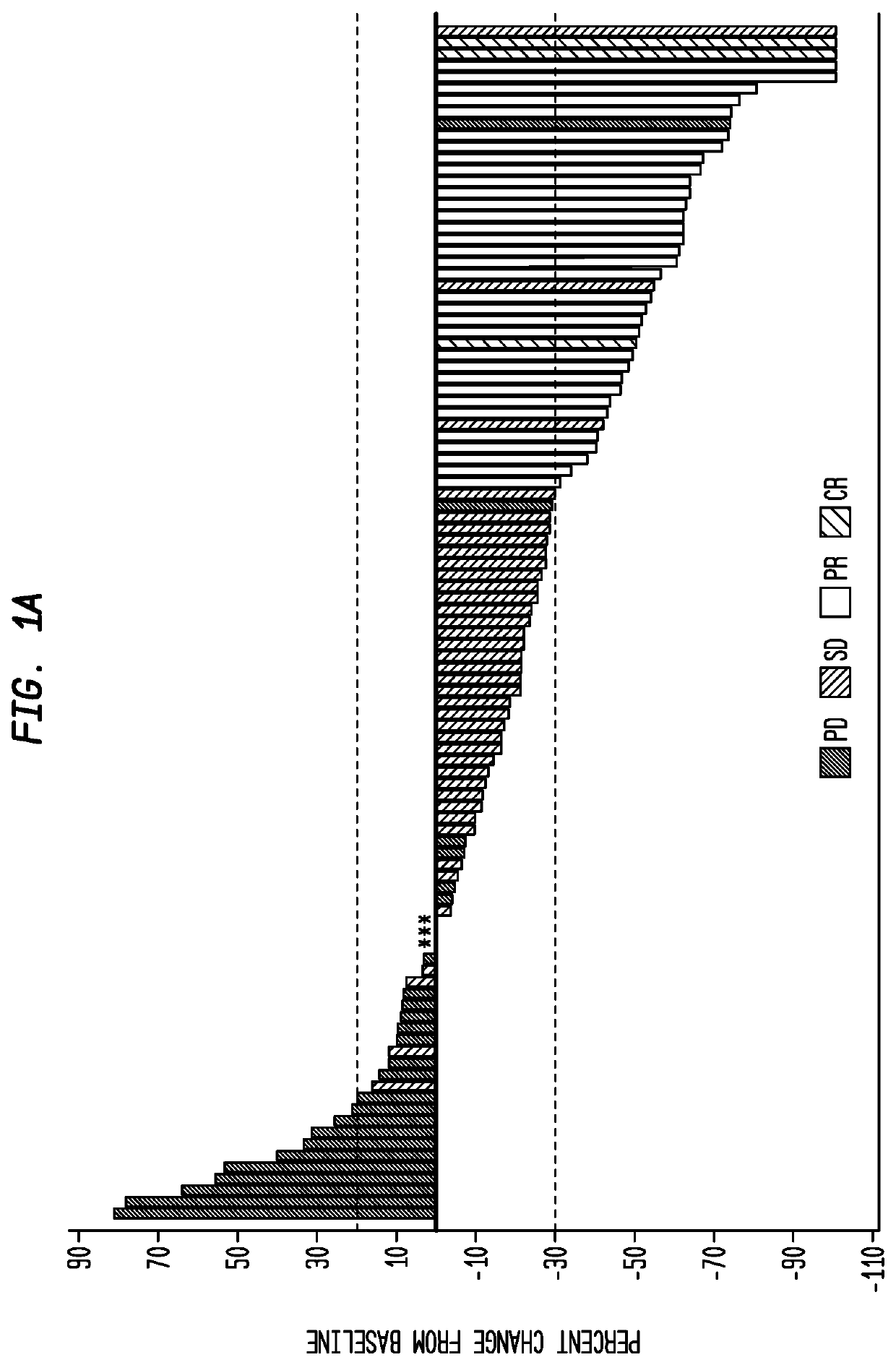
Therapy for metastatic urothelial cancer with the antibody-drug conjugate, sacituzumab govitecan (IMMU-132)
ActiveUS10413539B2Reducing certain severe side effectsReceive treatment wellImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsLymphatic SpreadAntibody fragments
The present invention relates to therapeutic ADCs comprising SN-38 attached to an anti-Trop-2 antibody or antigen-binding antibody fragment. The ADC may be administered at a dosage of between 4 mg / kg and 18 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg, most preferably 8 to 10 mg / kg. When administered at specified dosages and schedules, the ADC can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. Preferably, the ADC is administered in combination with one or more other therapeutic agents, such as a PARP inhibitor, a microtubule inhibitor, a Bruton kinase inhibitor or a PI3K inhibitor. Most preferably, the ADC is of use for treating a Trop-2 expressing cancer, such as metastatic urothelial cancer.
Owner:IMMUNOMEDICS INC
Subcutaneous administration of antibody-drug conjugates for cancer therapy
ActiveUS10799597B2Increase and decrease frequencyGood effectInorganic non-active ingredientsPharmaceutical delivery mechanismCD20Antiendomysial antibodies
The present invention relates to methods of cancer therapy using subcutaneous administration of antibody-drug conjugates (ADCs). Preferably, the ADC comprises an antibody that binds to Trop-2, CEACAM5, CEACAM6, CD20, CD22, CD30, CD46, CD74, Her-2, folate receptor, or HLA-DR. More preferably, the drug is SN-38. Subcutaneous administration is at least as effective as intravenous administration of the same ADC. Surprisingly, subcutaneous administration can be used without inducing unmanageable adverse local toxicity at the injection site. Subcutaneous administration is advantageous in requiring less frequent administration, substantially reducing the amount of time required for intravenous administration, and reducing the levels of systemic toxicities observed with intravenous administration. When administered at specified dosages and schedules, the ADCs can reduce solid tumors in size, reduce or eliminate metastases and are effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy.
Owner:IMMUNOMEDICS INC
Therapy of small-cell lung cancer (SCLC) with a topoisomerase-I inhibiting antibody-drug conjugate (ADC) targeting Trop-2
ActiveUS10744129B2Increase and decrease frequencyGood effectOrganic active ingredientsHeavy metal active ingredientsCarboplatinRecurrent Cancer
The present invention relates to treatment of SCLC with therapeutic ADCs comprising a drug attached to an anti-Trop-2 antibody or antigen-binding antibody fragment. Preferably, the drug is SN-38. More preferably, the antibody is an hRS7 antibody and the ADC is sacituzumab govitecan. The ADC may be administered at a dosage of between 4 mg / kg and 16 mg / kg, preferably 4, 6, 8, 9, 10, 12, or 16 mg / kg, mostly preferably 8 to 10 mg / kg. When administered at specified dosages and schedules, the ADC can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. Surprisingly, the ADC is effective to treat cancers that are refractory to or relapsed from irinotecan or topotecan. Preferably, the ADC is administered as a combination therapy with one or more other anti-cancer treatments, such as carboplatin or cisplatinum.
Owner:IMMUNOMEDICS INC
EFFICACY OF ANTI-HLA-DR ANTIBODY DRUG CONJUGATE IMMU-140 (hL243-CL2A-SN-38) IN HLA-DR POSITIVE CANCERS
ActiveUS20170360770A1Overcome tumorImprove targetingOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsLymphatic SpreadAntibody fragments
The present invention relates to therapeutic immunoconjugates comprising SN-38 attached to an anti-HLA-DR antibody or antigen-binding antibody fragment. The immunoconjugate may be administered at a dosage of between 3 mg / kg and 18 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg, more preferably 8, 10 or 12 mg / kg. When administered at specified dosages and schedules, the immunoconjugate can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. The methods and compositions are particularly useful for treating AML, ALL or multiple myeloma.
Owner:IMMUNOMEDICS INC
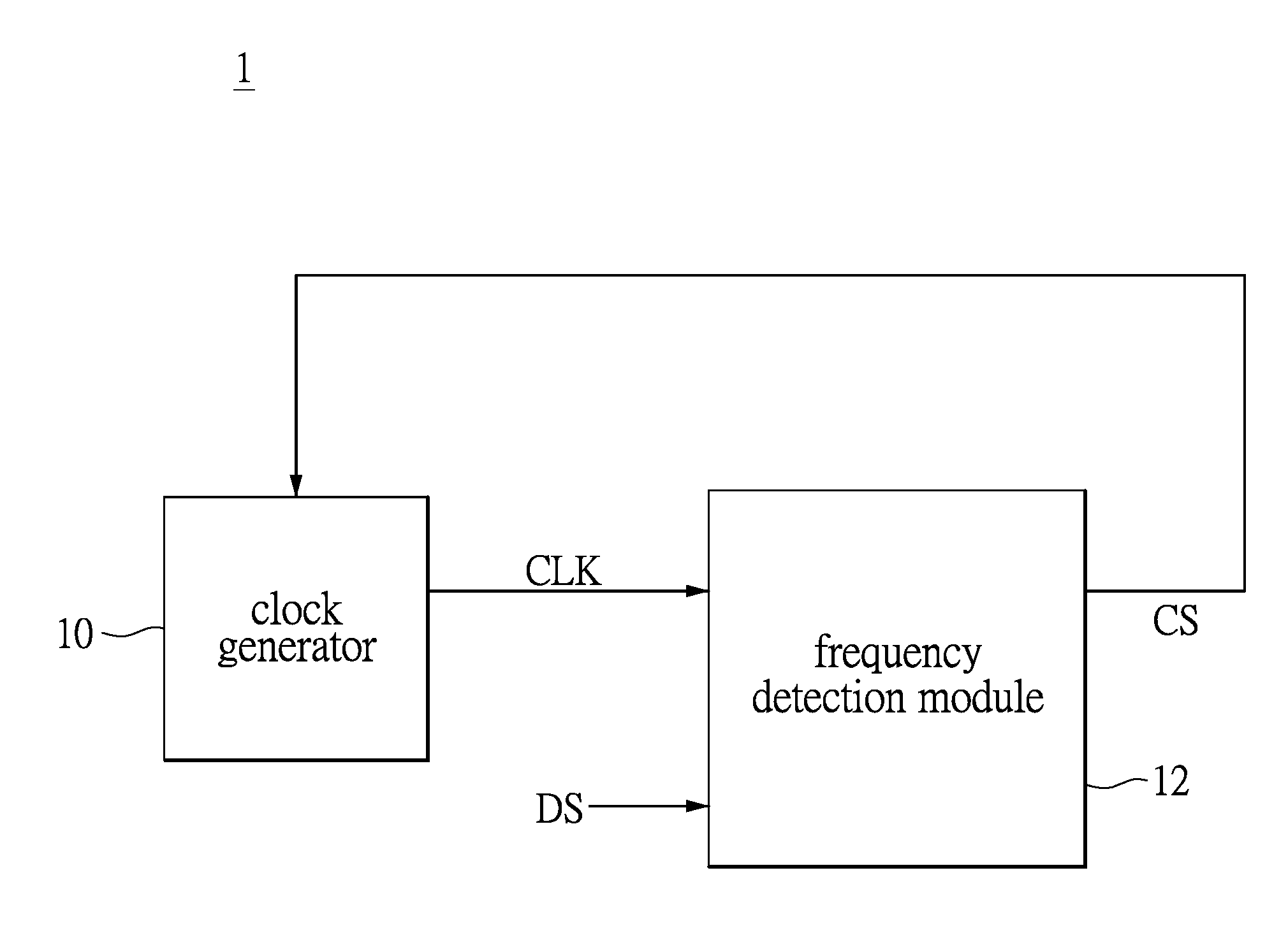
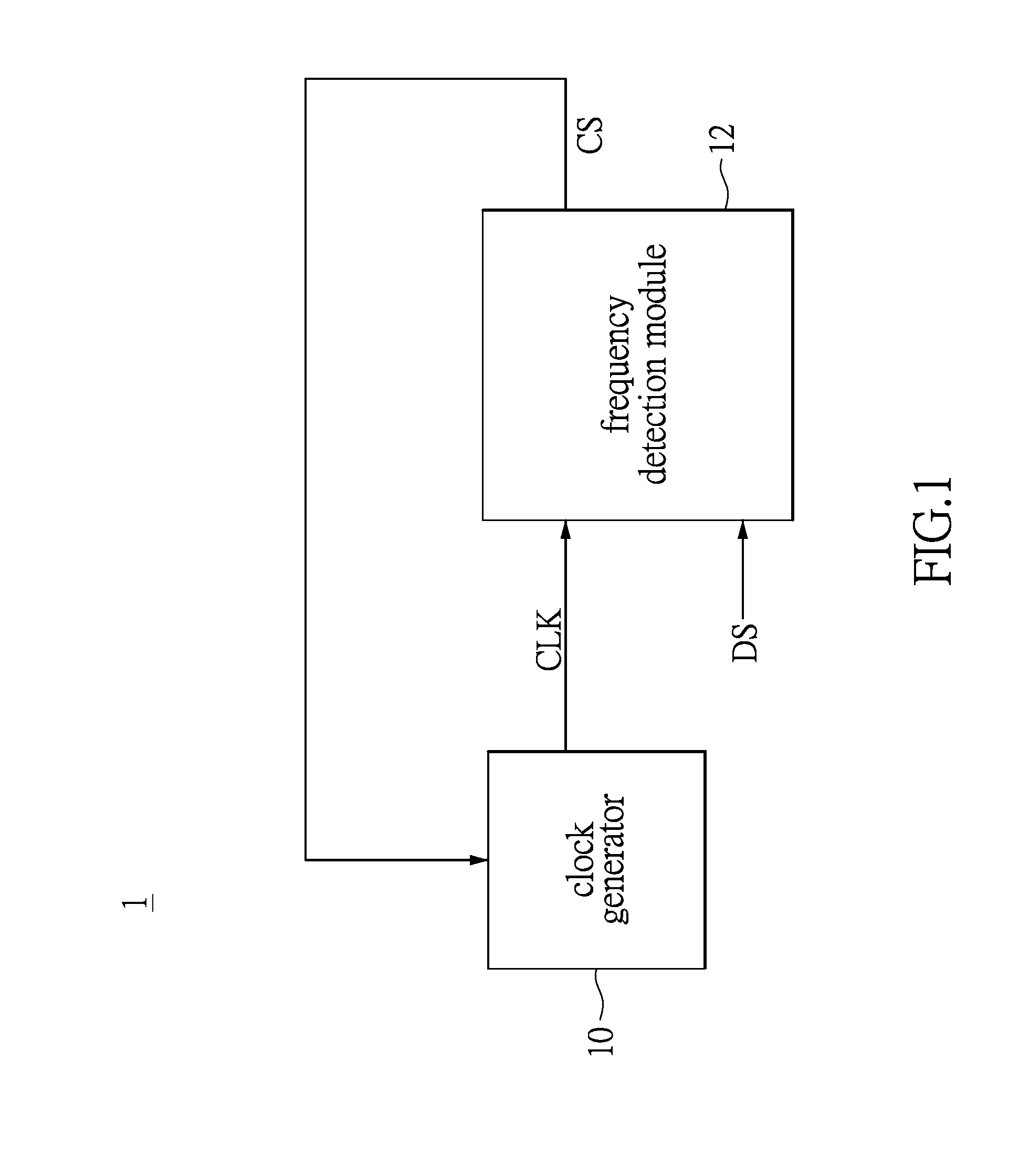
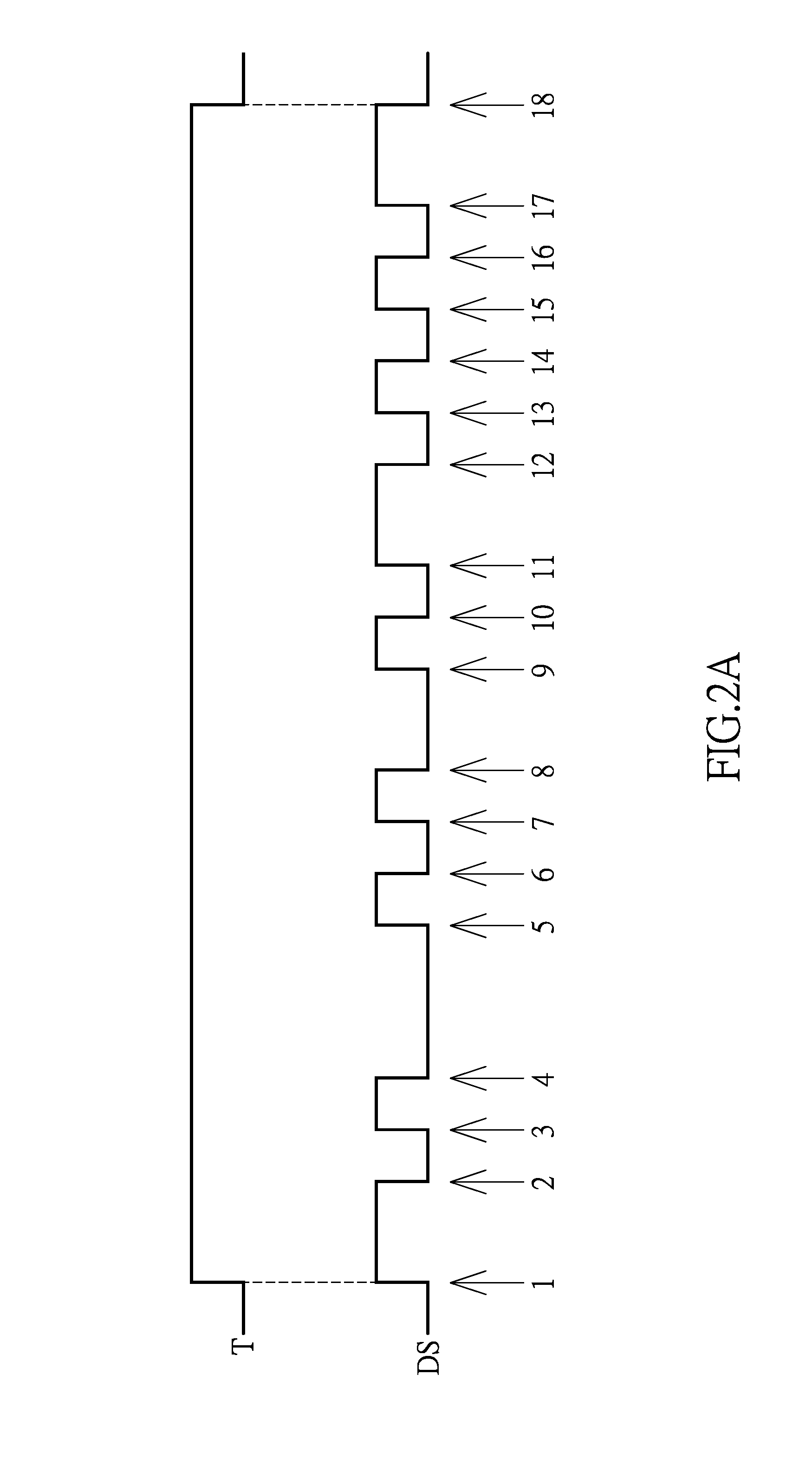
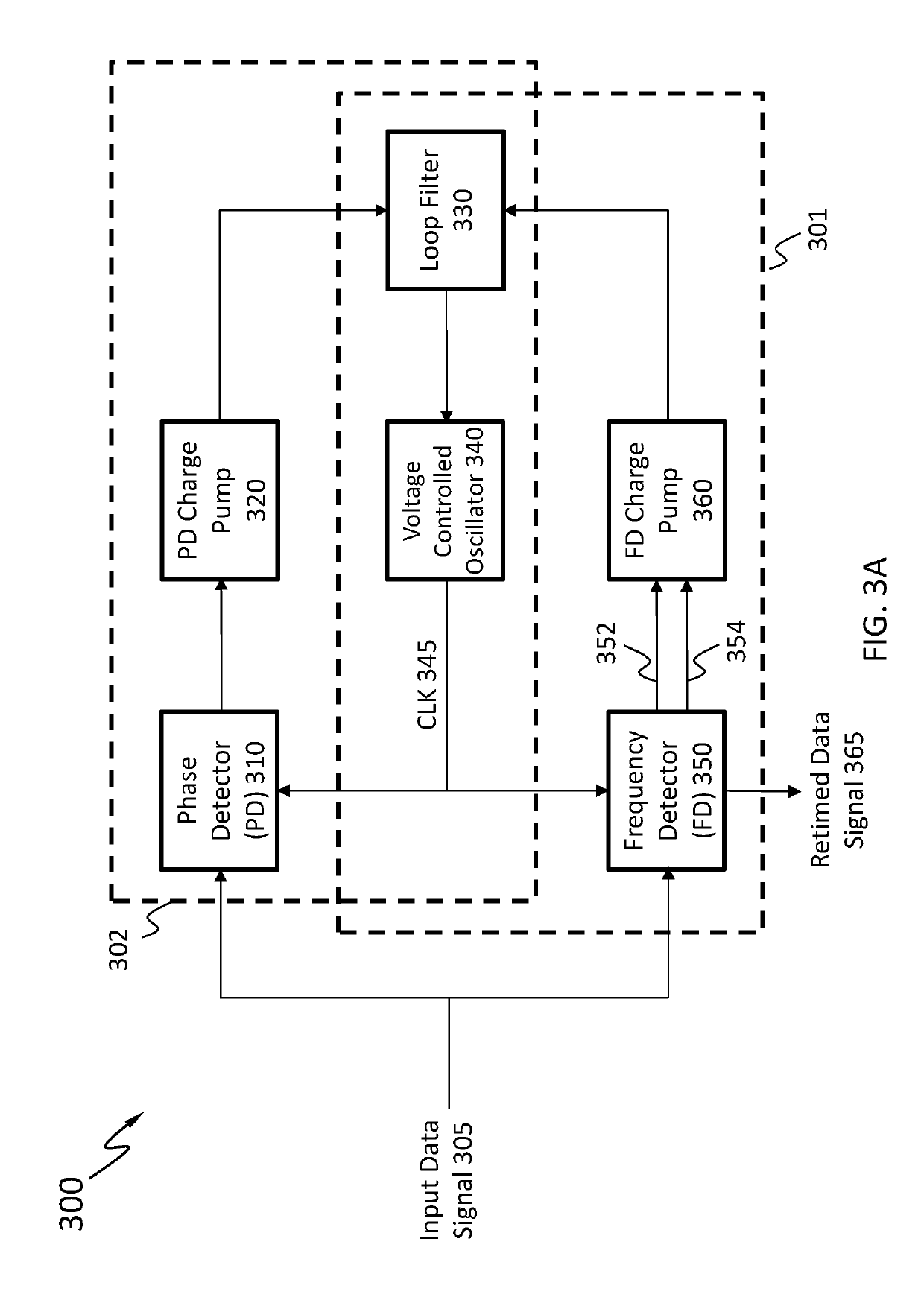
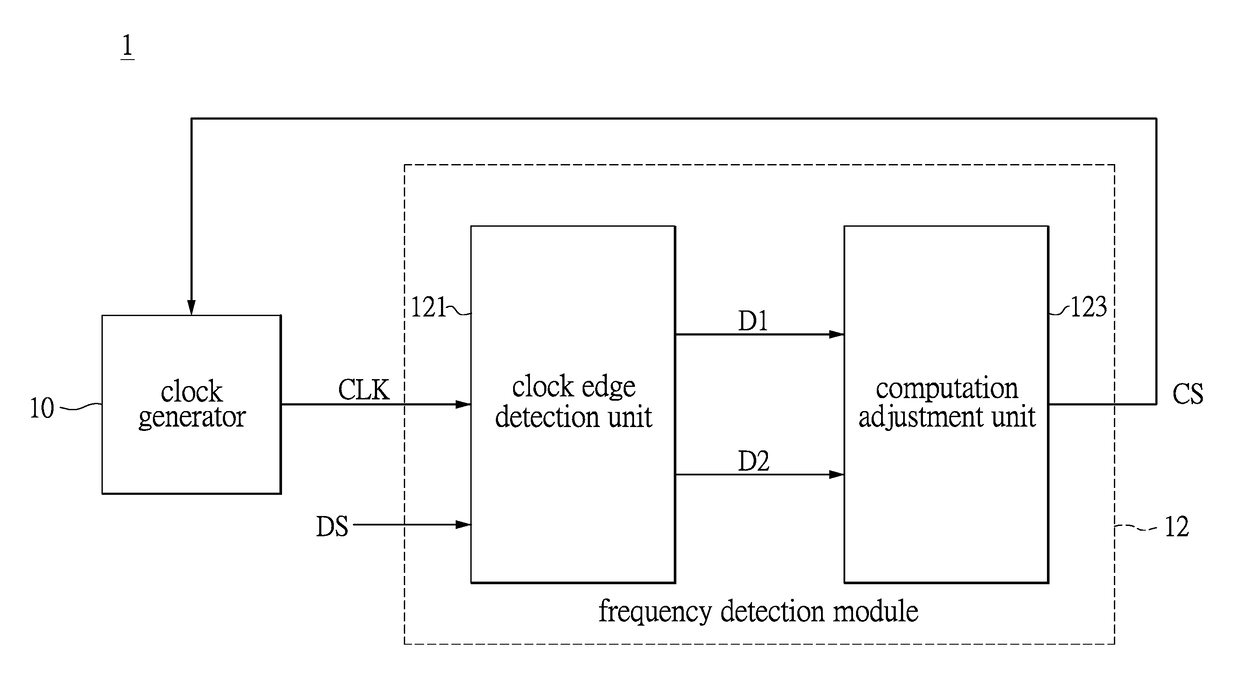
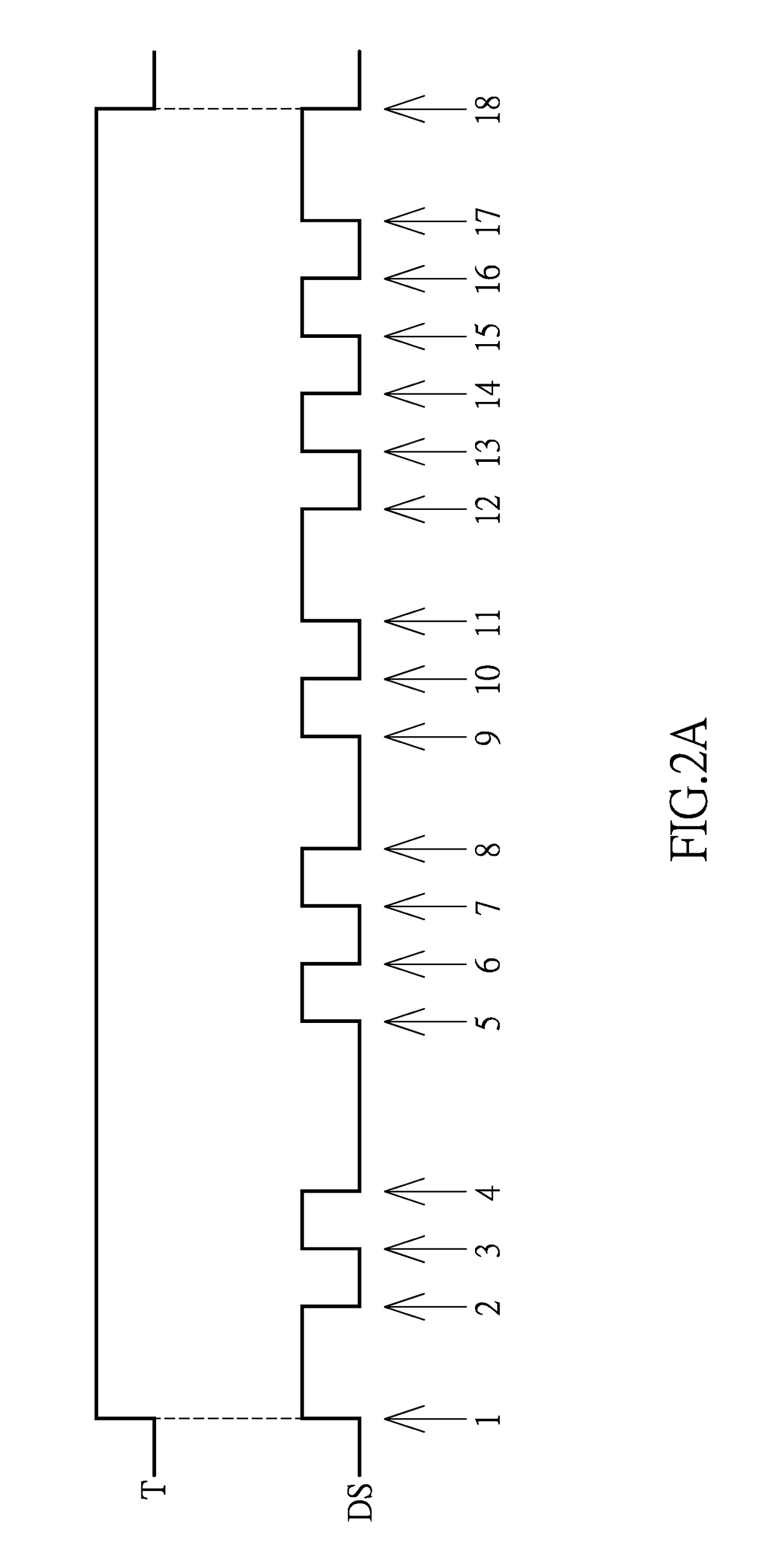
Clock and data recovery circuit and frequency detection method thereof
ActiveUS20160211964A1Increase and decrease frequencyAccurate detectionAngle demodulation by phase difference detectionSynchronising arrangementClock generatorEngineering
The present disclosure provides a crystal-less clock and data recovery (CDR) circuit and a frequency detection method thereof. The CDR circuit includes a clock generator and a frequency detection module. The clock generator is operable to generate a clock signal. The frequency detection module coupled to the clock generator is configured for outputting a control signal to the clock generator to increase or decrease the frequency of the clock signal according to a data signal received and a transition density.
Owner:REALTEK SEMICON CORP
Combination of ABCG2 inhibitors with sacituzumab govitecan (IMMU-132) overcomes resistance to SN-38 in Trop-2 expressing cancers
ActiveUS10954305B2Reducing certain severe side effectsReceive treatment wellOrganic active ingredientsRadioactive preparation carriersAntiendomysial antibodiesAntigen binding
The present invention relates to therapeutic ADCs comprising a drug attached to an anti-cancer antibody or antigen-binding antibody fragment. Preferably the drug is SN-38. More preferably the antibody or fragment thereof binds to Trop-2 and the therapy is used to treat a Trop-2 positive cancer. Most preferably the antibody is hRS7. The ADC is administered to a subject with a cancer in combination with an ABCG2 inhibitor. The combination therapy is effective to treat cancers that are resistant to drug alone and / or to ADC alone.
Owner:IMMUNOMEDICS INC
Treatment of high Trop-2 expressing triple negative breast cancer (TNBC) with sacituzumab govitecan (IMMU-132) overcomes homologous recombination repair (HRR) rescue mediated by Rad51
ActiveUS10918734B2Increase and decrease frequencyGood effectOrganic active ingredientsHeavy metal active ingredientsRAD51Recurrent Cancer
The present invention relates to treatment of Trop-2 positive cancers with the combination of anti-Trop-2 ADC and a Rad51 inhibitor. Preferably the drug conjugated to the antibody is SN-38, and the ADC is sacituzumab govitecan. The ADC may be administered at a dosage of between 4 mg / kg and 16 mg / kg, preferably 4, 6, 8, 9, 10, 12, or 16 mg / kg. When administered at specified dosages and schedules, the combination of ADC and Rad51 inhibitor can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. Surprisingly, the combination is effective to treat cancers that are refractory to or relapsed from irinotecan or topotecan.
Owner:IMMUNOMEDICS INC
Clock and data recovery circuit
ActiveUS20190245678A1Increase and decrease frequencyExclusive-OR circuitsPulse automatic controlControl signalData signal
An apparatus comprises a plurality of sampling circuits configured to receive a non-Non Return to Zero (non-NRZ) data signal; and a control circuit coupled to the plurality of sampling circuits, wherein the control circuit is configured to provide one or more control signals indicating whether to decrease or increase a frequency of a clock signal associated with the non-NRZ data signal based on the non-NRZ data signal.
Owner:PHOTONIC TECH SHANGHAI CO LTD
Clock and data recovery circuit and frequency detection method thereof
ActiveUS9686105B2Increase and decrease frequencyAccurate detectionAngle demodulation by phase difference detectionSynchronising arrangementTransition densityControl signal
The present disclosure provides a crystal-less clock and data recovery (CDR) circuit and a frequency detection method thereof. The CDR circuit includes a clock generator and a frequency detection module. The clock generator is operable to generate a clock signal. The frequency detection module coupled to the clock generator is configured for outputting a control signal to the clock generator to increase or decrease the frequency of the clock signal according to a data signal received and a transition density.
Owner:REALTEK SEMICON CORP
Combining anti-HLA-DR or anti-Trop-2 antibodies with microtubule inhibitors, PARP inhibitors, Bruton kinase inhibitors or phosphoinositide 3-kinase inhibitors significantly improves therapeutic outcome in cancer
ActiveUS11253606B2Reducing certain severe side effectsReceive treatment wellHeavy metal active ingredientsOrganic active ingredientsAntiendomysial antibodiesRadical radiotherapy
The present invention relates to combination therapy with drugs, such as microtubule inhibitors, PARP inhibitors, Bruton kinase inhibitors or PI3K inhibitors, with antibodies or immunoconjugates against HLA-DR or Trop-2. Where immunoconjugates are used, they preferably incorporate SN-38 or pro-2PDOX. The immunoconjugate may be administered at a dosage of between 1 mg / kg and 18 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg, more preferably 8 or 10 mg / kg. The combination therapy can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. Preferably, the combination therapy has an additive effect on inhibiting tumor growth. Most preferably, the combination therapy has a synergistic effect on inhibiting tumor growth.
Owner:IMMUNOMEDICS INC
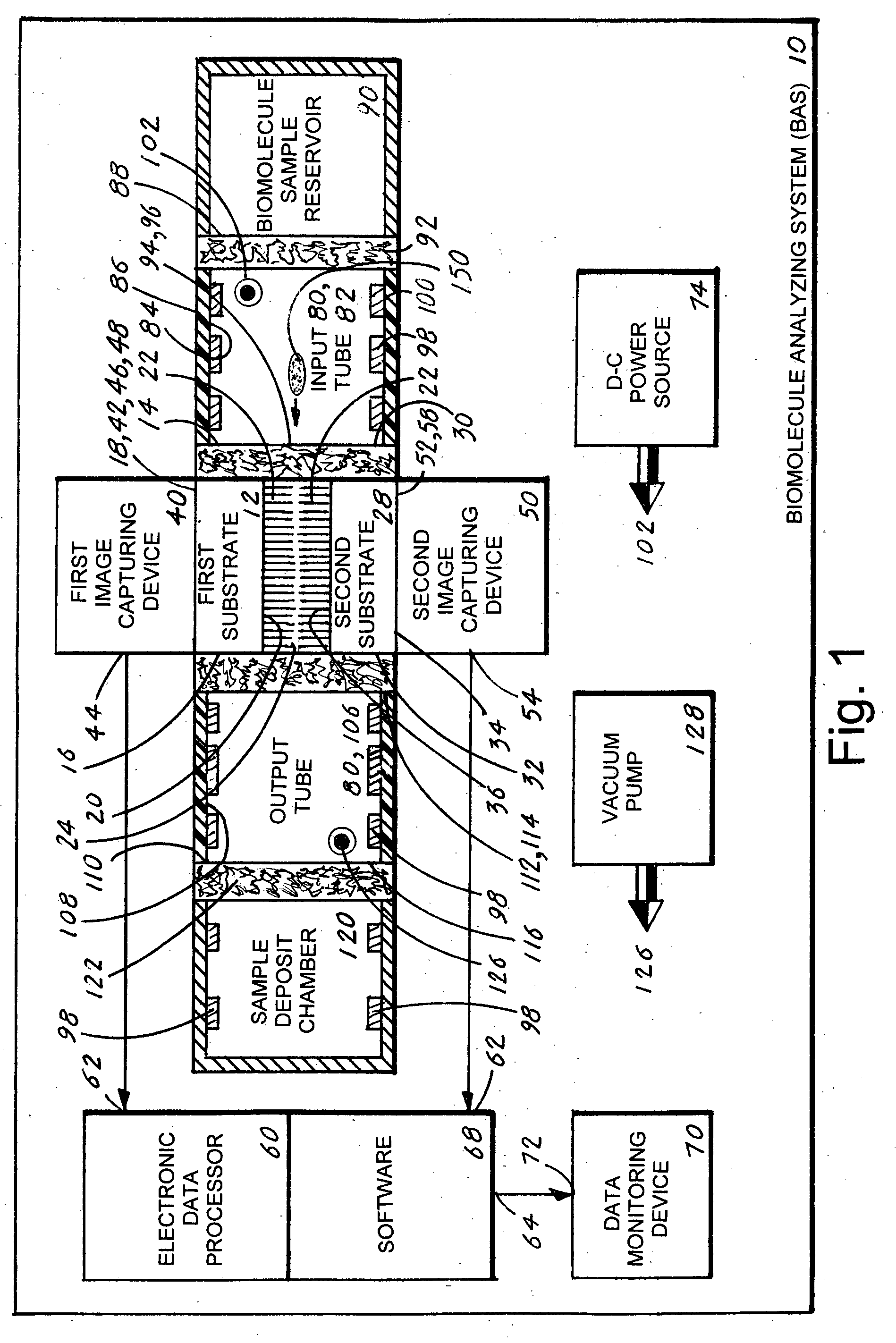
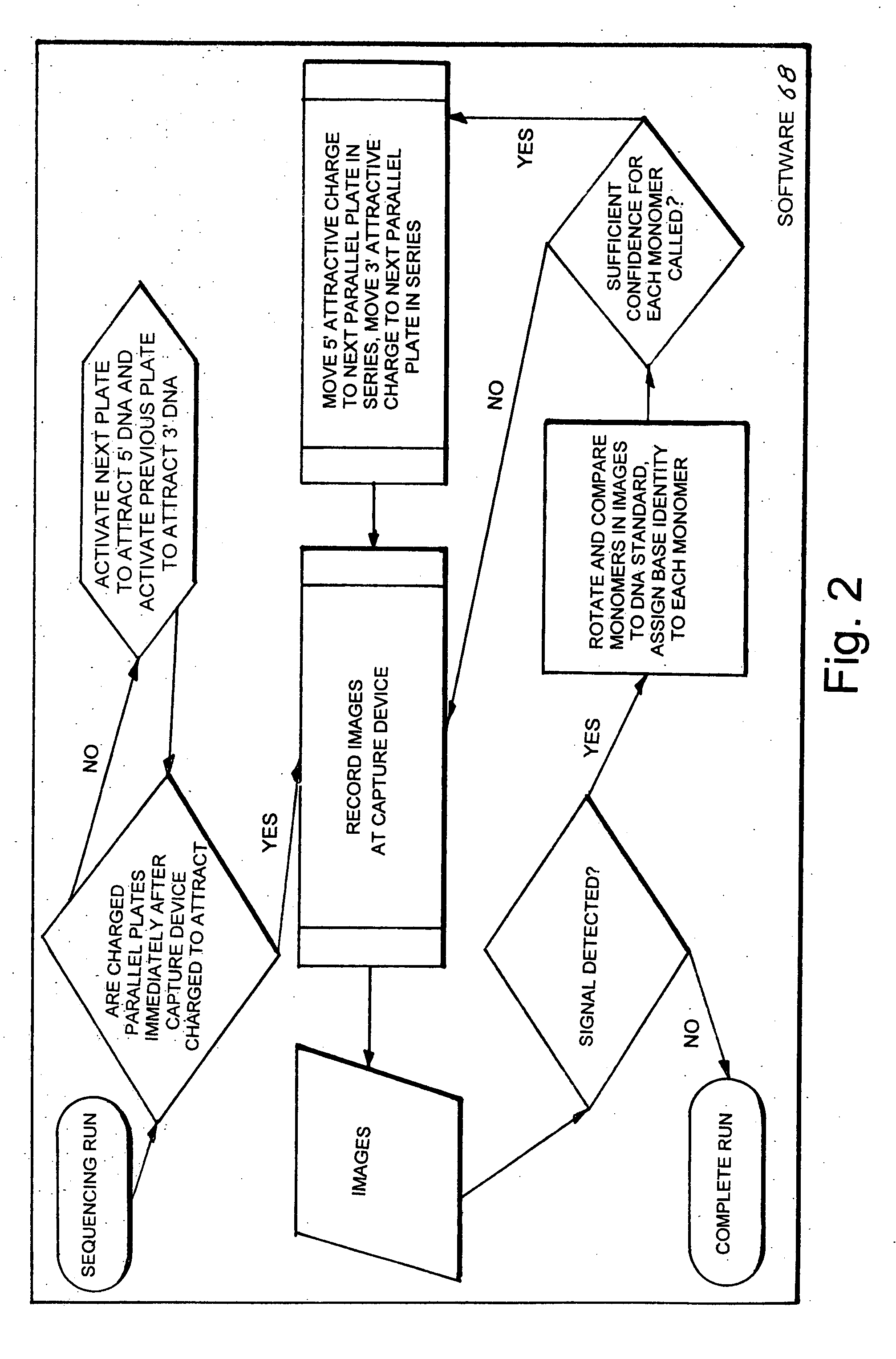
Biomolecule analyzing system
InactiveUS20090111174A1Increase and decrease frequencyRapid rateBioreactor/fermenter combinationsNanotechEngineeringElectronic data
A biomolecule analyzing system (10) that provides an expeditious, accurate and reliable method for analyzing a biomolecule (150). The system (10) includes two substrates (12,28) each having an inner edge (14,30), an outer edge (16,32) and an inner surfaces (20,36) from where extends a multiplicity of cilia (22). To the inner edges (14,30) is attached an input tube (82) that is also attached to a biomolecule sample reservoir (90). To the outer edges (16,32) is attached an output tube (106) that is also attached to a sample deposit chamber (120). The tubes (82,106) include a plurality of conductive plates (98) that are applied an electrical charge that causes the biomolecule (150) to traverse through the tubes (82,106). When the biomolecule (150) passes through the cilia (22) signals are produced that are applied to a pair of image capturing devices (40,50). Each device (40,50) produces a signal that is applied to an electronic data processor from where a three-dimensional image of the biomolecule (150) is produced and viewed on a data monitoring device (70).
Owner:PARKER JOHN A +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com