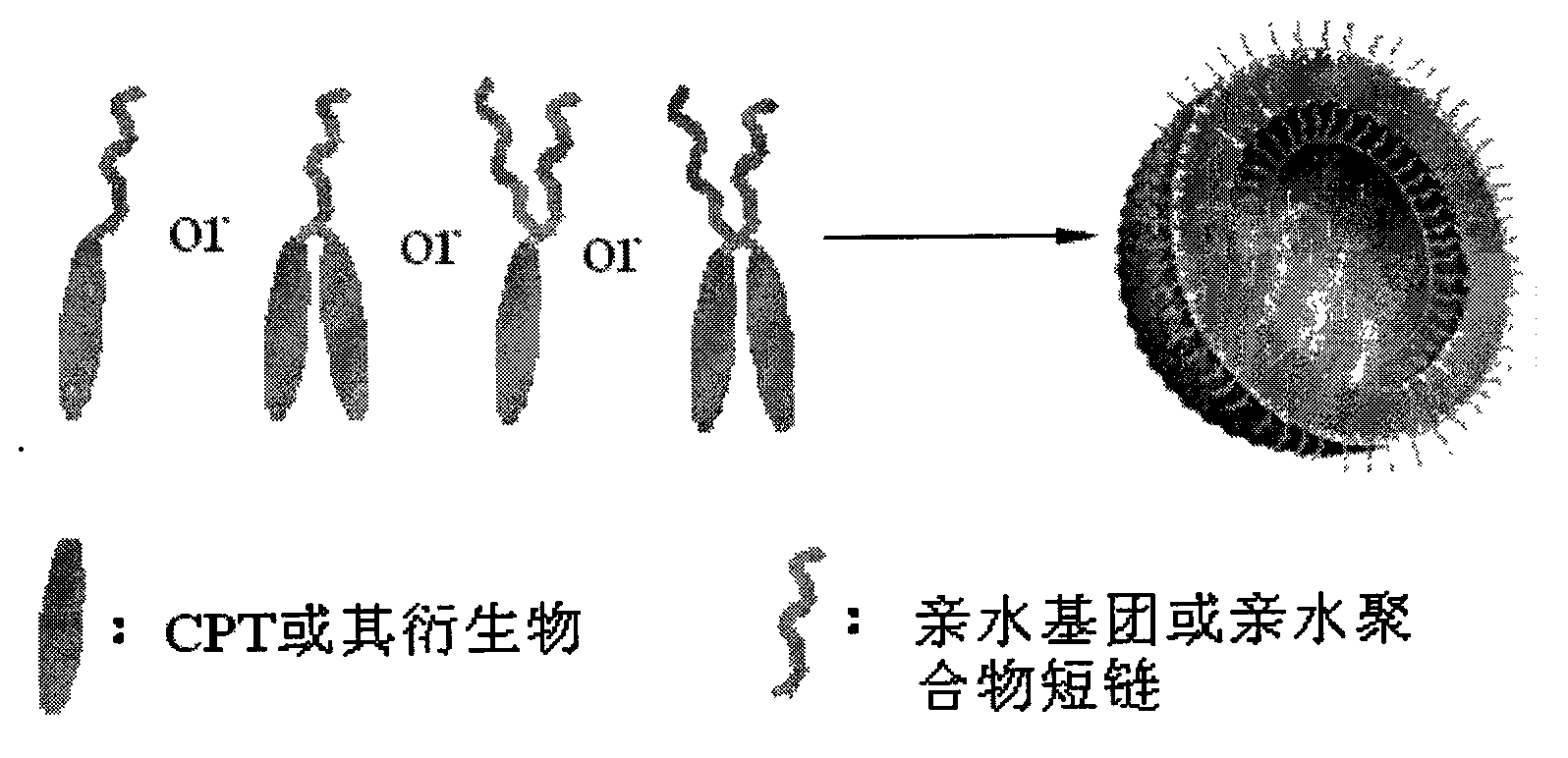
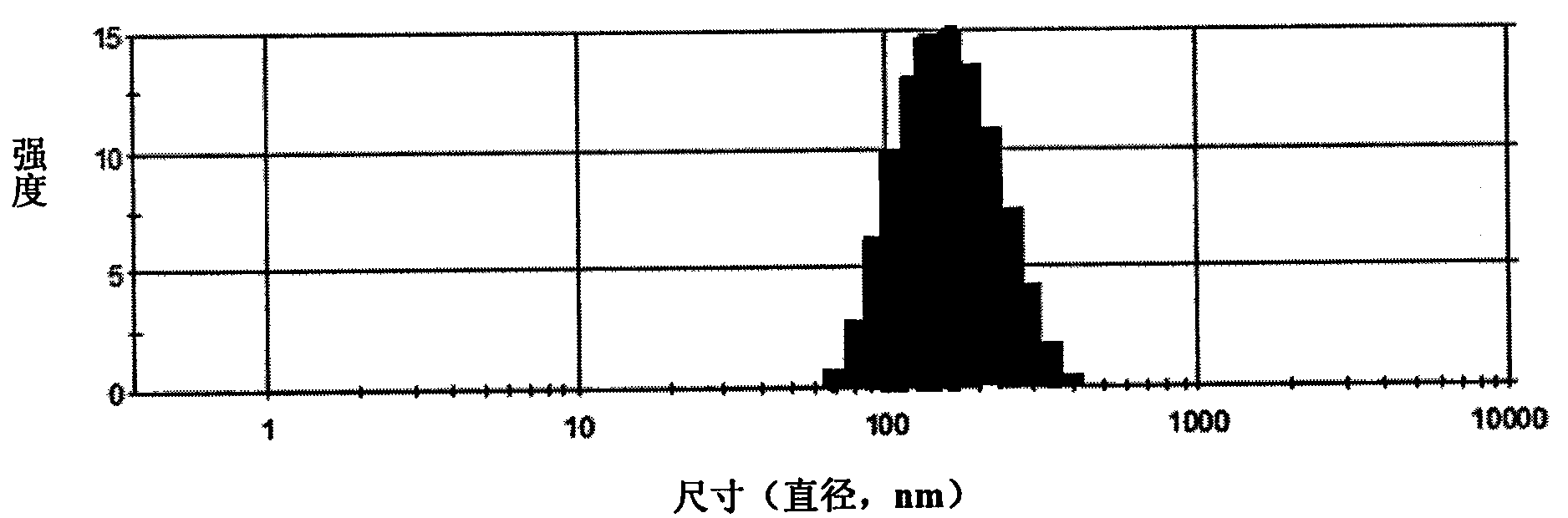
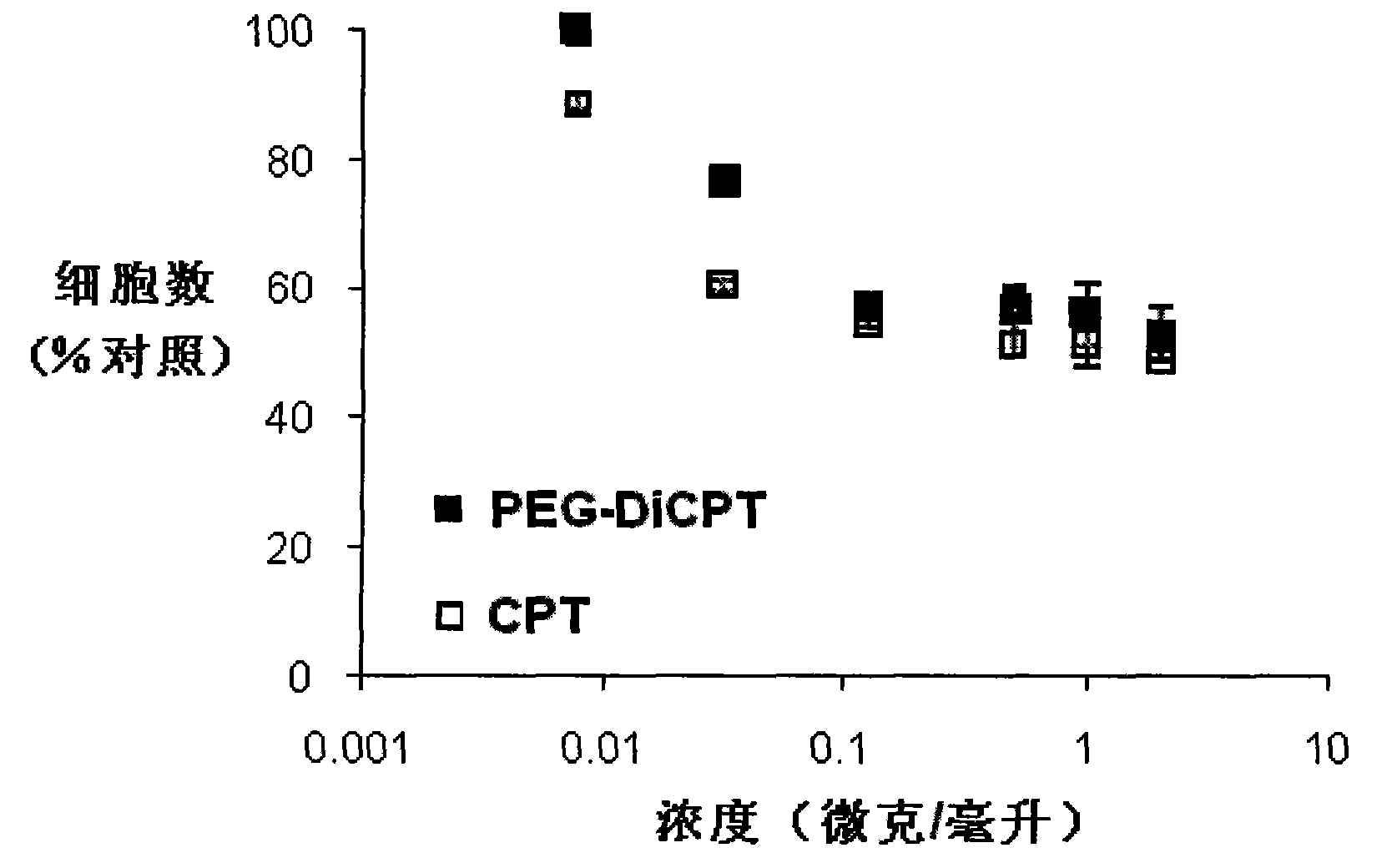
Camptothecin, self-emulsifying medicine precursor of derivative thereof and application thereof
A technology of self-emulsification and derivatives, applied in the field of biomedicine, can solve the problems of increased systemic toxicity and achieve the effects of long blood half-life, improved pharmaceutical effects, and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Synthesis of a PEG-linked self-emulsifying prodrug (PEG-diCPT) of two CPTs
[0042] (1) Synthesis of PEG-SH
[0043] 4.54g (10mmol) of poly(ethylene glycol) methyl ether acrylate (PEGMEA) was dissolved in 20mL of dry dichloromethane, and 1.92g (2mmol) of 98% 1,2-mercaptoethanol was added, Using 0.1 mL (0.72 mmol) of triethylamine as a catalyst, the reaction was stirred at room temperature for 24 h. After the reaction, the reactant was dialyzed with water to remove small molecules and organic solvents, the aqueous phase was washed with dichloromethane, and the organic phase was dried with anhydrous sodium sulfate and then vacuum-dried to obtain 4.61 g of product PEG-SH with a yield of 83.64%. The structural formula of PEG-SH is as follows:
[0044]
[0045] Among them, n=3-30.
[0046] (2) Synthesis of PEG with diacid at the end
[0047] Dissolve 2.2 g (4 mmol) PEG-SH and 0.52 g (4.5 mmol) fumaric acid in 5 mL dry N,N-dimethylformamide (DMF) with 0.01 mL...
Embodiment 2
[0058] Example 2 Synthesis of self-emulsifying prodrugs based on polyacrylic acid short chains
[0059] (1) Dissolve 1g (2.87mmol) of CPT and 0.34g (3.44mmol) of succinic anhydride in 5mL of DMSO, heat to 60°C, and react for 6 hours. After the reaction, pour the reaction solution into 50mL of acetone to precipitate, and filter through The solid product was collected and dried to give 1.09 g of product I with a yield of 85%. The structural formula of product I is as follows:
[0060]
[0061] (2) Dissolve 0.5g (1.11mmol) of the above product I and 0.154g (1.34mmol) of N-hydroxysuccinimide in DMSO, add 0.276g (1.34mmol) of N, N'-dicyclohexylcarbodi Imine (DCC) and 0.163g (1.34mmol) 4-dimethylamino must (DMAP, react overnight, filter and remove by-product. The filtrate is precipitated in ether, obtains 0.56g product II, and productive rate is 89%. Product II The structural formula is as follows:
[0062]
[0063] (3) 0.5g (0.89mmol) of the above-mentioned product II and ...
Embodiment 3
[0069] Example 3 Synthesis of glucose CPT self-emulsifying prodrug.
[0070] (1) Dissolve 1g (5.12mmol) of gluconic acid and 3.7g (61.6mmol) of ethylenediamine in 10mL of water, add 0.99g (5.12mmol) of 1-ethyl-(3-dimethylaminopropyl)-carbon Diimine (EDC) catalyzed the reaction for 12 hours. After the reaction, the reaction solution was poured into 100 mL of acetone to precipitate, and the solid product was collected by filtration. After drying, 1.11 g of product IV was obtained with a yield of 85%.
[0071] (2) Mix 0.3g (1.17mmol) of the above-mentioned product IV, 0.66g (1.17mmol) of the product II in the second step of Example 2 with 10ml DMSO, react for 3h, and pour the reaction solution into acetone to precipitate after the reaction. The solid product V was collected by filtration.
[0072]
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com