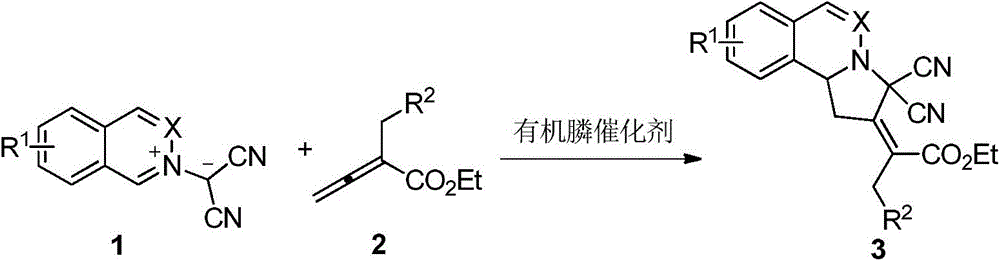
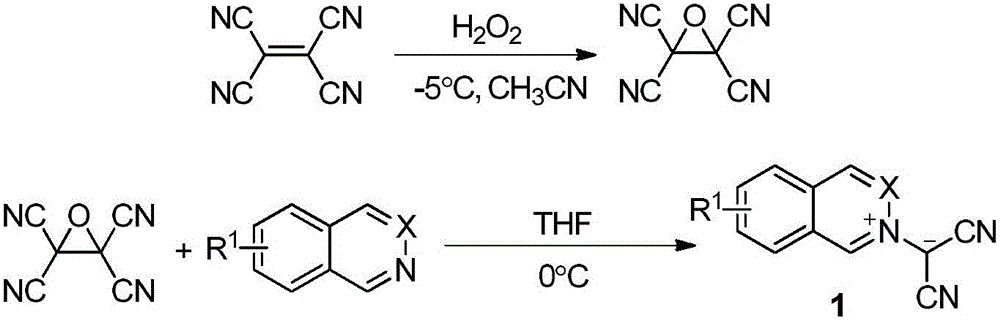
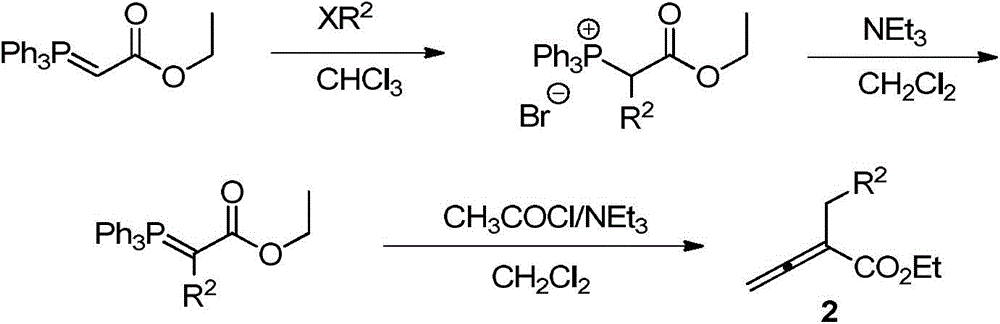
Phthalazinopyrrole compound and preparation method thereof
A compound and a technology for a synthesis method are applied in the field of phthalazinopyrrole compounds and their preparation, and achieve the effects of easy separation and purification, fast reaction speed and few side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
[0027] Put 0.0388g (0.2mmol) of compound 1a, 0.0607g (0.3mmol) of compound 2a and 1mL of dichloromethane into a dry 15mL Shrek tube, add 10.6mg (0.04mmol) of triphenylphosphine and mix well for cycloaddition into a reaction. In this reaction system, the molar ratio of Compound 1a to Compound 2a is 1:1.5, the organophosphorus catalyst accounts for 20% of the molar percentage of Compound 1a, stirred at 25° C. for 48 hours, concentrated through a column with a rotary evaporator (ethyl acetate Ester: Petroleum ether = 1:5, v / v), to obtain 63.4 mg of product 3aa, with a yield of 80%.
Embodiment 2
[0029]
[0030] Put 0.0388g (0.2mmol) of compound 1a, 0.0607g (0.3mmol) of compound 2a and 1mL of dichloromethane into a dry 15mL Shrek tube, add 0.04mmol of trimethylphosphine and mix well for cycloaddition reaction. In this reaction system, the molar ratio of compound 1a to compound 2a is 1: 1.5, the organophosphorus catalyst accounts for 20% of the molar percentage of compound 1a, stirred at 25°C for 2 hours, concentrated through a column with a rotary evaporator (ethyl acetate Ester:petroleum ether=1:5, v / v), to obtain 67.4mg of product 3aa, yield 85%.
Embodiment 3
[0032]
[0033] Put 0.0388g (0.2mmol) of compound 1a, 0.0607g (0.3mmol) of compound 2a and 1mL of dichloromethane into a dry 15mL Shrek tube, add 0.04mmol of dimethylphenylphosphine and mix well for cycloaddition reaction . In this reaction system, the molar ratio of compound 1a to compound 2a is 1: 1.5, the organophosphorus catalyst accounts for 20% of the molar percentage of compound 1a, stirred at 25°C for 2 hours, concentrated through a column with a rotary evaporator (ethyl acetate Ester:petroleum ether=1:5, v / v), to obtain 75.3mg of product 3aa, yield 95%.
[0034] It can be seen that the effect is very excellent when dimethylphenylphosphine is selected as the catalyst.
[0035] 3aa NMR
[0036] 1 HNMR (300MHz, CDCl 3)δ7.81(s, 1H), 7.50(td, J=7.4, 1.5Hz, 1H), 7.46-7.40(m, 1H), 7.39-7.31(m, 3H), 7.30-7.24(m, 1H) , 7.23-7.18(m, 2H), 7.11(d, J=7.4Hz, 1H), 4.54-4.23(m, 3H), 3.90(q, J=15.4Hz, 2H), 3.36(dd, J=16.4 , 5.8Hz, 1H), 3.04(ddd, J=16.4, 10.2, 1.4Hz, 1H), 1.37...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com