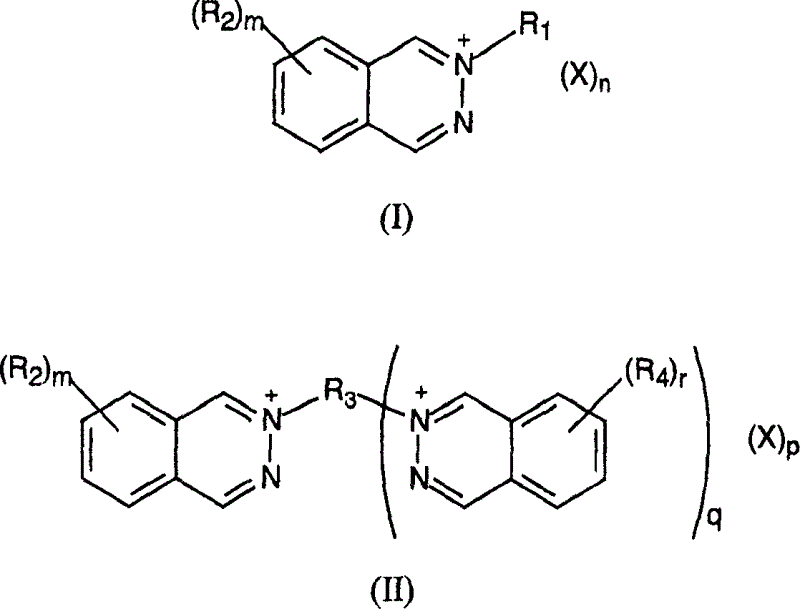
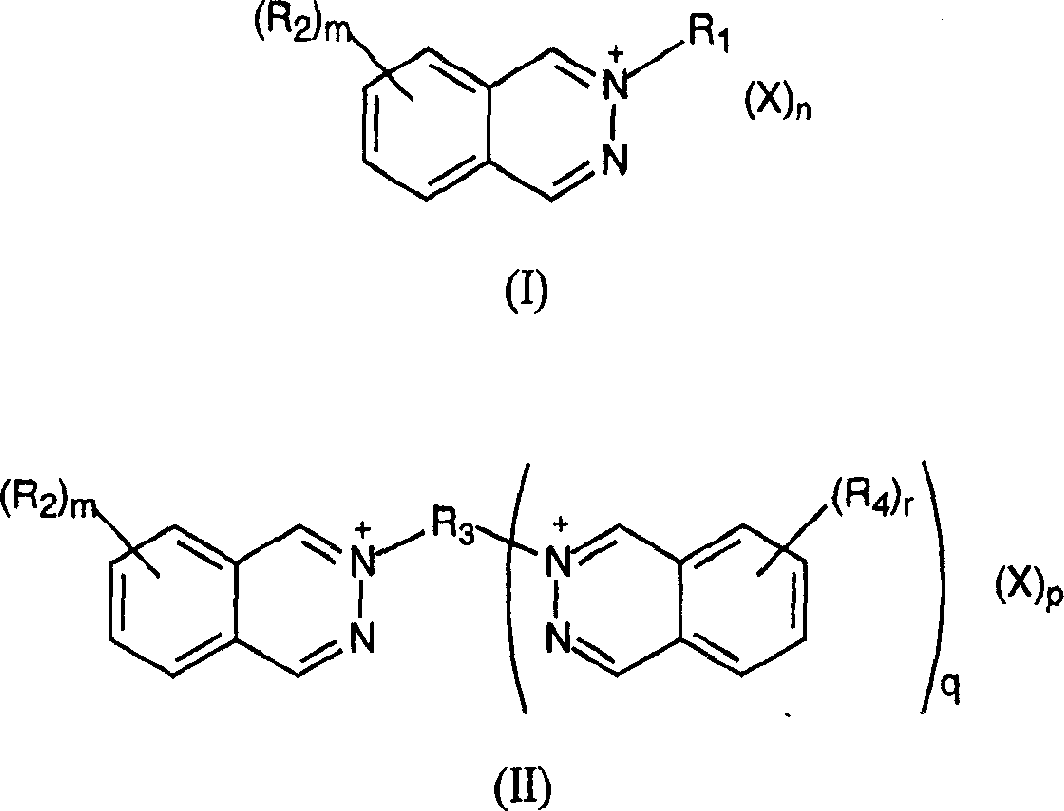
Heat developing emulsion and material contg. 2,3-phthalazine compound
A kind of naphthalene, compound technology, applied in the field of thermally developable compositions and imaging materials, can solve the problem of poor washout latitude and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0307] The preparation method of compound II-1 is as follows: 95.97g (0.457mol) bisvinylsulfonylmethane is dissolved in 700ml warm methanol, and the insoluble matter is removed by filtration; 121.73g (0.935mmol) 2,3-naphthyridine is dissolved In 250 ml methanol, this solution was filtered and then added to the previous solution. Sufficient methanol was added to bring the solution to a final volume of 1400 ml and the solution was left to crystallize at room temperature. After 5 h the product was filtered, washed with 50 ml of methanol and dried to obtain 212.98 g of compound II-1.
[0308] The structures of all compounds were confirmed by proton NMR and mass spectral data.
[0309] Preparation of benzotriazole silver salt dispersion:
[0310] To the stirred reaction vessel was added 85 g of alkali-processed gelatin, 25 g of phthalylated gelatin, and 2000 g of deionized water. A solution was prepared containing 185 g of benzotriazole, 1405 g of deionized water and 680 g of 2....
Embodiment 1-3
[0338] The examples shown in Table II below show that adding the phthalazine compound of the present invention to the photothermographic material greatly improves the sensitivity of the material. Moreover, after prolonging the flushing time from 15s to 20s, the increment of Dmin is small, from which it can be seen that the thermal flushing tolerance of 2,3-naphthyridine compounds is better.
[0339] All samples were developed by heating at 150°C for 15 or 20 seconds, and the resulting black images were obtained.
[0340] "PHZ" refers to phthalazine. This compound is outside the scope of the present invention and is used as a comparative example.
[0341] Table II
[0342] Control sample 1 Control sample 2 Example 1 Example 2 Example 3
[0343]Toner Compound None PHZ I-1 II-1 I-2
[0344] rinse time
[0345] 15s D min 0.263 0.304 0.293 0.280 0.278
[0346] D. max 2.18...
Embodiment 4-8
[0360] The examples shown in Table III below show that different capping groups R 1 and R 2 Substituents and counter ions X - The 2,3-naphthyridine compound of the present invention has better film stability after natural aging at 21° C. and 40% relative humidity for 7 days.
[0361] All samples were developed by heating at 150°C for 20 seconds, and black images were obtained.
[0362] "PHZ" refers to 2,3-naphthyridine, while "6-CH 3 PHZ" refers to 6-methyl-2,3-naphthyridine. These two compounds do not belong to the scope of the present invention, and they are used as comparison
[0363] Example.
[0364] Table III
[0365] Control sample 2 Control sample 3 Example 4 Example 5 Example 6 Example 7 Example 8
[0366] Toner compound PHZ 6-CH 3 PHZ I-1 I-3 I-5 I-6 I-7
[0367] Initial value (new system) D min 0.329 0.367 0.306 0.297 0.261 0.281 0.315
[0368] D. max ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com