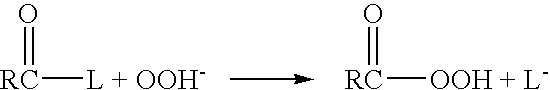
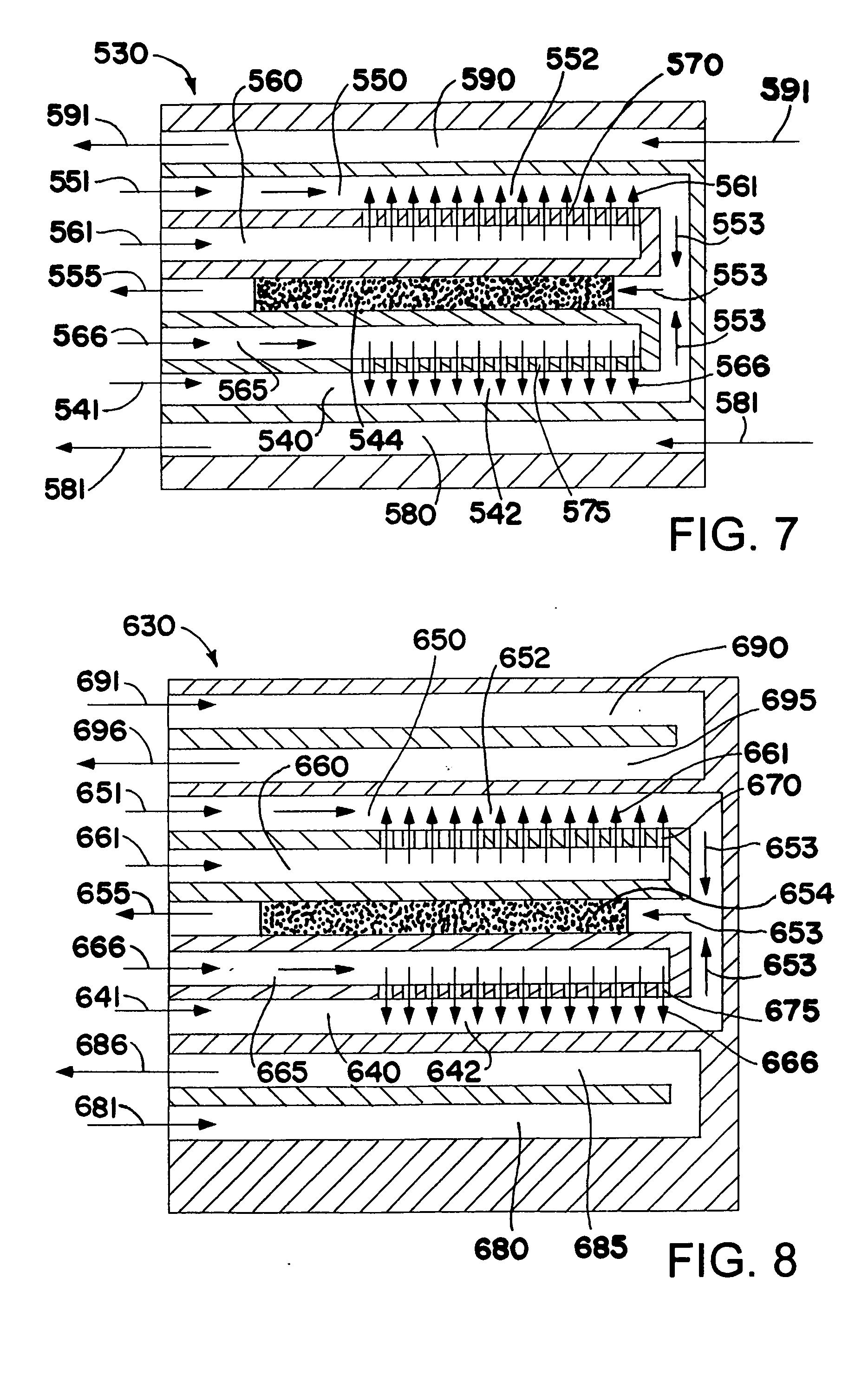
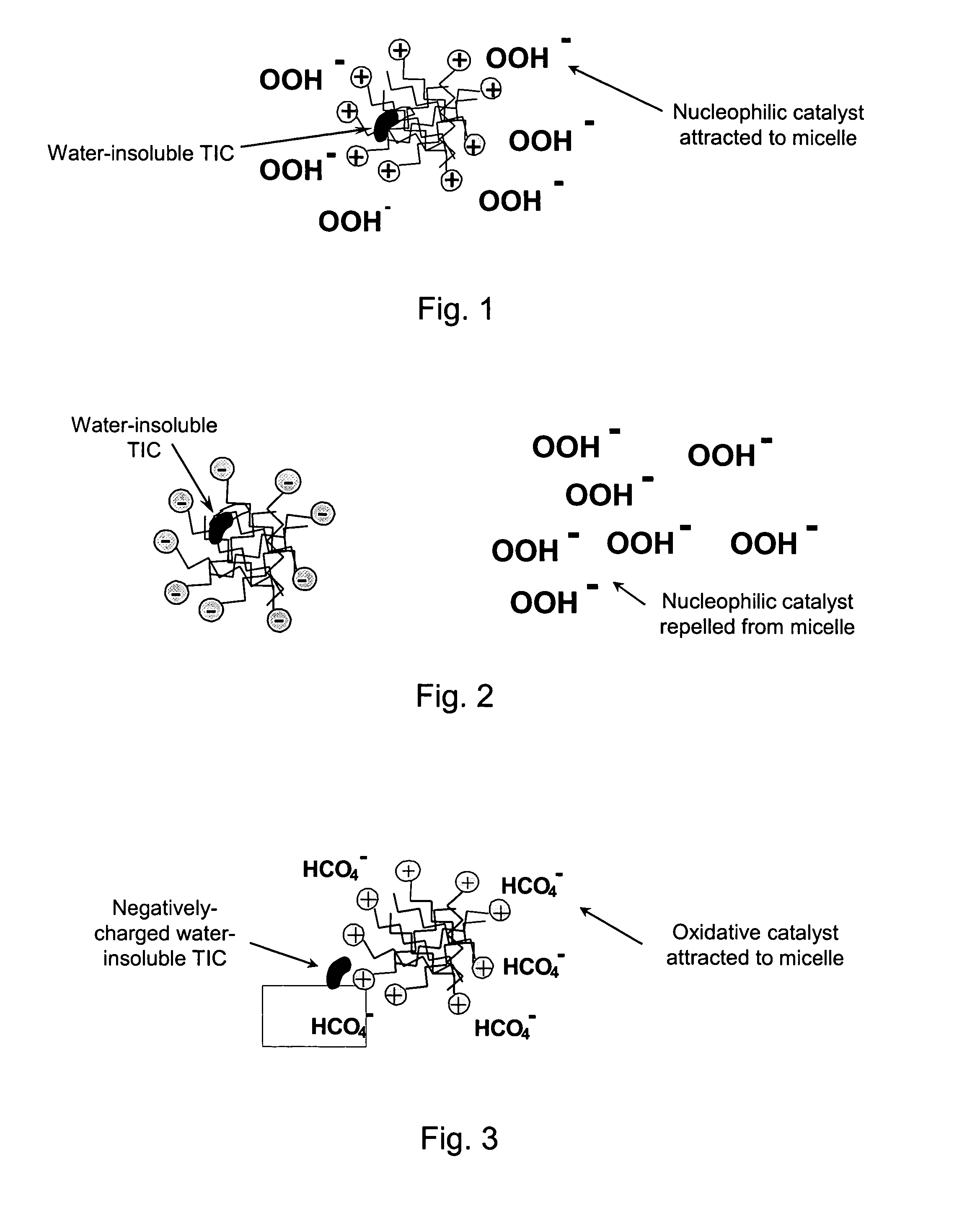
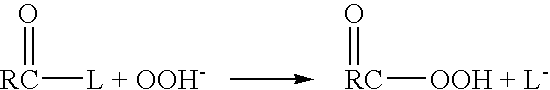
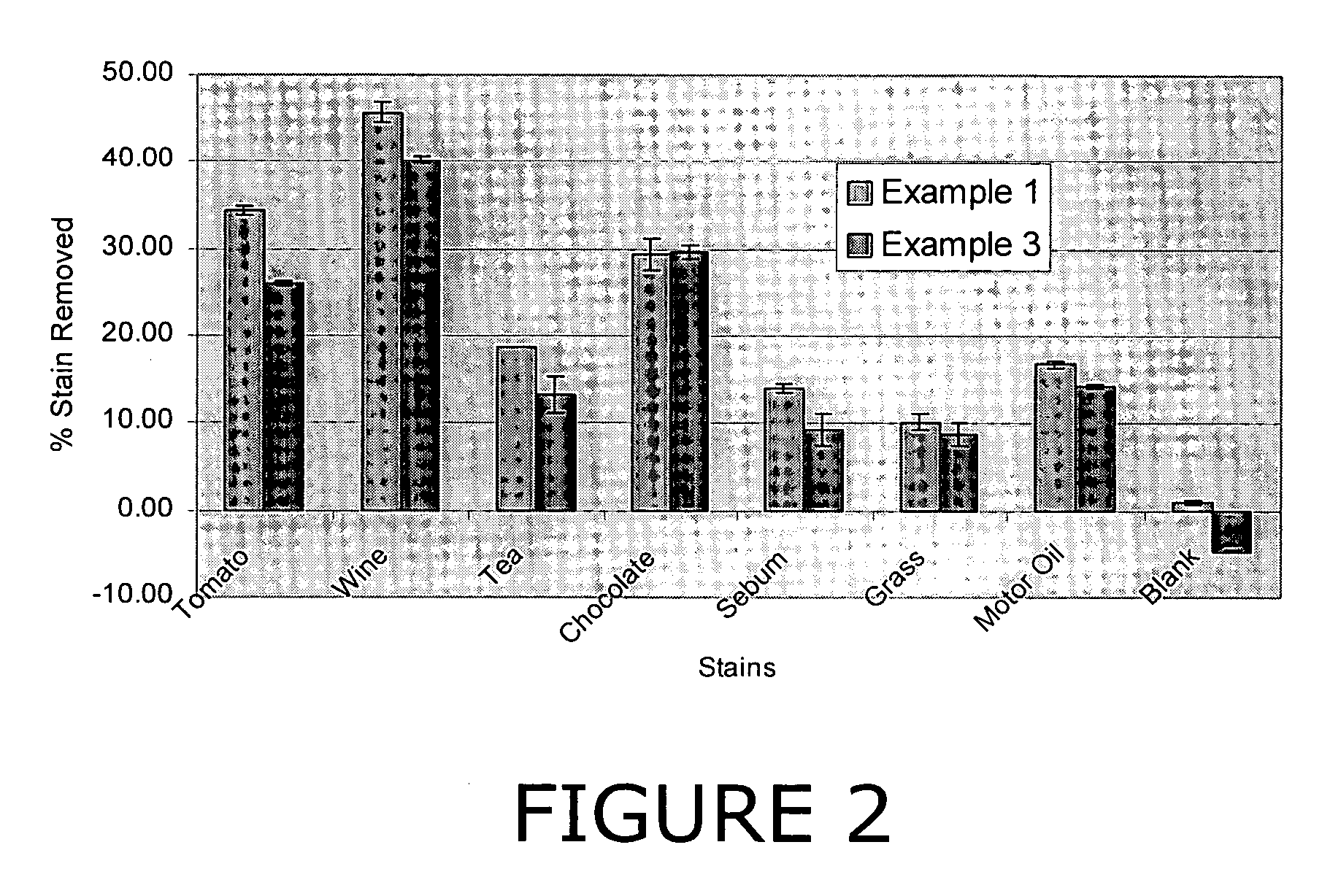
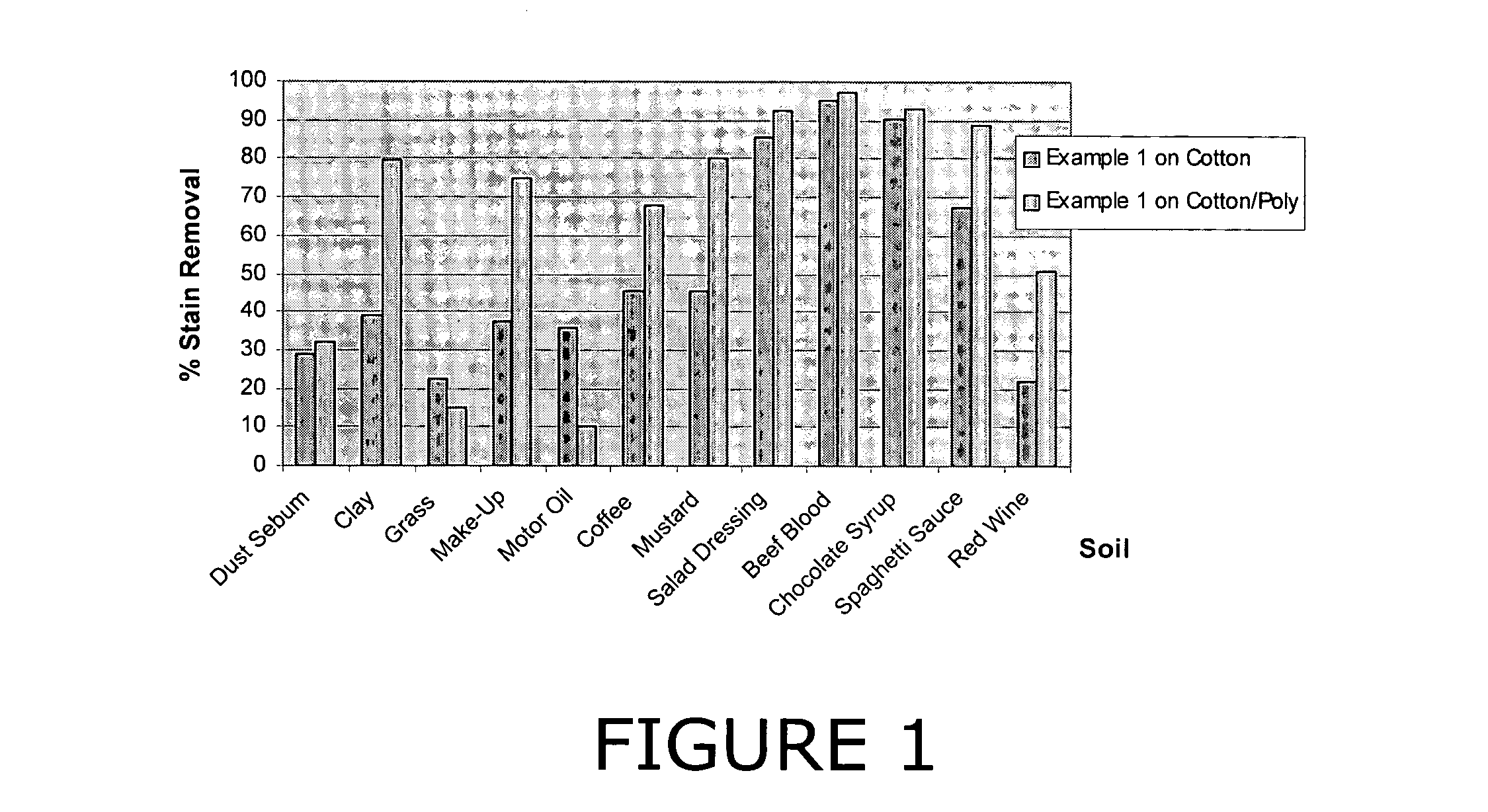
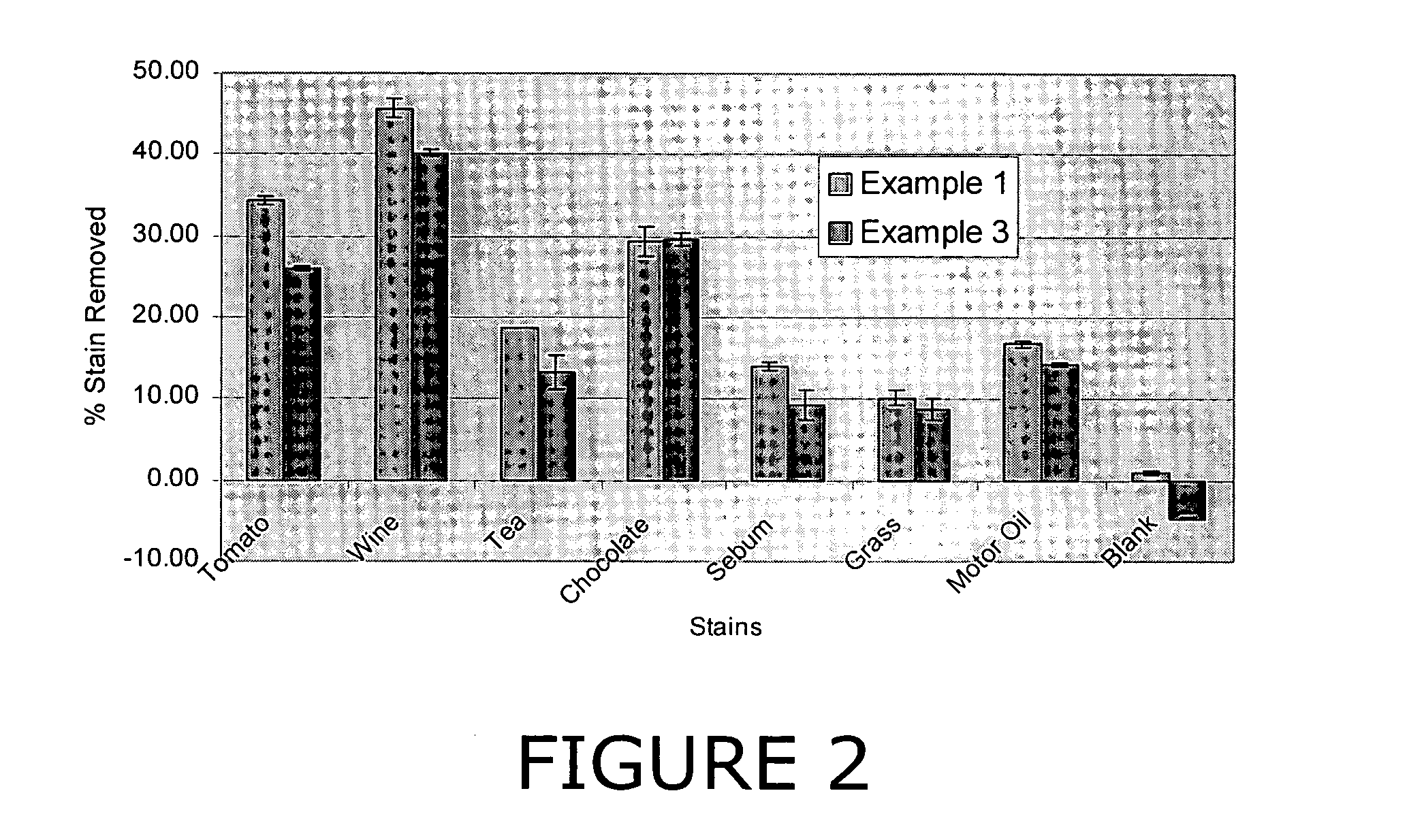
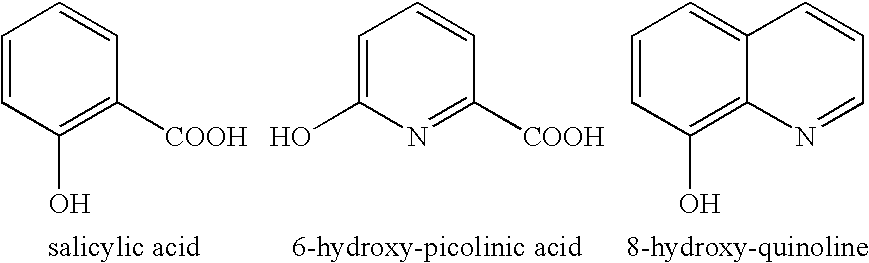
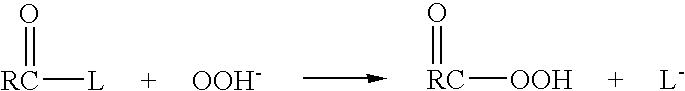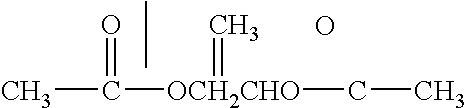Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
306results about "Hydrogen peroxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
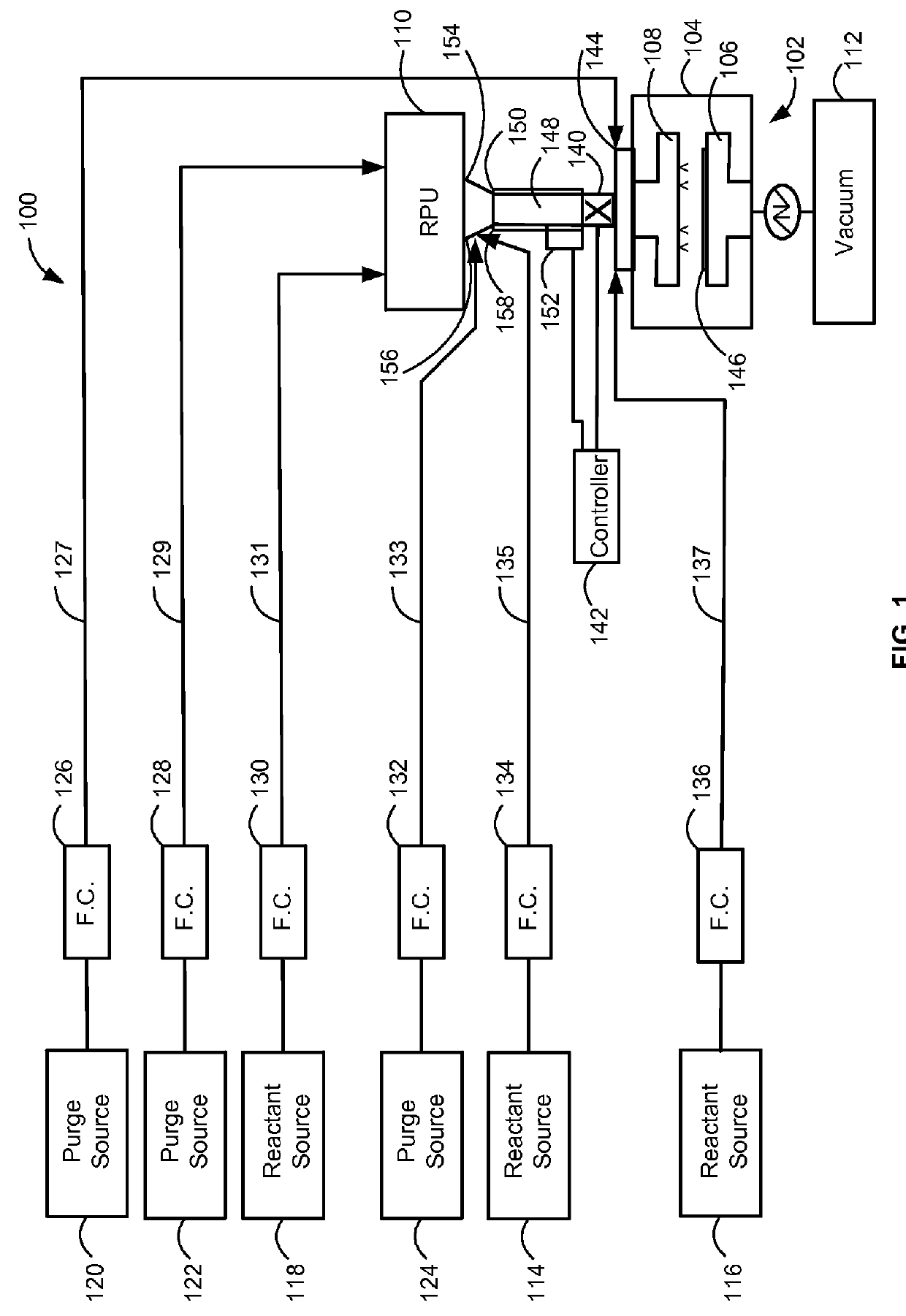
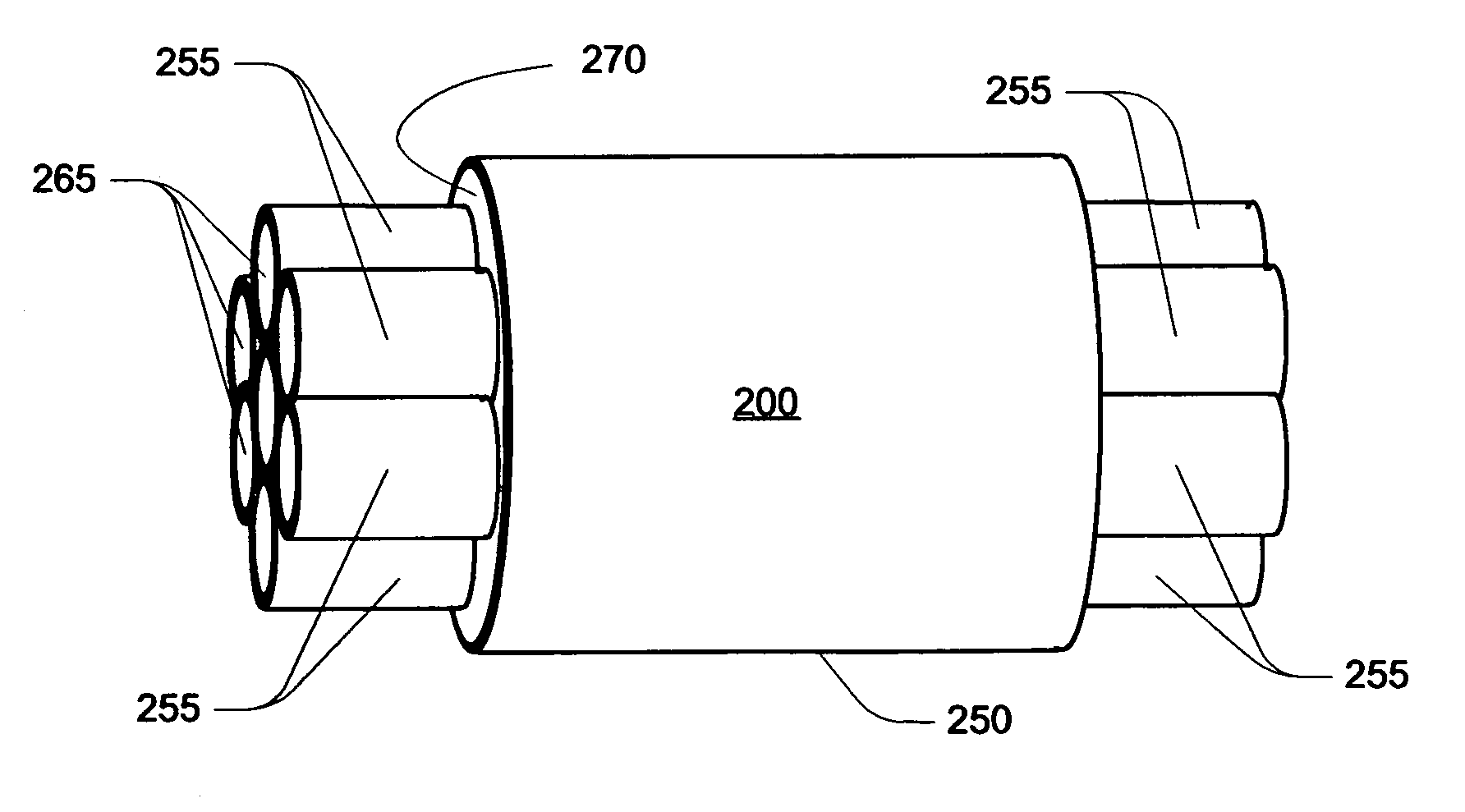
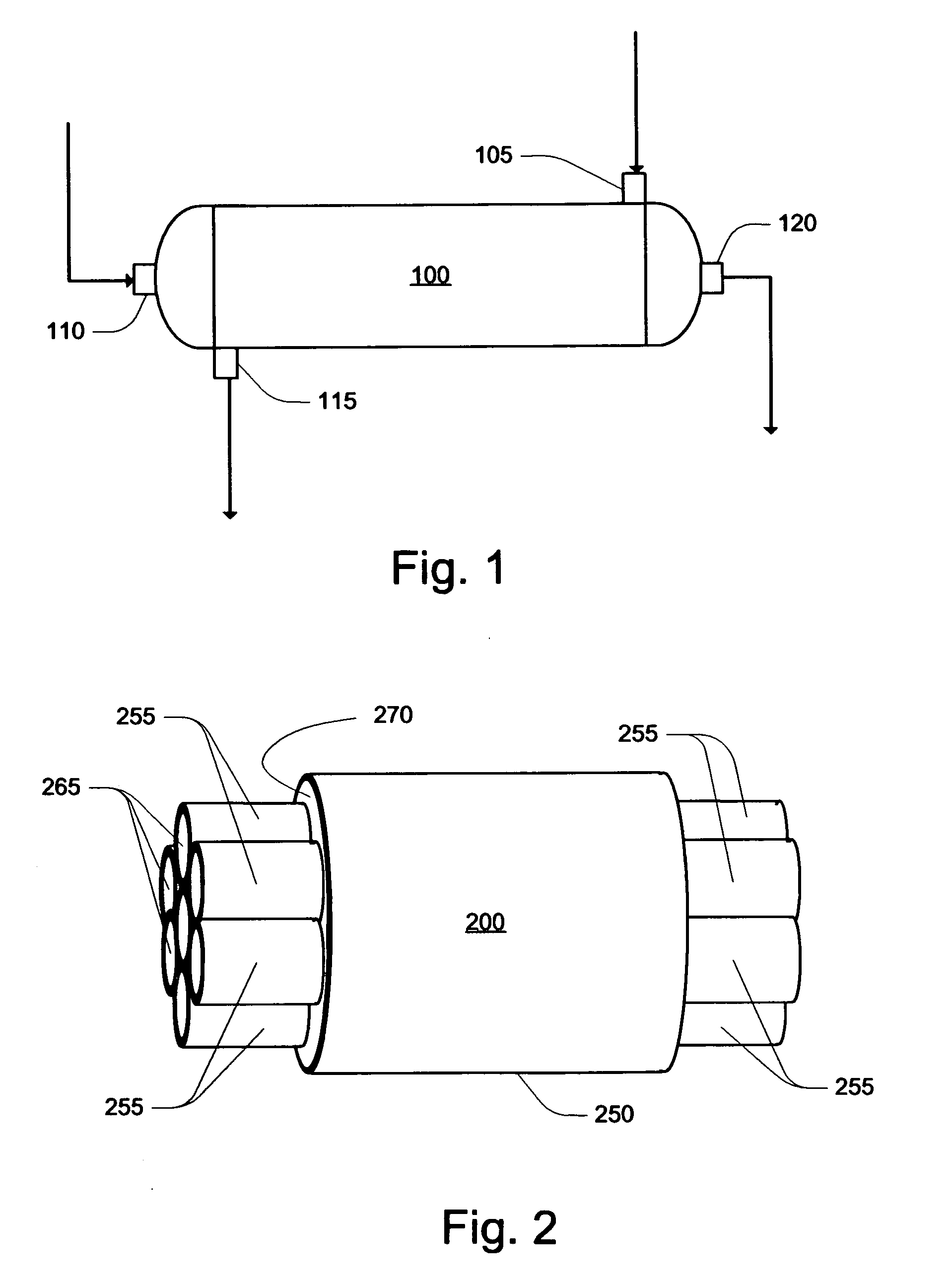
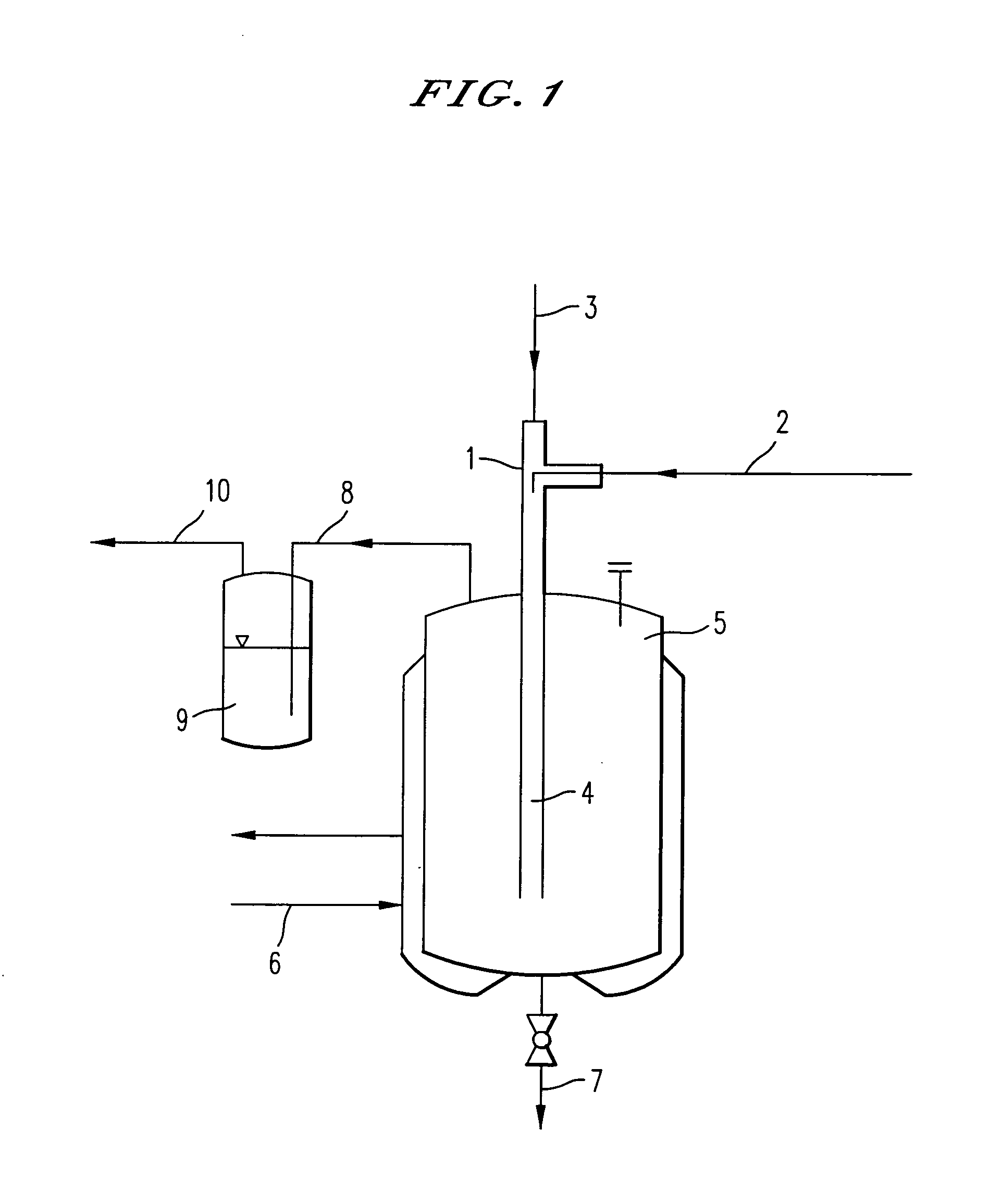
Method and system for in situ formation of gas-phase compounds
ActiveUS20160051964A1Process can be usedProcess control/regulationCell electrodesCompound aGas phase
A system and method for providing intermediate reactive species to a reaction chamber are disclosed. The system includes an intermediate reactive species formation chamber fluidly coupled to the reaction chamber to provide intermediate reactive species to the reaction chamber. A pressure control device can be used to control an operating pressure of the intermediate reactive species formation chamber, and a heater can be used to heat the intermediate reactive species formation chamber to a desired temperature.
Owner:ASM IP HLDG BV
Catalyst and process for direct catalytic production of hydrogen peroxide, (H2O2)
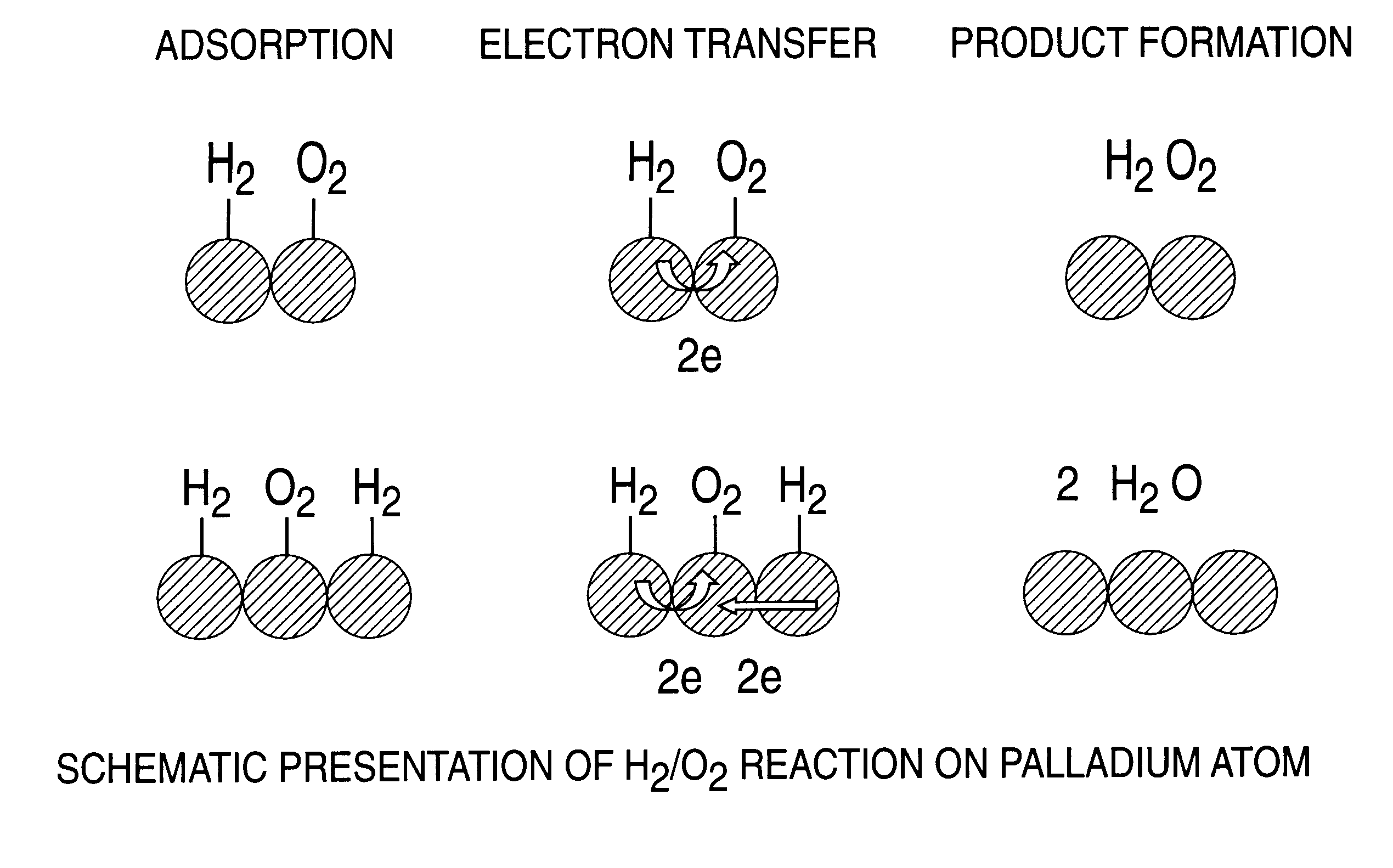
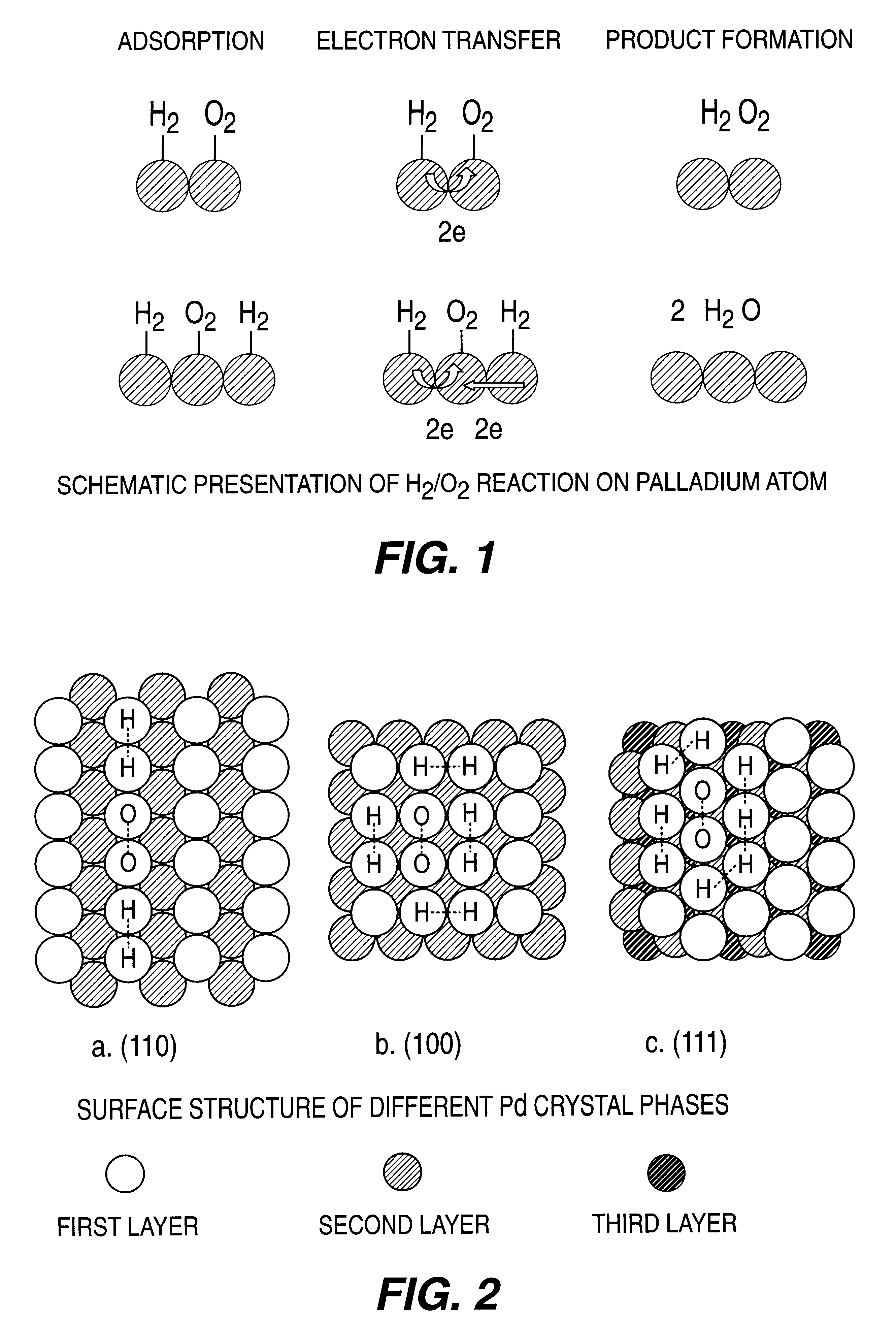
InactiveUS6168775B1High catalytic activityHigh activityHydrogen peroxideCatalyst activation/preparationParticulatesHydrogen
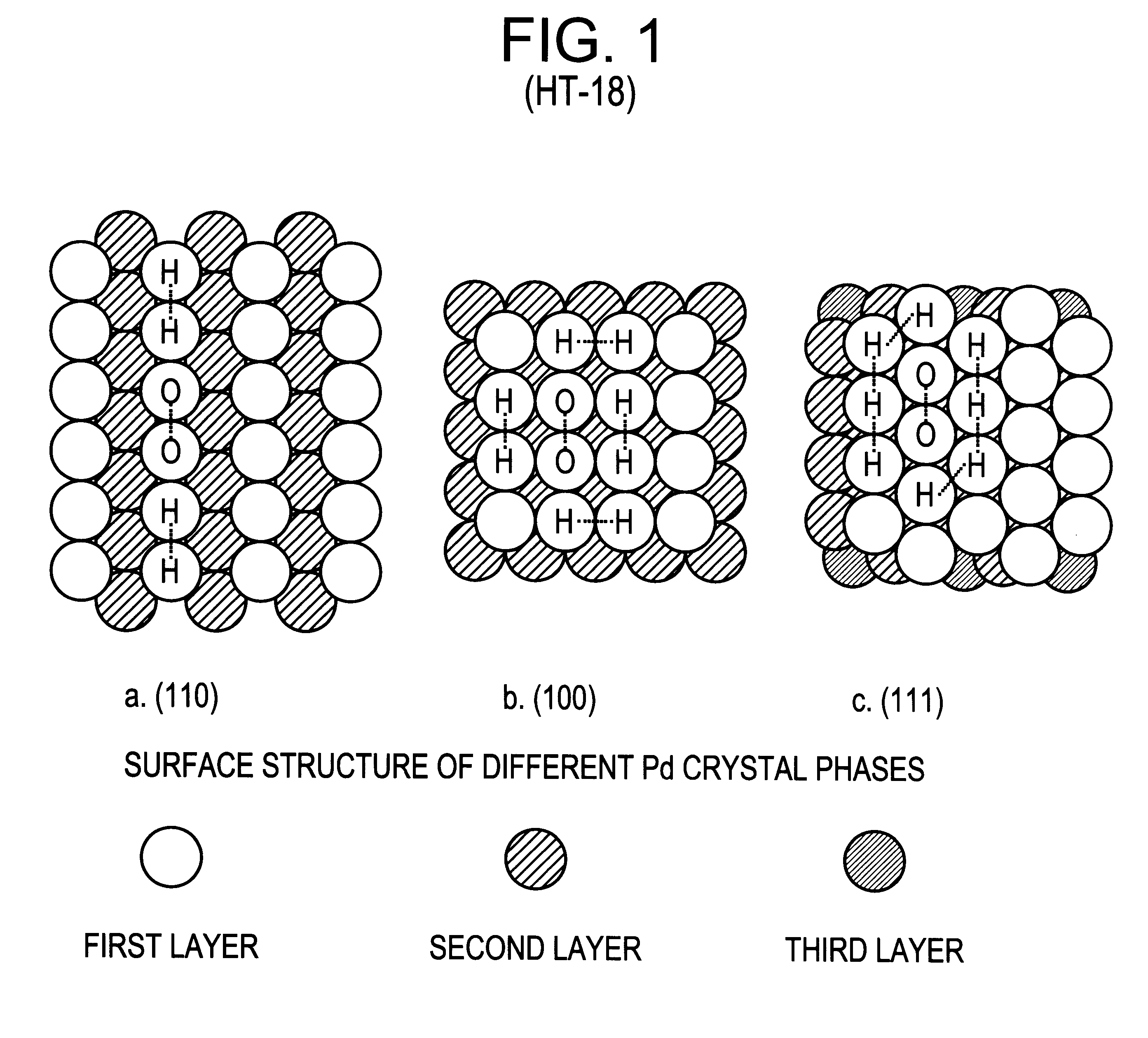
A particulate supported noble metal phase-controlled catalyst material having 5-1000 mum surface area of 50-500 m2 / gm is provided for use in direct catalytic production of hydrogen peroxide (H2O2) product from hydrogen and oxygen-containing feedstreams. The catalyst is made by depositing phase controlled crystals of a noble metal such as palladium on a suitable particulate support material such as carbon black, by utilizing a precursor solution of the metal and a suitable control ionic polymer having molecular weight of 300-8000 such as sodium polyacrylate in a selected metal to polymer molar ratio of 1:0.1 to 1:10, which procedure provides desired phase control of the noble metal atoms to form widely dispersed minute noble metal crystals on the support material. The invention includes methods for making the catalyst, and also a process for utilizing the catalyst to directly produce high yields of hydrogen peroxide (H2O2) product from hydrogen and oxygen-containing gaseous feedstreams.
Owner:BORAL IP HLDG
Film coating composition for whitening teeth
PCT No. PCT / CN97 / 00004 Sec. 371 Date Sep. 17, 1998 Sec. 102(e) Date Sep. 17, 1998 PCT Filed Jan. 20, 1997 PCT Pub. No. WO97 / 25968 PCT Pub. Date Jul. 24, 1997The invention relates to a tooth-whitening varnish composition, comprising 6-20% of carbamide peroxide, 2-9% of film forming agent and 77-88% of volatile organic solvent, based on the total weight of the composition. The volatile organic solvent is selected from ether, ethylacetate, ethyl alcohol, or acetone. The film forming agent is artificial or natural material selected from cellulose, polyvinyl, butyral, coumarone resin or shellac. The composition can rapidly form films on dry tooth surfaces, and a remarkable tooth-whitening effect can be obtained.
Owner:IVOCLAR VIVADENT INC
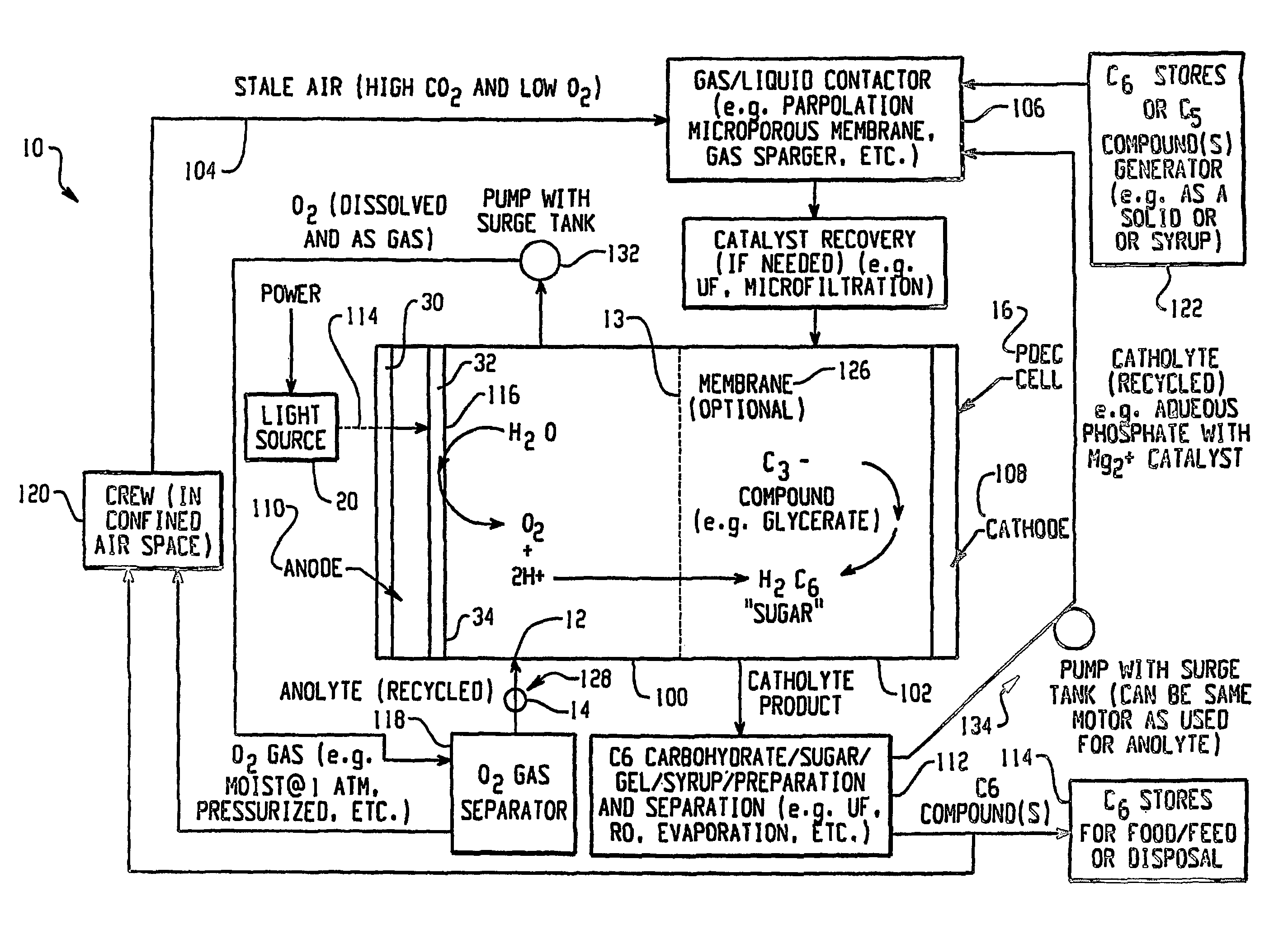
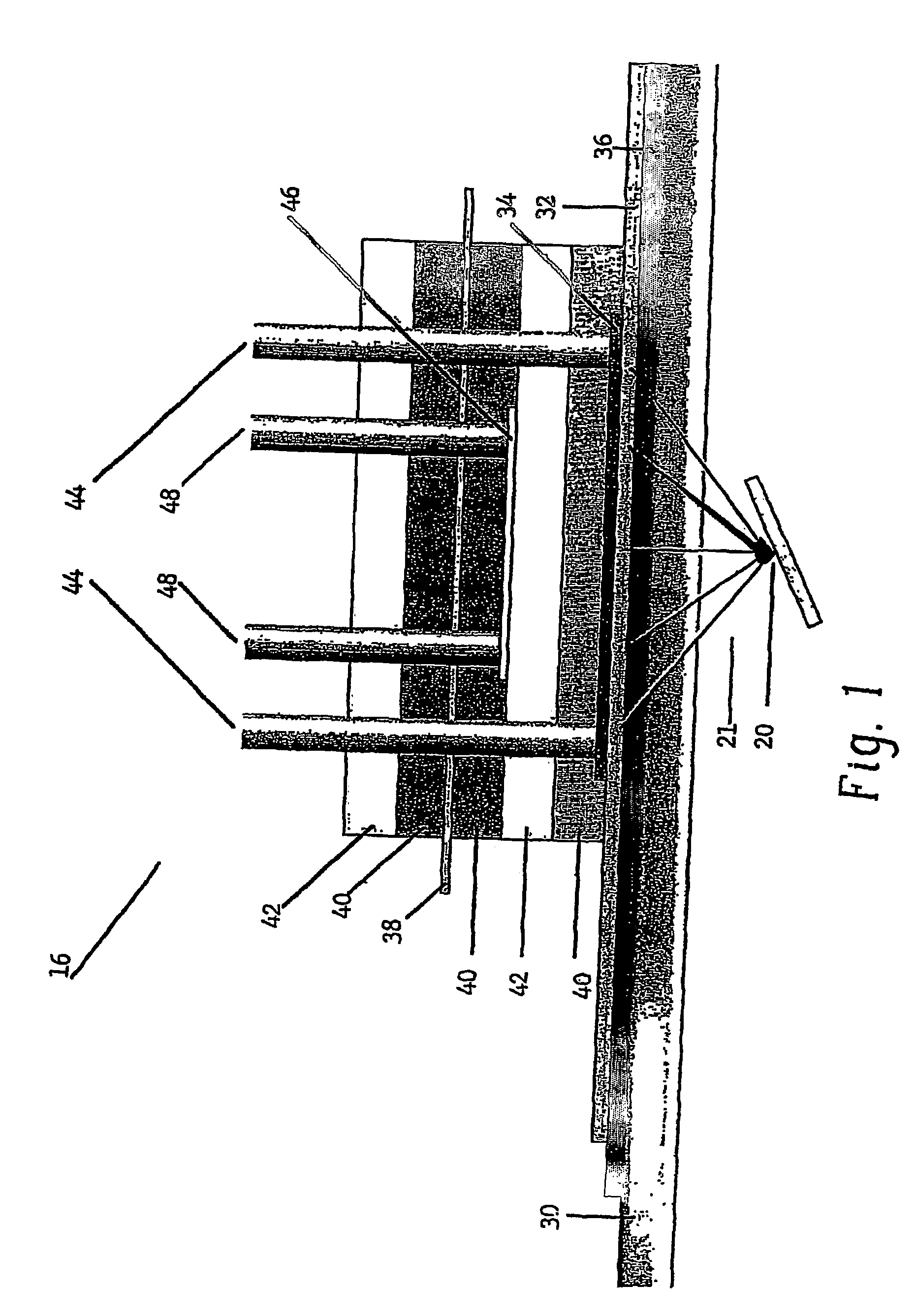
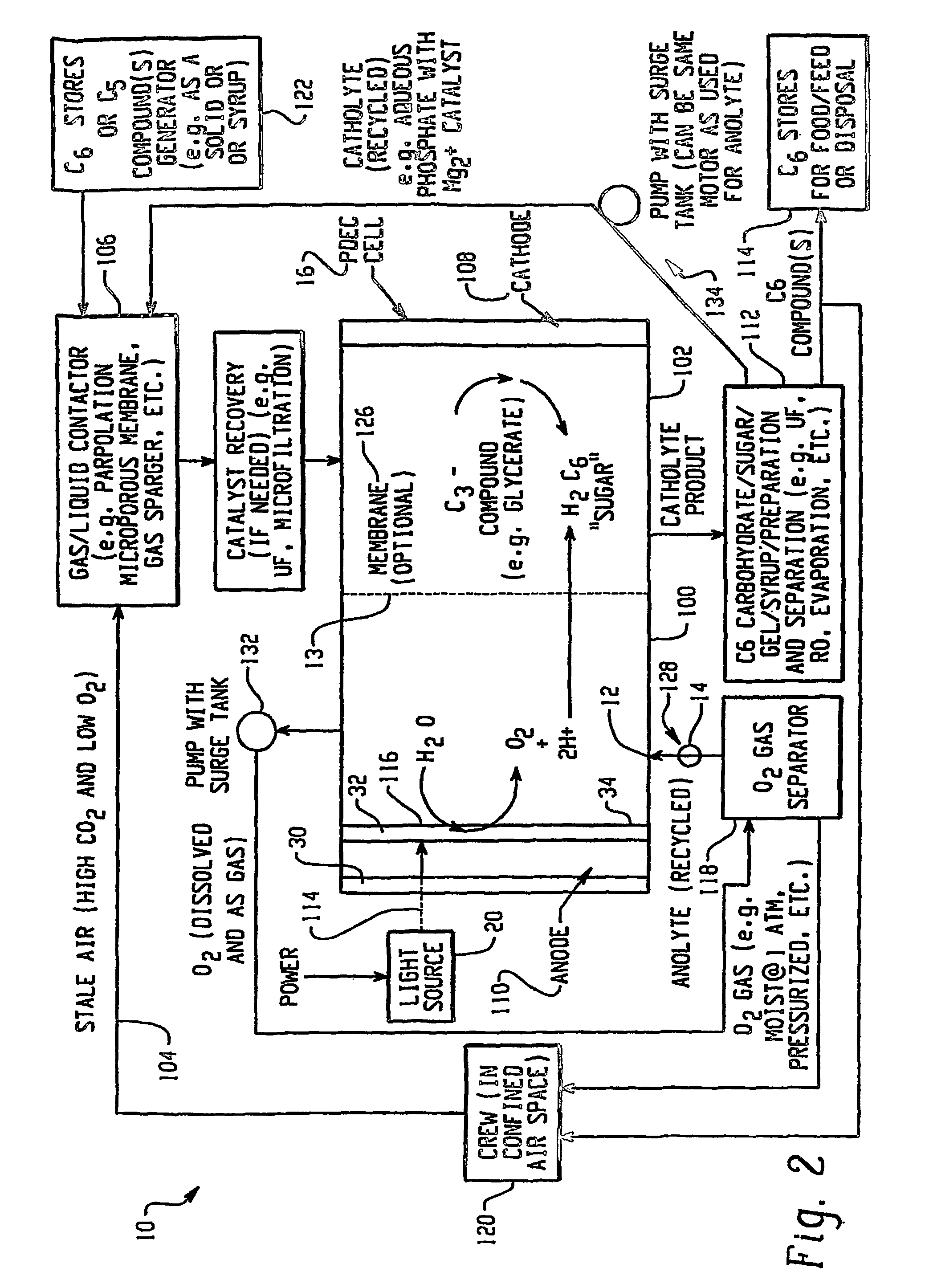
Photolytic oxygenator with carbon dioxide and/or hydrogen separation and fixation
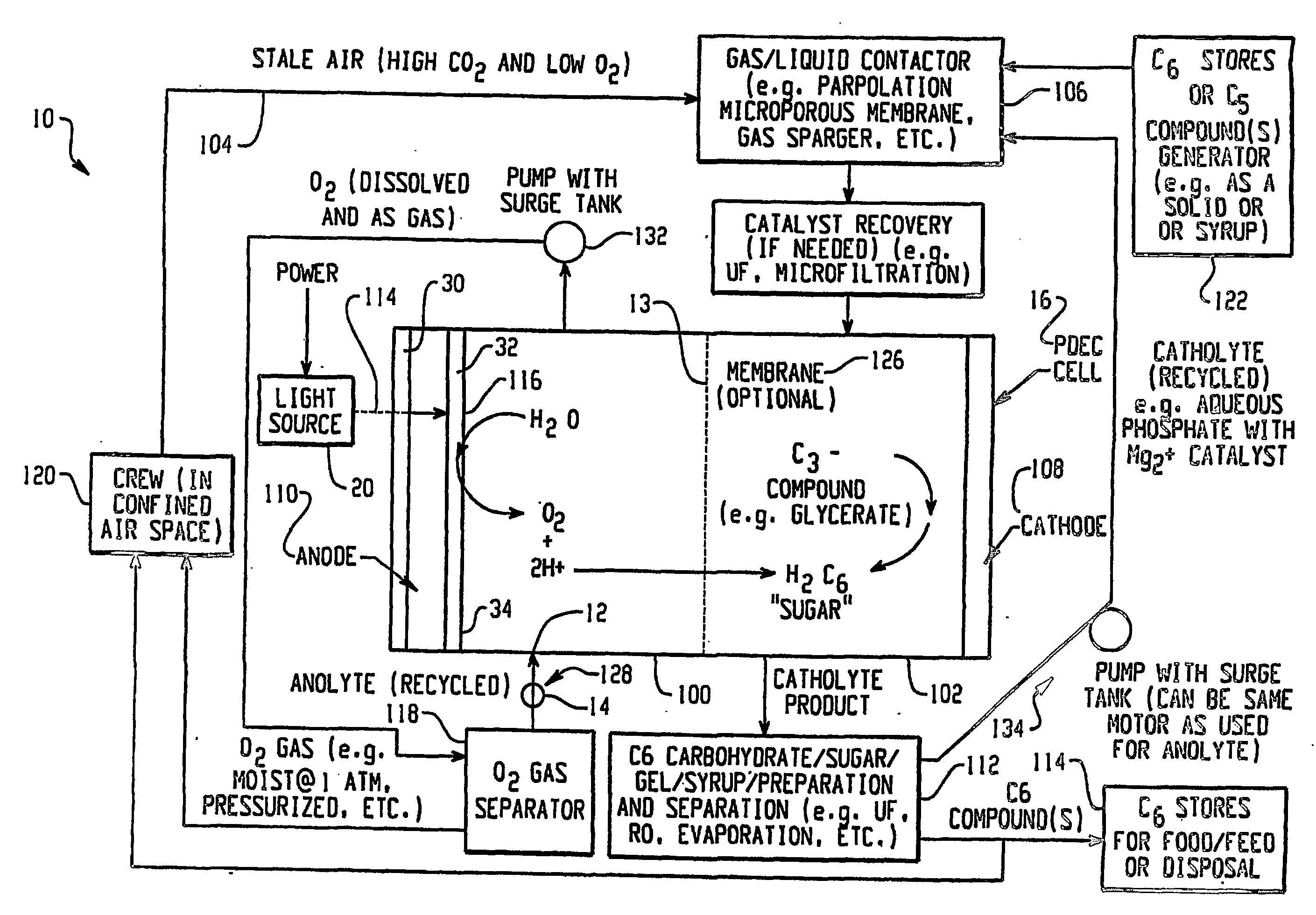
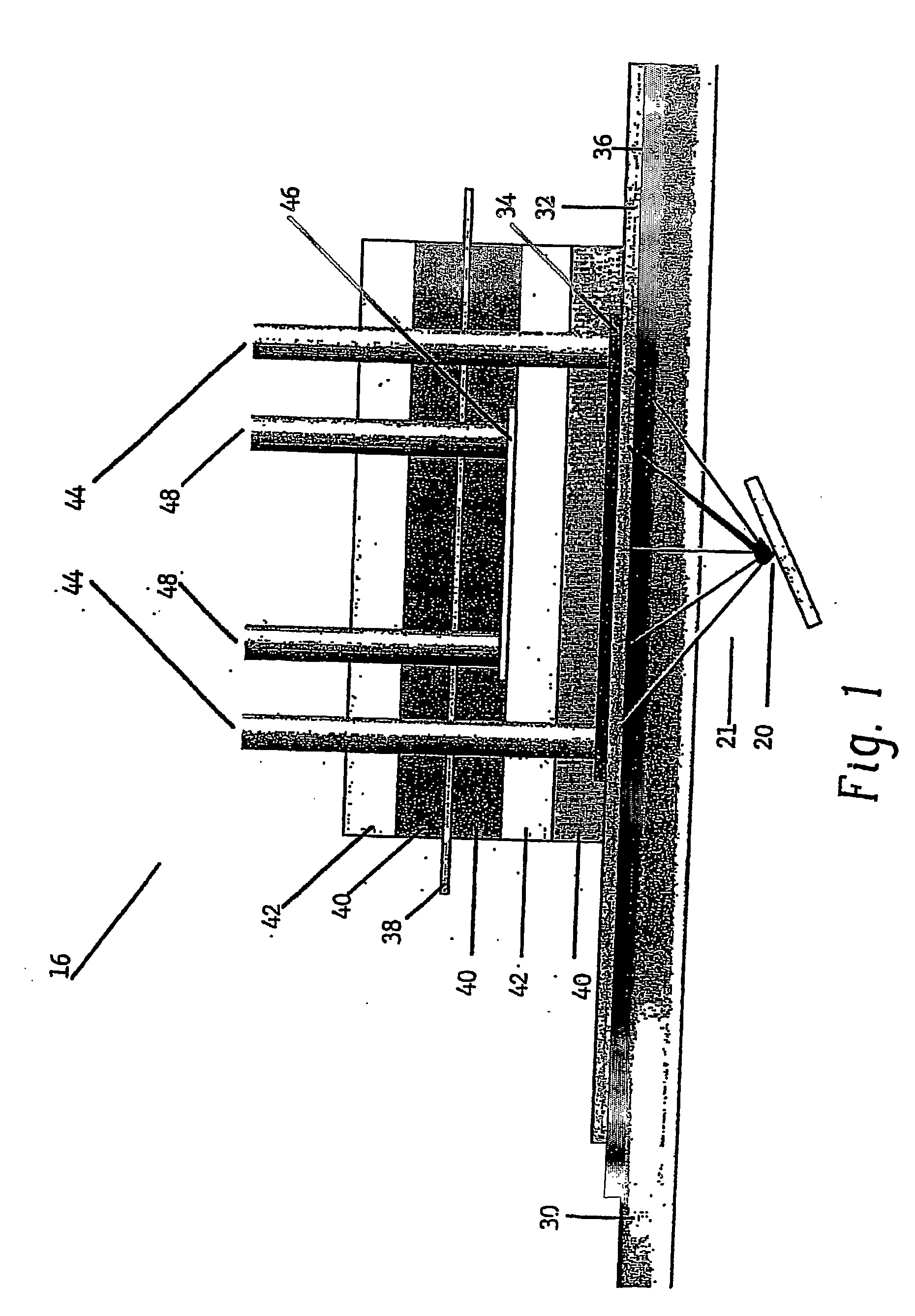
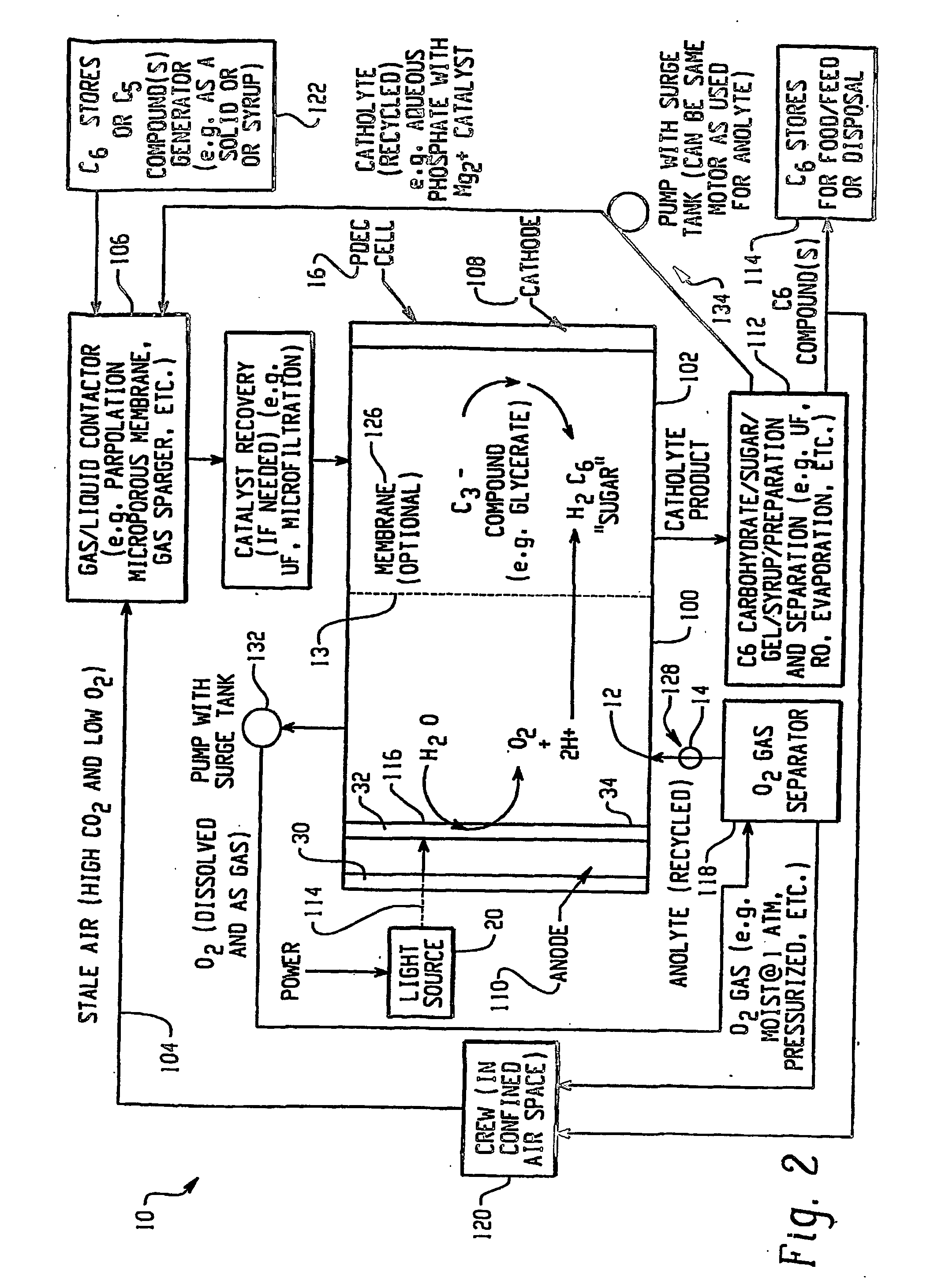
Apparatus for oxygenating an enclosed space as well as removing carbon dioxide from the enclosed space. The apparatus comprises a photolytic cell (16) having an anode compartment with a photo-active surface having the ability to convert water to oxygen; a cathode compartment having the ability to convert carbon dioxide and hydrogen to a solid or liquid medium; and a light source (20) for providing light photons (21) to said photolytic cell and activating the photo-reactive surface.
Owner:BATTELLE MEMORIAL INST
Integrated process and dual-function catalyst for olefin epoxidation
The invention discloses a dual-functional catalyst composition and an integrated process for production of olefin epoxides including propylene oxide by catalytic reaction of hydrogen peroxide from hydrogen and oxygen with olefin feeds such as propylene. The epoxides and hydrogen peroxide are preferably produced simultaneously in situ. The dual-functional catalyst comprises noble metal crystallites with dimensions on the nanometer scale (on the order of <1 nm to 10 nm), specially dispersed on titanium silicalite substrate particles. The dual functional catalyst catalyzes both the direct reaction of hydrogen and oxygen to generate hydrogen peroxide intermediate on the noble metal catalyst surface and the reaction of the hydrogen peroxide intermediate with the propylene feed to generate propylene oxide product. Combining both these functions in a single catalyst provides a very efficient integrated process operable below the flammability limits of hydrogen and highly selective for the production of hydrogen peroxide to produce olefin oxides such as propylene oxide without formation of undesired co-products.
Owner:HEADWATERS TECH INNOVATION GRP
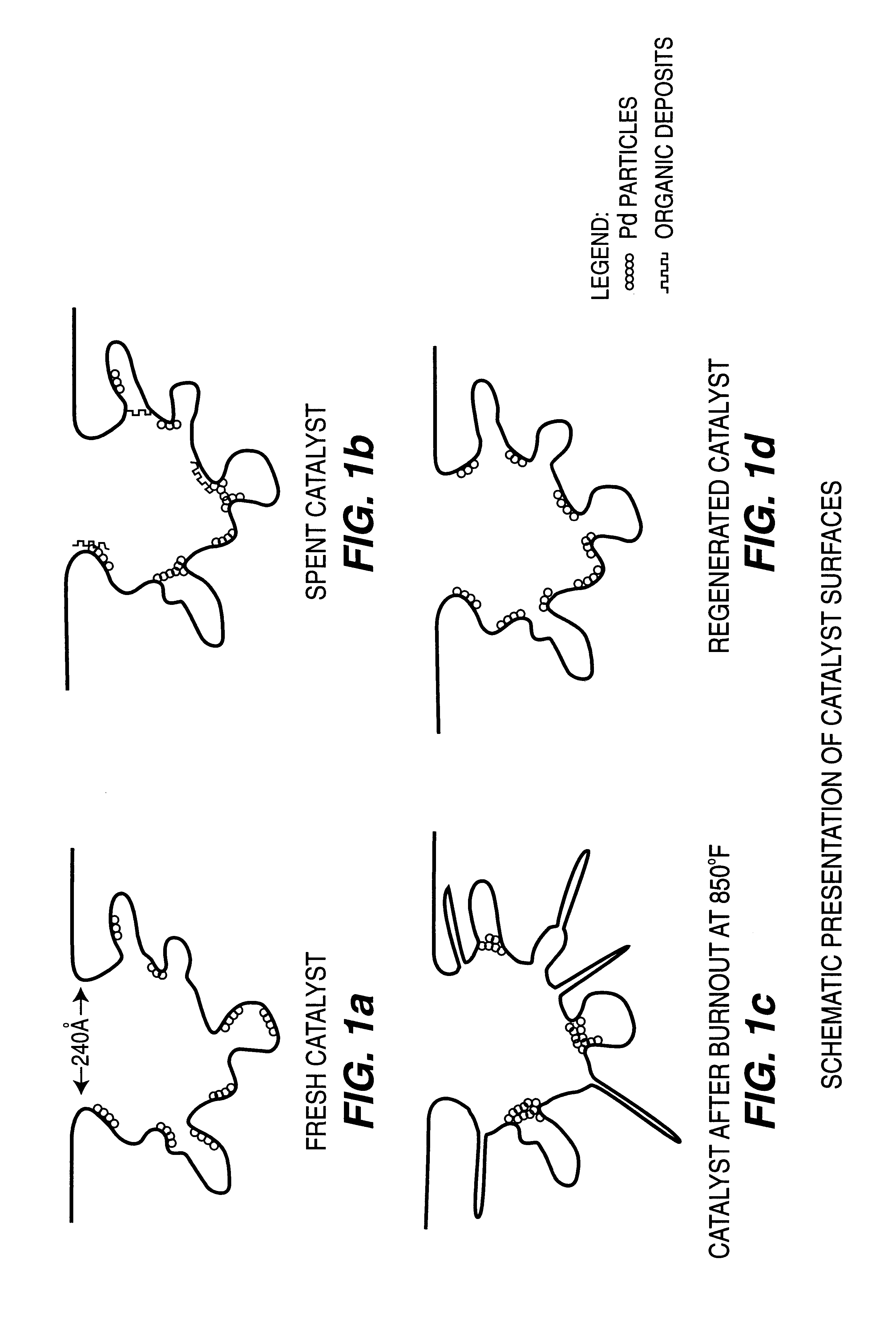
Regeneration of used supported noble metal catalysts
InactiveUS6740615B2Efficient removalImproves expositionHydrogen peroxideOther chemical processesPalladium catalystMetal particle
A method for regenerating used supported noble metal catalysts, which method includes solvent cleaning the used catalyst by contact with a suitable organic liquid cleaning solvent such as alcohols, ketones and such to remove organic deposits from the catalyst, followed by drying and calcining at elevated temperature to remove any remaining organic deposits from the catalyst, then treating the catalyst with an organo-metallic complex forming agent having ionization constant pK1 greater than about 2.5, such as glycolic acid and the like. The organic-metallic complex forming agent acts to break down large clusters of noble metal particles such as palladium (Pd) and redistributes the metal particles on the catalyst support such as alumina (Al2O3) in the same or other larger pores, so as to increase catalyst surface area and catalytic activity to provide a catalytic activity level at least 80% or even exceeding that of the fresh catalyst. This regeneration method is particularly useful for regenerating used supported palladium catalysts utilized for hydrogenation of ethyl anthraquinone (EAQ) for producing hydrogen peroxide (H2O2) product.
Owner:POROCEL INT LLC +1
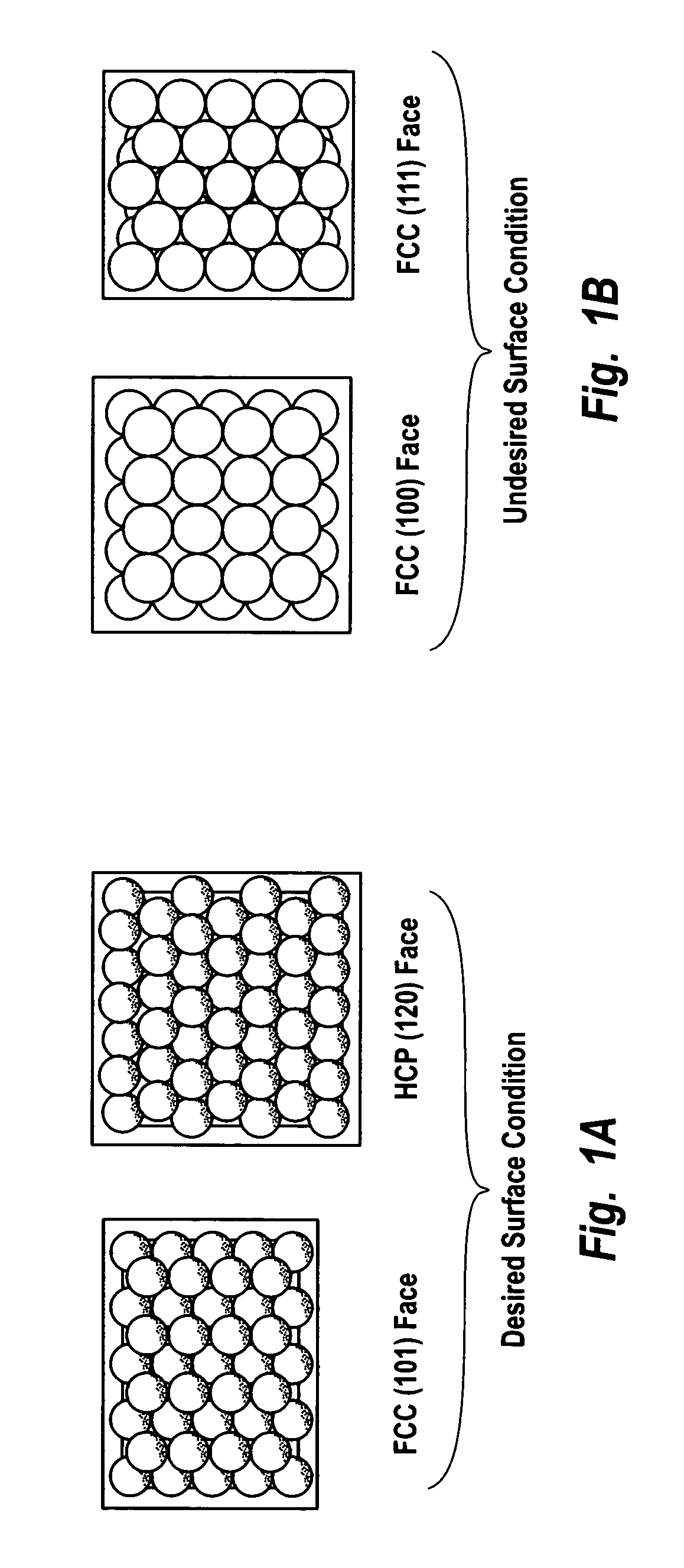
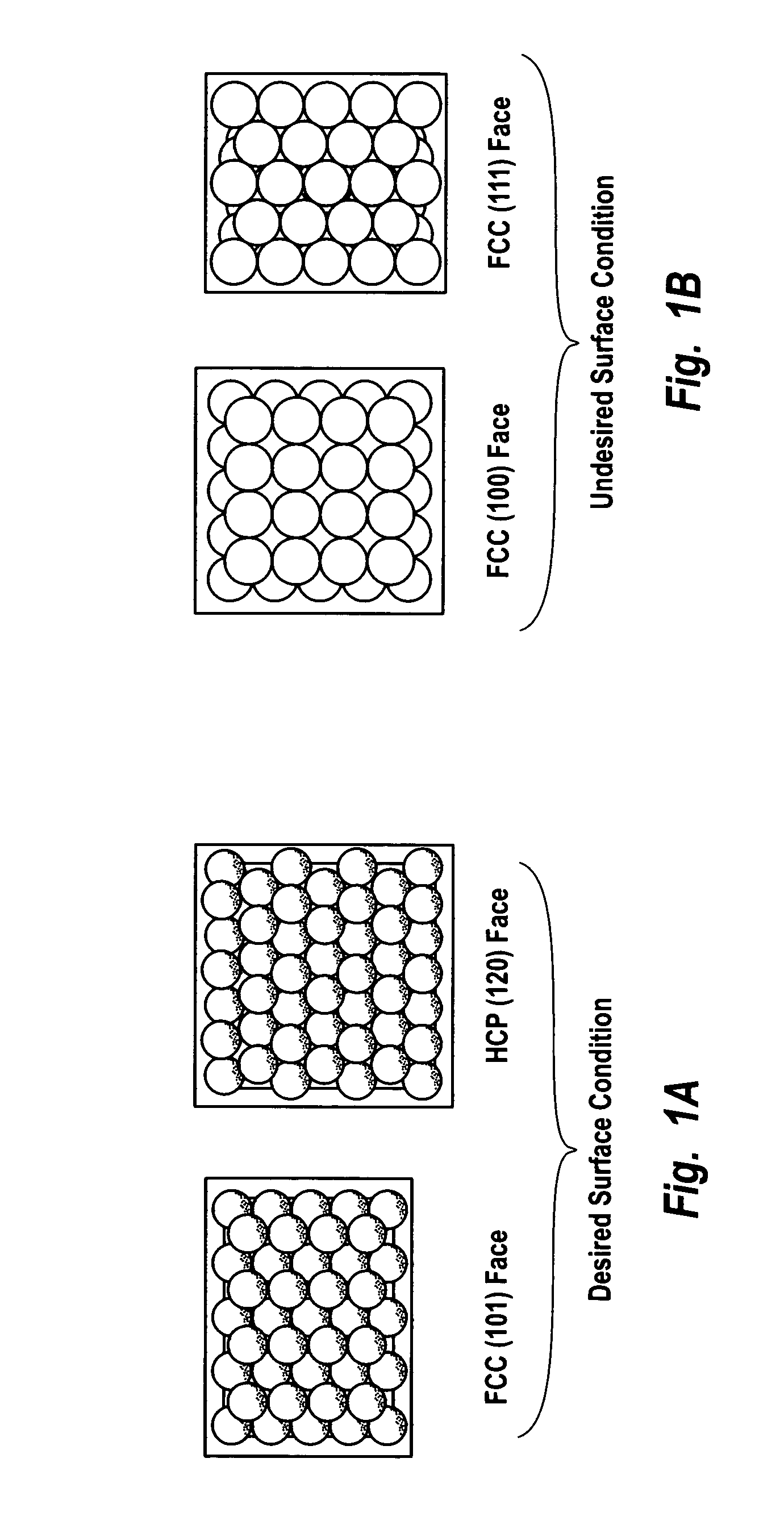
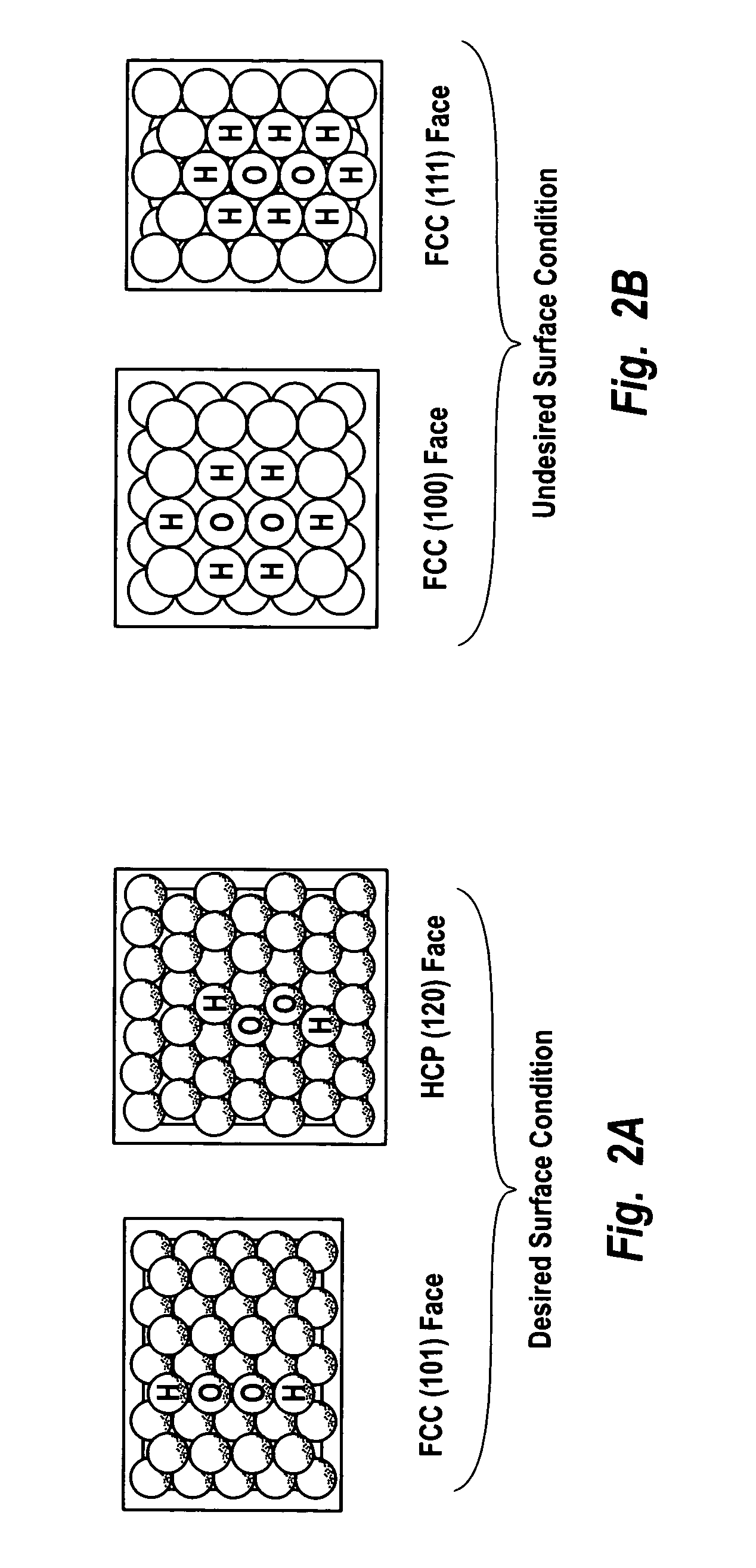
Supported catalysts having a controlled coordination structure and methods for preparing such catalysts
ActiveUS7011807B2Reduce molecular weightReduce the possibilityHydrogen peroxideHydrogenHydrogenOrganic fluid
Supported reactive catalysts having a controlled coordination structure and methods for their production are disclosed. The supported catalysts of the present invention are useful for the preparation of hydrogen peroxide with high selectivity in addition to other chemical conversion reactions. The supported catalyst comprises catalyst particles having top or outer layer of atoms in which at least a portion of the atoms exhibit a controlled coordination number of 2. The catalyst and methods may be used for the concurrent in situ and ex situ conversion of organic compounds. In addition, a process is provided for catalytically producing hydrogen peroxide from hydrogen and oxygen feeds by contacting them with the catalysts of the invention and a suitable organic liquid solvent having a Solvent Selection Parameter (SSP) between 0.14×10−4 and 5.0×10−4.
Owner:BORAL IP HLDG
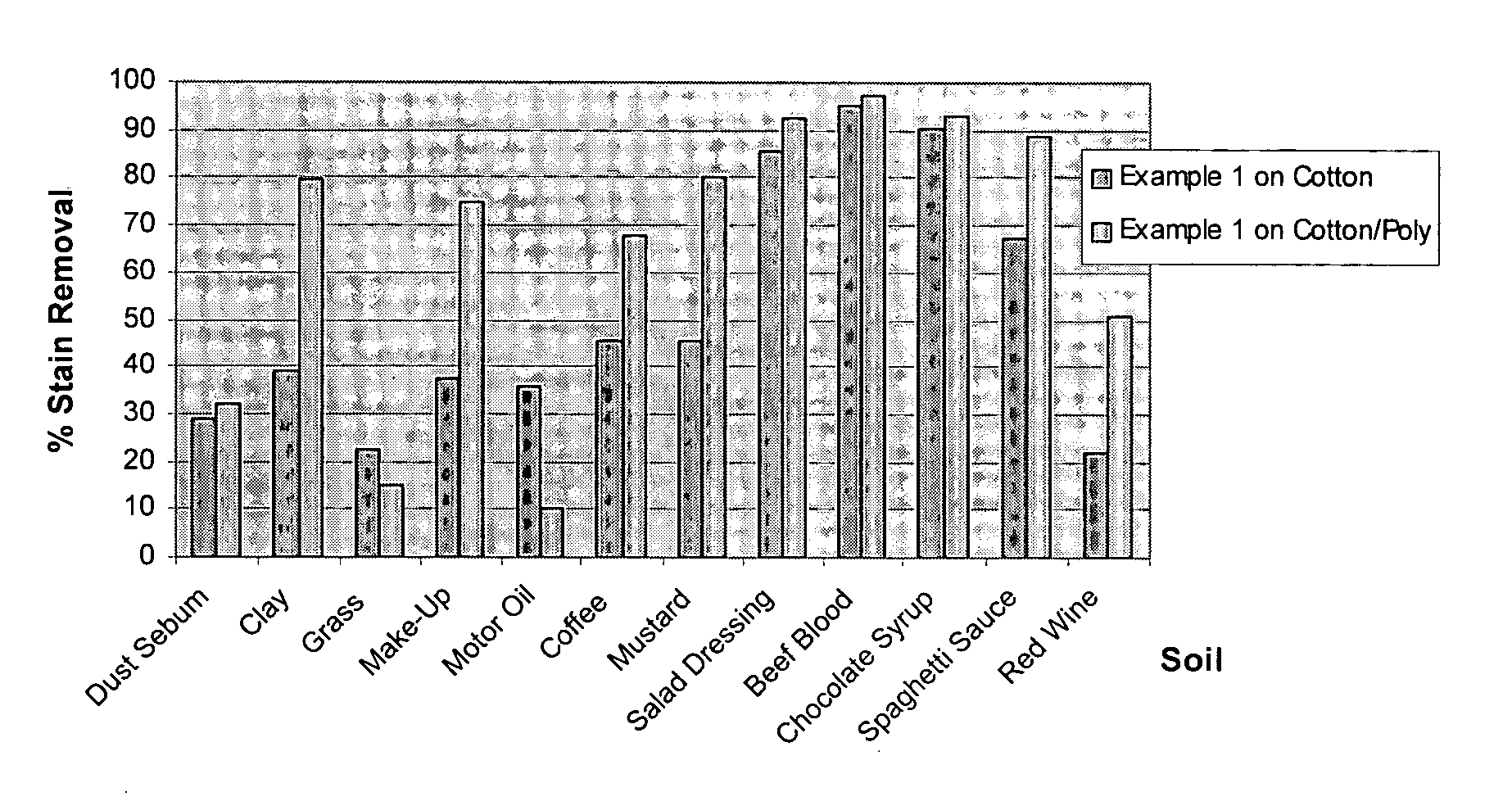
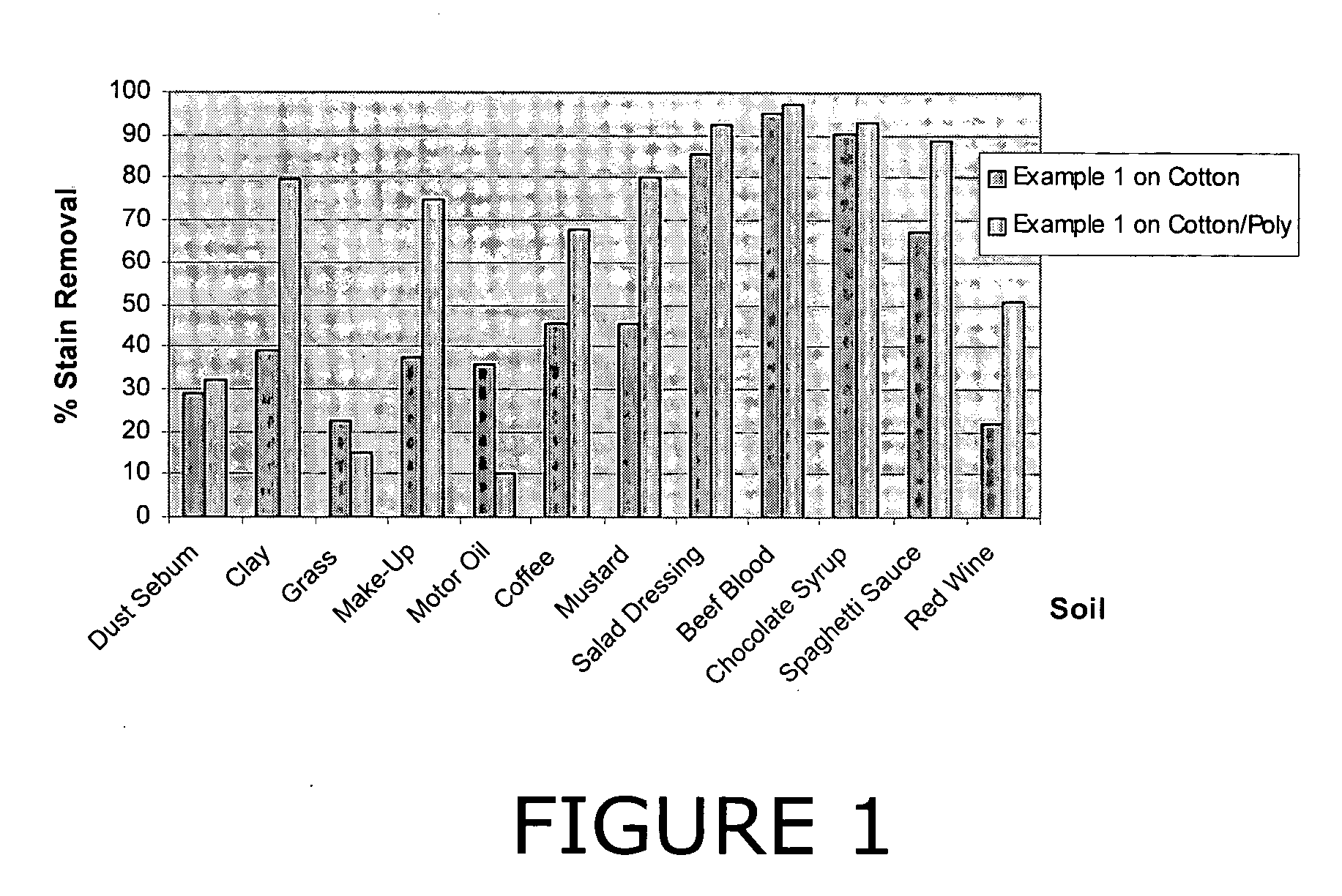
Decontamination formulations for disinfection and sterilization
Aqueous decontamination formulations that neutralize biological pathogens for disinfection and sterilization applications. Examples of suitable applications include disinfection of food processing equipment, disinfection of areas containing livestock, mold remediation, sterilization of medical instruments and direct disinfection of food surfaces, such as beef carcasses. The formulations include at least one reactive compound, bleaching activator, inorganic base, and water. The formulations can be packaged as a two-part kit system, and can have a pH value in the range of 7-8.
Owner:NAT TECH & ENG SOLUTIONS OF SANDIA LLC
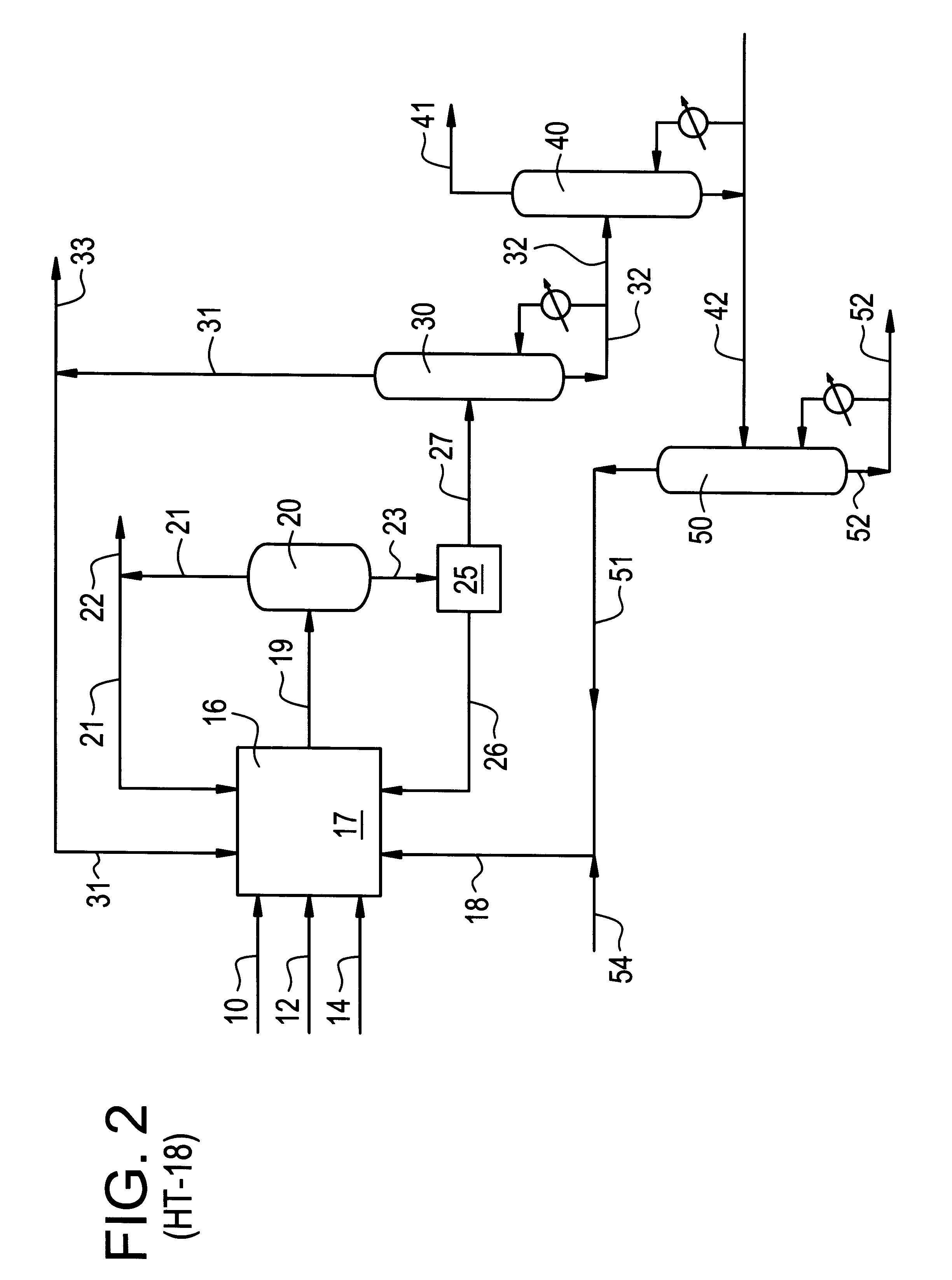
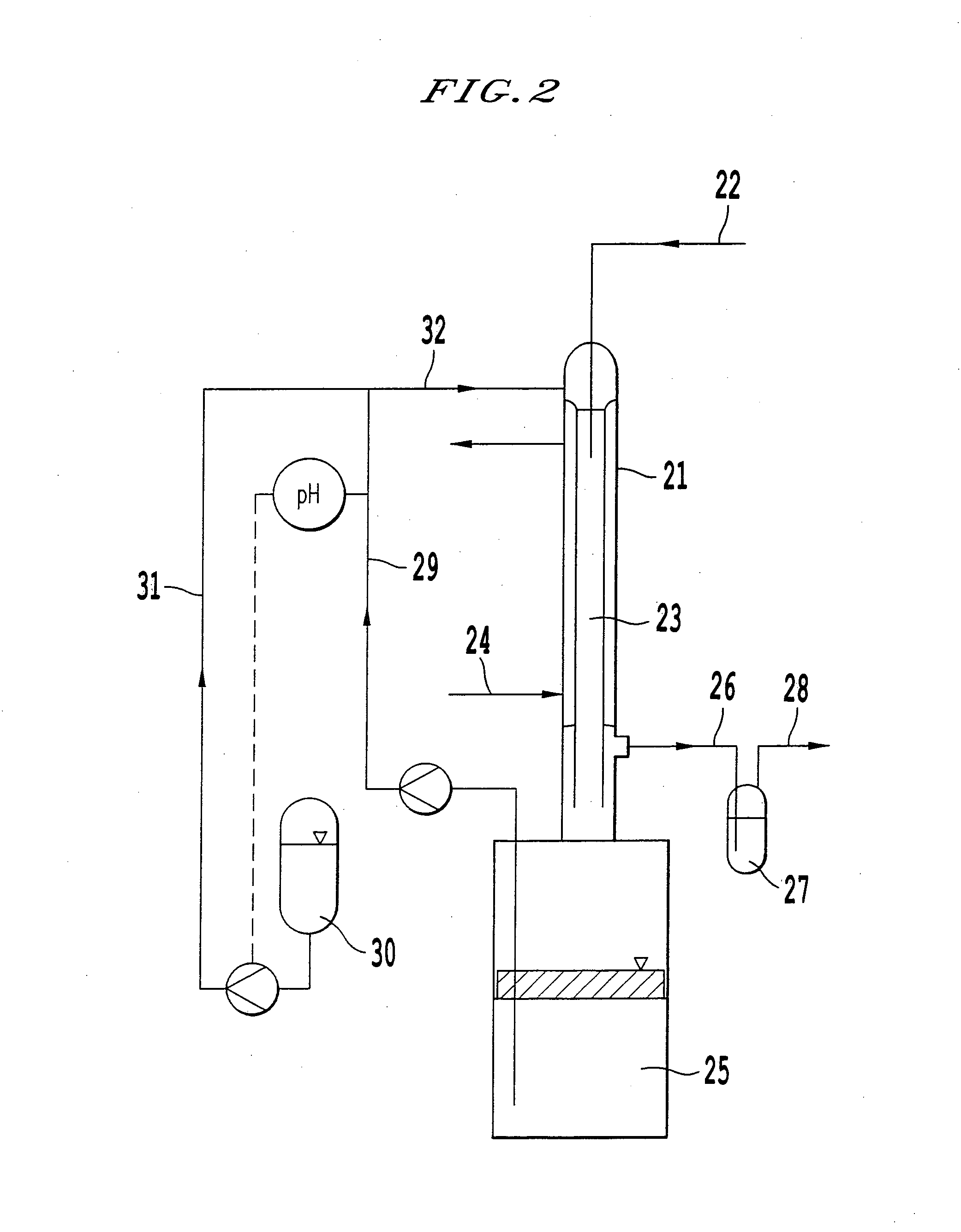
Process for producing hydrogen peroxide using microchannel technology
This invention relates to a process for making hydrogen peroxide in a microchannel reactor. The process comprises flowing a process feed stream and a staged addition feed stream in a process microchannel in contact with each other to form a reactant mixture comprising O2 and H2, and contacting a catalyst with the reactant mixture in the process microchannel to convert the reactant mixture to a product comprising hydrogen peroxide; transferring heat from the process microchannel to a heat exchanger; and removing the product from the process microchannel.
Owner:VELOCYS CORPORATION
Reactive formulations for a neutralization of toxic industrial chemicals
InactiveUS7125497B1Efficiently neutralizedHydrogen peroxideLiquid degasificationBoron trichlorideMalathion
Decontamination formulations for neutralization of toxic industrial chemicals, and methods of making and using same. The formulations are effective for neutralizing malathion, hydrogen cyanide, sodium cyanide, butyl isocyanate, carbon disulfide, phosgene gas, capsaicin in commercial pepper spray, chlorine gas, anhydrous ammonia gas; and may be effective at neutralizing hydrogen sulfide, sulfur dioxide, formaldehyde, ethylene oxide, methyl bromide, boron trichloride, fluorine, tetraethyl pyrophosphate, phosphorous trichloride, arsine, and tungsten hexafluoride.
Owner:NAT TECH & ENG SOLUTIONS OF SANDIA LLC
Nanoparticles of cerium oxide having superoxide dismutase activity
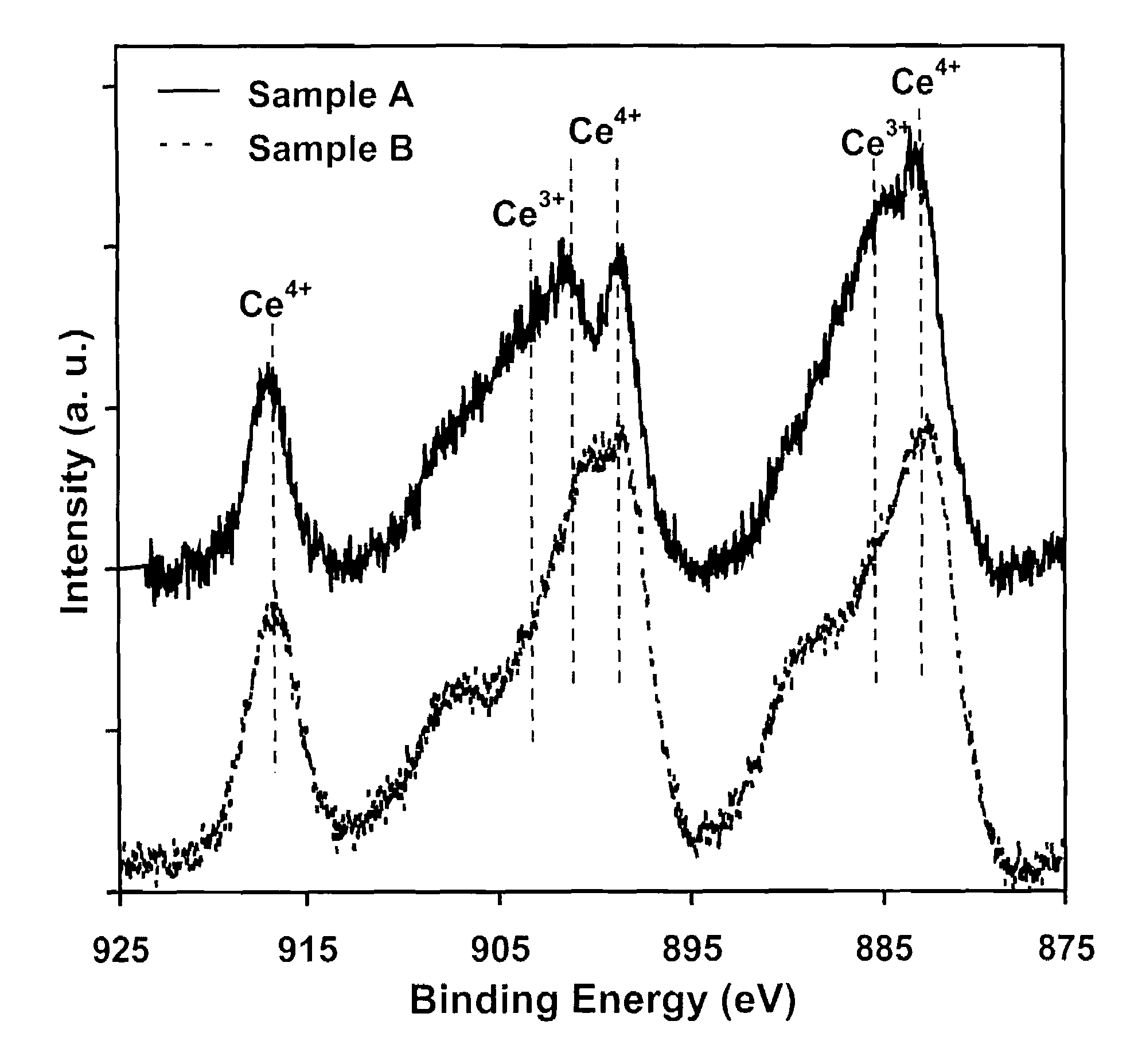
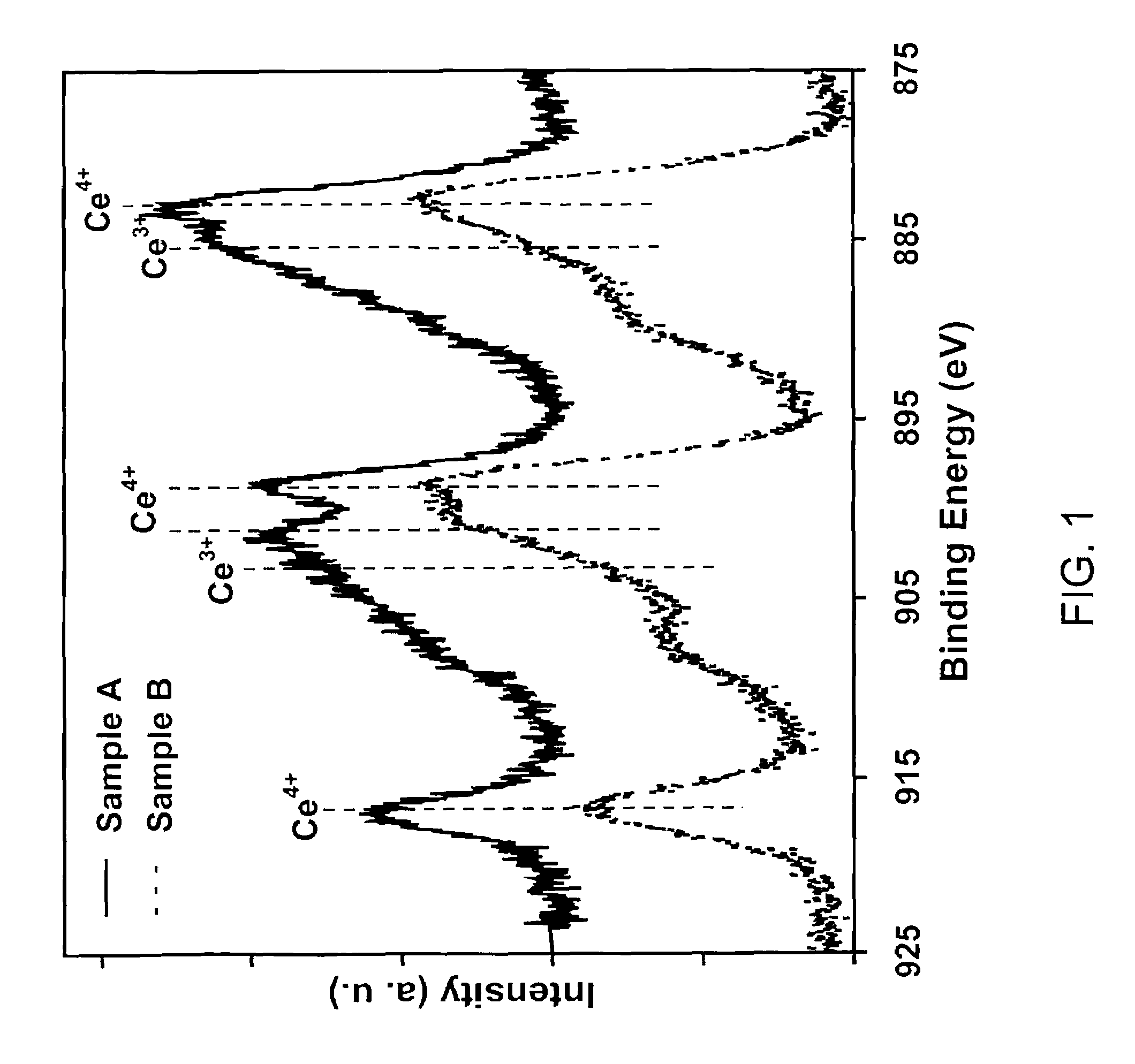
ActiveUS7504356B1Extend your lifeEffectively scavenge reactive molecular speciesHydrogen peroxideMaterial nanotechnologyOxygen vacancyCerium oxide
A synthetic catalyst providing superoxide dismutase activity consists essentially of monodispersed nanoparticles of cerium oxide having a crystal lattice containing cerium in mixed valence states of Ce3+ and Ce4+ wherein the Ce4+ valence state predominates and containing an enhanced Ce3+ / Ce4+ ratio and an effective number of oxygen vacancies in the crystal lattice so as to increase catalytic efficiency. A method of making the synthetic catalyst includes dissolving hydrous Ce(NO3)3 in water so as to form a solution, stirring the solution, adding hydrogen peroxide, heating and stopping when the solution develops a light yellow color.
Owner:UNIV OF CENT FLORIDA RES FOUND INC
Decontamination formulations for disinfection and sterilization
Owner:NAT TECH & ENG SOLUTIONS OF SANDIA LLC
Photolytic oxygenator with carbon dioxide and/or hydrogen separation and fixation
InactiveUS7883610B2Safely vented to the atmosphereAdvanced technologyHydrogen peroxideCellsLiquid mediumHydrogen
Apparatus for oxygenating an enclosed space as well as removing carbon dioxide from the enclosed space. The apparatus comprises a photolytic cell (16) having an anode compartment with a photo-active surface having the ability to convert water to oxygen; a cathode compartment having the ability to convert carbon dioxide and hydrogen to a solid or liquid medium; and a light source (20) for providing light photons (21) to said photolytic cell and activating the photo-reactive surface.
Owner:BATTELLE MEMORIAL INST
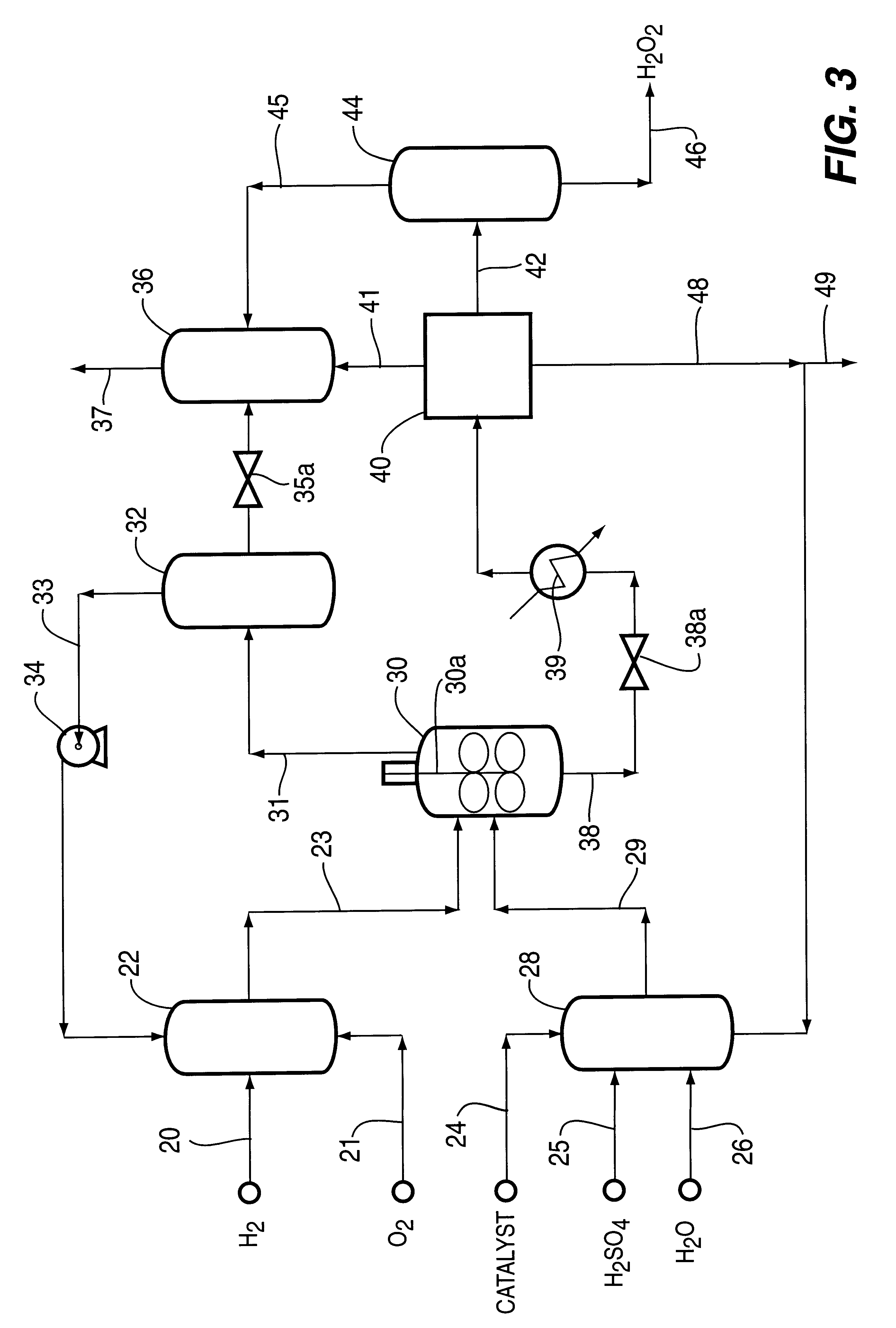
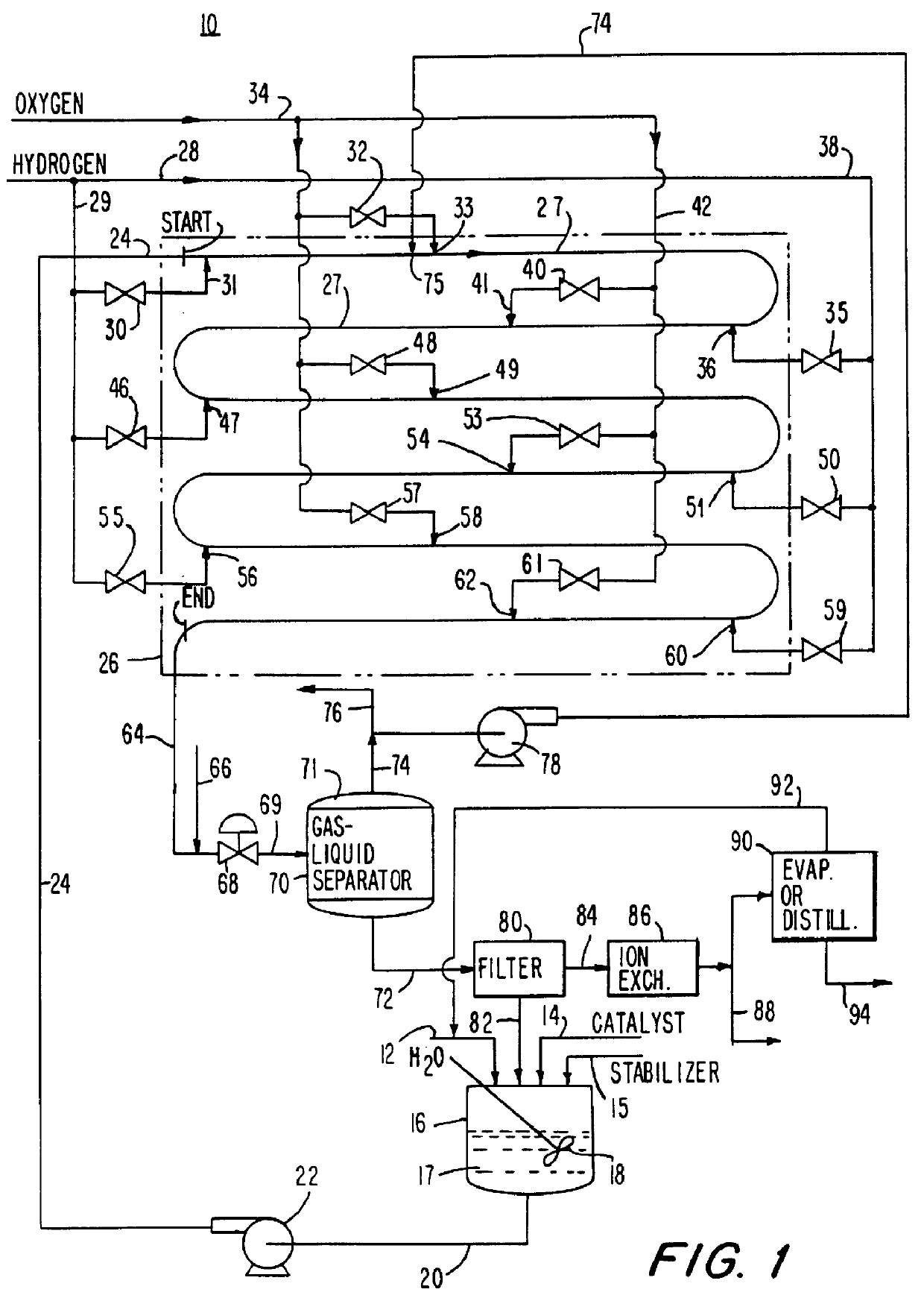
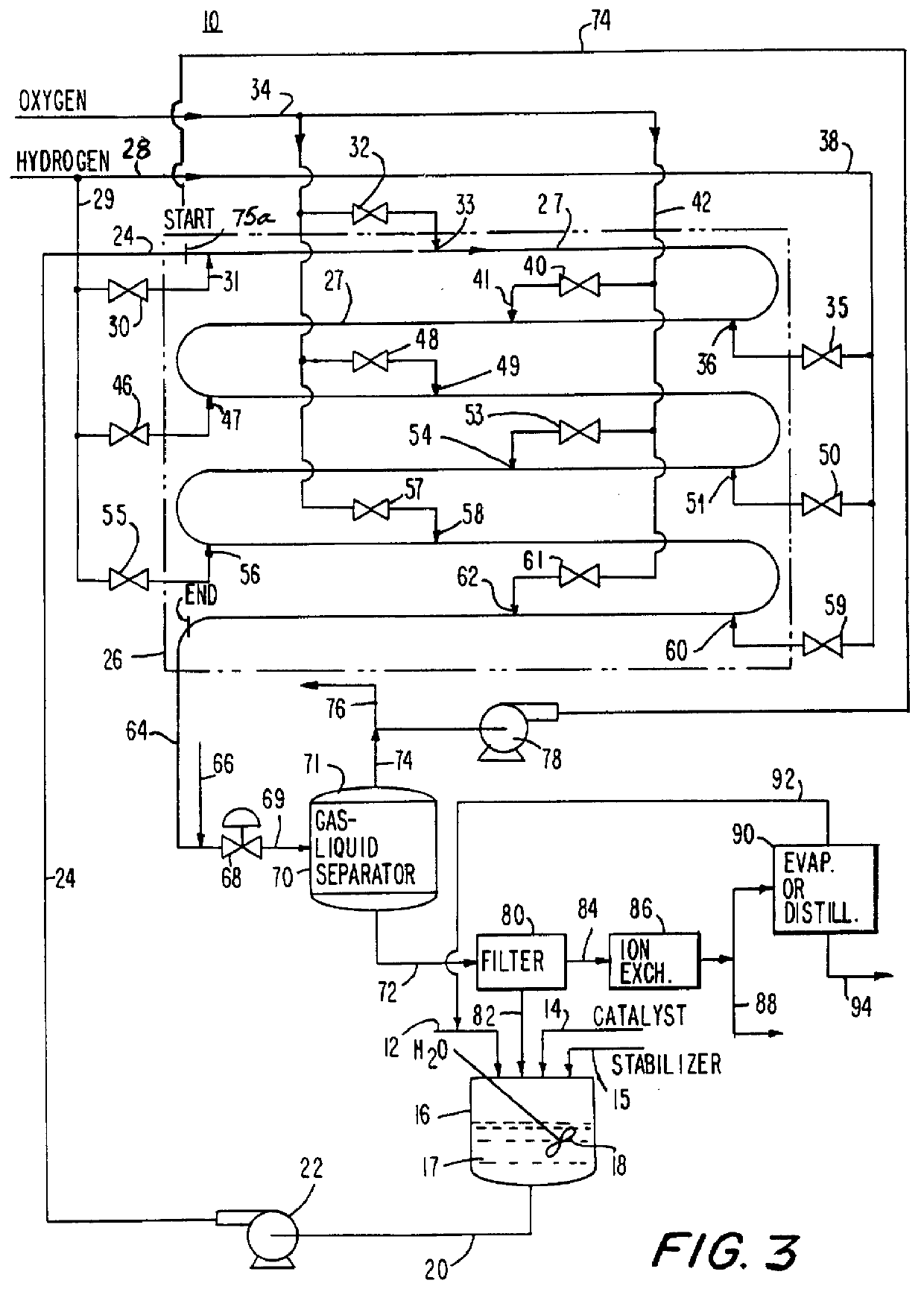
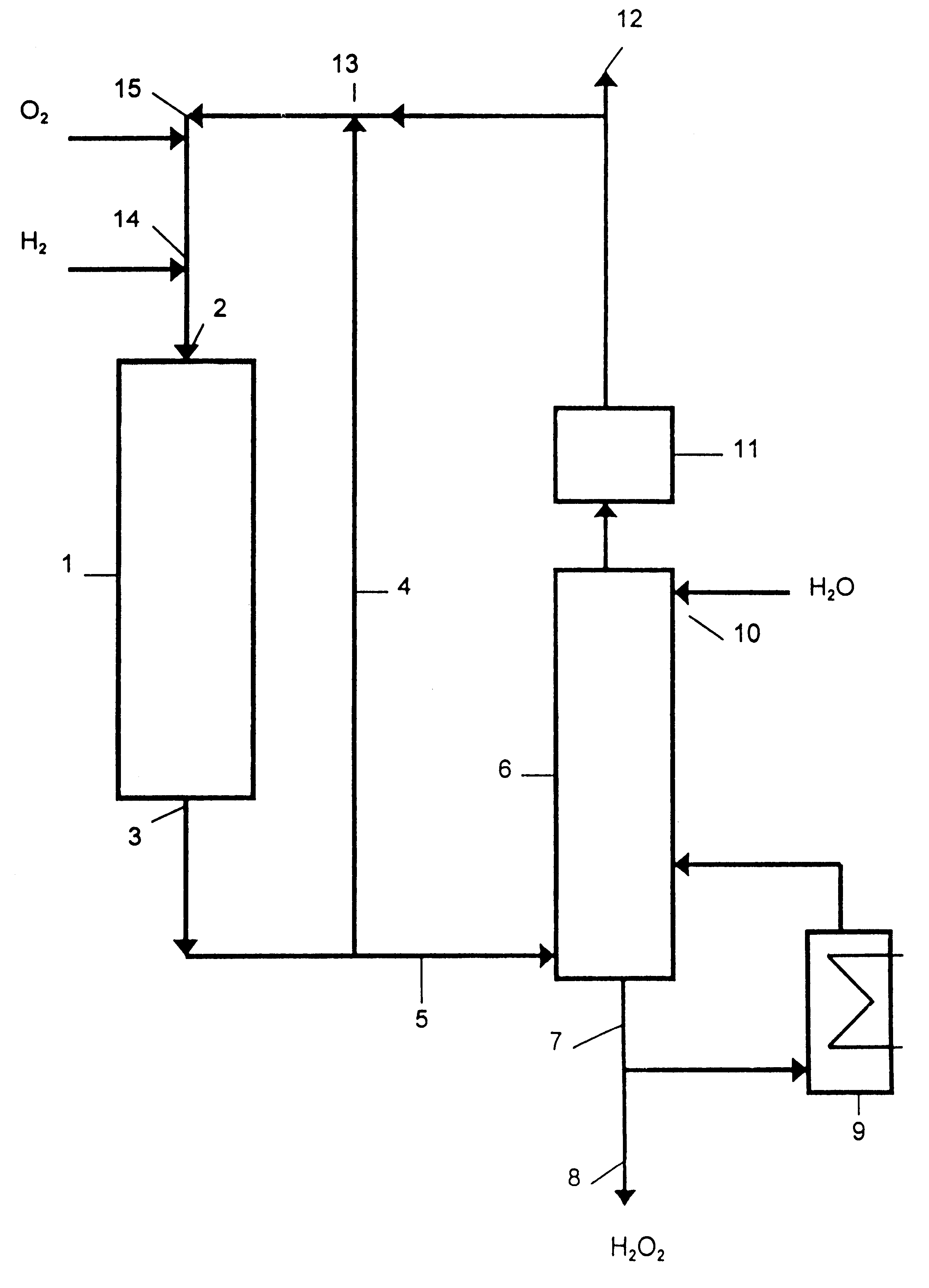
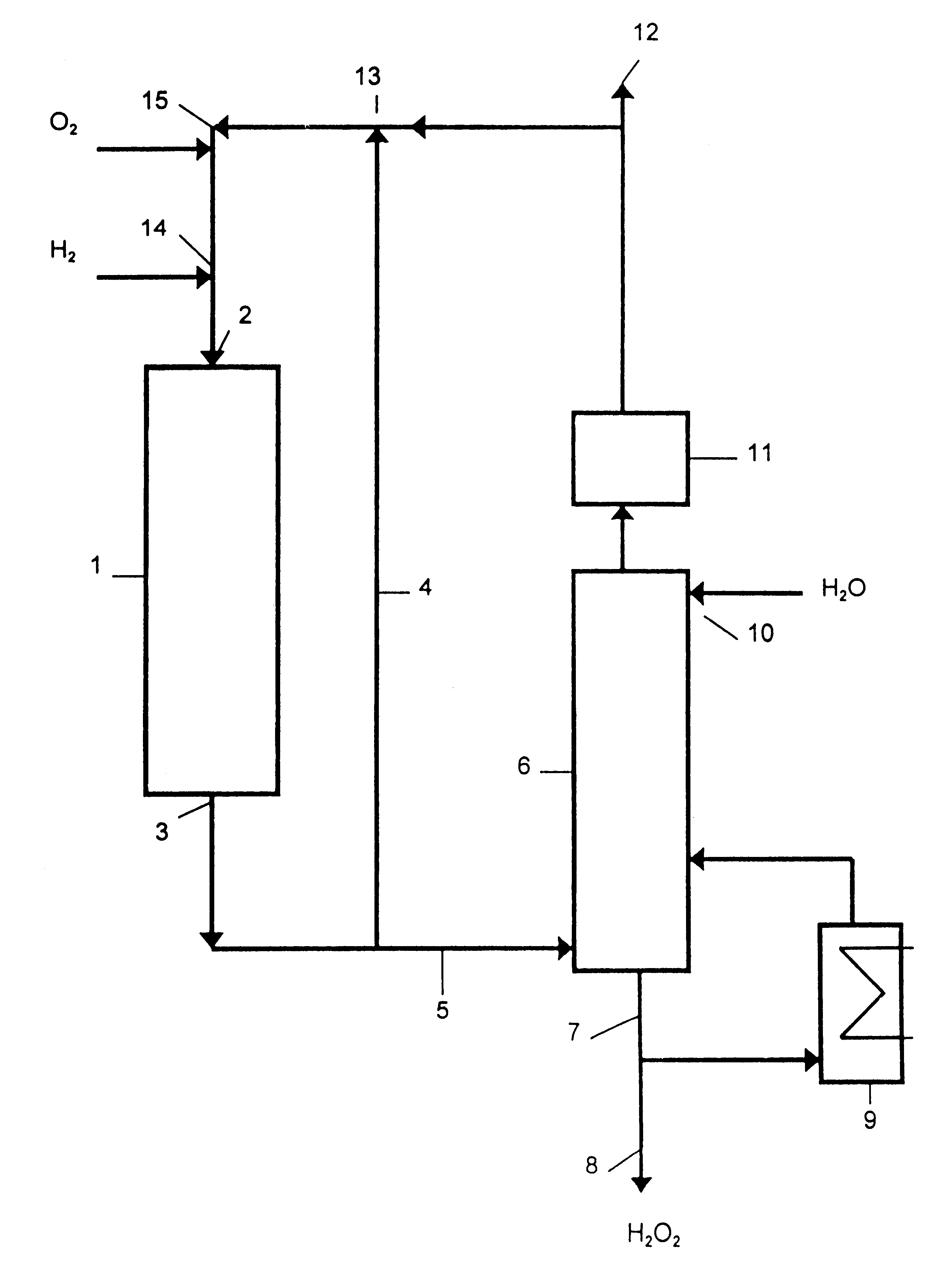
Method for producing hydrogen peroxide from hydrogen and oxygen
InactiveUS6042804ASafe and efficientFull utilization of entire volume of reactorHydrogen peroxideGas-gas reaction processesPtru catalystLiquid medium
The invention relates to a method and apparatus for safely producing hydrogen peroxide by injecting dispersed minute bubbles of hydrogen and oxygen into a rapidly flowing liquid medium. The minute bubbles are surrounded by the liquid medium of sufficient volume for preventing an explosive reaction between the hydrogen and oxygen. The liquid medium is formed of an acidic aqueous solution and a Group VIII metal catalyst. Hydrogen is sparged into the flowing medium for dissolution of the hydrogen in the medium. Oxygen bubbles are reacted with the dissolved hydrogen for producing hydrogen peroxide. Preferably, the liquid medium has a velocity of at least 10 feet per second for providing a bubbly flow regime in the reactor. The invention allows the direct combination of oxygen and hydrogen while preventing propagation of an explosive condition within the reactor. The method and apparatus provide for the safe production of hydrogen peroxide with low manufacturing costs.
Owner:PRINCETON ADVANCED TECH
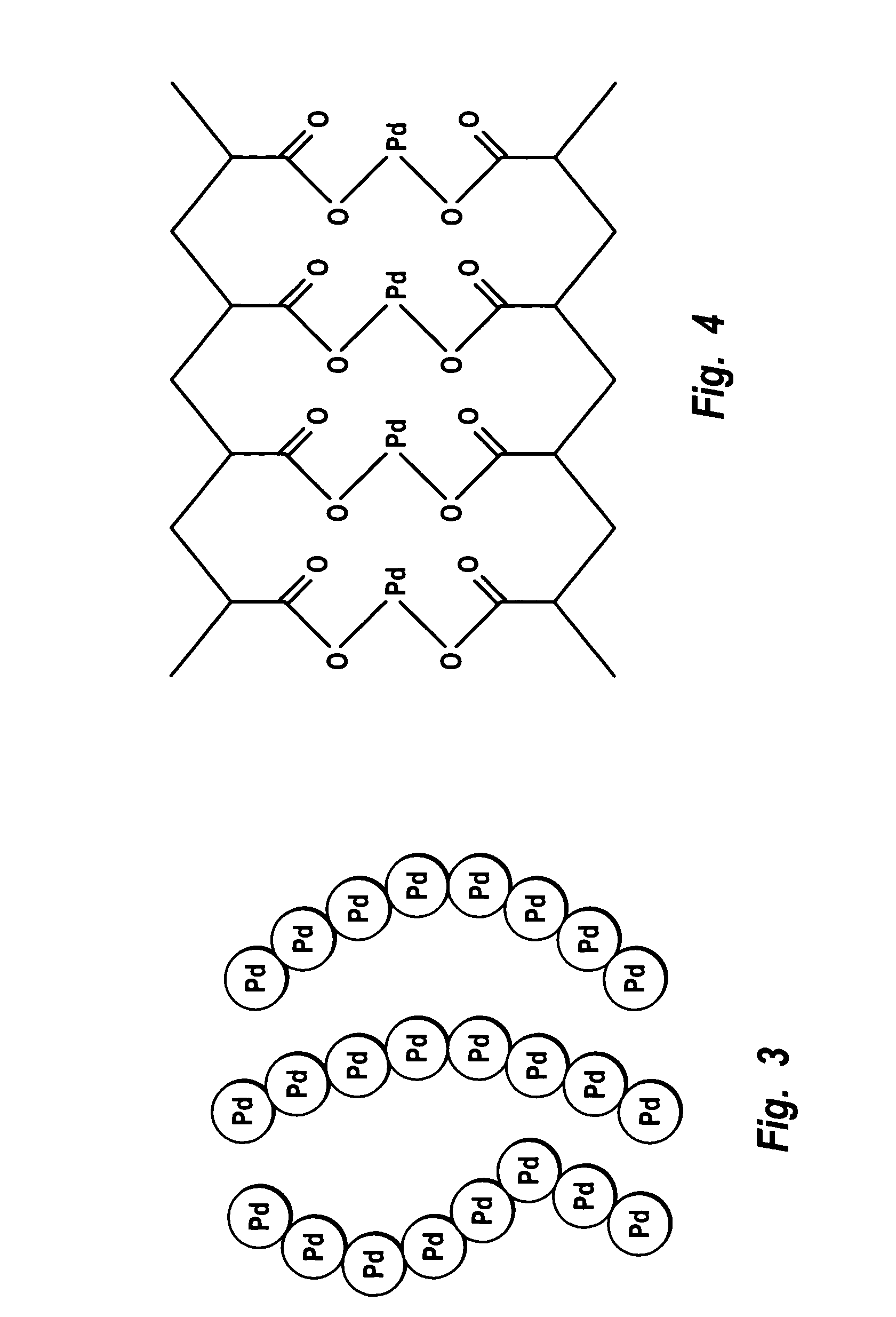
Membrane process for the production of hydrogen peroxide by non-hazardous direct oxidation of hydrogen by oxygen using a novel hydrophobic composite Pd-membrane catalyst
The above objects are achieved by providing a novel process for the production of hydrogen peroxide, by the non-hazardous direct oxidation of hydrogen by oxygen to hydrogen peroxide, without the formation of an explosive H2 and O2 gas mixture, using a novel tubular hydrophobic composite Pd-membrane catalyst, represented by a formula:Wherein: IPM is an inorganic porous membrane, permeable to all gases and vapors, in a form of tube having a thickness of at least 0.5 mm and internal diameter of at least 0.6 cm; Mx Pd1-x is a metal alloy, permeable only to hydrogen gas, deposited on the inner side of IPM; Pd is a palladium metal; M is a metal selected from copper, silver, gold, noble metals other than palladium, or a mixture of two or more thereof; x is a mole fraction of the metal M in the metal alloy (MxPd1-x) in the range from about 0.03 to about 0.6; (a) is a weight of the metal alloy per unit area of IPM in the range from about 5.0 g.m-2 to about 500 g.m-2; SOMF is a surface oxidized thin metal film comprising palladium which is permeable only to hydrogen, deposited on the metal alloy (Mx Pd1-x); (b) is a thickness of SOMF in the range from about 0.05 mum to about 5.0 mum; HPM is a hydrophobic polymer membrane permeable to hydrogen and oxygen gases and also to vapors of water and hydrogen peroxide but not to liquid water or aqueous solution; and (c) is a weight of the HPM per unit area of SOMF in the range from about 0.2 g.m-2 to about 40 g.m-2.
Owner:COUNCIL OF SCI & IND RES
Hydrophobic multicomponent catalyst useful for direct oxidation of hydrogen to hydrogen peroxide
InactiveUS6346228B1High selectivitySelectivity for in directHydrogen peroxideCatalyst protectionHydrogenHydrophobic polymer
The present invention relates to a novel hydrophobic multicomponent catalyst useful in the direct oxidation of hydrogen to hydrogen peroxide and to a method for the preparation of such catalyst. More specifically, this invention relates to a novel hydrophobic muticomponent catalyst comprising a hydrophobic polymer membrane deposited on a Pd containing acidic catalyst, useful for the direct oxidation of hydrogen by oxygen to hydrogen peroxide, an a method for preparing the same.
Owner:COUNCIL OF SCI & IND RES
Process for producing hydrogen peroxide
InactiveUS6210651B1Increase response rateHigh selectivityHydrogen peroxideCatalyst carriersStationary phaseHydrogen
The invention relates to a process for continuously producing hydrogen peroxide comprising the steps of feeding hydrogen and oxygen containing gas to a reactor provided with a catalyst; contacting the hydrogen and oxygen gas with the catalyst and thereby forming hydrogen peroxide, and; withdrawing hydrogen peroxide containing gas from the reactor; wherein the catalyst comprises a solid catalytically active material at least partially covered with a layer of a stationary phase different from the catalytically active material.
Owner:AKZO NOBEL NV
Supported catalysts having a controlled coordination structure and methods for preparing such catalysts
ActiveUS20050014635A1Reduce molecular weightReduce the possibilityHydrogen peroxideHydrogenHydrogenOrganic fluid
Supported reactive catalysts having a controlled coordination structure and methods for their production are disclosed. The supported catalysts of the present invention are useful for the preparation of hydrogen peroxide with high selectivity in addition to other chemical conversion reactions. The supported catalyst comprises catalyst particles having top or outer layer of atoms in which at least a portion of the atoms exhibit a controlled coordination number of 2. The catalyst and methods may be used for the concurrent in situ and ex situ conversion of organic compounds. In addition, a process is provided for catalytically producing hydrogen peroxide from hydrogen and oxygen feeds by contacting them with the catalysts of the invention and a suitable organic liquid solvent having a Solvent Selection Parameter (SSP) between 0.14×10−4 and 5.0×10−4.
Owner:BORAL IP HLDG
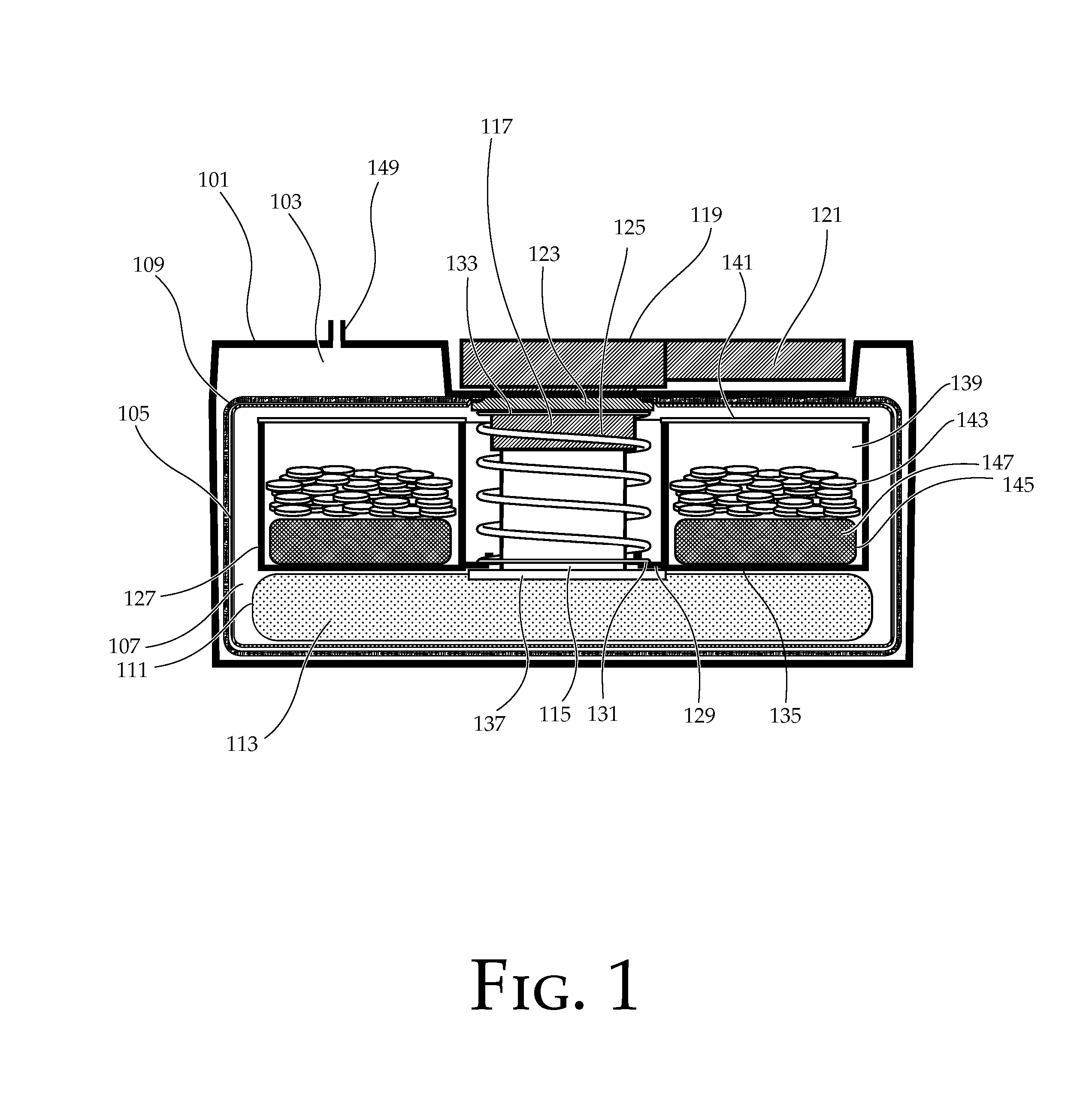
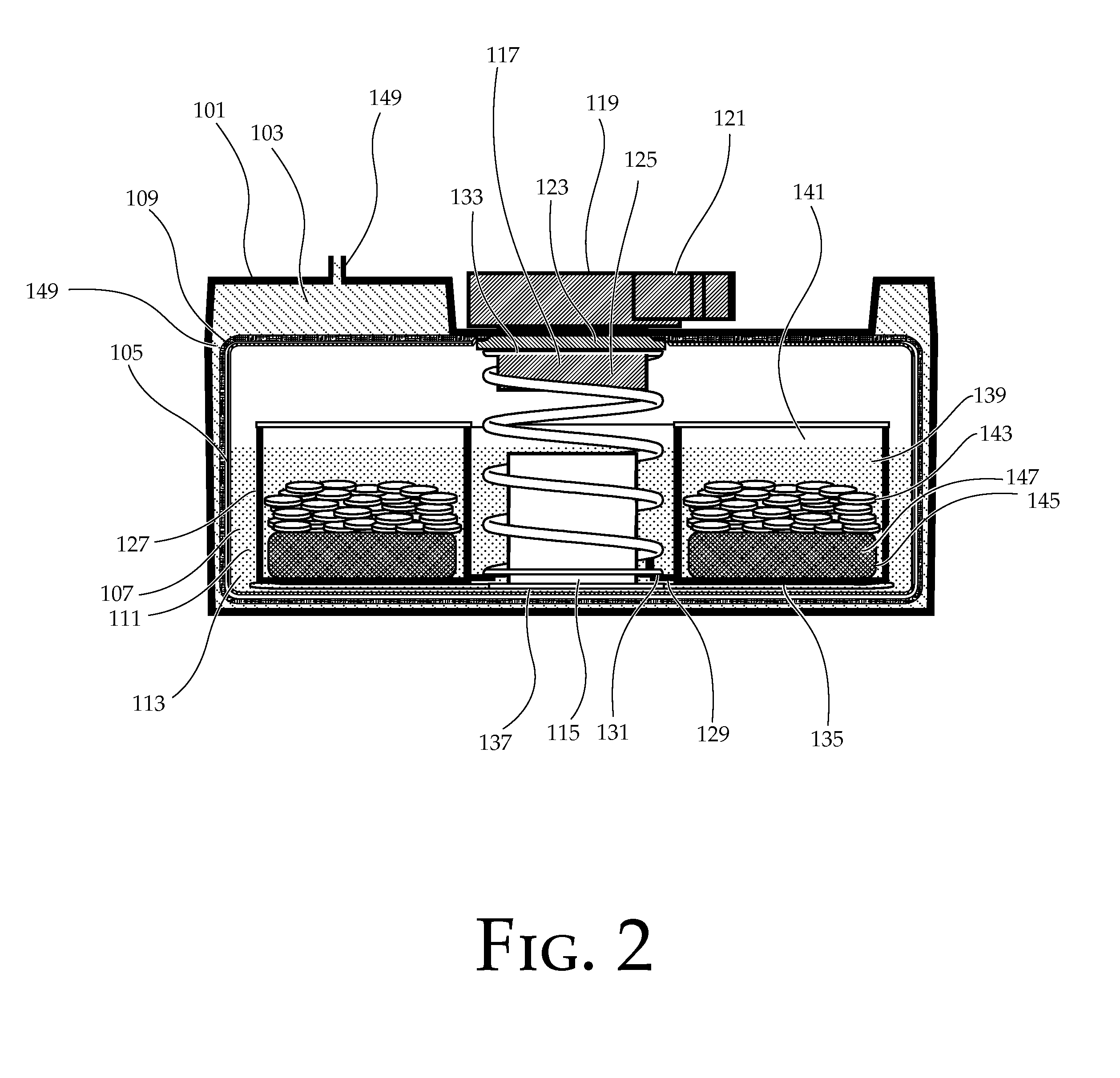
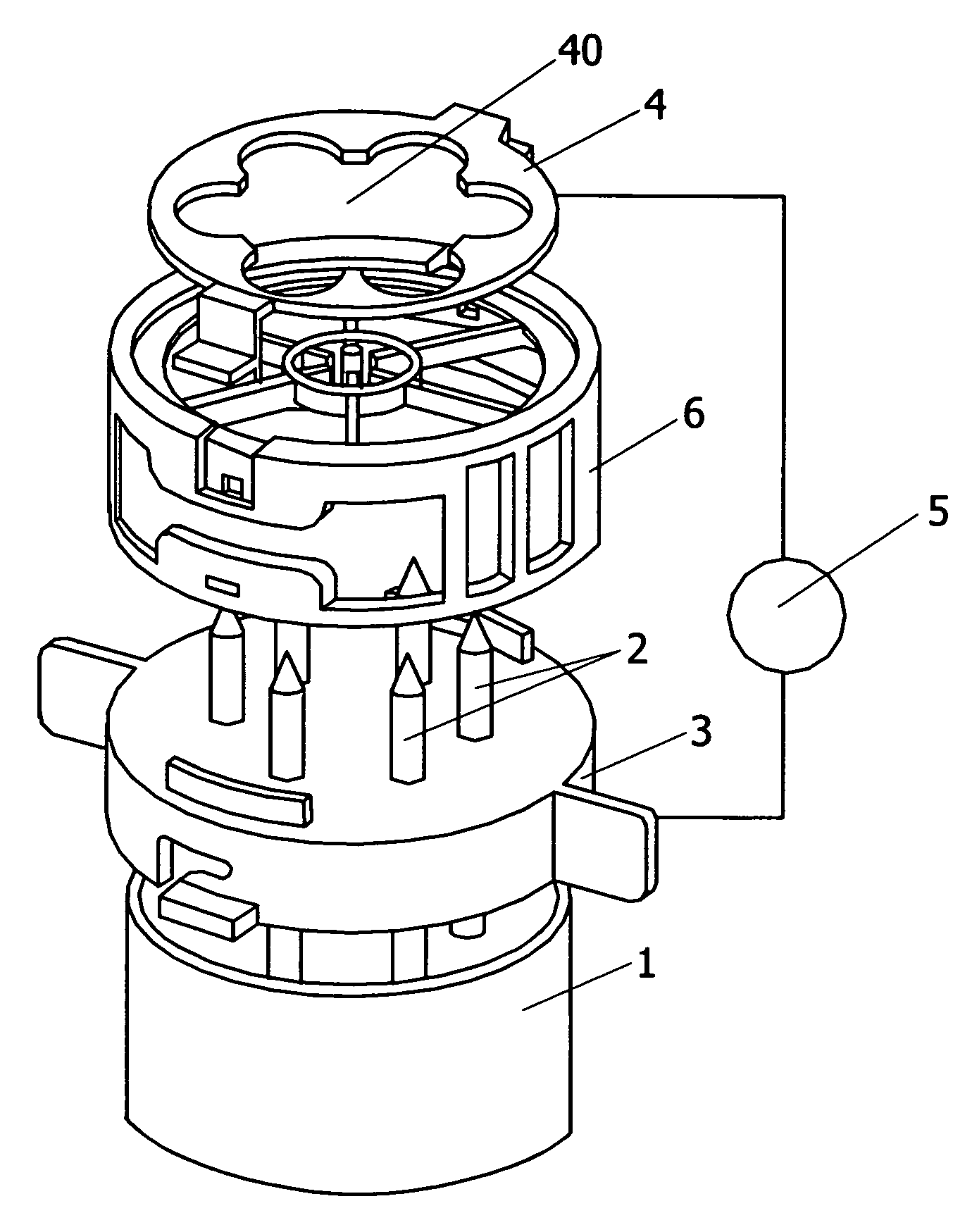
Portable chemical oxygen generator
A portable apparatus is provided which generates high purity breathable oxygen. The oxygen is produced in a reaction chamber from the reaction between an oxygen generating composition and a water / catalyst solution. The reaction chamber comprises a first compartment containing an oxygen peroxide adduct and a temperature stabilizing material formulated into controlled release tablets and a second sealed compartment containing a catalyst dispersed in an aqueous solution. The temperature stabilizing material undergoes an endothermic reaction upon dissolution in the solution. The tablets dissolve in the solution releasing hydrogen peroxide at a constant rate. The hydrogen peroxide is further decomposed into oxygen and water from contact with the catalyst. A first membrane made from a hydrophobic and gas permeable material, lets oxygen through while containing the reaction materials. A second membrane made of hydrophilic superabsorbent material and impregnated with catalyst particles absorbs any unreacted hydrogen peroxide letting high purity oxygen through.
Owner:VIRGINIA COMMONWEALTH UNIV
Methods and Compositions for Controlled and Sustained Production and Delivery of Peroxides and/or Oxygen for Biological and Industrial Applications
InactiveUS20090169630A1Reduce chanceProtects against oxidative damageCosmetic preparationsBiocideOxygenAdduct
Methods and compositions for the controlled and sustained release of peroxides or oxygen to aqueous environments (e.g. a patient's body or circulatory system, or for other applications) or non-aqueous environments, include a material coating or encapsulating hydrogen peroxide, inorganic peroxides or peroxide adducts. In the case of peroxide adducts, and particularly in one type of embodiment, the peroxide adducts should be able to permeate the material, but water, hydrogen peroxide and inorganic peroxides should be able to permeate the material. The methods and compositions that allow the release of oxygen, H2O2 or inorganic peroxides from peroxide adducts with movement of these moieties across a selectively permeable barrier into, preferably, an aqueous environment. In the case of hydrogen peroxide, it can be acted upon by catalase or other enzymes, or be simply degraded, or are otherwise acted upon by enzymes or catalysts embedded in the selectively permeable barrier to produce, for example, O2. Alternatively, hydrogen peroxide or inorganic peroxides can be delivered selectively to a site of action of cleaning, disinfecting or other applications.
Owner:VIRGINIA COMMONWEALTH UNIV
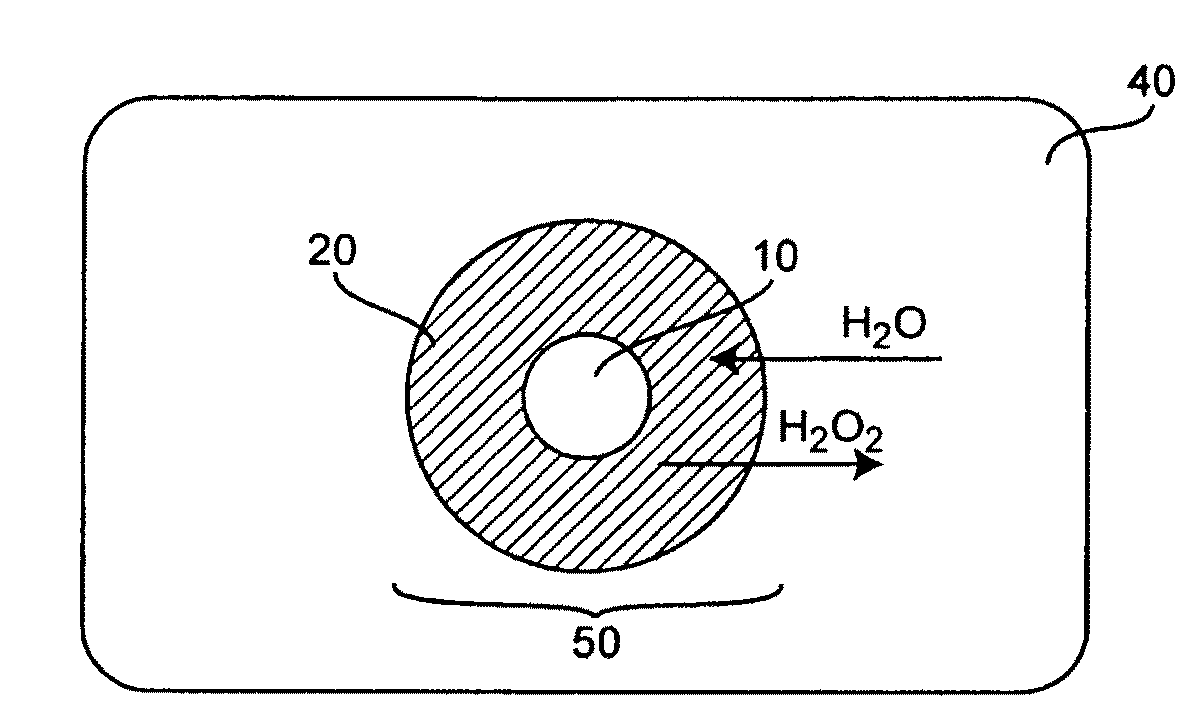
Concentration of hydrogen peroxide
Methods for concentrating hydrogen peroxide solutions have been described. The methods utilize a polymeric membrane separating a hydrogen peroxide solution from a sweep gas or permeate. The membrane is selective to the permeability of water over the permeability of hydrogen peroxide, thereby facilitating the concentration of the hydrogen peroxide solution through the transport of water through the membrane to the permeate. By utilizing methods in accordance with the invention, hydrogen peroxide solutions of up to 85% by volume or higher may be generated at a point of use without storing substantial quantities of the highly-concentrated solutions and without requiring temperatures that would produce explosive mixtures of hydrogen peroxide vapors.
Owner:NASA
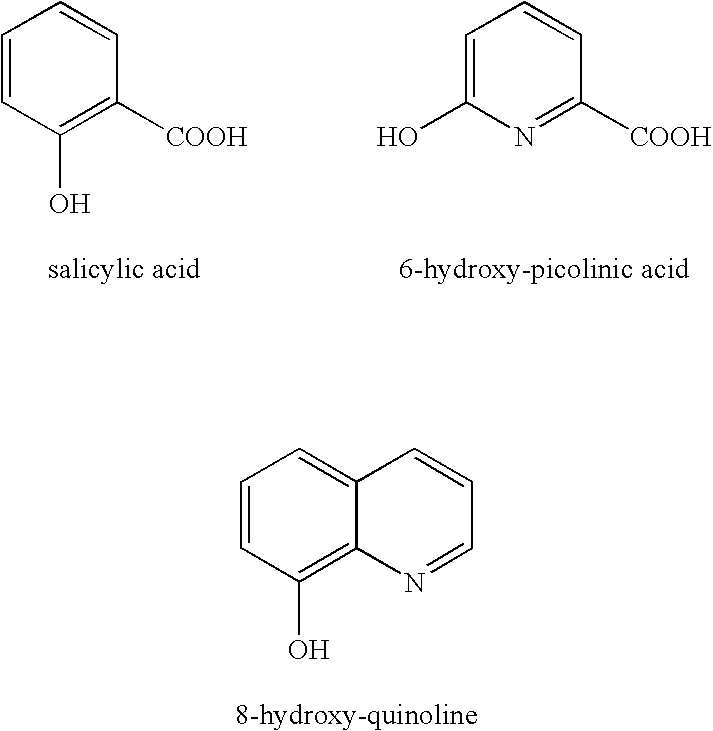
Method for the preparation of reactive compositions containing hydrogen peroxide
The subject invention provides a potentially economically viable method for the preparation of hydrogen peroxide (H2O2) in deep eutectic solvents (DES). H2O2 is then used for the destruction of small to large quantities of sulfur and nitrogen mustards and lewisite, their homologous / analogues, and similar chemical warfare agents at ambient conditions in DES without producing any toxic by-products. Furthermore, H2O2 has been used for the destruction of small to large quantities of halogenated hydrocarbons, their homologous / analogues, and similar hazardous chemicals at ambient conditions. H2O2 can be formed by either the electrochemical reduction of oxygen in DES in the presence of water or by dissolving Group 1 (alkali metals) or Group 2 (alkaline earth metals) superoxides, e.g. potassium superoxide, in DES in the presence of water, with / without chemicals used for the enhancement of the solubility of the metal superoxide in the DES, e.g. crown ethers.
Owner:KING SAUD UNIVERSITY
Liquid detergent formulation with hydrogen peroxide
ActiveUS20070087954A1Inorganic/elemental detergent compounding agentsHydrogen peroxideNon ionicOxygen
A stabilized basic aqueous liquid detergent composition is disclosed. The composition comprises hydrogen peroxide, a hydrogen peroxide stabilizer or a mixture of hydrogen peroxide stabilizers, an anionic surfactant or mixture of anionic surfactants, a non-ionic surfactant or mixture of anionic surfactants, and a builder or a mixture of builders. The composition maintains a homogeneous single phase, maintains active oxygen level, and gives a high level of cleaning performance.
Owner:ARKEMA INC
Process for making hydrogen peroxide
InactiveUS20050201925A1Easy to prepareEasy to useHydrogen peroxideOrganic-compounds/hydrides/coordination-complexes catalystsHydrogenSolvent
A process for making hydrogen peroxide directly from hydrogen and oxygen is disclosed. The process comprises reacting the gases in a solvent in the presence of a catalyst comprising a polymer-encapsulated transition metal. Polymer-encapsulated transition metal catalysts are easy to prepare and use, they are easy to recover and reuse, and they provide good conversions to hydrogen peroxide.
Owner:LYONDELL CHEM TECH LP
Liquid detergent formulation with hydrogen peroxide
A stabilized basic aqueous liquid detergent composition is disclosed. The composition comprises hydrogen peroxide, a hydrogen peroxide stabilizer or a mixture of hydrogen peroxide stabilizers, an anionic surfactant or mixture of anionic surfactants, a non-ionic surfactant or mixture of anionic surfactants, and a builder or a mixture of builders. The composition maintains a homogeneous single phase, maintains active oxygen level, and gives a high level of cleaning performance.
Owner:ARKEMA INC
Process for the separation of chlorosilanes from gas streams
Chlorosilanes are continuously removed from a gas stream in an apparatus in which the gas stream is treated in a first stage with water vapor in the gas phase, and in a second stage with a liquid, aqueous phase.
Owner:EVONIK DEGUSSA GMBH
Pulverulent mixtures containing hydrogen peroxide and hydrophobized silicon dioxide
InactiveUS20090252815A1Solve the stability is not highBiocideCosmetic preparationsControlled releaseTopical medication
The present invention is directed to pulverulent mixtures comprising hydrogen peroxide and hydrophobized, pyrogenically prepared silicon dioxide powder, preferably with a methanol wettability of at least 40. The pulverulent mixtures exhibit good storage stability and can be used for the controlled release of hydrogen peroxide and / or oxygen. The invention also includes methods of making these pulverulent mixtures and methods of using the mixtures in detergents, cleaning compositions, topical medications, antimicrobials and other products.
Owner:EVONIK DEGUSSA GMBH
Charged water particle, and method for creating environment where mist of charged water particle is dispersed
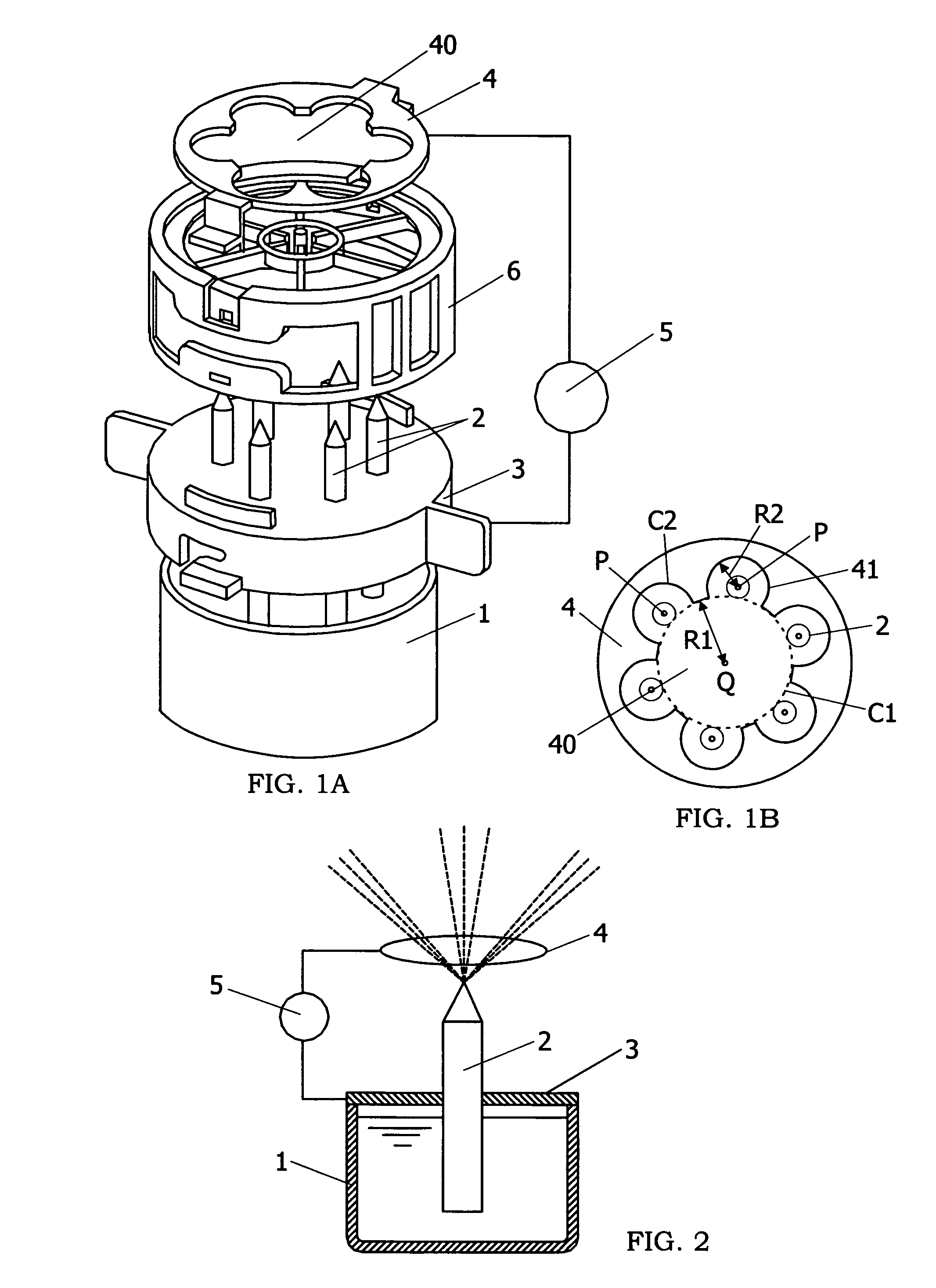
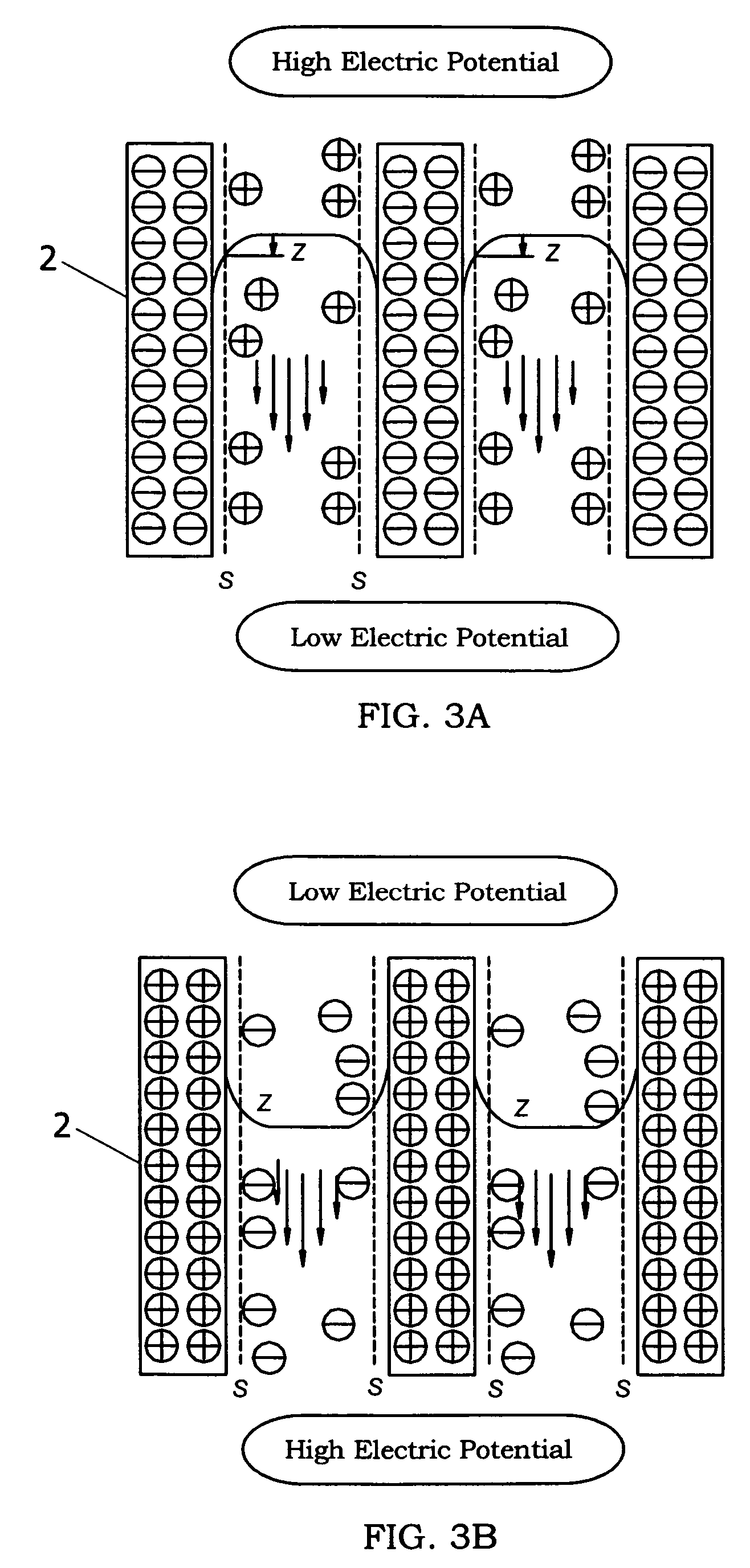
ActiveUS7473298B2Effectively inactivate bacteriaGood moisturizing effectHydrogen peroxideBurnersSuperoxideFine particulate
Owner:MATSUSHITA ELECTRIC WORKS LTD
Stabilization of alkaline hydrogen peroxide
ActiveUS20050226800A1Hydrogen peroxideInorganic/elemental detergent compounding agentsPyrophosphateAqueous solution
A stabilized basic aqueous hydrogen peroxide composition is disclosed. The composition contains about 0.3 wt % to about 15 wt % of hydrogen peroxide; water; and a stabilizer system that contains about 10 ppm to about 1% of a stannate stabilizer; 10 ppm to about 1% of a phosphonic acid chelating agent or a mixture of phosphonic acid chelating agents; and 10 ppm to about 1% of an aromatic chelating agent or a mixture of aromatic chelating agents. The composition comprises less than about 10 ppm pyrophosphate, preferably no pyrophosphate; and has a pH greater than 7.0, preferably about 8.0 to about 10.5.
Owner:ARKEMA INC
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com