High performance liquid phase detection method of homopiperazine
A high-performance liquid phase and detection method technology, applied in the field of liquid chromatography detection, can solve the problems of low accuracy, cumbersome operation, difficult to control, etc., and achieve the effects of high precision, good reproducibility and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
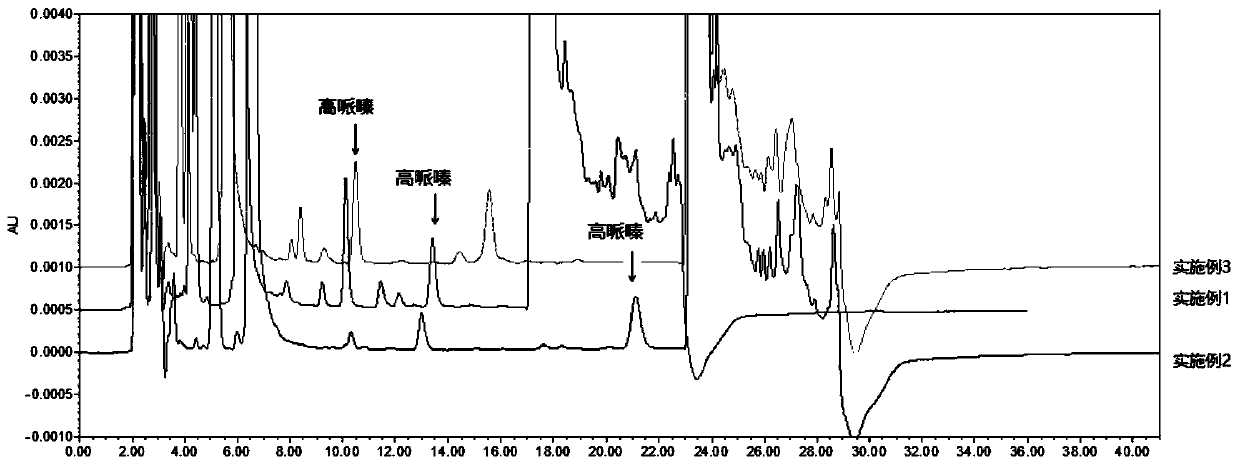
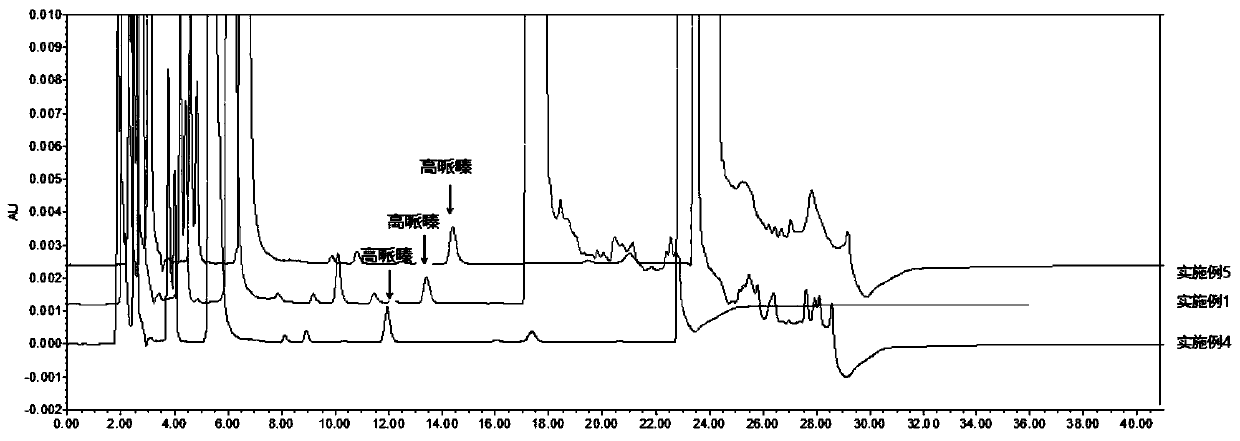
[0048] Chromatographic conditions use octadecylsilane bonded silica gel as filler; use 0.02mol / L sodium acetate trihydrate (pH=5.0)-acetonitrile (75:25) as mobile phase to adopt gradient elution; detection wavelength is 248nm, column The temperature was 30°C, and the injection volume was 10 μl.
[0049] Determination method: Precisely measure the appropriate amount of this product and homopiperazine reference substance, add water to dissolve and quantitatively dilute to make a solution containing about 0.6mg of fasudil hydrochloride and 0.6μg of homopiperazine in every 1ml, as the test solution; Another appropriate amount of homopiperazine reference substance was taken, accurately weighed, dissolved in water and quantitatively diluted to make a solution containing about 1.2 μg of homopiperazine per 1 ml, as the reference substance solution.
[0050] Sample derivation: Accurately measure 10 μl of the test solution, place it in a dry derivation tube with a diameter of 6 mm and a...
Embodiment 2
[0052] Chromatographic conditions: use octadecylsilane bonded silica gel as filler; use 0.02mol / L sodium acetate trihydrate (pH=5.0)-acetonitrile (78:22) as mobile phase, use isocratic elution; detection wavelength is 248nm, column temperature 30°C, injection volume 10μl.
[0053] Determination method: Precisely measure the appropriate amount of this product and homopiperazine reference substance, add water to dissolve and quantitatively dilute to make a solution containing about 0.6mg of fasudil hydrochloride and 0.6μg of homopiperazine in every 1ml, as the test solution; Another appropriate amount of homopiperazine reference substance was taken, accurately weighed, dissolved in water and quantitatively diluted to make a solution containing about 1.2 μg of homopiperazine per 1 ml, as the reference substance solution.
[0054]Sample derivation: Accurately measure 10 μl of the test solution, place it in a dry derivation tube with a diameter of 6 mm and a height of 50 mm, add 70...
Embodiment 3
[0056] Chromatographic conditions: use octadecylsilane bonded silica gel as filler; use 0.02mol / L sodium acetate trihydrate (pH=5.0)-acetonitrile (72:28) as mobile phase, use isocratic elution; 1.0ml per minute, the detection wavelength is 248nm, the column temperature is 30°C, and the injection volume is 10μl.
[0057] Determination method: Precisely measure the appropriate amount of this product and homopiperazine reference substance, add water to dissolve and quantitatively dilute to make a solution containing about 0.6mg of fasudil hydrochloride and 0.6μg of homopiperazine in every 1ml, as the test solution; Another appropriate amount of homopiperazine reference substance was taken, accurately weighed, dissolved in water and quantitatively diluted to make a solution containing about 1.2 μg of homopiperazine per 1 ml, as the reference substance solution.
[0058] Sample derivation: Accurately measure 10 μl of the test solution, place it in a dry derivation tube with a diame...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com