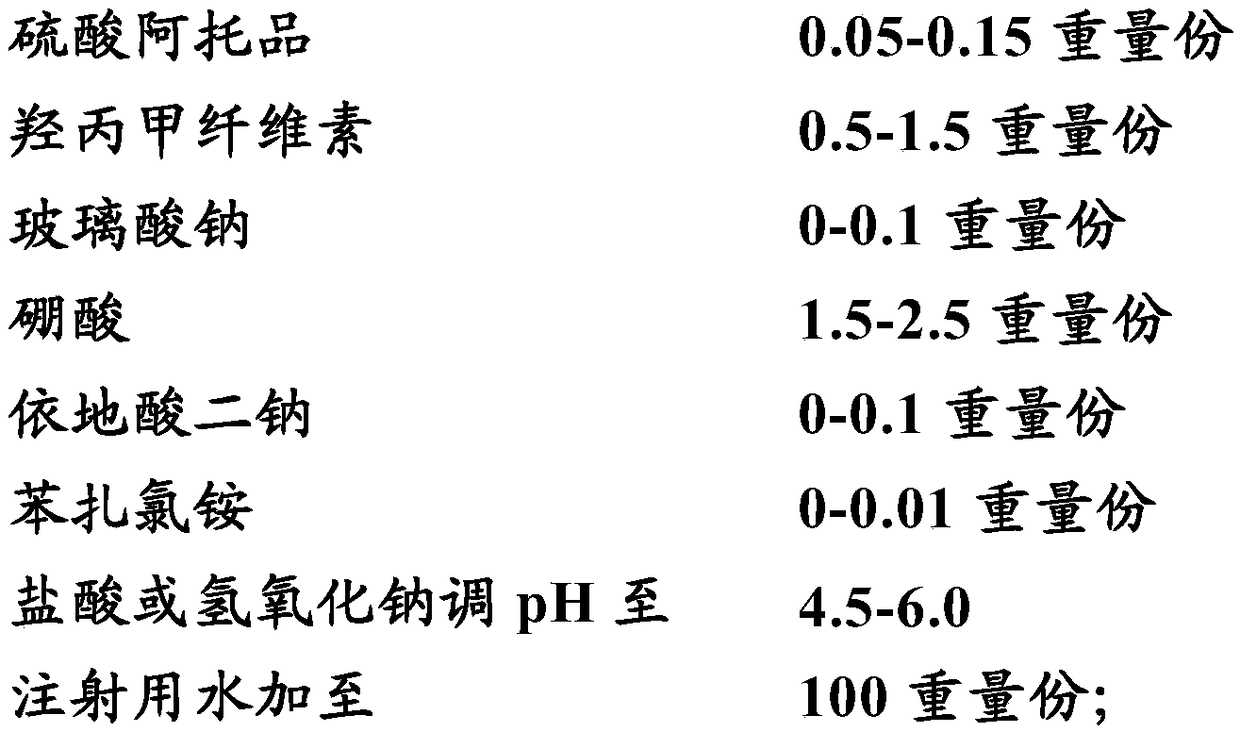
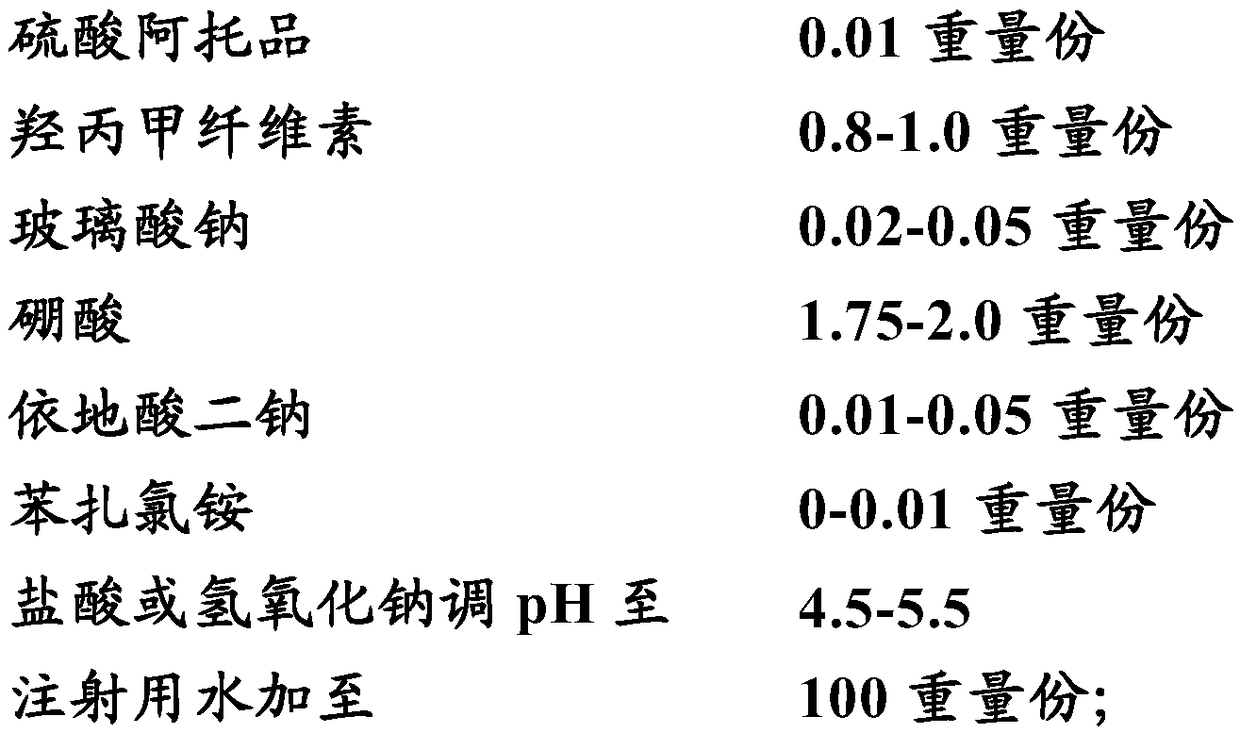
Medical composition for preventing and treating NITM and pharmaceutical application of medical composition
A composition and drug technology, applied in the field of medicine, can solve problems such as no effective treatment or prevention means, and insufficient attention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Example 1: NITM treatment experiment
[0109] 1. Experimental drugs
[0110] Prepare 0.01% atropine sulfate eye drops with 0.9% saline, and adjust the pH to 6.0 with hydrochloric acid. Used for administration in the test group.
[0111] 2. Experimental method
[0112] Select 30 myopia patients aged 6-18 years. The selected eyes have diopters ranging from -0.50DS to -5.00DS, astigmatism ≤-1.0DC; initial NITM value ≥-0.25D; intraocular pressure is normal, not exceeding 21mmHg. Randomly divided into 2 groups, 15 cases in each group. The test group was dripped with the aforementioned experimental drugs, and the control group was dripped with 0.9% normal saline; each group was administered once a day, 1 drop per eye each time, and dripped into the conjunctival sac before going to bed for 6 months. Perform NITM detection at the set time, record the initial NITM value, NITM attenuation time, and the equivalent spherical power of the glasses worn to the best vision. The initial NIT...
Embodiment 2
[0135] Example 2: Study on the effect and side effects of different concentrations of atropine sulfate eye drops on NITM
[0136] 1. Experimental drugs
[0137] Use 0.9% normal saline to prepare eye drops of atropine sulfate with concentrations of 0.001%, 0.005%, 0.01%, 0.02%, 0.05%, 0.1%, 0.2%, and adjust the pH to 5.5 with hydrochloric acid. Used for administration in the test group.
[0138] 2. Experimental method
[0139] Select 120 myopia patients aged 6-18 years. The selected eyes have diopters ranging from -0.50DS to -5.00DS, astigmatism ≤-1.0DC; initial NITM value ≥-0.25D; intraocular pressure is normal, not exceeding 21mmHg. Randomly divided into 8 groups with 15 cases in each group. The 7 test groups were dripped with 0.9% saline solution of 0.001%, 0.005%, 0.01%, 0.02%, 0.05%, 0.1%, 0.2% atropine sulfate eye drops, and the control group was dripped with 0.9% saline; each group They were administered once a day, 1 drop per eye each time, and dropped into the conjunctiva...
Embodiment 3
[0154] Example 3: Study on the effect of atropine sulfate eye drops with different pH values on NITM
[0155] 1. Experimental drugs
[0156] 0.9% sodium chloride solution was used to prepare 0.01% atropine sulfate eye drops, and the pH value was adjusted to 4.0, 4.5, 5.0, 5.5, 6.0, 6.5 with hydrochloric acid. Used for administration in the test group.
[0157] 2. Experimental method
[0158] Select 105 myopia patients aged 6-18 years. The selected eyes have diopters ranging from -0.50DS to -5.00DS, astigmatism ≤-1.0DC; initial NITM value ≥-0.25D; intraocular pressure is normal, not exceeding 21mmHg. Randomly divided into 7 groups, 15 cases in each group. The 6 test groups were instilled with the previously prepared atropine solution (pH values were 4.0, 4.5, 5.0, 5.5, 6.0, 6.5), and the control group was instilled with 0.9% saline; each group was administered once a day, each time 1 drop per eye, instill in the conjunctival sac before going to bed for 2 weeks. NITM detection...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com