Quality standard of Sinopanax formosanus pills
A quality standard and technology of ginseng dripping pills, applied in the field of quality standards of Huashan ginseng dripping pills, can solve the problems of difference in reproducibility of results, complicated operation, lengthy operation time, etc., and achieve shortened operation time, simple operation steps, and repeatability. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Determining detectable target components
[0042] The core structures of several components of tropanes are the same, and the only difference is the difference in the substituent groups. Their chemical properties are very similar, so we chose liquid chromatography as the experimental scheme. Two main problems to be solved in our experiment It is a screening determination to determine the target components and experimental conditions that can be detected.
[0043] Select the object or combination by examining the degree of separation and content level. Preliminary experiments have found that the total alkaloids in Huashan Ginseng Dripping Pills mainly contain atropine, scopolamine and anisodamine. Therefore, these three components are used as the object of investigation, as shown in the table 1
[0044] Table 1 Results of separation investigation
[0045] Resolution (r)
content level
>1.5
can be detected
Scopolam...
Embodiment 2
[0051] Embodiment 2: Determine the best experimental conditions
[0052] 1. Instruments and reagents
[0053] ① Instrument
[0054] HP1100 liquid chromatograph, VWD ultraviolet-visible detector, HP Chemstation chemical workstation, Sartorius R200D electronic analytical balance; KQ-400DB numerical control ultrasonic cleaner
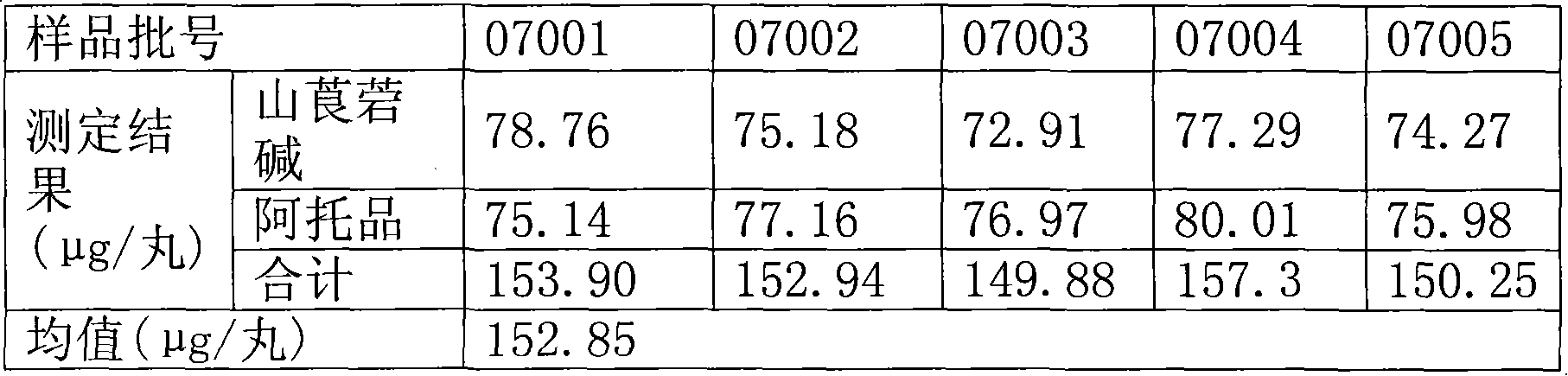
[0055] ②Reagent: standard product: anisodamine hydrobromide: purchased from China Institute for the Control of Pharmaceutical and Biological Products, batch number: 0051-94097; atropine sulfate: purchased from China Institute for the Control of Pharmaceutical and Biological Products, batch number: 200510. Reagent: acetonitrile for chromatography pure; sodium lauryl sulfate is analytically pure; water is double distilled water; sample: Huashan ginseng dripping pills, provided by our factory, batch numbers are: experiment 06001, 07001, 07002, 07003, 07004, 07005.
[0056] 2. Methods and results
[0057] Chromatographic conditions and system suitability te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com