Recombinant bacterium for producing sulfo-recombinant hirudin as well as preparation method and application of recombinant bacterium
A technology of recombinant hirudin and recombinant bacteria, applied in the field of genetic engineering, can solve the problems of reduced activity, increased pressure of downstream purification, increased production cost and difficulty, etc., to achieve the effect of improving activity and reducing the pressure of subsequent purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
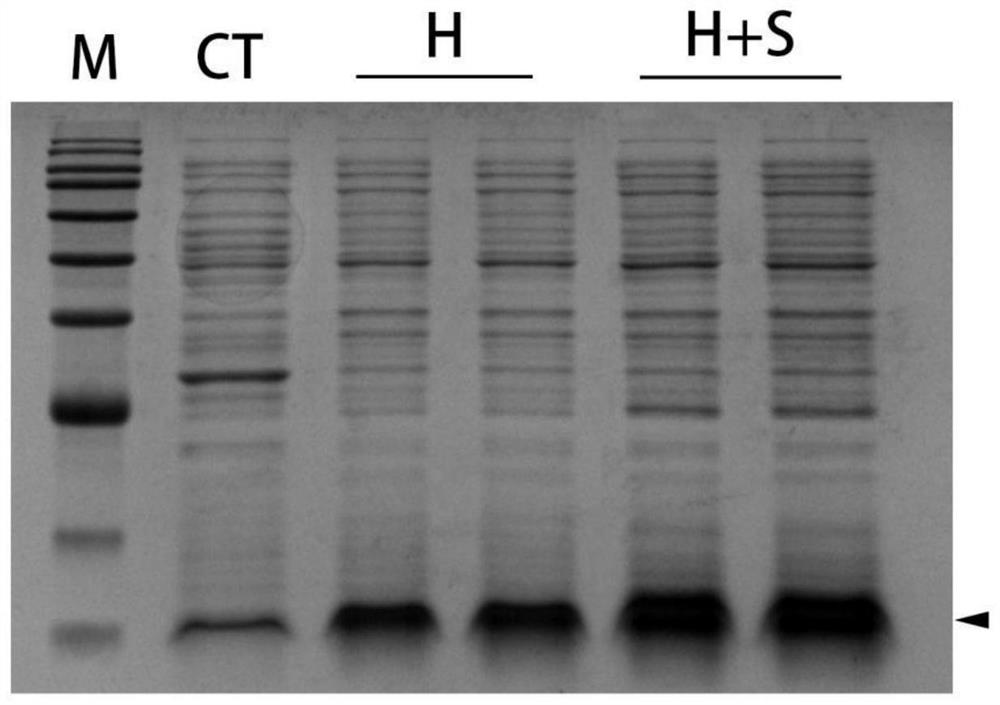
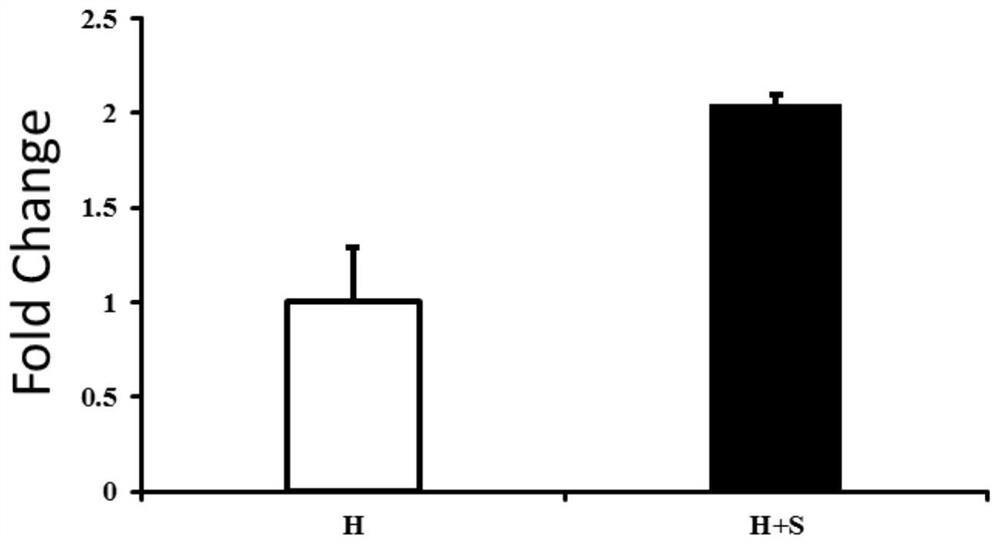
Image
Examples
Embodiment 1
[0028] Example 1. Preparation of recombinant bacteria for producing sulfo-recombinant hirudin.
[0029] The recombinant bacterium prepared in this embodiment overexpresses the sulfonylase gene and the hirudin gene, the nucleotide sequence of the hirudin gene is as shown in SEQ ID No.1, and the sulfonylase gene, the nucleoside The acid sequence is shown in SEQ ID No.4, and the starting strain is Escherichia coli. The build method is as follows:
[0030] 1) Hirudin gene expression vector construction: adding a nucleotide sequence encoding a periplasmic secreted peptide to the 5' end of the hirudin gene to obtain a hirudin gene with a periplasmic secreted peptide, and then connecting it to a plasmid vector, Obtain the expression vector expressing hirudin; The specific method is as follows:
[0031] The nucleotide sequence encoding the periplasmic secretory peptide shown in SEQ ID No.5 was added to the 5' end of the hirudin gene shown in SEQ ID No.1, and the obtained sequence wa...
Embodiment 2
[0041] Embodiment 2. repeat embodiment 1, and the difference with embodiment 1 is, the hirudin gene described in the present embodiment, nucleotide sequence is as shown in SEQ ID No.2, compared with embodiment 1, present embodiment In the hirudin gene, the 181st base G was replaced with the base T, and the 183rd base A was replaced with the base C. Refer to Example 1 for the remaining steps.
Embodiment 3
[0042] Embodiment 3. Repeat Example 1, the difference from Example 1 is that the hirudin gene described in this example, the nucleotide sequence is as shown in SEQ ID No.3, compared with Example 1, the present example In the hirudin gene, the 181st base G is replaced with a base T, the 183rd base A is replaced with a base C, the 184th base G is replaced with a base T, and the 186th base A is replaced with a base c. Refer to Example 1 for the remaining steps.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com