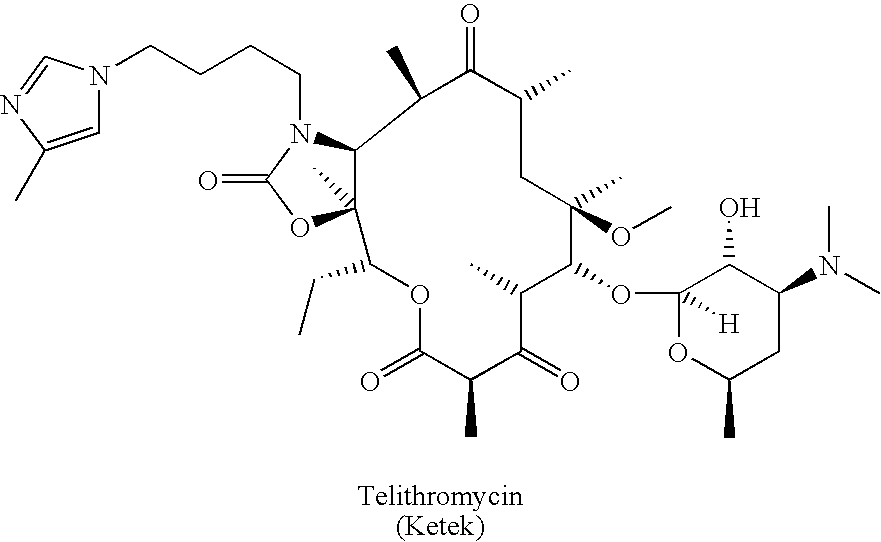
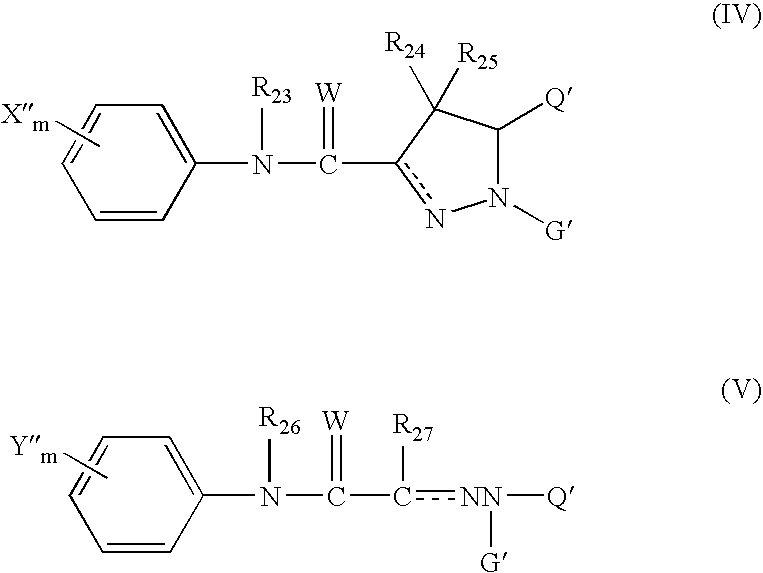
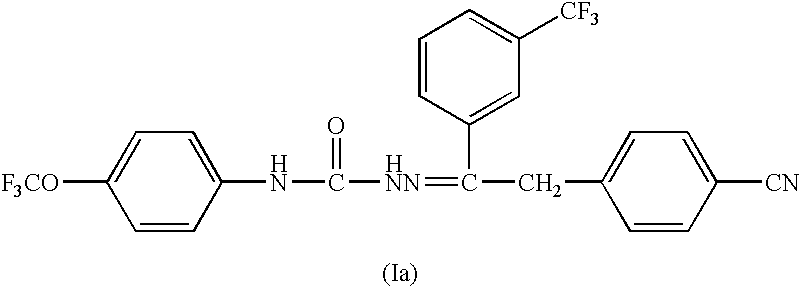
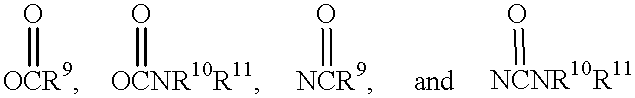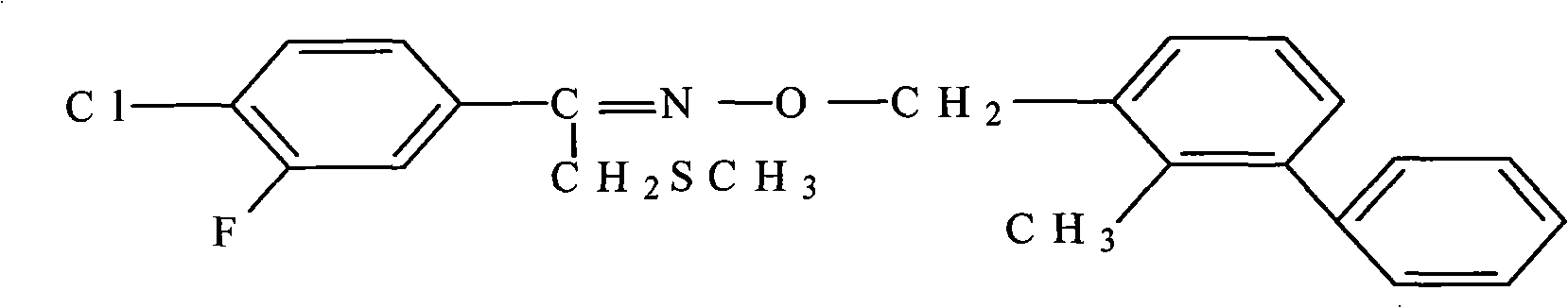Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
412 results about "Macrocyclic lactone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Synergistic Mixtures of Anthranilamide Invertebrate Pest Control Agents
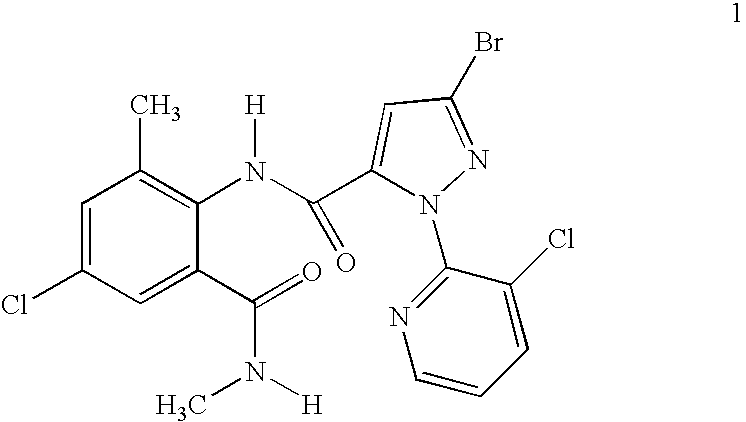
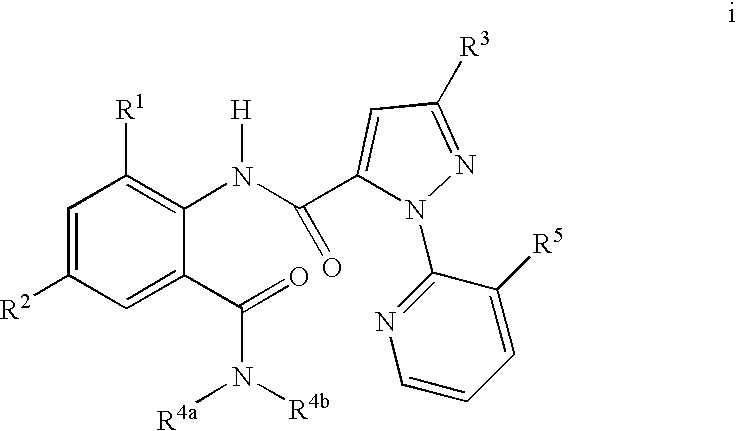
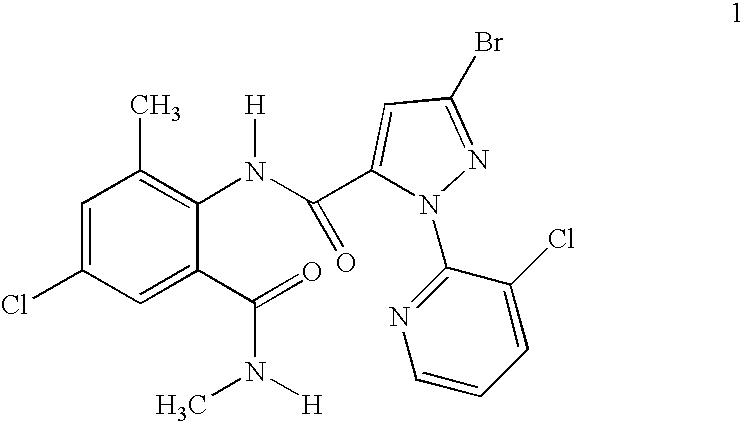
Disclosed are mixtures and compositions for controlling invertebrate pests relating to combinations comprising (a) 3-bromo-N-[4-chloro-2-methyl-6-[(methylamino)carbonyl]phenyl]-1-(3-chloro-2-pyridinyl)-1H-pyrazole-5-carboxamide, and its N-oxides, and suitable salts thereof and a component (b) wherein the component (b) is at least one compound or agent selected from neonicotinoids, cholinesterase inhibitors, sodium channel modulators, chitin synthesis inhibitors, ecdysone agonists, lipid biosynthesis inhibitors, macrocyclic lactones, GABA-regulated chloride channel blockers, juvenile hormone mimics, ryanodine receptor ligands, octopamine receptor ligands, mitochondrial electron transport inhibitors, nereistoxin analogs, pyridalyl, flonicamid, pymetrozine, dieldrin, metaflumizone, biological agents, and suitable salts of the foregoing. Also disclosed are methods for controlling an invertebrate pest comprising contacting the invertebrate pest or its environment with a biologically effective amount of a mixture or composition of the invention.
Owner:FMC CORP
Spot-on formulations for combating parasites
InactiveUS6962713B2Toxic effectsEffective and lasting destructionBiocideDead animal preservationAntiparasiticAntiparasite agent
In particular this invention provides for spot-on compositions for the treatment or prophylaxis of parasite infestations in mammals or birds which comprise:(1) a composition comprising(A) an effective amount of a 1-phenylpyrazole derivative; and / or(B) an effective amount of a macrocyclic lactone antihelmintic or antiparasitic agent;(2) an acceptable liquid carrier vehicle; and(3) optionally, a crystallization inhibitor.The invention also provides for a method of treating parasitic infestations or for the prophylaxis of parasite infestations in mammals or birds which comprises topically applying to said mammal treating parasitic infestations or for the prophylaxis of parasite infestations in mammals or birds which comprises topically applying to said mammal or bird an effective amount of a composition according to the present invention.
Owner:MERIAL SAS
Water soluble nanoparticles and method for their production
InactiveUS20050191359A1Readily bioavailableLow costPowder deliveryBiocidePolymer scienceNanoparticle
Hydrophilic dispersions of stable nano-sized particles are provided comprising: (a) a water-insoluble or water-soluble active compound, wherein said active compound is selected from the group consisting of a macrolide antibiotic, donepezil hydrochloride, an azole compound and a taxane; and (b) an amphiphilic polymer which wraps said active compound in a non-crystalline manner to form a nano-sized molecular entity in which no valent bonds are formed.
Owner:SOLUBEST
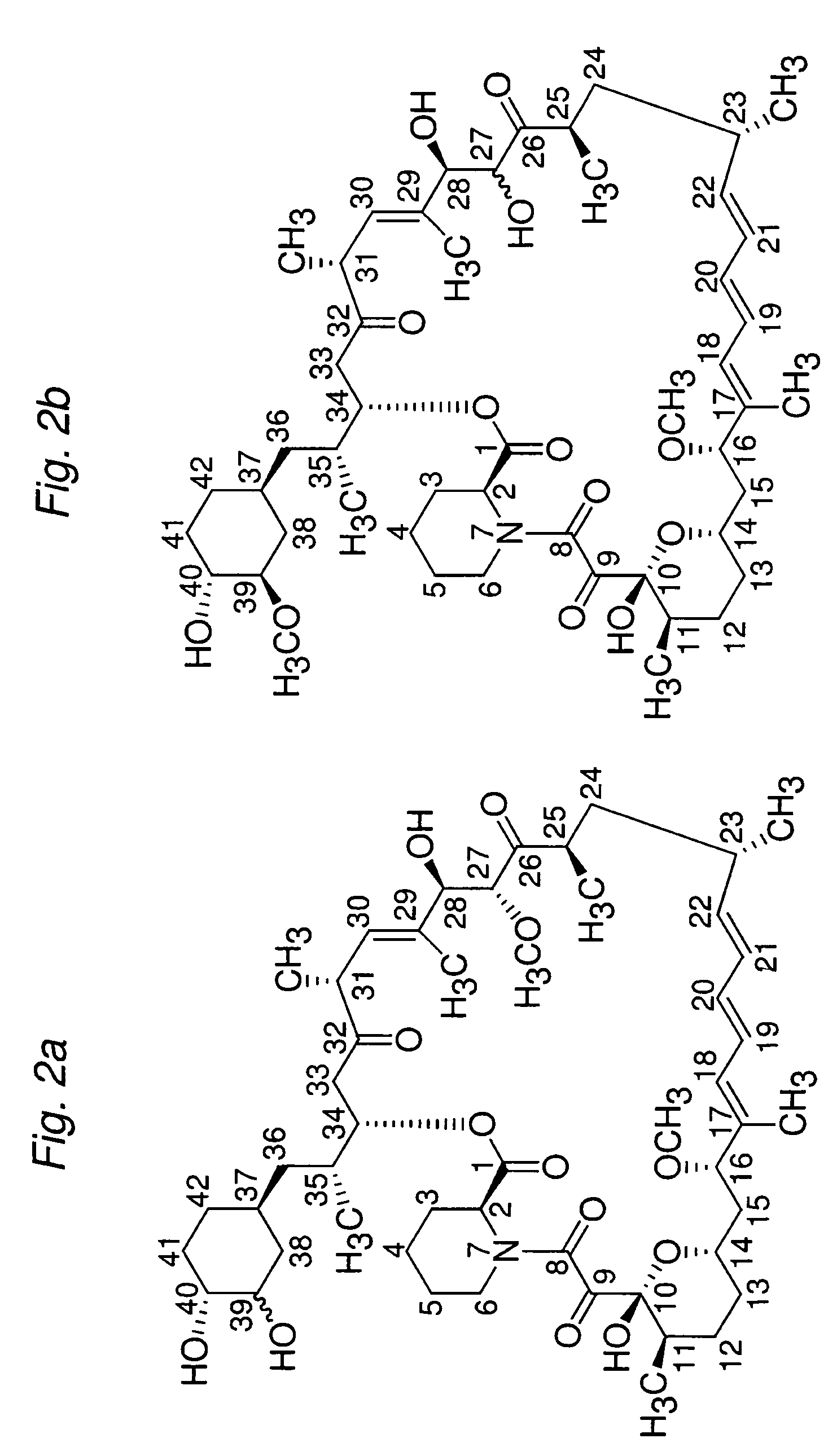
Macrocyclic lactone compounds and their production process
InactiveUS6187568B1Antibacterial agentsMicroorganism based processesMacrocyclic lactoneActinoplanes sp.
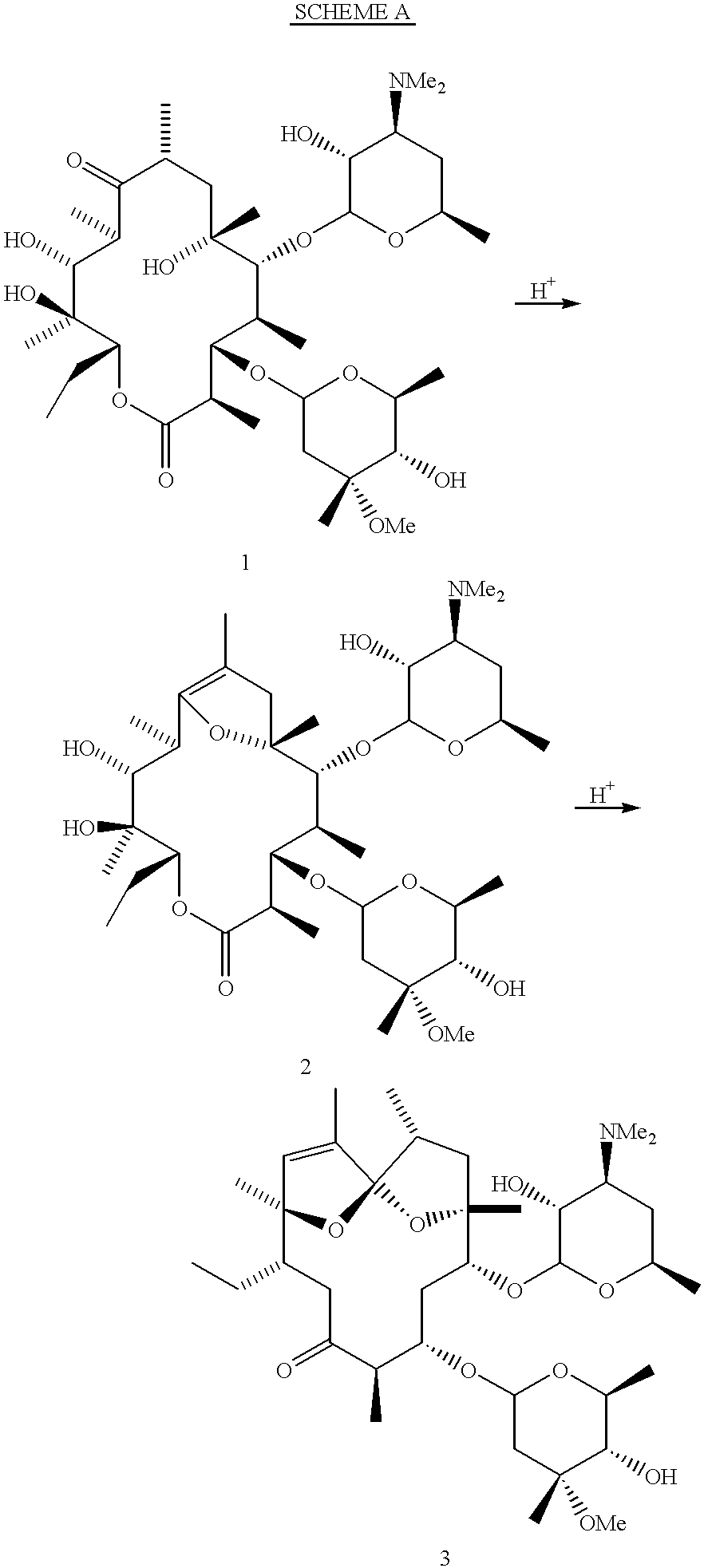
This invention provides a process for producing a macrocyclic lactone compound, which comprises cultivating Actinoplanes sp. FERM BP-3832, in the presence of L-proline, L-hydroxyproline or L-nipecotic acid, and then isolating a macrocylic lactone compound from the fermentation broth. The compounds produced by this process include a compound of the following formula:The present invention also relates to a pharmaceutical composition comprising the same, which is useful as immunosuppressive, antimycotic, antitumor agent or the like.
Owner:PFIZER INC
Macrocyclic lactone compounds and methods for their use
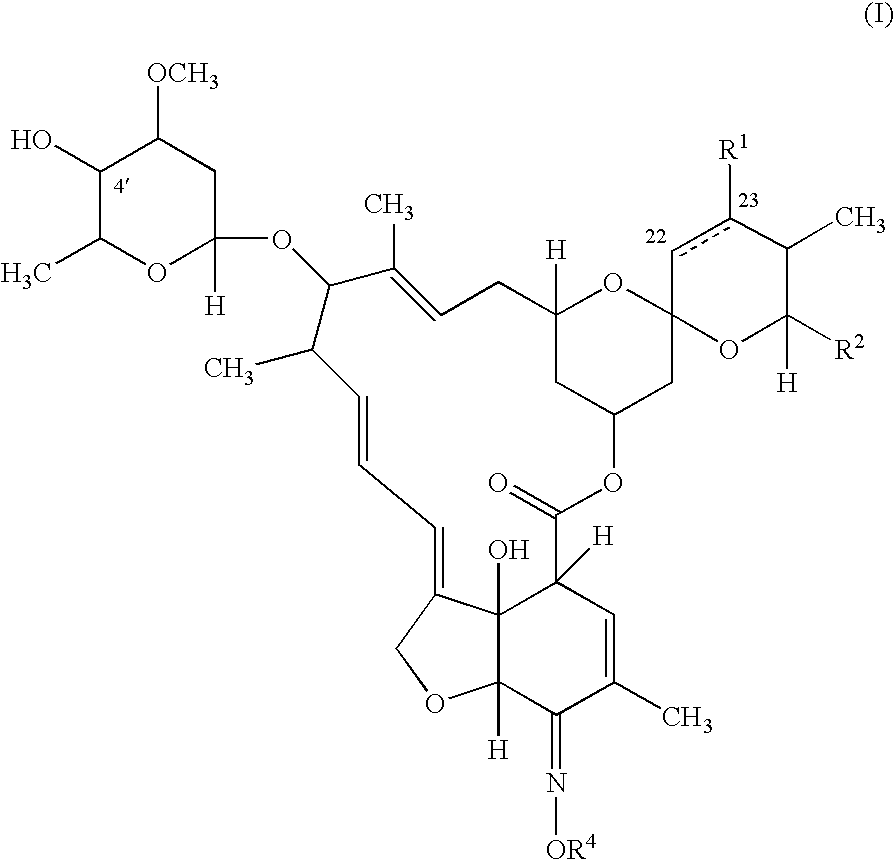
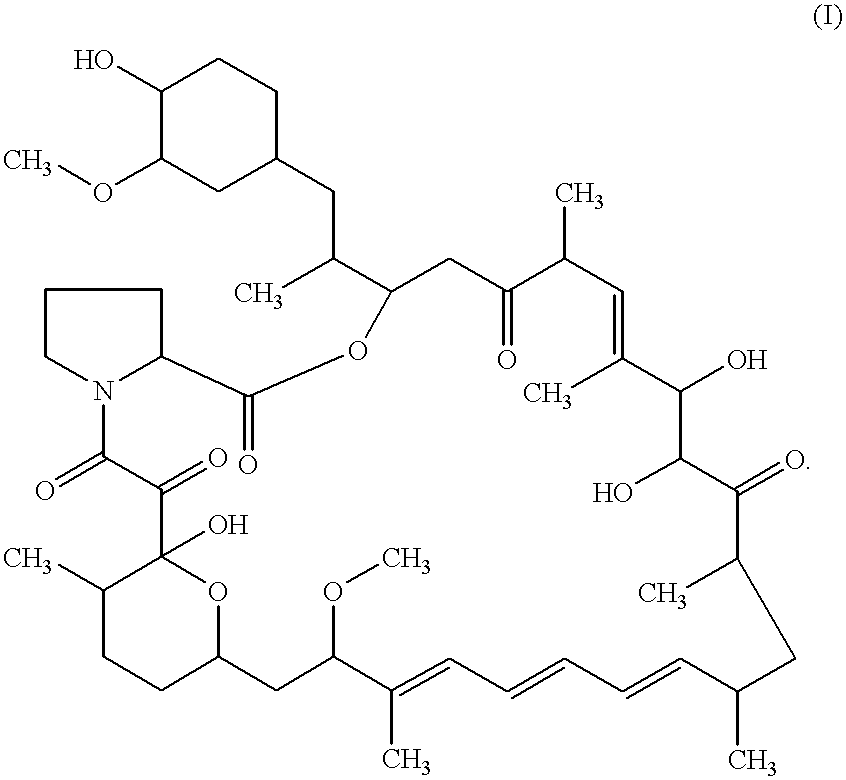
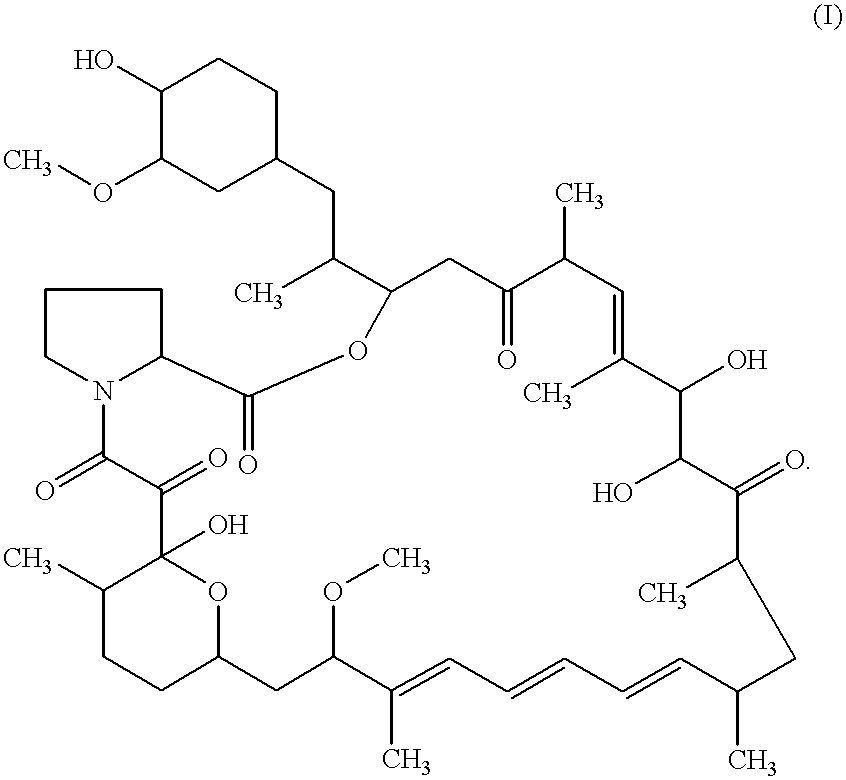
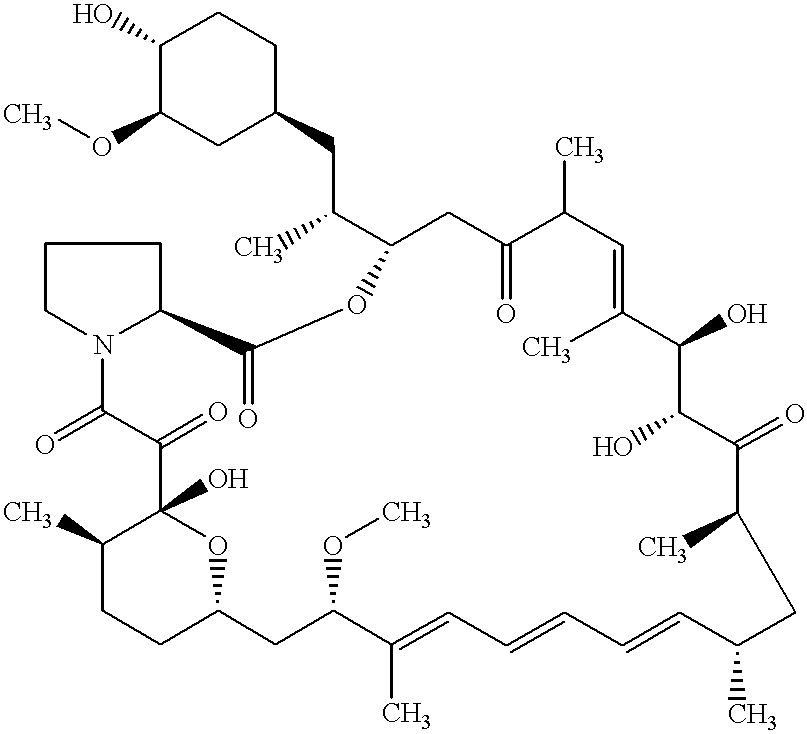
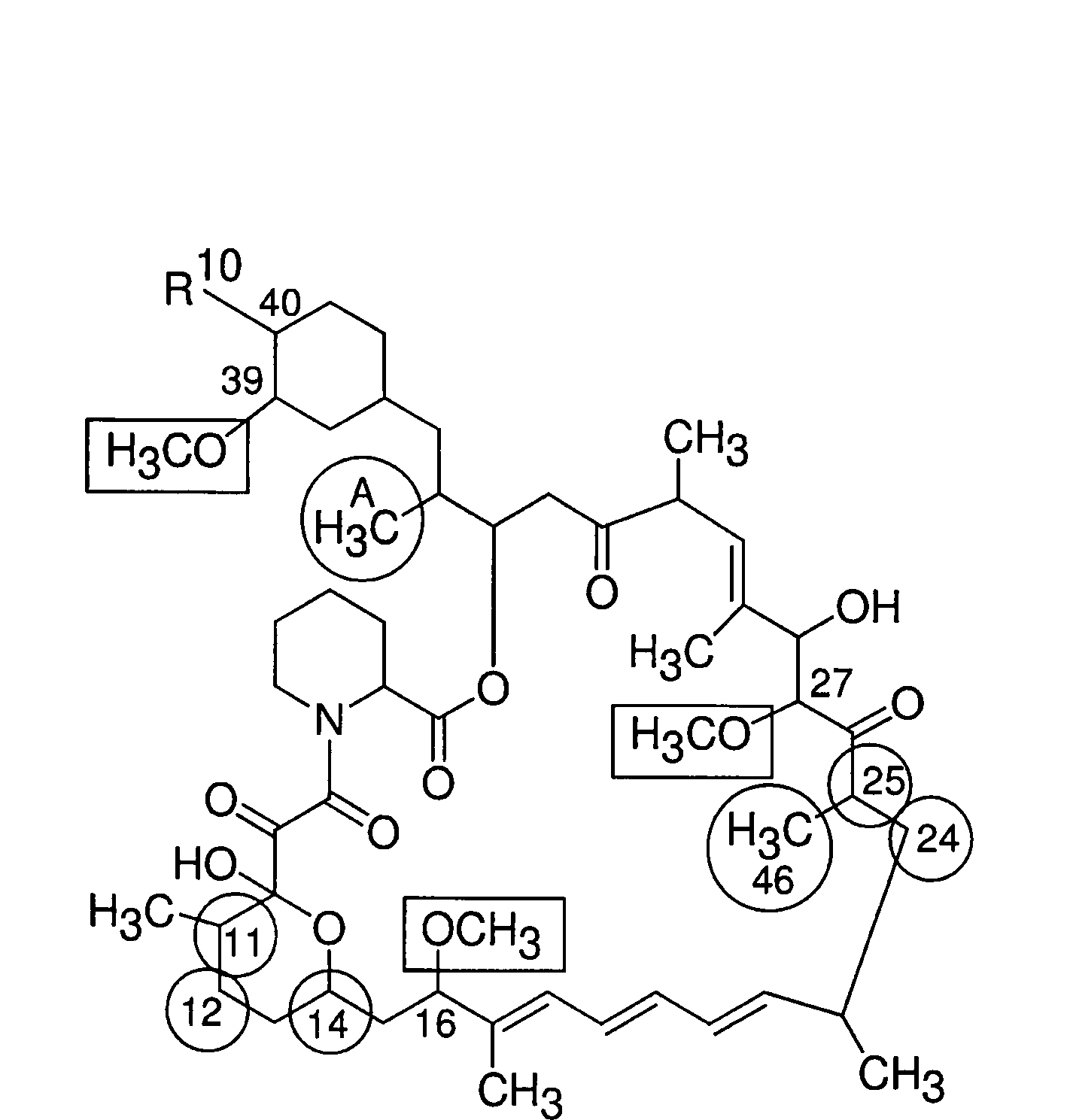
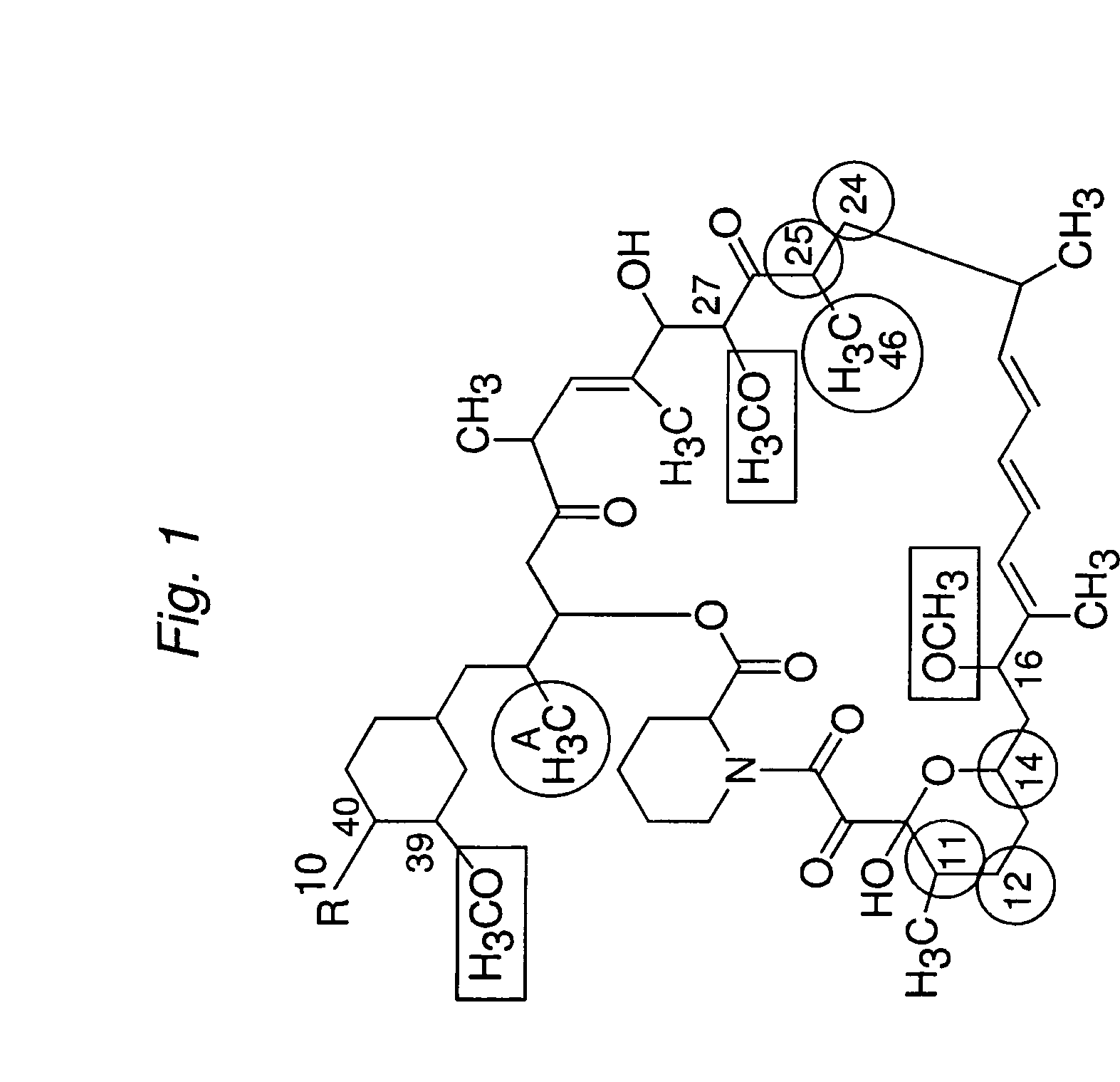
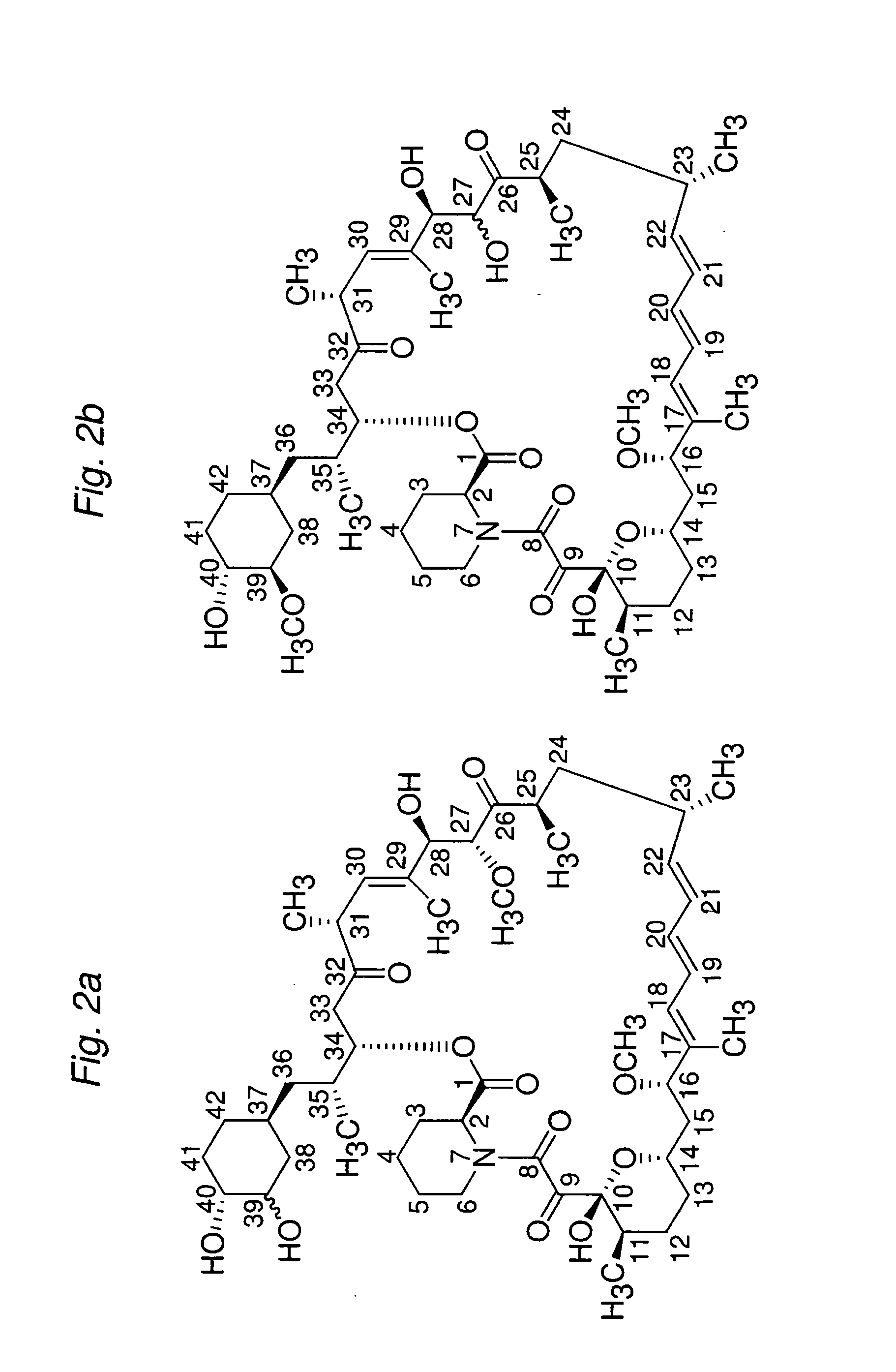
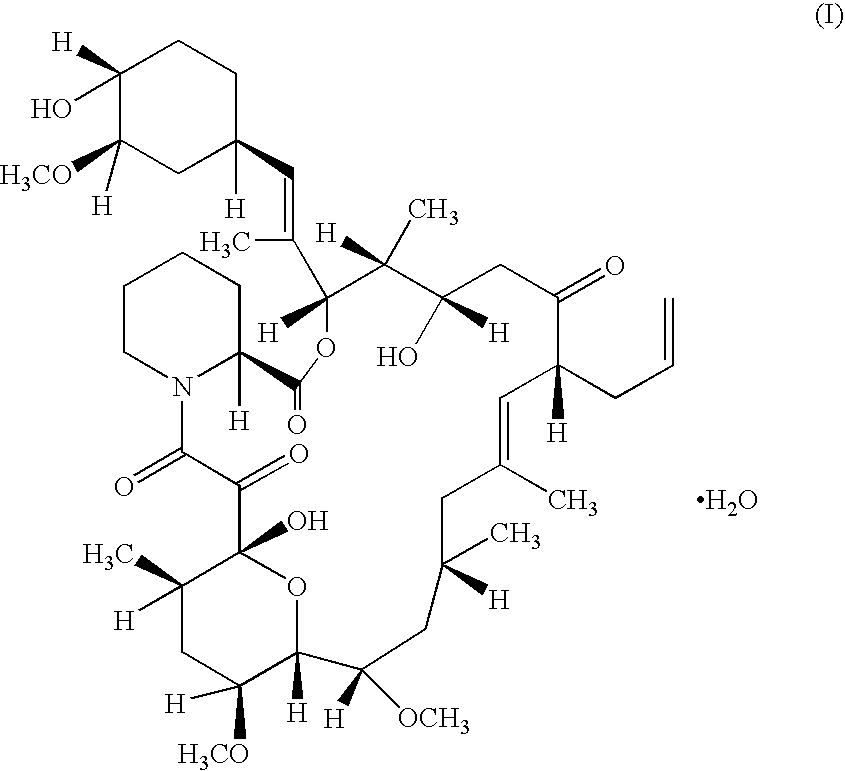
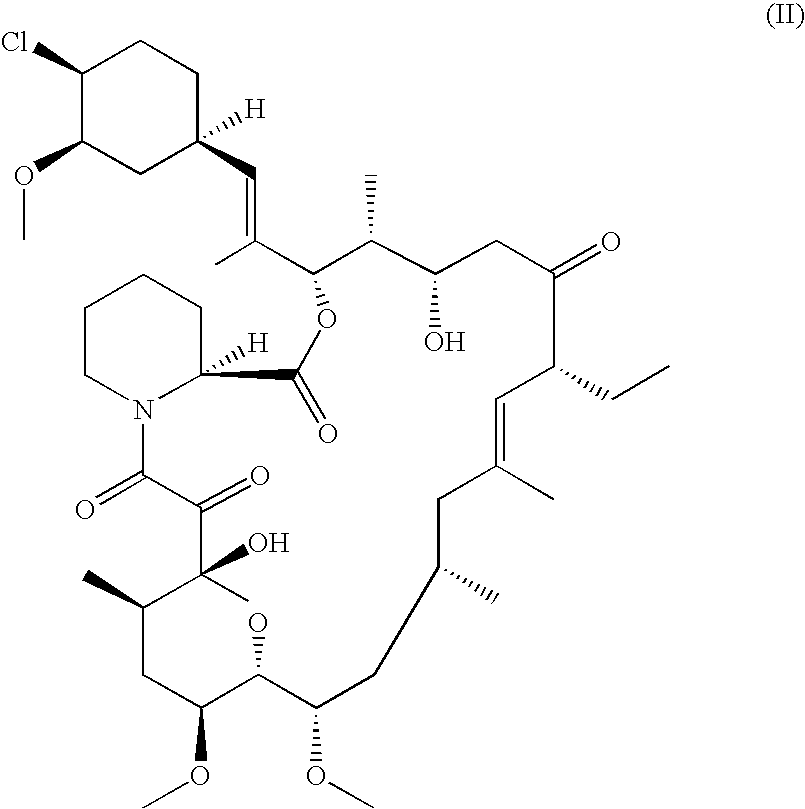
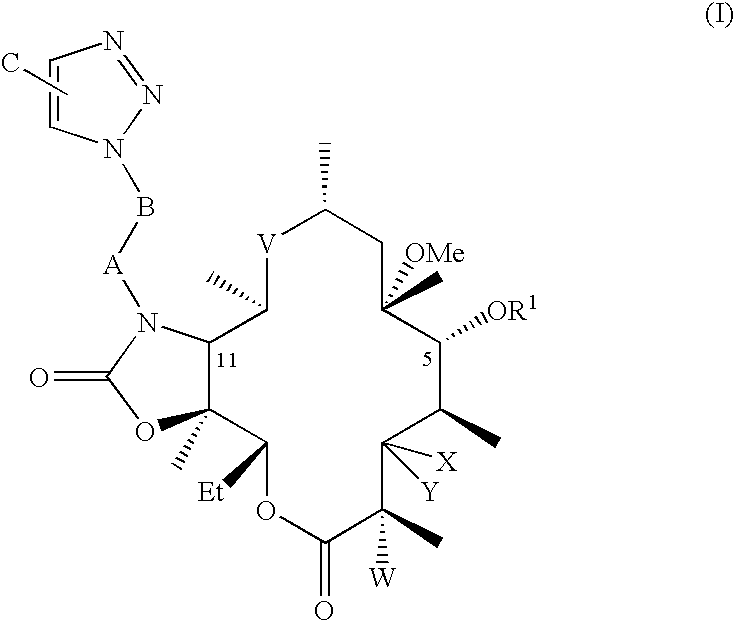
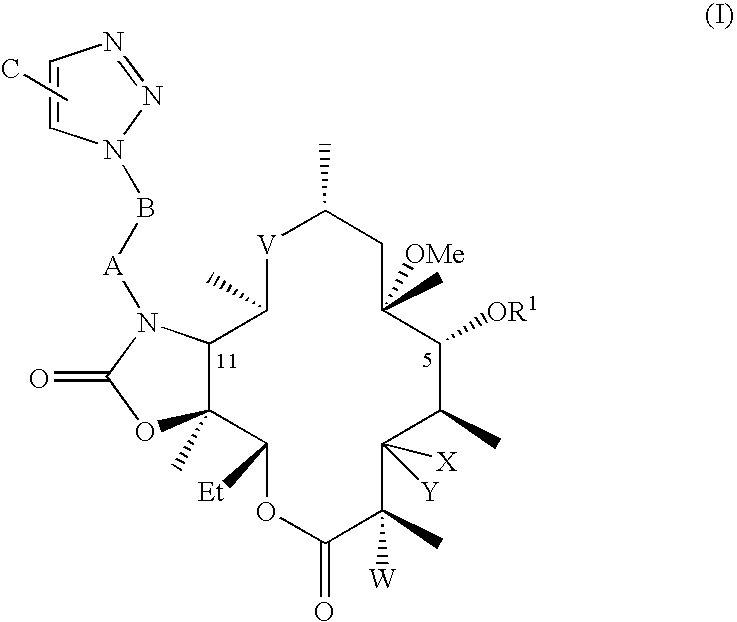
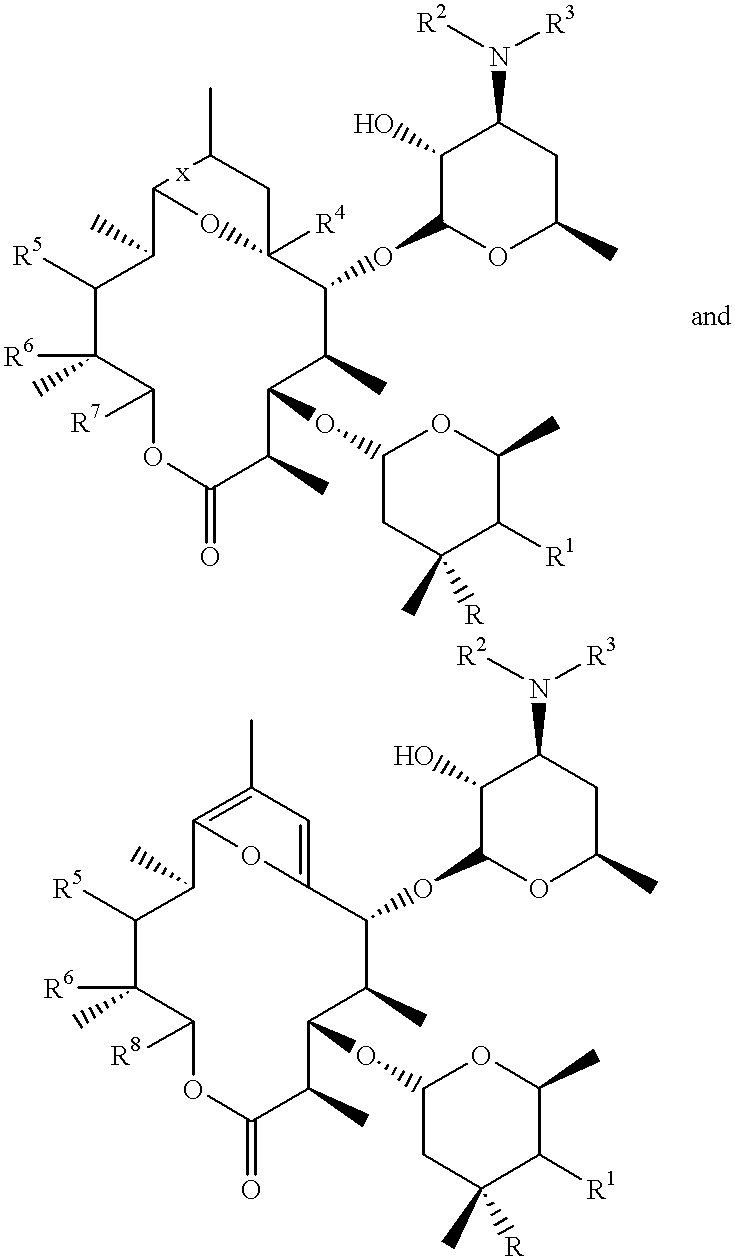
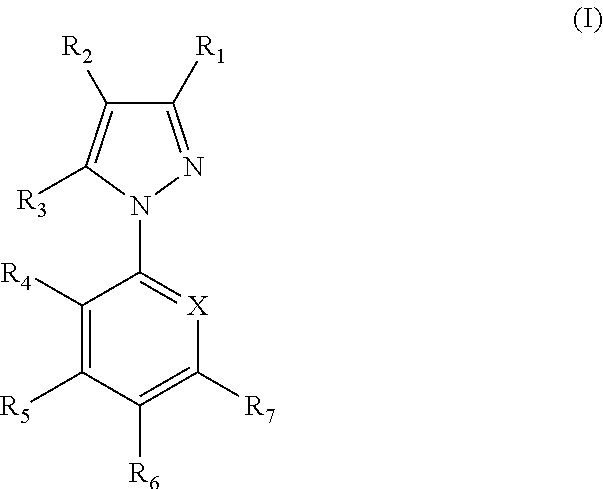
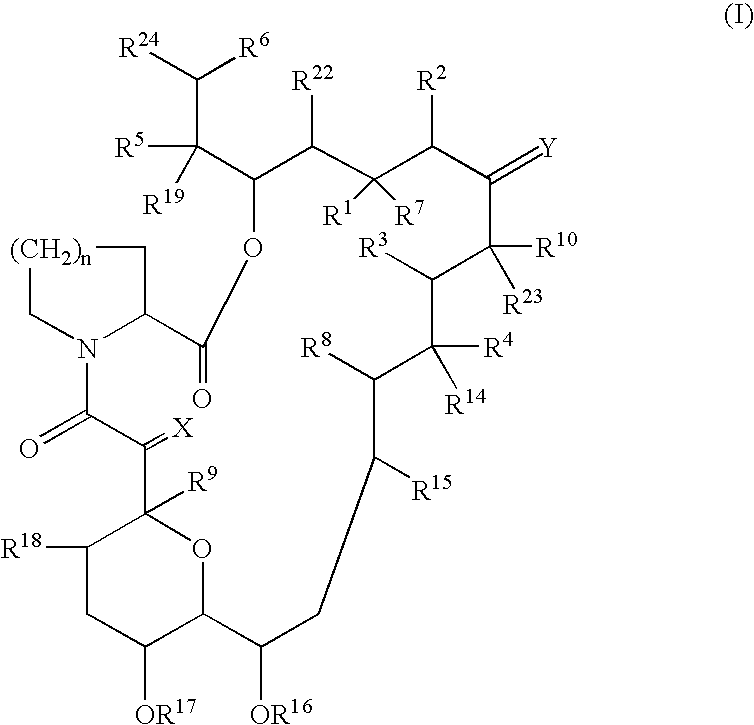
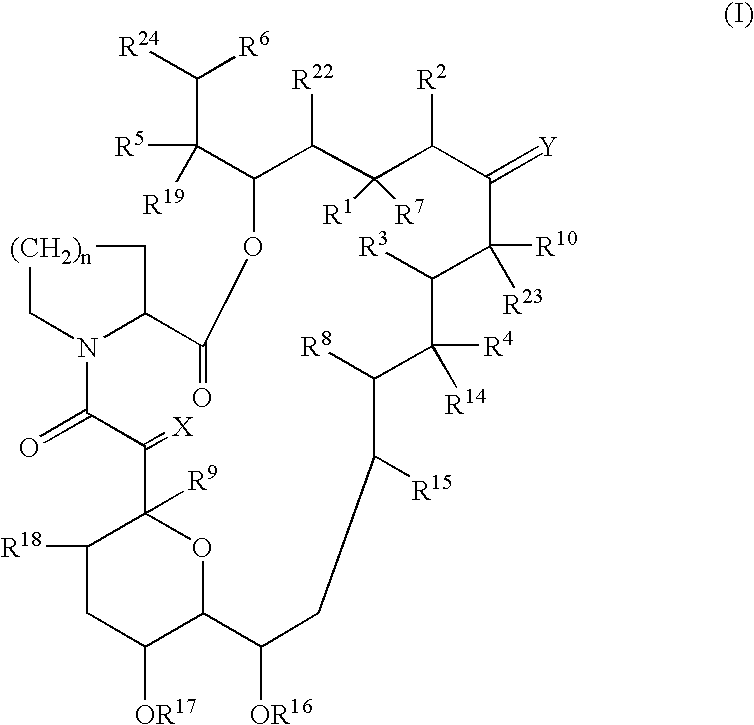
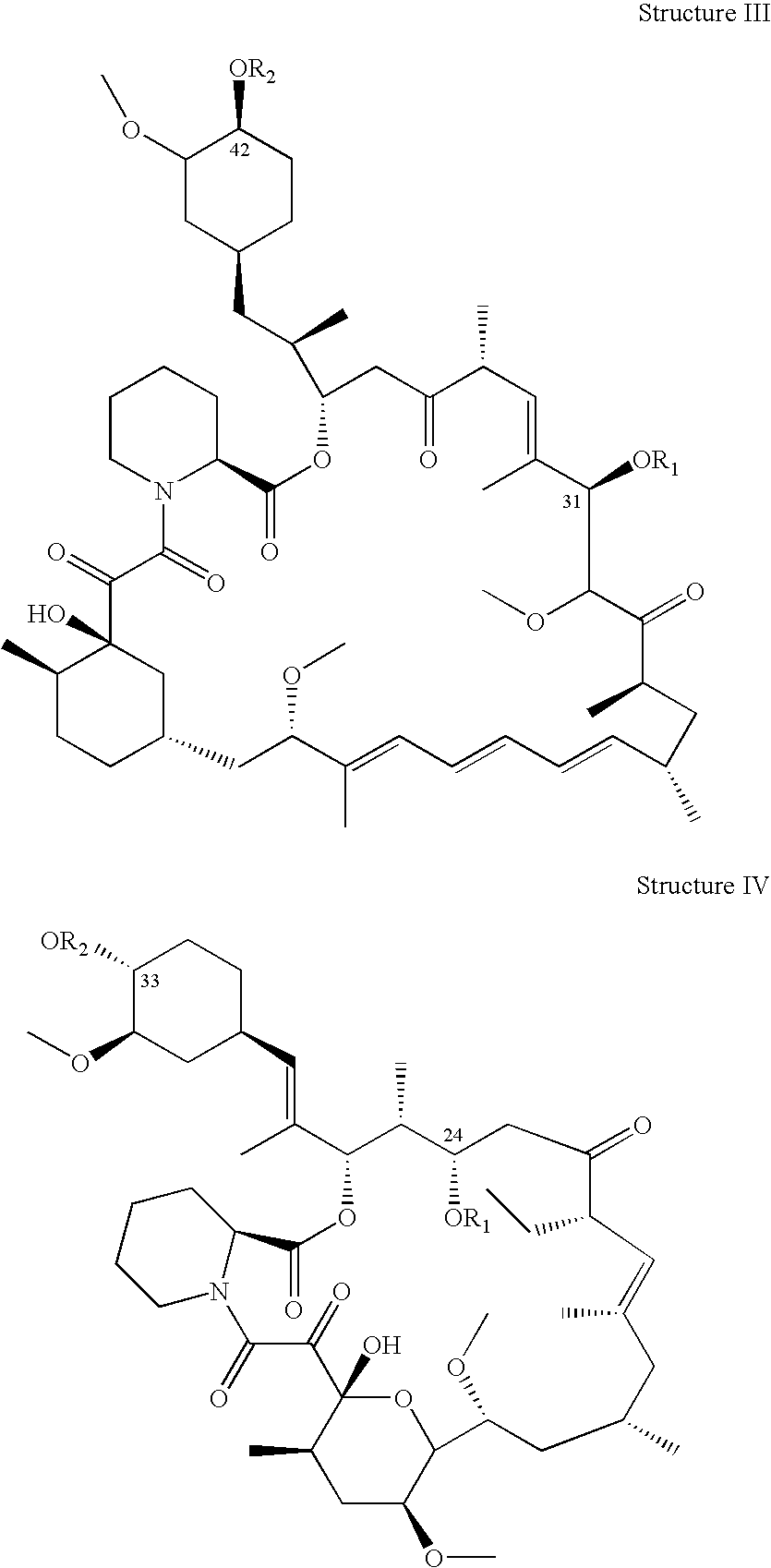
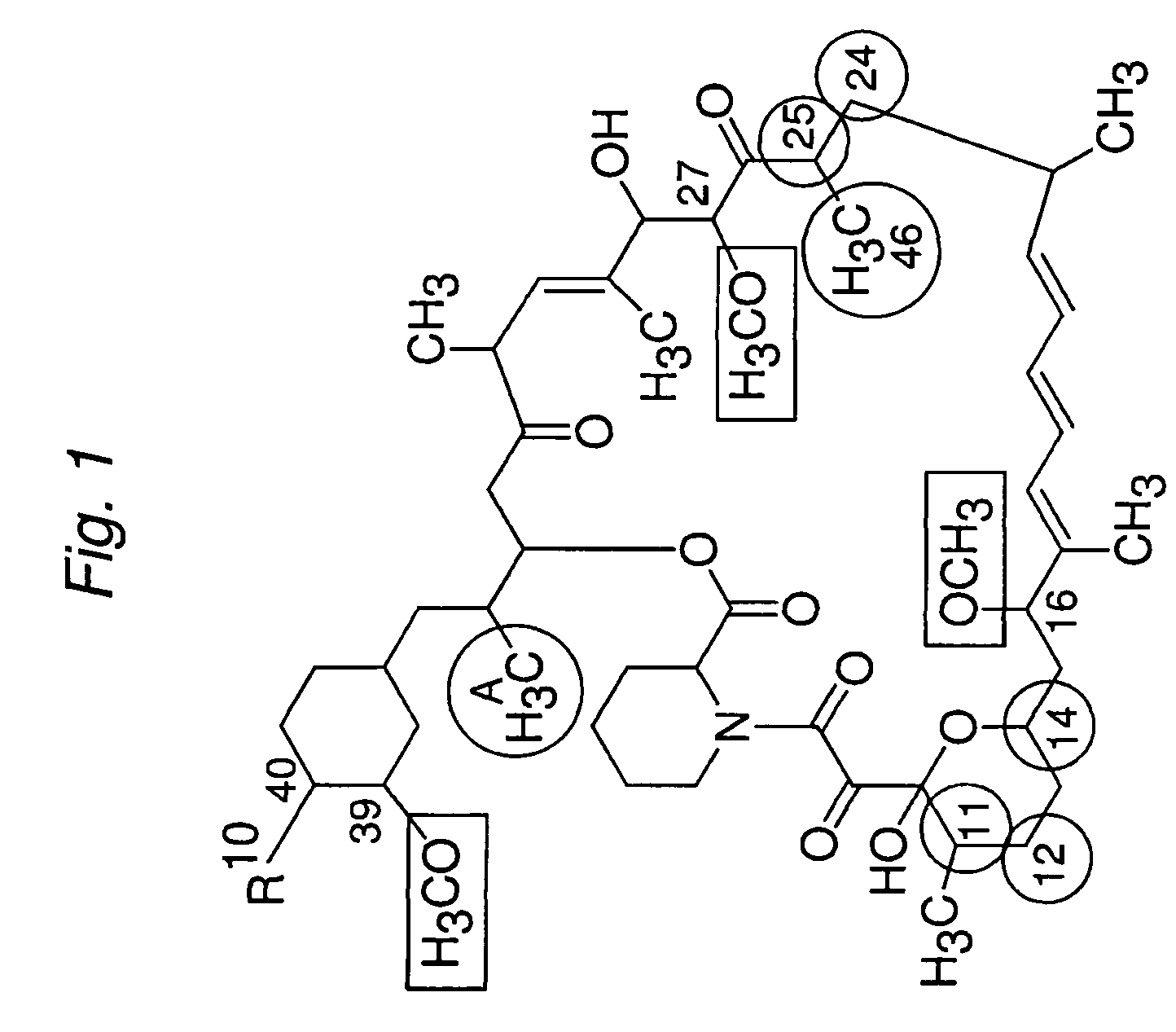
The present invention provides a pharmaceutical composition comprising a pharmaceutically acceptable excipient and a compound of the formula:wherein R1, R2, R3, R5, R6 and R8 are each independently a member selected from the group consisting of H, C1-6 alkyl and OH; R4, R7 and R9 are each independently selected from the group consisting of C1-6 alkoxy and OH; R10 is a member selected from the group consisting of H, —OH, —OP(O)Me2,—O—(CH2)n—OH and —O—(CH2)m—O—(CH2)o— CH3, wherein subscripts n and m are each independently from 2 to 8 and subscript o is from 1 to 6; each of L1 and L4 are independently selected from the group consisting of:each of L2 and L3 are independently selected from the group consisting of:salts, hydrates, isomers, metabolites and prodrugs thereof.
Owner:ELIXIR MEDICAL CORP
Pharmaceutical gel formulations
A pharmaceutical gel composition is provided comprising (a) a therapeutically effective amount of one or more active pharmaceutical ingredients comprising one or more macrolide related immunosuppressants or pharmaceutically acceptable salts or esters thereof; (b) one or more gel forming agents; and (c) an effective amount of one or more skin penetration enhancers capable of percutaneous delivery of the macrolide related immunosuppressant through the skin. Also provided is a process for its preparation and methods for delivering a macrolide related immunosuppressant through the skin of a mammal in order to treat conditions situated on and beneath the skin.
Owner:GLENMARK PHARMACEUTICALS LIMITED
Anthelmintic macrocyclic lactone compositions
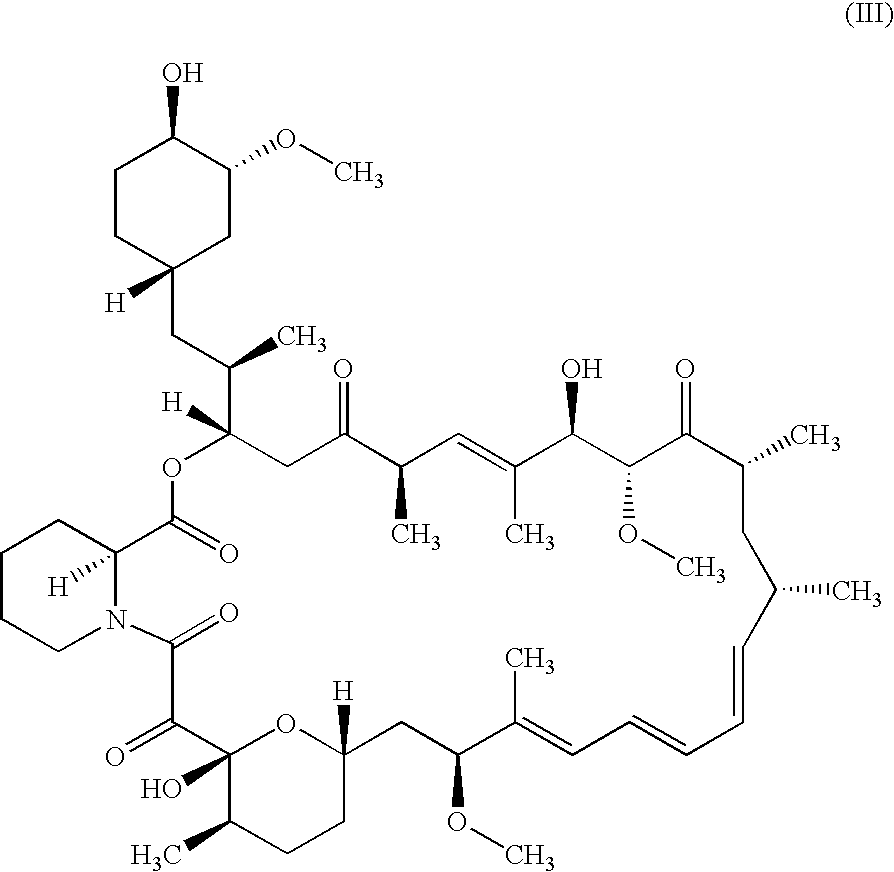
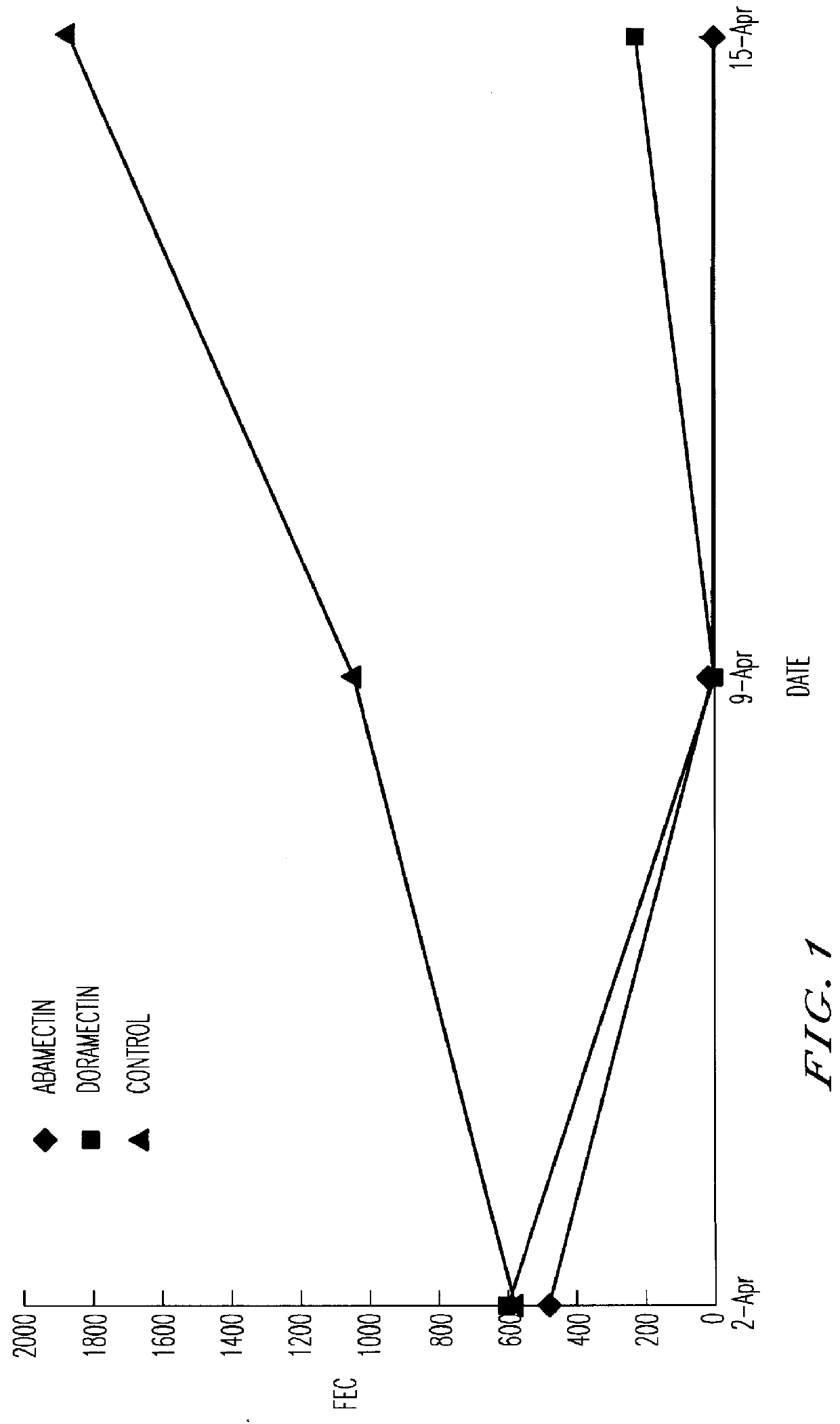
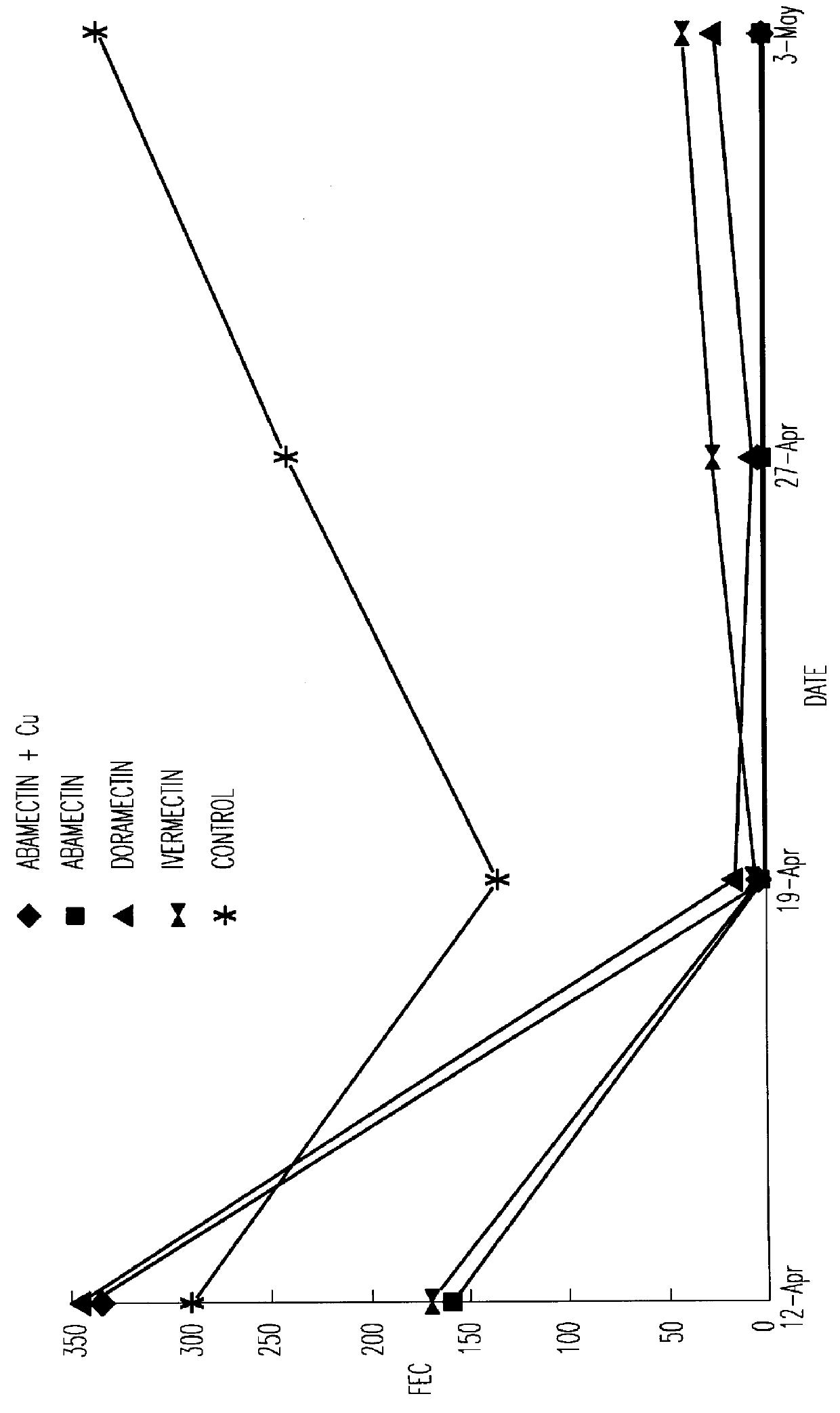
PCT No. PCT / NZ96 / 00099 Sec. 371 Date Mar. 25, 1998 Sec. 102(e) Date Mar. 25, 1998 PCT Filed Sep. 19, 1996 PCT Pub. No. WO97 / 11709 PCT Pub. Date Apr. 3, 1997A composition comprising an anthelmintic chosen from the class of macrocyclic lactones including but not limited to the avermectins, ivermectin, doramectin, abamectin, milbemycin and moxidectin, together with a vegetable oil and a co-solvent chosen from the group comprising alcohols having four or more carbons atoms. The compositions of the inventions may be formulated as injections, drenches or for topical administration and are suitable for treating helminthiasis in animals.
Owner:MERIAL INC
Process for the preparation of macrolide antibacterial agents
ActiveUS20100216731A1Effective treatment and preventionExcellent activityAntibacterial agentsBiocideMacrolide resistanceStereochemistry
Owner:TETARD INC
Rate-controlled delivery of macrolides
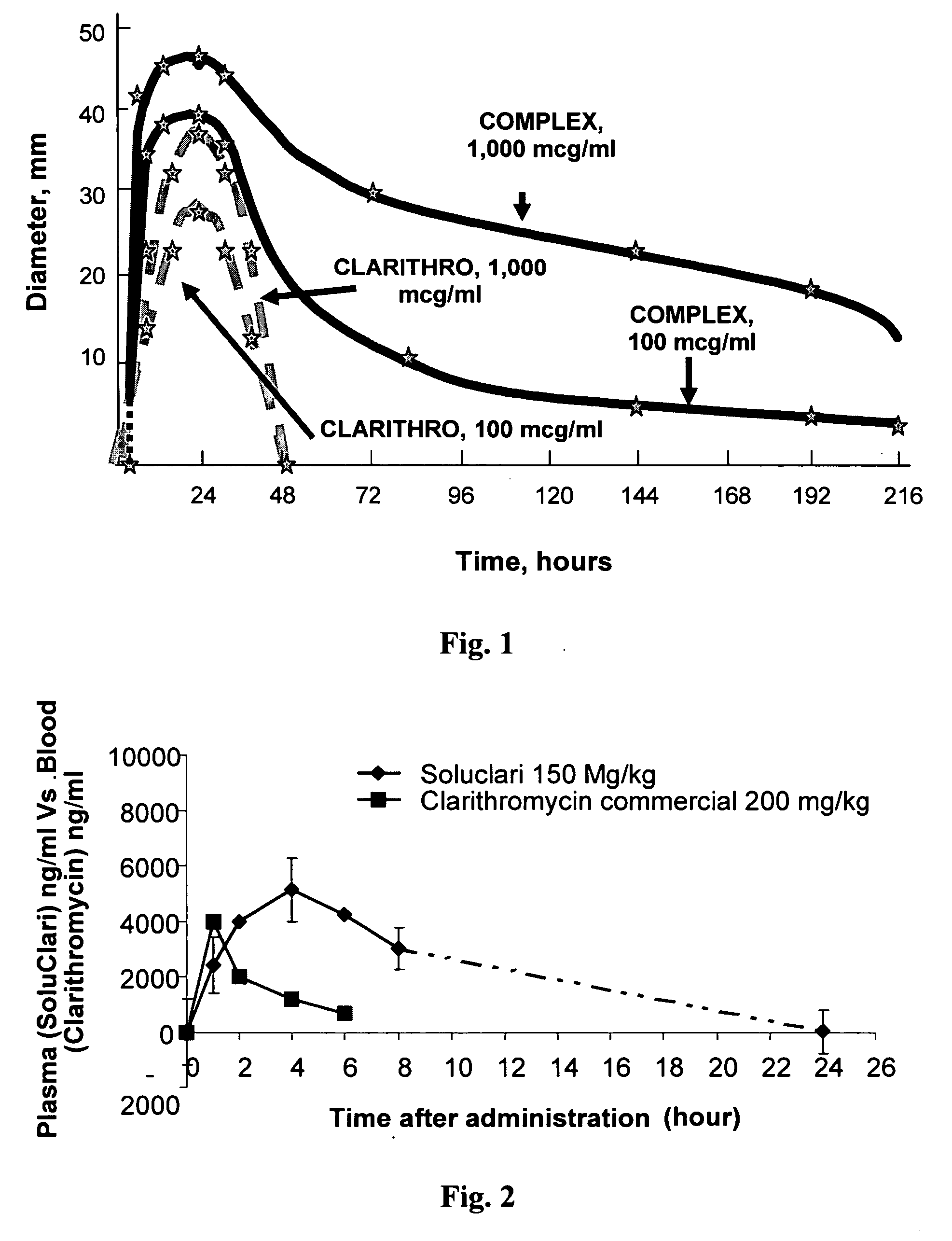
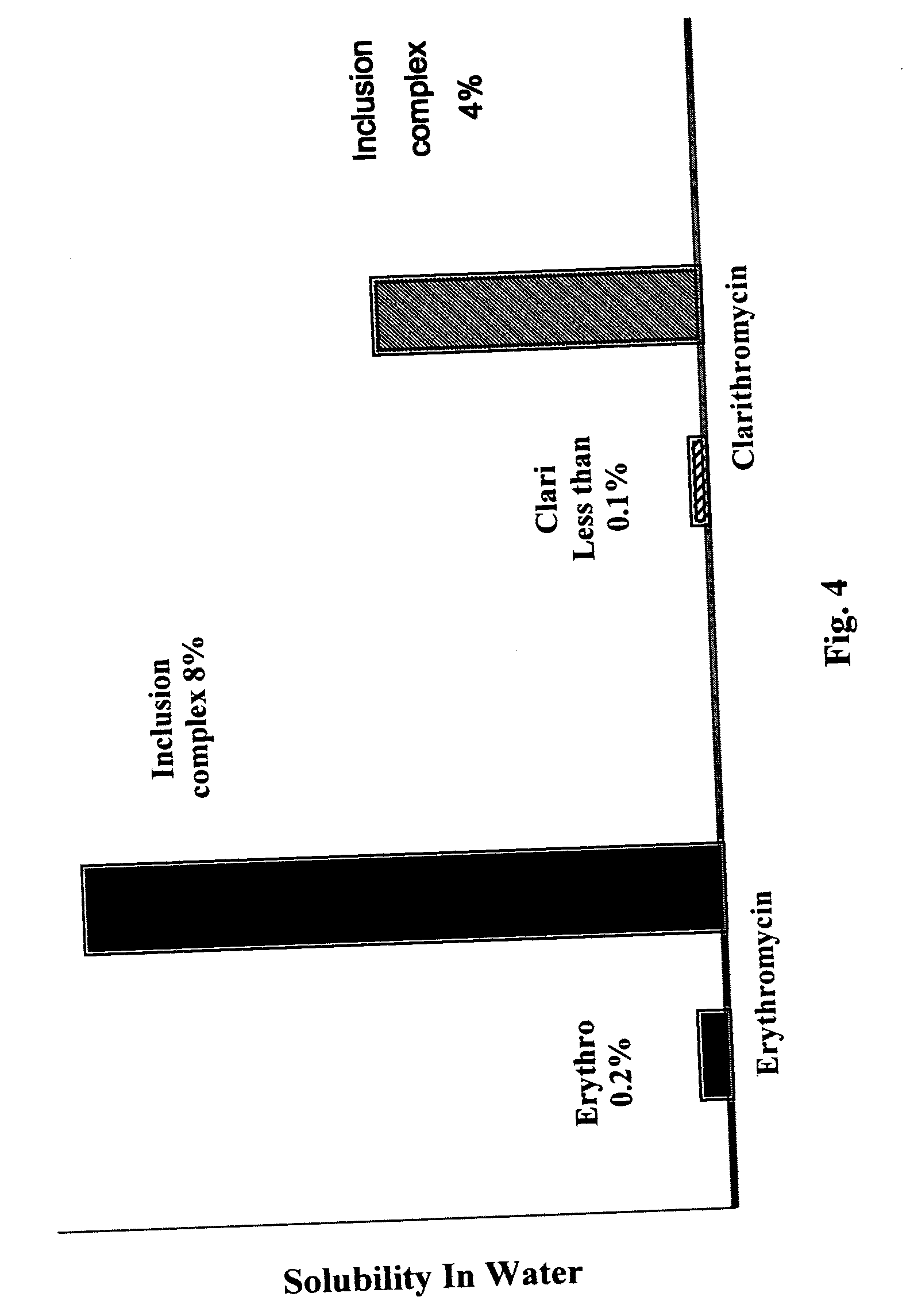
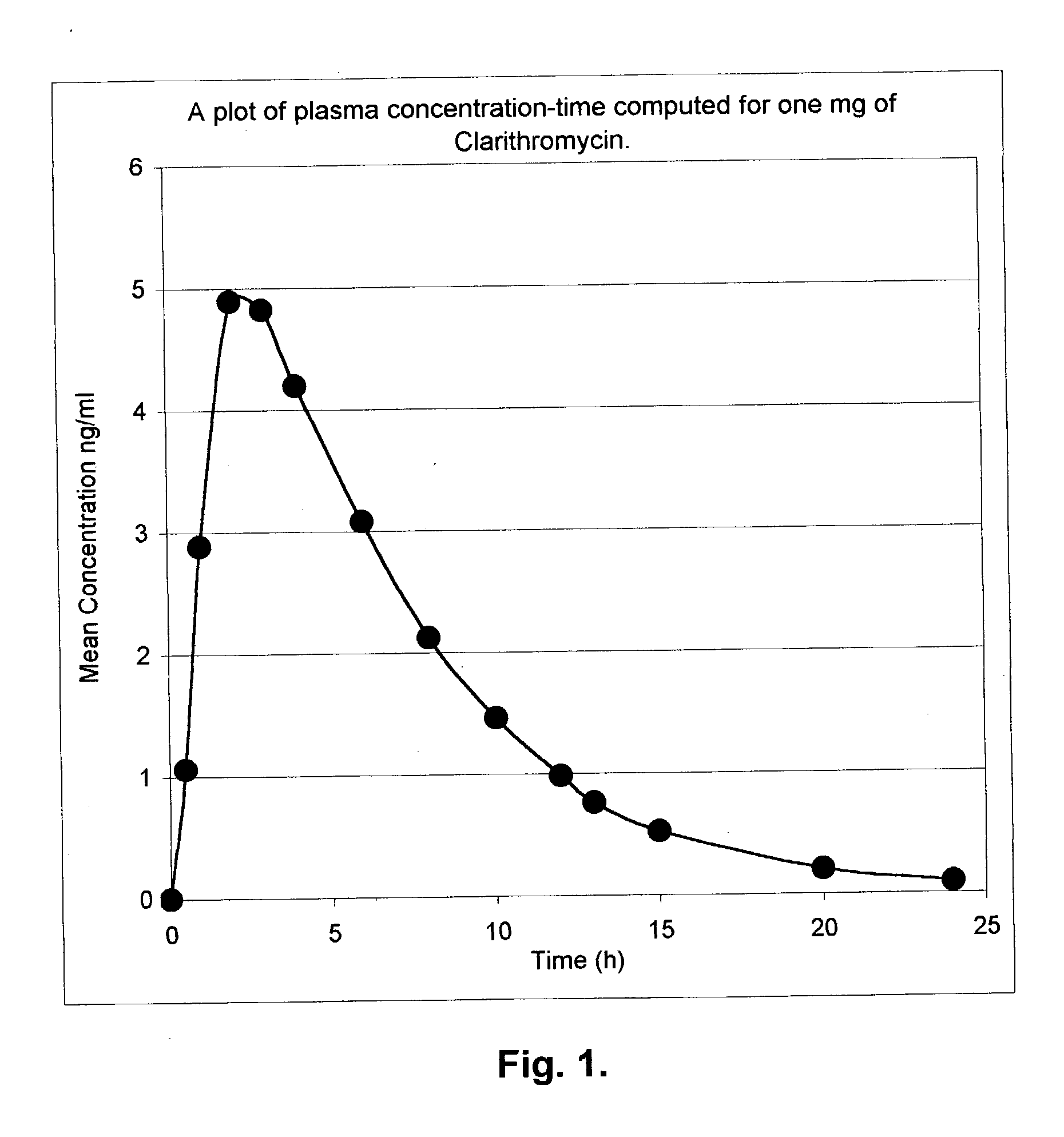
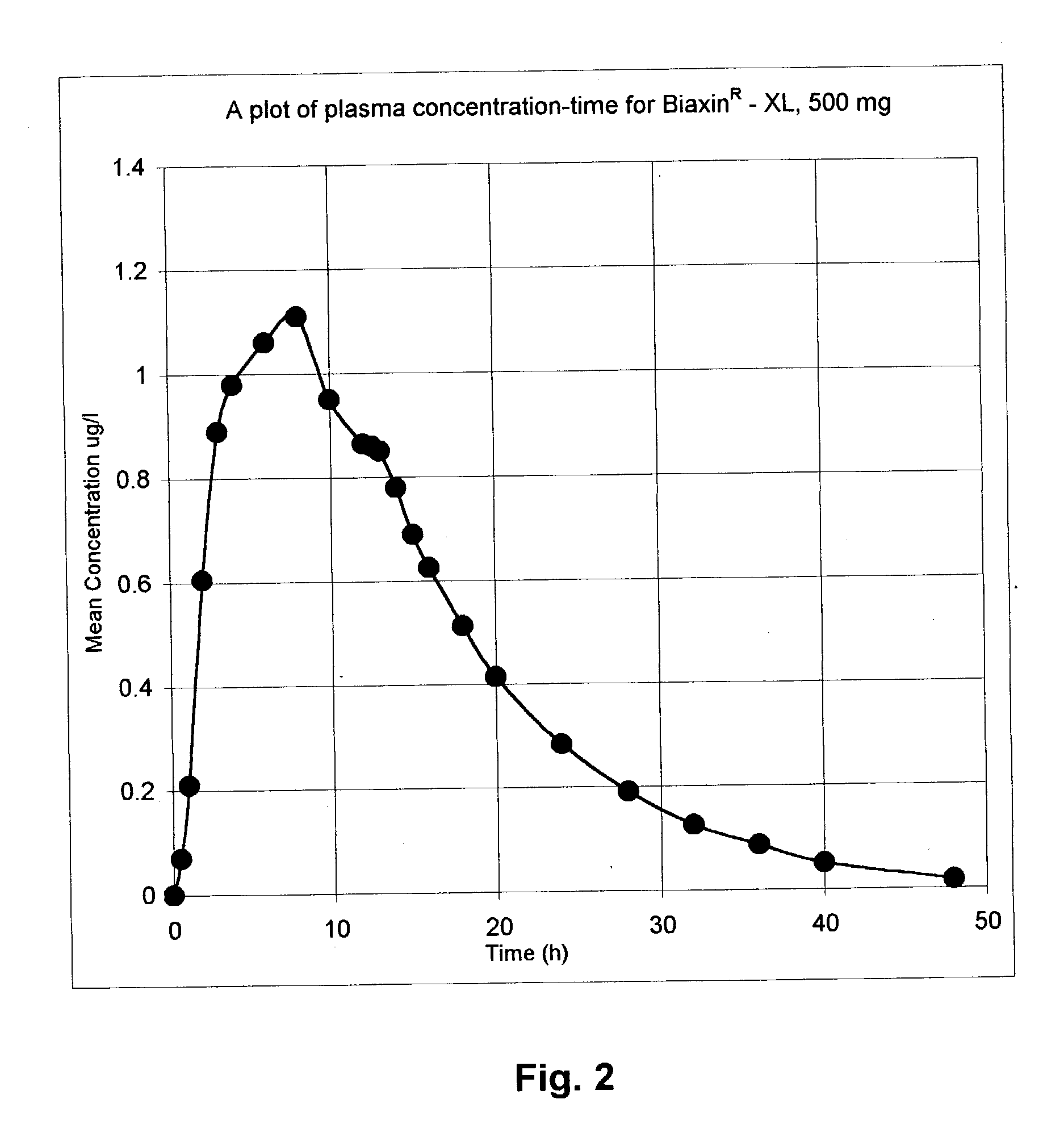
InactiveUS20030091627A1EffectiveEliminate the problemBiocideCarbohydrate active ingredientsCyclodextrinClarithromycin
There is disclosed the formulation of a poorly soluble macrolide antibiotic, such as clarithromycin together with beta-cyclodextrin, and optionally a dicarboxylic acid wherein the particles of the formulation are prepared using microfluidization techniques in a particle size in the range of from 5 to 15 microns.
Owner:SHARMA VINAY
Pharmaceutical ointment formulations
A pharmaceutical ointment composition is provided comprising (a) a therapeutically effective amount of one or more active pharmaceutical ingredients comprising one or more macrolide related immunosuppressants or pharmaceutically acceptable salts or esters thereof; (b) an ointment base; and (c) an effective amount of one or more skin penetration enhancers capable of percutaneous delivery of the macrolide related immunosuppressant through the skin. Also provided is a process for its preparation and methods for delivering a macrolide related immunosuppressant or a pharmaceutically acceptable salt or ester thereof through the skin of a mammal in order to treat conditions situated on and beneath the skin.
Owner:GLENMARK PHARMACEUTICALS LIMITED
Microcapsules containing macrolide lactones abamectin, milbemectin, avermectins, milbemycins, emamectins, ivermectins and mectins in general
Microencapsulated formulations of macrolide lactones (abamectin, milbemectin, milbemycins emamectin, avermectins, ivermectins) wherein the active ingredient is protected from UV-degradation, with exceptional release characteristics resembling those of an emulsion concentrate or, if desired, of long-lasting effect; further with appropriate rheological properties, and with reduced toxicity. The invention provides a unique microencapsulation process for the chemical stability and biological activity of mectins, e.g. abamectin, and provides microcapsules of mectins to be used in formulations CS, WG / CS, ZC, EC / CS and any formulation type containing microcapsules and combination with other biologically active ingredients.
Owner:GAT MICROENCAPSULATION AG
Preparation and utility of substituted erythromycin analogs
InactiveUS20070281894A1Decreased inter-individual variation in plasma levelsSignificant clinical effectAntibacterial agentsBiocideChemical synthesisMicroorganism
The present disclosure is directed to novel macrolide antibiotics of Formula 1 and pharmaceutically acceptable salts and prodrugs thereof; and the chemical syntheses and medical uses of these novel macrolide antibiotics for the treatment and / or management of infections caused by various aerobic and anaerobic gram-positive and gram-negative microorganisms as well as various mycobacteria.
Owner:AUSPEX PHARMA INC
Motilide compounds
The present invention provides novel macrolide compounds of the formulas 1 wherein: R is hydroxyl or methoxy; R.sup.1 is selected from the group consisting of hydrogen, hydroxyl, halide, NH.sub.2, OR.sup.9, 2 where R.sup.9 is C.sub.1-C.sub.10 alkyl, C.sub.2-C.sub.10 alkenyl, C.sub.2-C.sub.10 alkynyl, aryl or heteroaryl and R.sup.10 and R.sup.11 are each independently hydrogen, C.sub.1-C.sub.10 alkyl, C.sub.2-C.sub.10 alkenyl, C.sub.2-C.sub.10 alkynyl, or aryl; R.sup.2 and R.sup.3 are each independently selected from the group consisting of hydrogen, C.sub.1-C.sub.10 alkyl, C.sub.2-C.sub.10 alkenyl, C.sub.2-C.sub.10 alkynyl, aryl, alkylaryl, alkenylaryl, alkynylaryl or R.sup.2 and R.sup.3 together form a cycloalkyl or a cycloaryl moiety; R.sup.4 is hydrogen or methyl; R.sup.5 is hydrogen, hydroxyl, oxo, or together with R.sup.6 and the carbons to which they are attached form a cyclic carbonate; R.sup.6 is hydrogen, hydroxyl, OR.sup.12 where R.sup.12 is C.sub.1-C.sub.10 alkyl, C.sub.2-C.sub.10 alkenyl, C.sub.2-C.sub.10 alkynyl, or together with R.sup.5 and the carbons to which they are attached form a cyclic carbonate; R.sup.7 is methyl, C.sub.3-C.sub.10 alkyl, C.sub.2-C.sub.10 alkenyl, C.sub.2-C.sub.10 alkynyl, alkylaryl, alkenylaryl, alkynylaryl, amidoalkylaryl, amidoalkenylaryl, or amidoalkynylaryl; R.sup.8 is C.sub.1-C.sub.10 alkyl, C.sub.2-C.sub.10 alkenyl, C.sub.2-C.sub.10 alkynyl, alkylaryl, alkenylaryl, alkynylaryl, amidoalkylaryl, amidoalkenylaryl, or amidoalkynylaryl; and, x is a single or a double bond.
Owner:KOSAN BIOSCI
Parasiticidal compositions comprising multiple active agents, methods and uses thereof
ActiveUS20110245191A1Superior broad spectrum efficacyBiocideDead animal preservationActive agentMacrocyclic lactone
This invention relates to compositions for combating ectoparasites and endoparasites in animals, comprising at least one 1-arylpyrazole, at least one macrocyclic lactone, at least one insect growth regulator, and at least one anthelmintic compound in combination with a pharmaceutically acceptable carrier. This invention also provides for an improved methods for eradicating, controlling, and preventing parasite infections and infestations in an animal comprising administering the compositions of the invention to the animal in need thereof.
Owner:MERIAL INC
Macrolide compositions having improved taste and stability
InactiveUS20090232744A1Improve stabilityBad tasteBiocideDispersion deliverySodium acetateSodium lactate
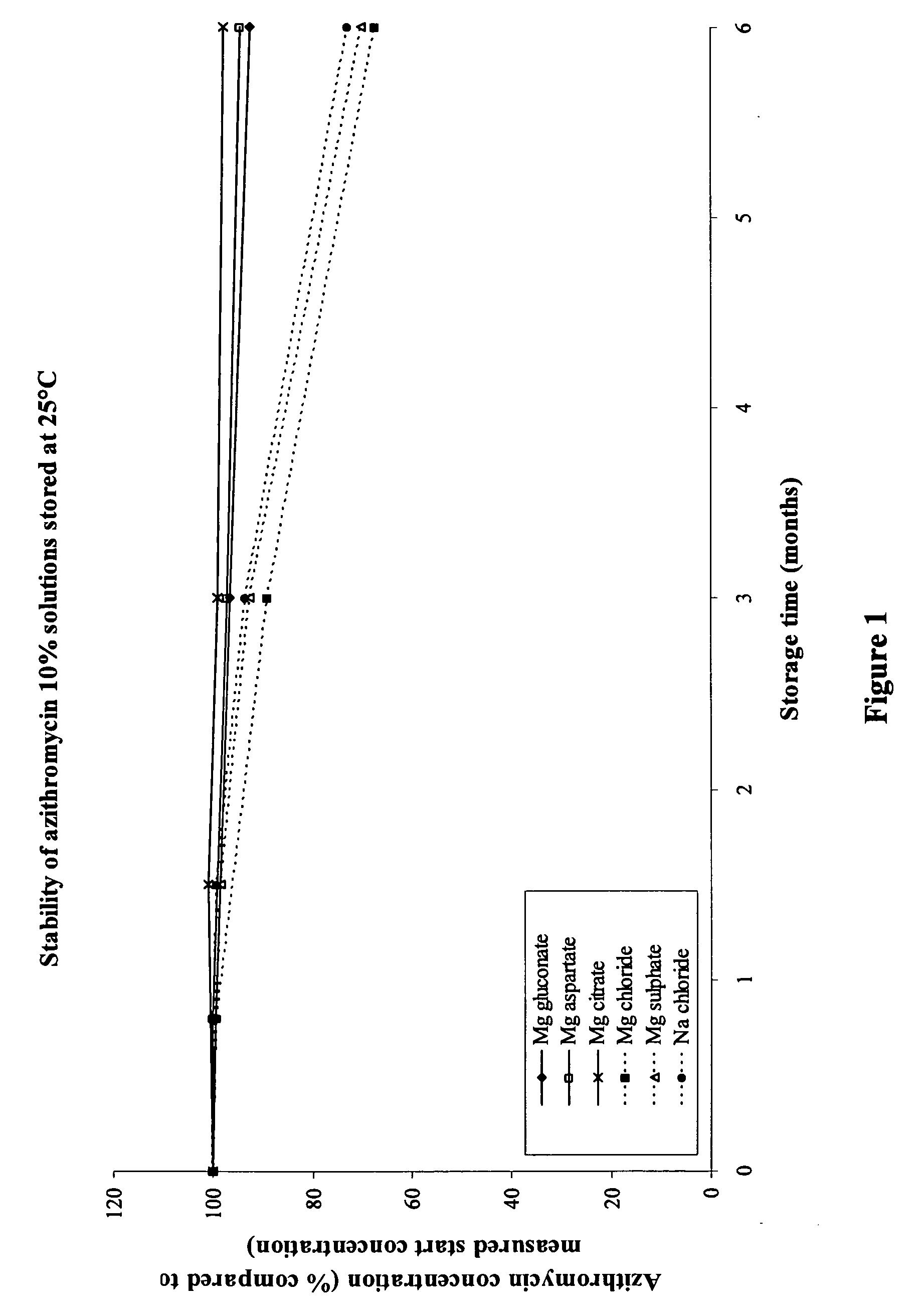
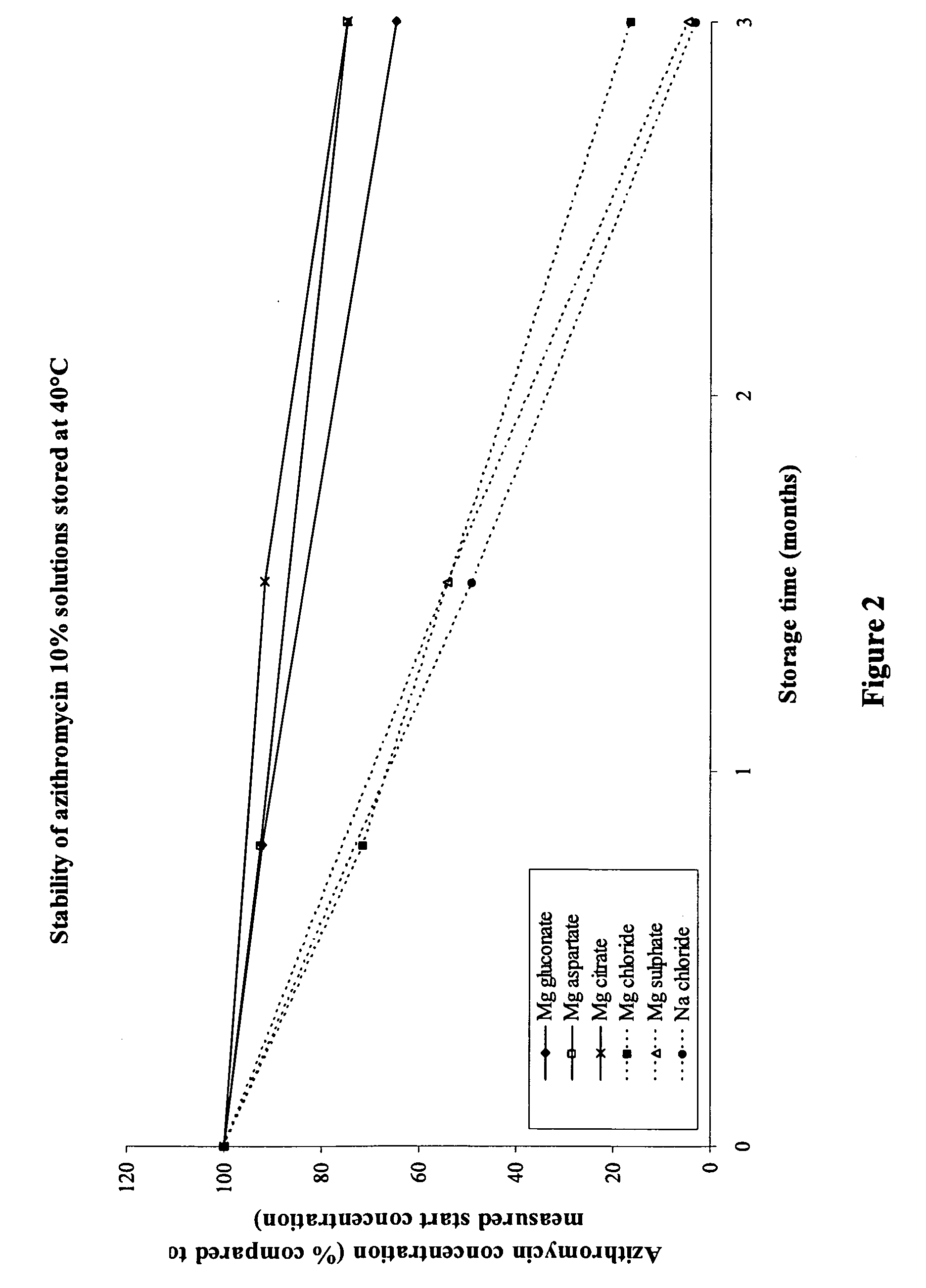
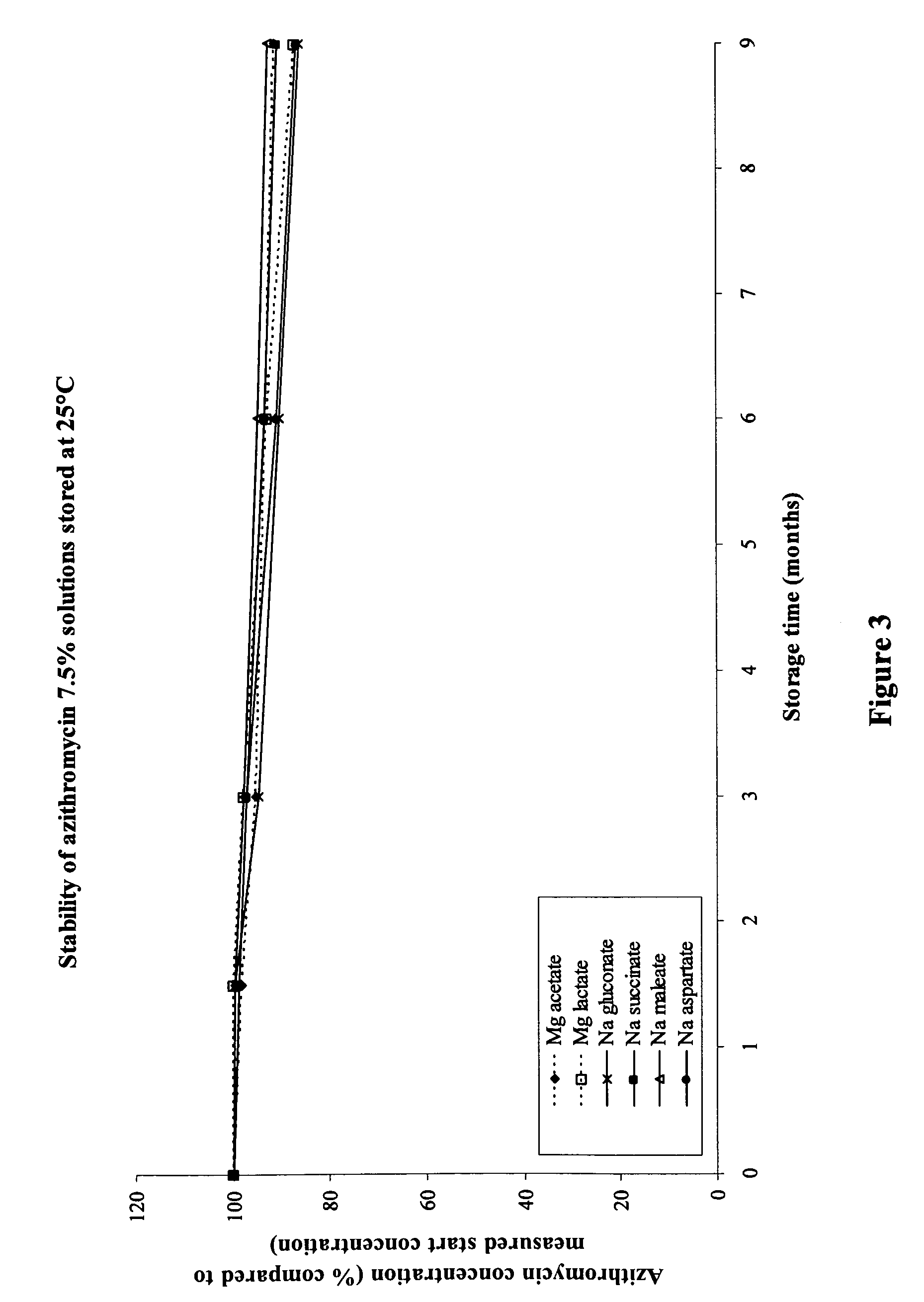
The invention provides an aqueous pharmaceutical composition for administration as an aerosol to the respiratory tract, nose or oropharyngeal region comprising (i) a macrolide having a poor taste and poor chemical stability in aqueous solution; (ii) at least one salt selected from the group consisting of sodium gluconate, sodium aspartate, sodium acetate, sodium lactate, sodium succinate, sodium maleate, magnesium gluconate, magnesium aspartate, magnesium citrate, magnesium acetate, magnesium lactate, magnesium succinate, and magnesium maleate; or mixtures thereof and (iii) a taste-masking agent different from said salt; wherein (a) the concentration of said macrolide in the composition is in the range of about 0.25 wt.-% to about 15 wt.-%; (b) the molar ratio of said macrolide:said salt is in the range from about 1:0.5 to about 1:100; (c) the pH of the composition is in the range of about 3 to 9; and (d) the osmolality of the composition is in the range of about 150 mOsmol / kg to about 1500 mOsmol / kg. The invention further provides a method of generating an aerosol, preferably by means of a nebuliser, which uses such an aqueous pharmaceutical composition. The macrolide may be used alone or in combination with other drugs. The composition is suitable to treat inflammatory disorders and / or infections of the respiratory tract. It has an improved taste and stability.
Owner:PARI PHARMA GMBH
Use of macrolide compounds for the treatment of dry eye
InactiveUS7063857B1Superior improving effect on dry eye symptomsGood treatment effectPill deliveryCapsule deliveryMacrolide resistancePharmacology
Owner:SUCAMPO
Pyridyl substituted ketolide antibiotics
Antimicrobial macrolide and ketolide compounds are provided having formulas XII: as well as pharmaceutically acceptable salts, esters or prodrugs thereof; pharmaceutical compositions comprising such compounds; methods of treating bacterial infections by the administration of such compounds; and processes for the preparation of the compounds.
Owner:NOVARTIS AG
Antibiosis streptomycete
InactiveCN101463332AHigh activityFunction increaseBiocideBacteriaIntellectual propertyField experiment
The invention relates to an antibiotic streptomyces, fermentation product of which can inhibit broad spectrum fungus and protozoan, and field experiment can control crop fungus plant disease and root-knot nematode. The antibiotic streptomyces has good effect, no pollution nuisance and no residue. The antifungal agent has extremely low hazardness for the people and good performance and development prospect for medical and agricultural use. The invention is named as SIM001 antibiotic streptomyces which is Streptomyces antibioticus subspecies xi an, and is preserved as patent in the depositary institution appointed by patent office of state intellectual property office; the preservation data is April 14th, 2005, the name of the depositary institution is CGMCC, and the number of preservation is CGMCC No.1349. The whole cell hydrolysate of the antibiotic streptomyces contains L, L-DAP (Diaminopimelic acid) and no characteristic glucide; the cytoderm belongs to I type and glucide type C; one of the main antibacterial materials is polyene macrocyclic ketolide which belongs to the broad spectrum antifungal substance.
Owner:刘昶志
Method of purifying moxidectin through crystallization
Methods for the purification of the macrolide moxidectin result in higher purity levels than can often otherwise be obtained. The crystalline moxidectin is then used in a wide variety of pharmaceutical and veterinary applications, including the prevention, treatment and control of parasites in plants, animals and humans.
Owner:ZOETIS W LLC
Methods for Alzheimer's disease treatment and cognitive enhance
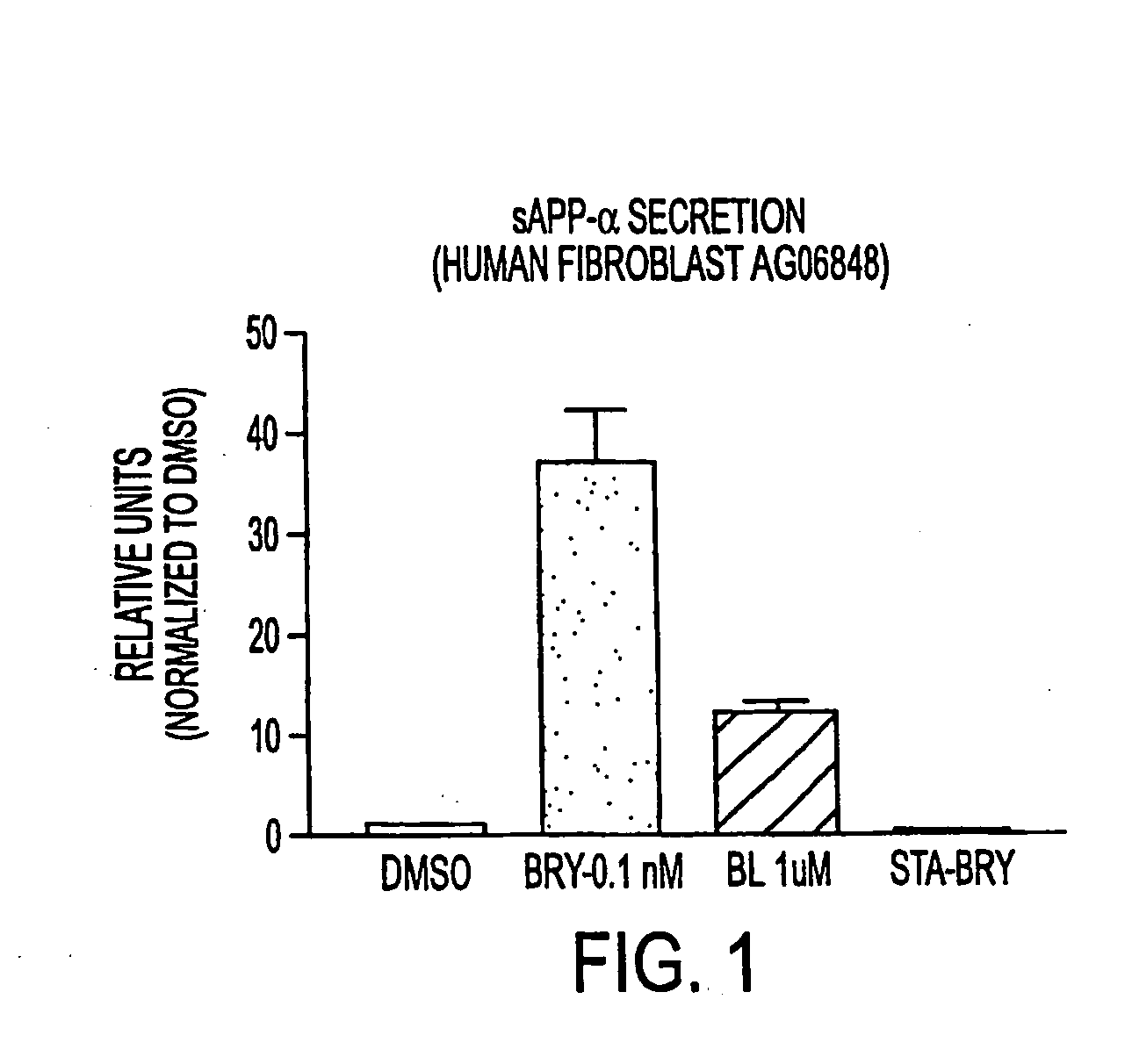
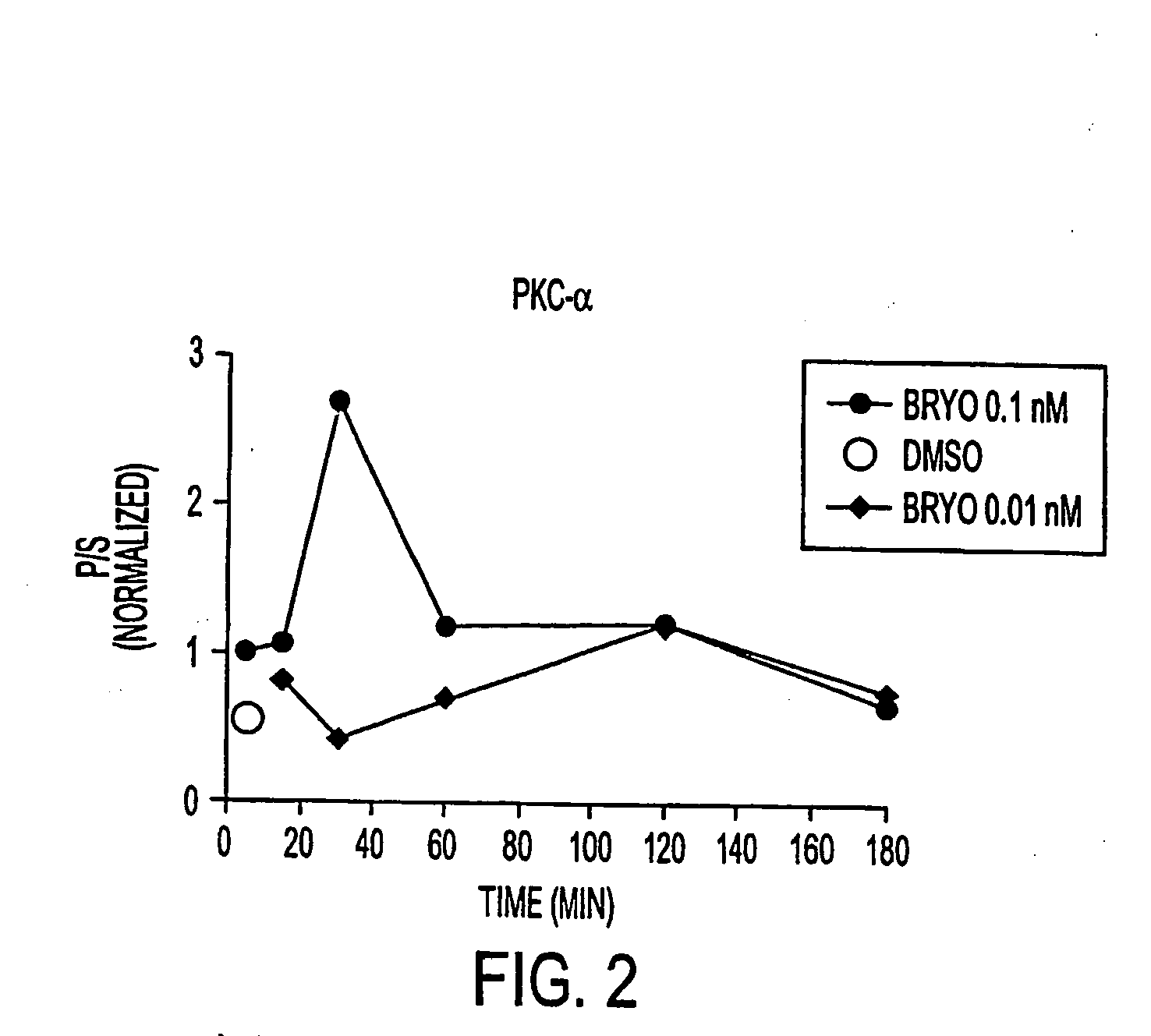
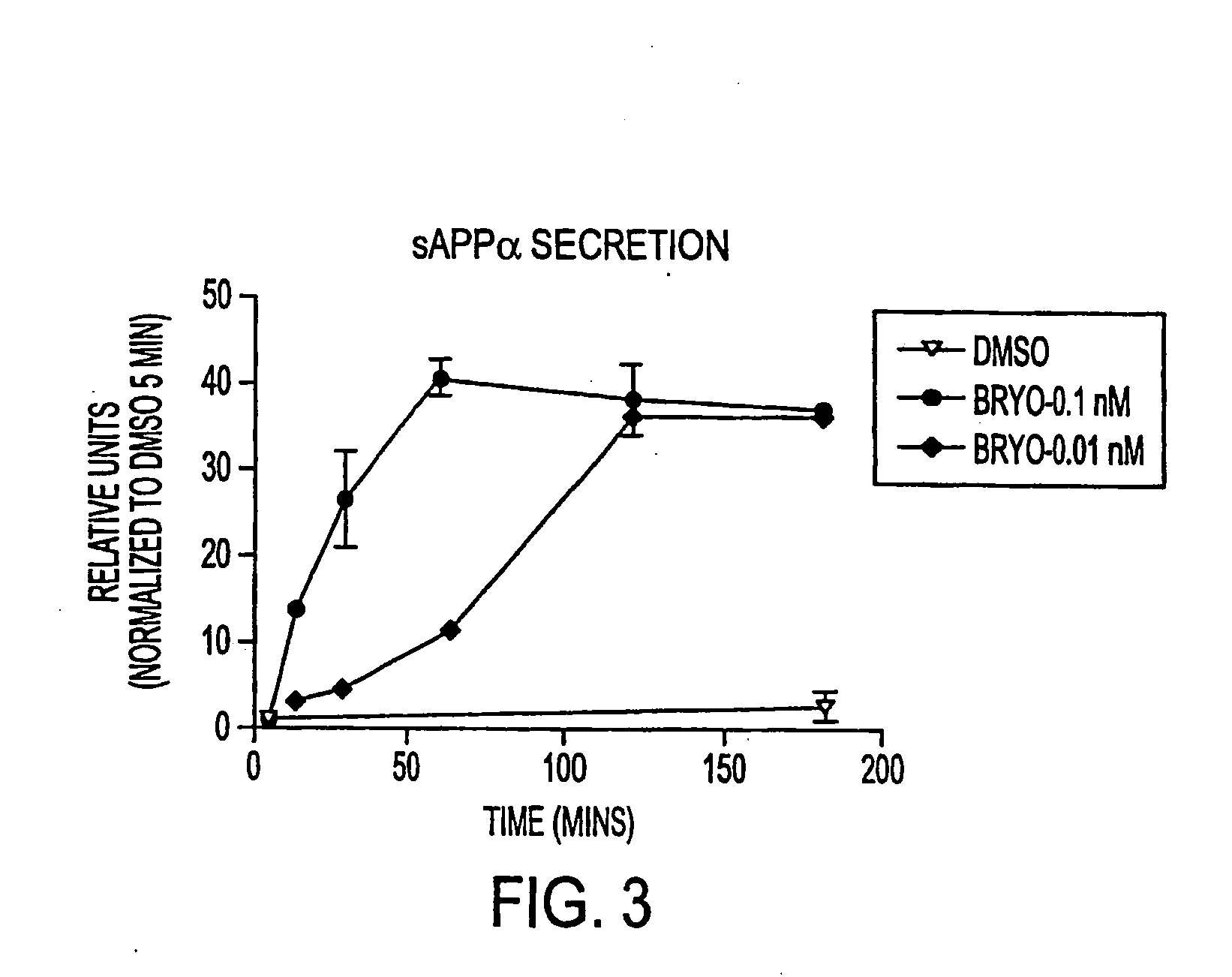
InactiveUS20050065205A1Reduces myalgiaIncreased Tolerated DoseBiocideNervous disorderDepressantBryostatin I
The present invention relates to compositions comprising a combination of PKC activators and PKC inhibitors and methods to modulate α-secretase activity; improve or enhance cognitive ability; and / or reduce neurodegeneration in individuals suffering from diseases that impair cognitive ability, particularly Alzheimer's Disease. The invention also relates to methods for improving or enhancing cognitive ability. The present invention also provides methods for increasing the generation of non-amyloidogenic soluble APP (sAPP) comprising the activation of protein kinase C (PKC) in the brain and inhibiting PKC in peripheral tissues. Macrocyclic lactones (i.e. bryostatin class and neristatin class) are preferred PKC activators and Vitamin E is a preferred PKC inhibitor for use in the inventive composition.
Owner:COGNITIVE RES ENTERPRISES INC
Anti-infective agents useful against multidrug-resistant strains of bacteria
The invention relates to novel methods for using macrolide anti-infective agents. The macrolide anti-infective agents demonstrate antibacterial activity against multi-drug resistant strains of bacteria and, in particular, methicillin-resistant Staphylococcus aureus (MRSA). Methods for inhibiting the activity of multi-drug resistant bacterial organisms and methods for treating a bacterial infection caused by such organisms are described herein.
Owner:ABBVIE INC
Pharmaceutical cream formulations
InactiveUS20050249757A1BiocidePharmaceutical delivery mechanismMacrolide resistanceMacrocyclic lactone
A pharmaceutical cream composition is provided comprising (a) a therapeutically effective amount of one or more active pharmaceutical ingredients comprising one or more macrolide related immunosuppressants or pharmaceutically acceptable salts or esters thereof; (b) one or more cream forming agents; and (c) an effective amount of one or more skin penetration enhancers capable of percutaneous delivery of the macrolide related immunosuppressant through the skin. Also provided is a process for its preparation and methods for delivering a macrolide related immunosuppressant through the skin of a mammal in order to treat conditions situated on and beneath the skin.
Owner:GLENMARK PHARMACEUTICALS LIMITED
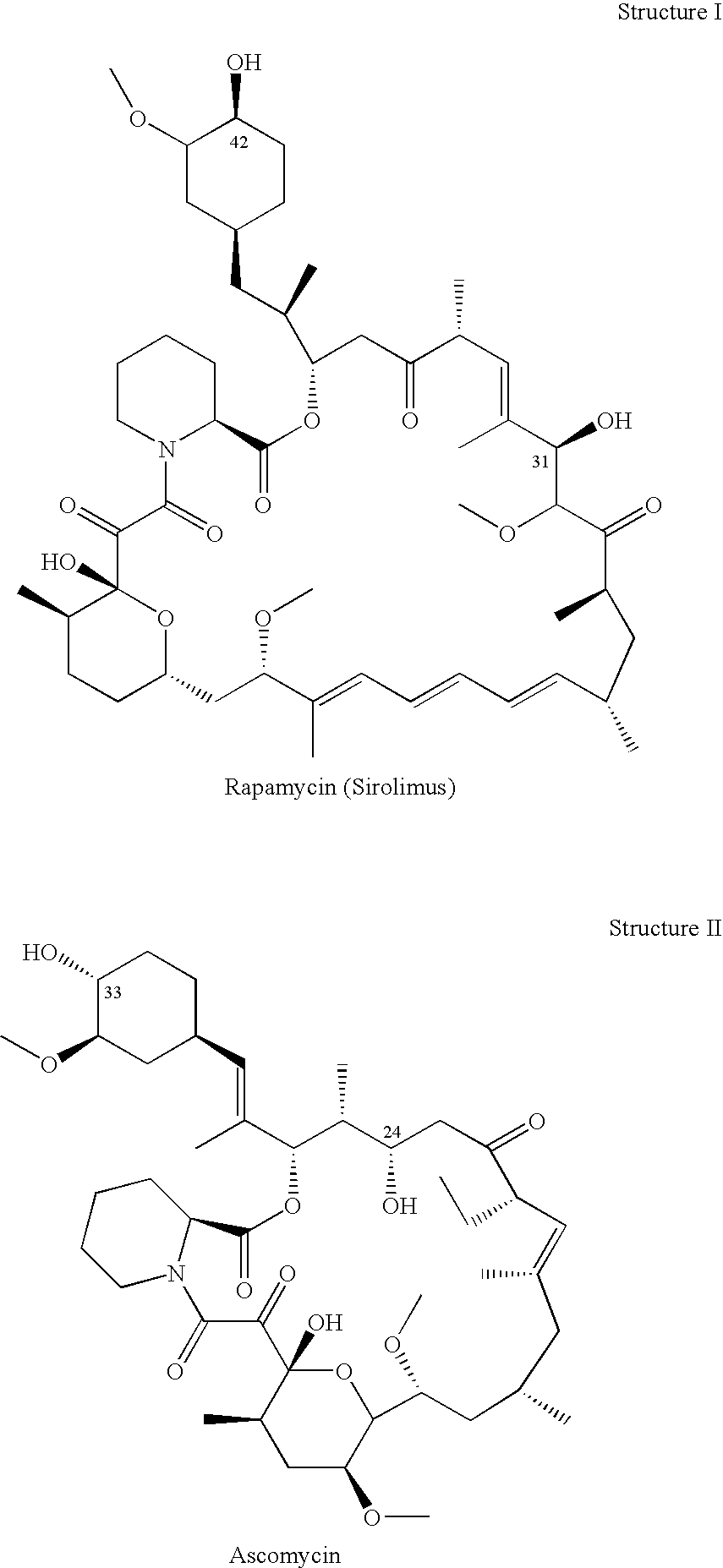
Non-Invasive Ocular Delivery of Rapamycin
Methods and systems for preventing or treating various ocular conditions are disclosed and described. In one aspect, for example, a method for noninvasively delivering a water insoluble macrolide into an eye of a subject for treatment of an ocular condition is provided. Such a method may include administering non-invasively a water soluble form of the macrolide directly into an eye of a subject having or at risk for having the ocular condition, wherein the water soluble form is converted into the water insoluble macrolide in the eye in order to treat the ocular condition.
Owner:HIGUCHI JOHN W
Benzimidazole non-aqueous compositions
InactiveUS20070128239A1Broaden spectrumWide range of activitiesBiocideDispersion deliveryTriclabendazoleCompound (substance)
The present invention provides a stable veterinary oral composition which comprises one or more surfactants, a water-miscible solvent, optionally an oil and an effective amount of each of a benzimidazole antihelmintic compound, such as triclabendazole and a macrocyclic lactone, such as moxidectin. Said composition is useful for treating and controlling endo- and ectoparasitic infection and infestation in a homeothermic animal.
Owner:ZOETIS SERVICE LLC
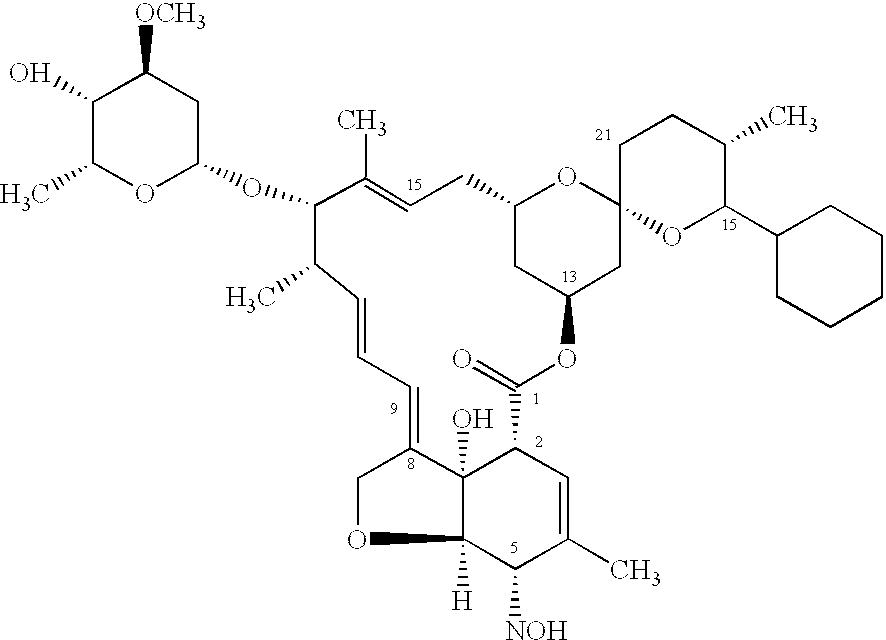
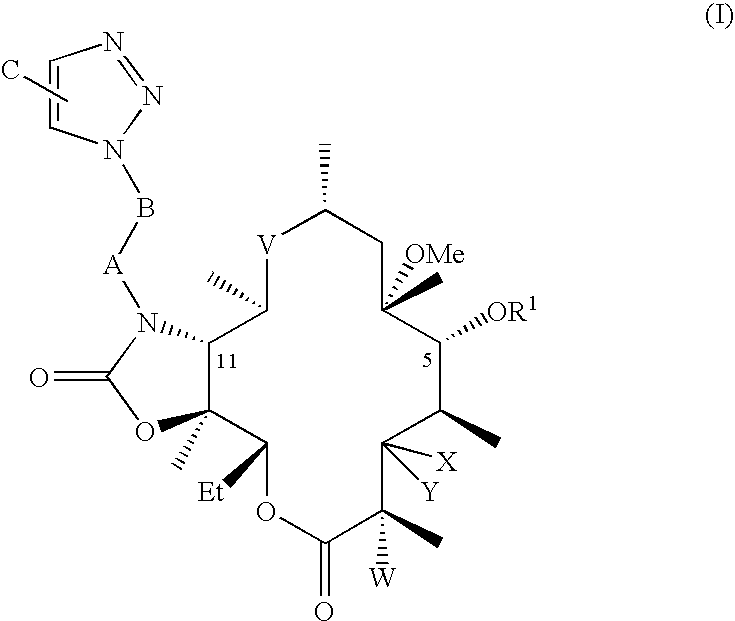
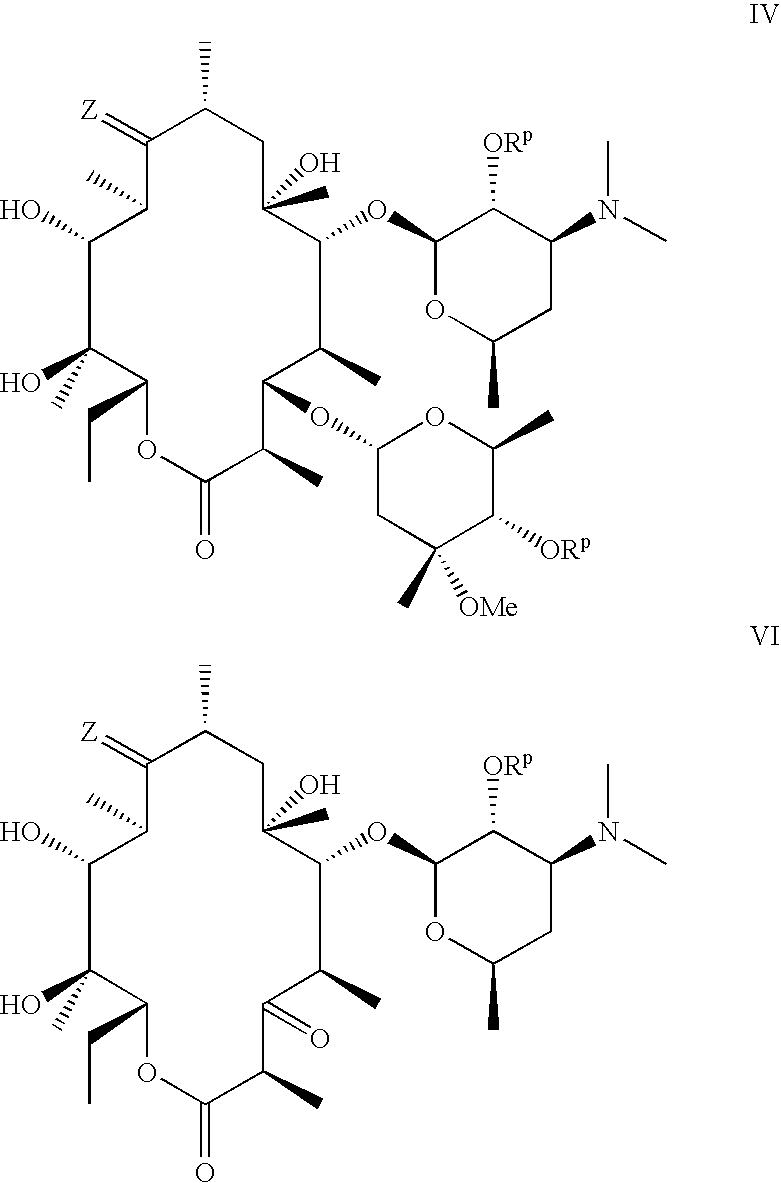
Macrocyclic lactone compounds and methods for their use
A method of treating an ophthalmic condition or disease by administering a compound disclosed herein is provided. The compound can be administered systemically or locally and in a variety of ways, such as via a temporary device, an implant, an injection or an eye drop. The compound can also be administered with an additional therapeutic agent.
Owner:ELIXIR MEDICAL CORP
Compounds, compositions and methods for treatment of inflammatory diseases and conditions
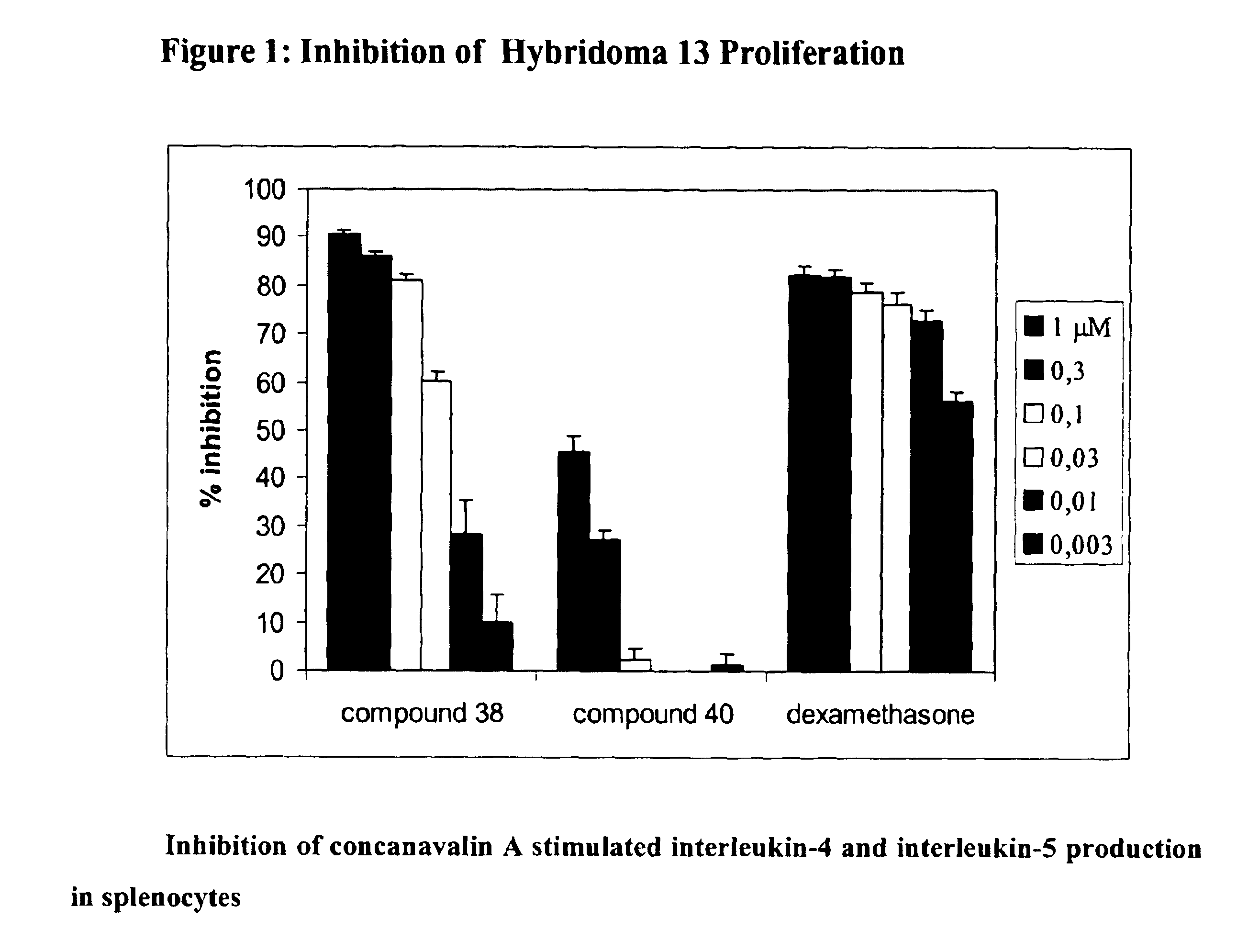
The present invention relates (a) to new compounds represented by Formula I: wherein M represents a macrolide subunit (macrolide moiety) derived from macrolide possessing the property of accumulation in inflammatory cells, S represents a steroid subunit (steroid moiety) derived from steroid drug with anti-inflammatory activity and L represents a linker molecule linking M and S, (b) to their pharmacologically acceptable salts, prodrugs and solvates, (c) to processes and intermediates for their preparation, and (d) to their use in the treatment of inflammatory diseases and conditions in humans and animals. Such compounds inhibit many cytokines and immune mediators involved in immune responses which cause inflammation, allergy, or alloimmunity, including without limitation IL-1, 2, 4, 5, 6, 10, 12, GMCSF, ICAM, and TNF-α. Importantly, anti-inflammatory steroids exert a direct anti-inflammatory effect through binding to the glucocorticosteroid receptor.
Owner:GLAXOSMITHKLINE ISTRAZIVACKI CENTAR ZAGREB D O O
Macrocyclic lactone compounds and methods for their use
A method of inhibiting smooth muscle cell proliferation or cytokine production in a subject, comprising administering a compound disclosed herein to the subject, is provided. The compound can be administered systemically, locally, or a combination thereof. For example, the compound can be locally delivered from a temporary device or an implant, such as a vascular prosthesis.
Owner:ELIXIR MEDICAL CORP
Synergistic insecticidal compositions
InactiveUS20080125420A1Improve protectionOrganic active ingredientsBiocideSodium Channel AntagonistsCarbamate
The present invention provides a synergistic insecticidal composition comprising as essential active ingredients a neuronal sodium channel antagonist in combination with one or more compounds selected from the group consisting of pyrethroids, pyrethroid-type compounds, recombinant nucleopolyhedroviruses capable of expressing an insect toxin, organophosphates, carbamates, formamidines, macrocyclic lactones, amidinohydrazones, GABA antagonists and acetylcholine receptor ligands.Also provided are methods for synergistic insect control and crop protection.
Owner:TREACY MICHAEL FR +4
Insecticidal composition of sufluoxime
The present invention discloses a combination of macrolides insecticide and insecticide of Sufluoxime, wherein the macrolides insecticide is spinosad, avermectin, the derivative thereof, etc., and the Sufluoxime is 1-(3-fluoro-4-chlorophenyl)-2-methylthioethanone-O-(2-hydroxymethyl-3-methyl) aether. The various surfactants and additives such as carrier, thickening agent, stabilizing agent, antifreeze agent, antifoaming agent, adhesive and the like are added to prepare an insecticidal composition in dosage forms of missible oil, aqueous emulsion, microemulsion, suspending agent, granular formulation, water dispersible powder or dispersible granula, etc. The insecticidal composition has characteristics of evident synergism, resistance delay, widening the spectrum of insect disinfestation, etc., and has disinfestation effect to the destructive insect in the disinfestation range of two normal insecticides.
Owner:HUNAN CHEM RES INST
Synergistic insecticidal compositions
InactiveUS20080125421A1Improve protectionOrganic active ingredientsBiocideSodium Channel AntagonistsCarbamate
Owner:TREACY MICHAEL FR +4
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com