Methods and compositions for control of fetal growth via modulation of relaxin
a technology of relaxin and fetal growth, which is applied in the direction of peptide/protein ingredients, immunological disorders, metabolism disorders, etc., can solve the problems of significant perinatal morbidity and mortality, interfering with the normal vascular development of the placenta, and failure of the fetus to exhibit a normal growth rate, so as to reduce the risk or incidence of spontaneous abortion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0259] The effect of relaxin administration on tissues thought to be targeted by relaxin during pregnancy and on the health and well-being of neonates born to mothers treated with relaxin was tested in female marmoset monkeys. Relaxin was administered during the period that spanned ovulation, implantation, and early pregnancy of the monkeys.
[0260] Animals. Female marmosets (Callithrix jacchus) were young, healthy breeders in a captive breeding colony and were housed in pairs with males under controlled environmental conditions. Under these conditions, pregnancy occurs in 90% of receptive females per cycle. Animal weights and general health were recorded immediately prior to the start of the study;
[0261] Relaxin. The recombinant human relaxin used in the study is identical to the naturally occurring, mature product of the human relaxin H2 gene and contains a 24 residue A chain and a 29 residue B chain. It was used at a concentration of either 1.5 mg / mL or 5.0 mg / mL and was formulat...
example 2
[0267] The effect of relaxin administration on (1) tissues thought to be targeted by relaxin during pregnancy and (2) on the health and well-being of neonates born to mothers treated with relaxin was tested in the female Old World cynomolgus monkey, Macaca fascicularis. Endocrine signals and the timing of implantation in these monkeys are quite similar to that seen in humans. Ovarian hormone production during the luteal phase of the cycle is similar in the macaque and humans, and both cycles end with sloughing of the endometrium. Relaxin levels in the circulation during the luteal phase and peri-implantation period approximate 50 pg / mL and 40 pg / mL in humans and macaques, respectively. Implantation occurs nine days after fertilization in the macaque while it occurs approximately two days earlier in humans, but trophoblast rescue of the corpus luteum occurs similarly in both species with increased chorionic gonadotropin production three days after implantation. Enhanced relaxin and p...
example 3
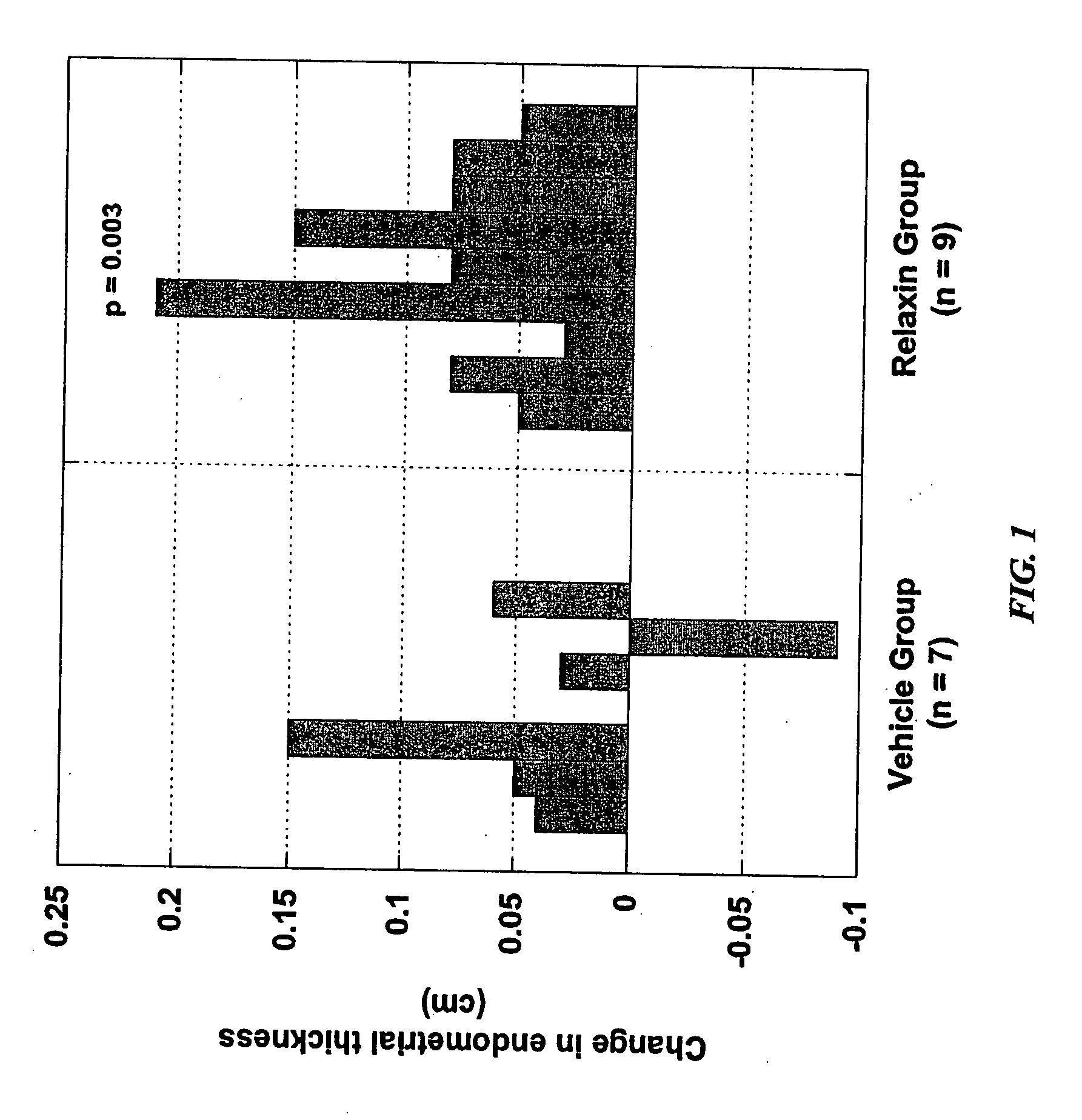
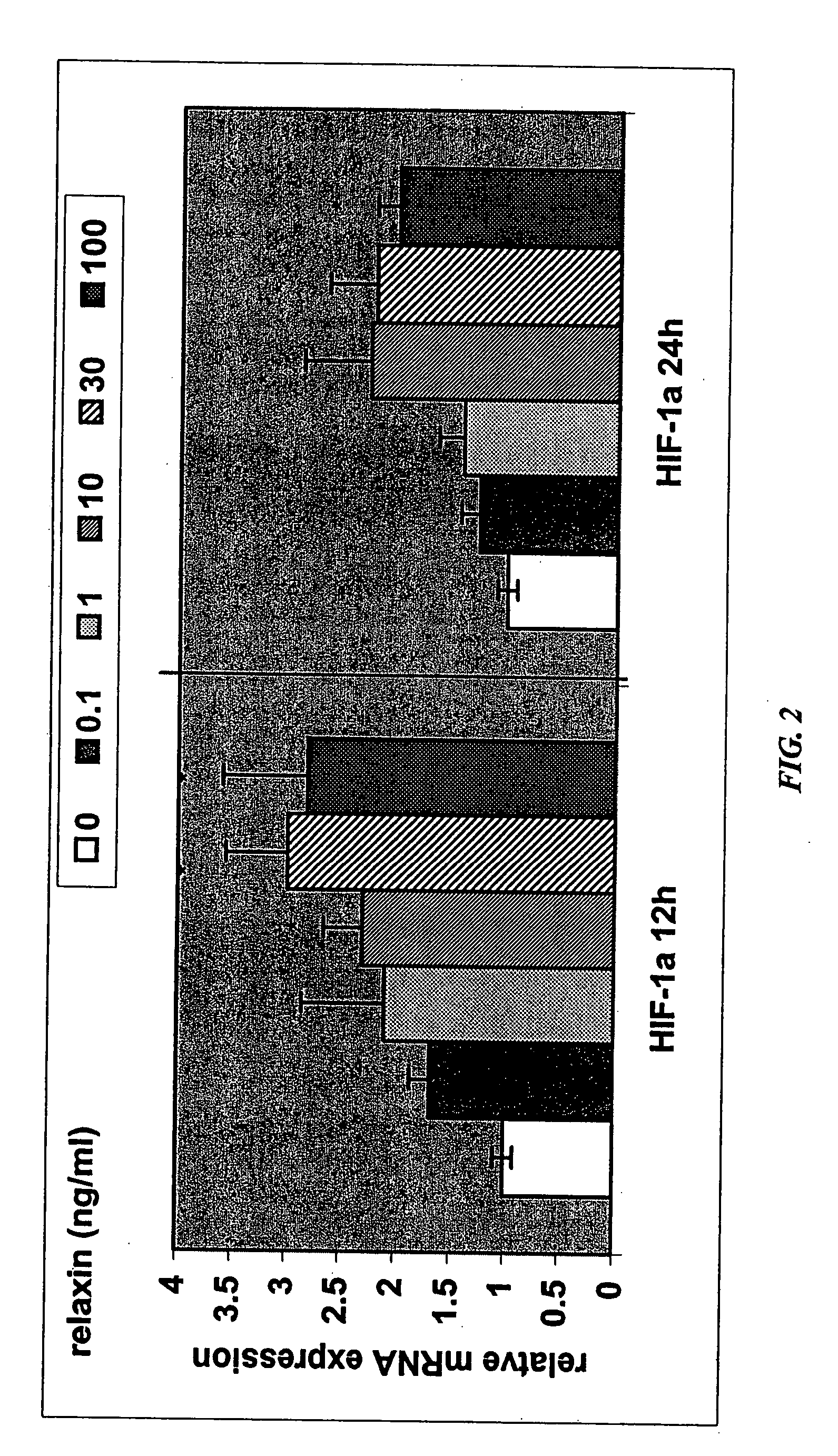
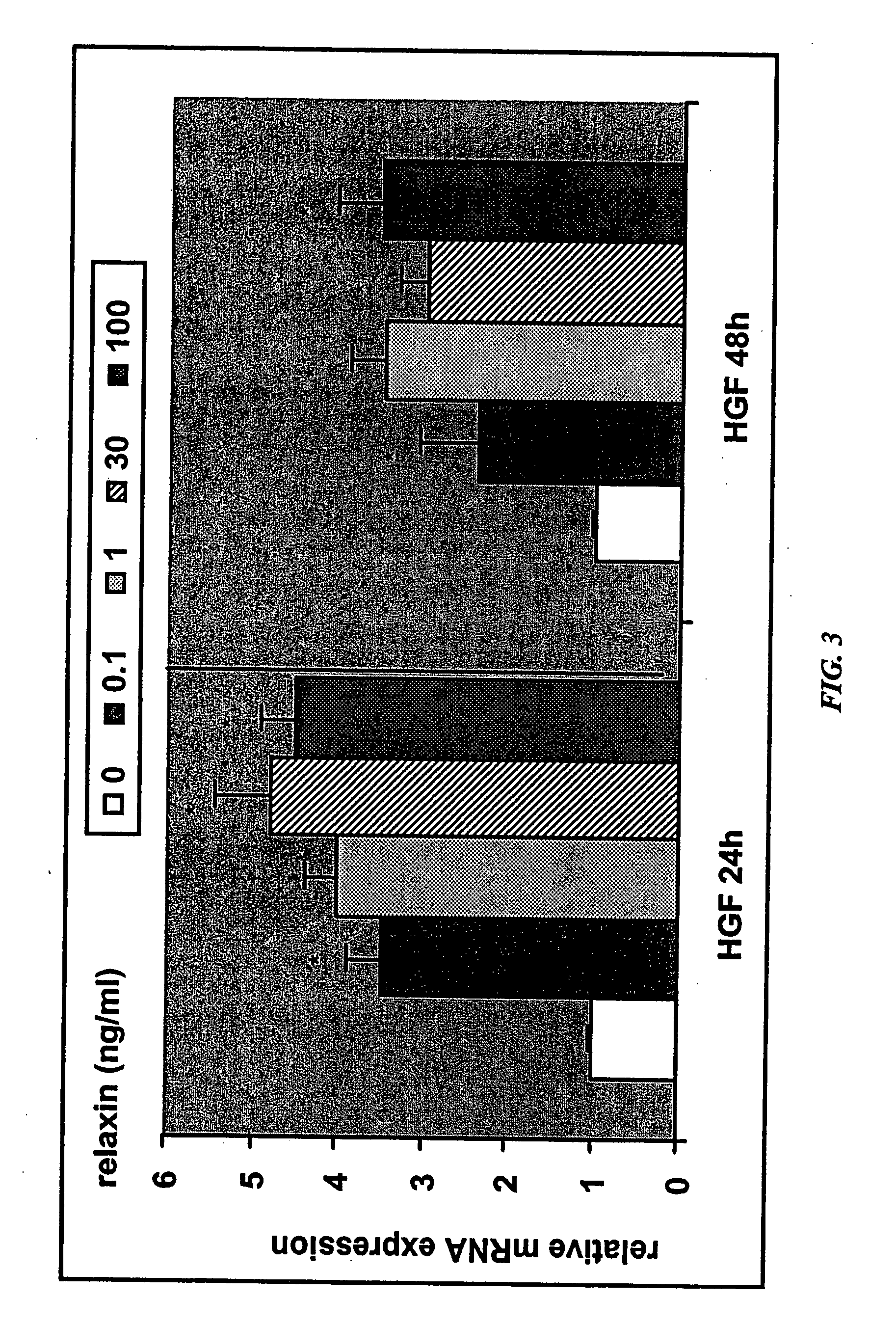
[0301] A decrease in placental hepatocyte growth factor (HGF) expression has been associated with SGA, IUGR, and preeclampsia (Dokras et al., 2001, Biol Reprod 65:1278-1288; Kauma et al., J Clin Endocrinol Metab 84:4092-4096). HIF-1α is a known upregulator of VEGF expression, as well as other angiogenic cytokines, and is also involved in increasing expression of glucose transporter-1, which is involved in glucose uptake by the developing placenta. Relaxin upregulation of HIF-1α protein expression may make HIF-1α available for activation under hypoxic conditions.
[0302] The data in this Example show that relaxin induces molecules that are deficient in IUGR and preeclampsia. Specifically, the data show that (1) relaxin increases the expression of HGF in endometrial stromal cells, as detected by quantitative PCR and ELISA; and (2) relaxin increases expression of HIP-1α in endometrial stromal cells under normoxic conditions.
[0303] Real-time quantitative RT-PCR determination of abundanc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| real time quantitative RT- | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com