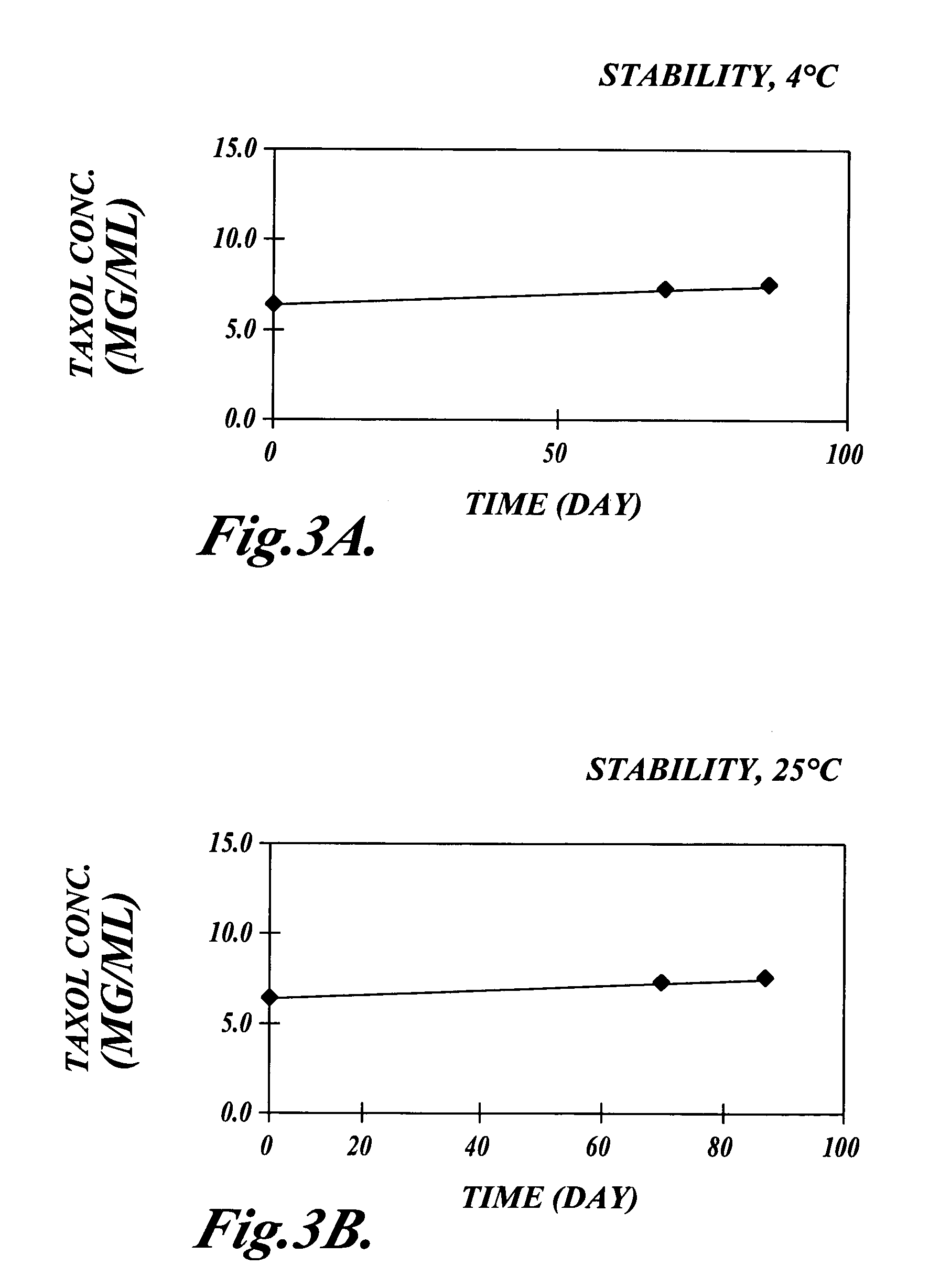
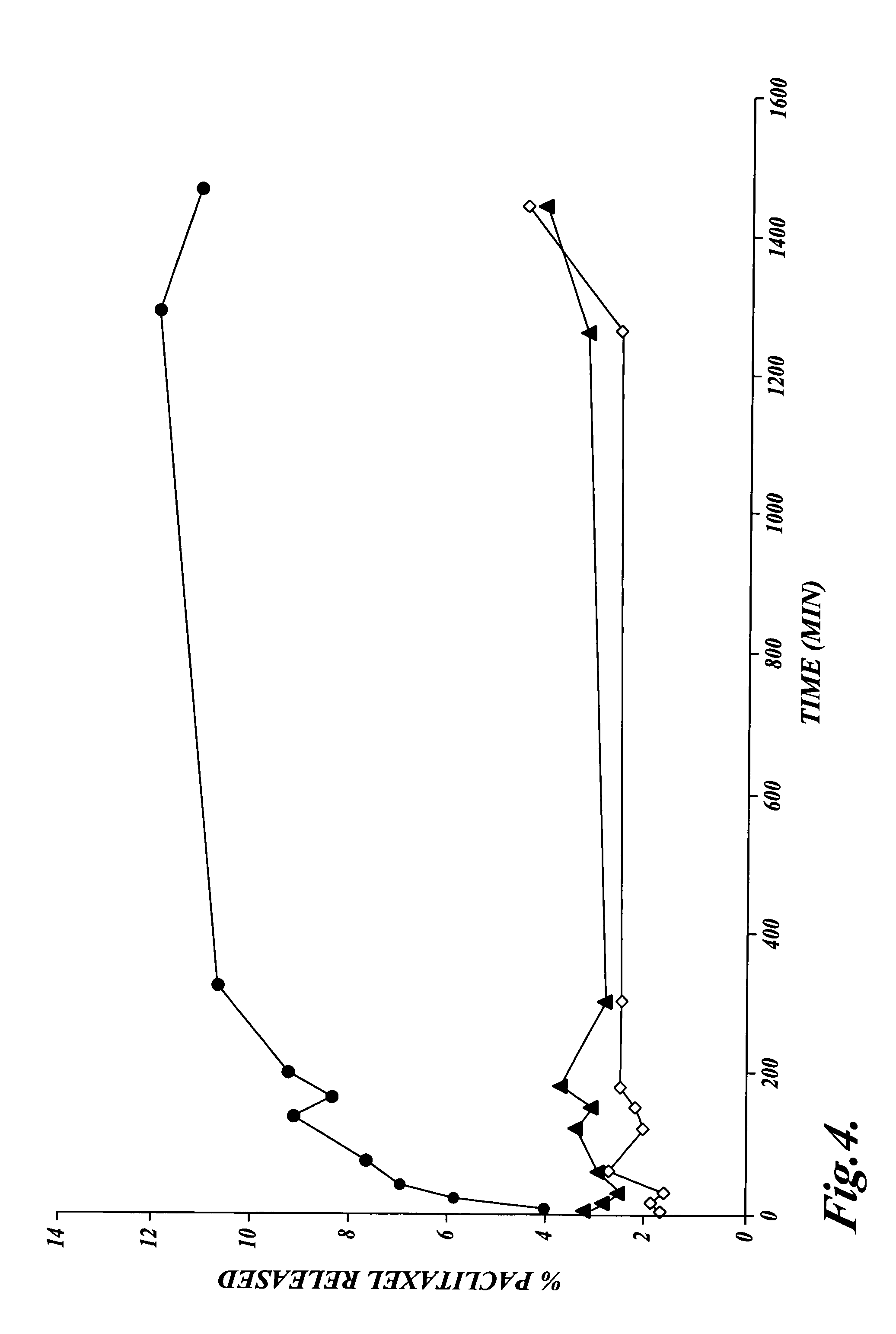
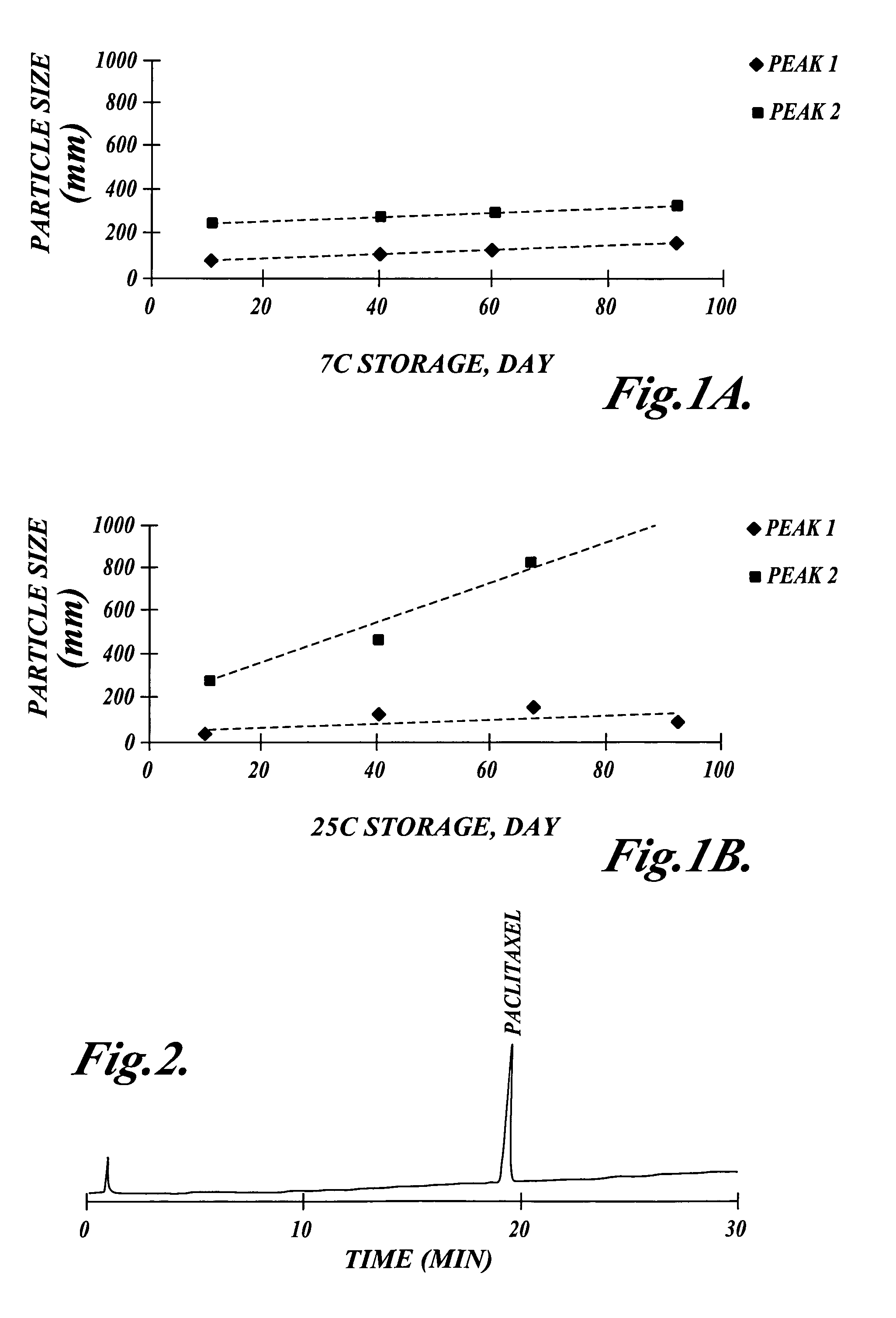
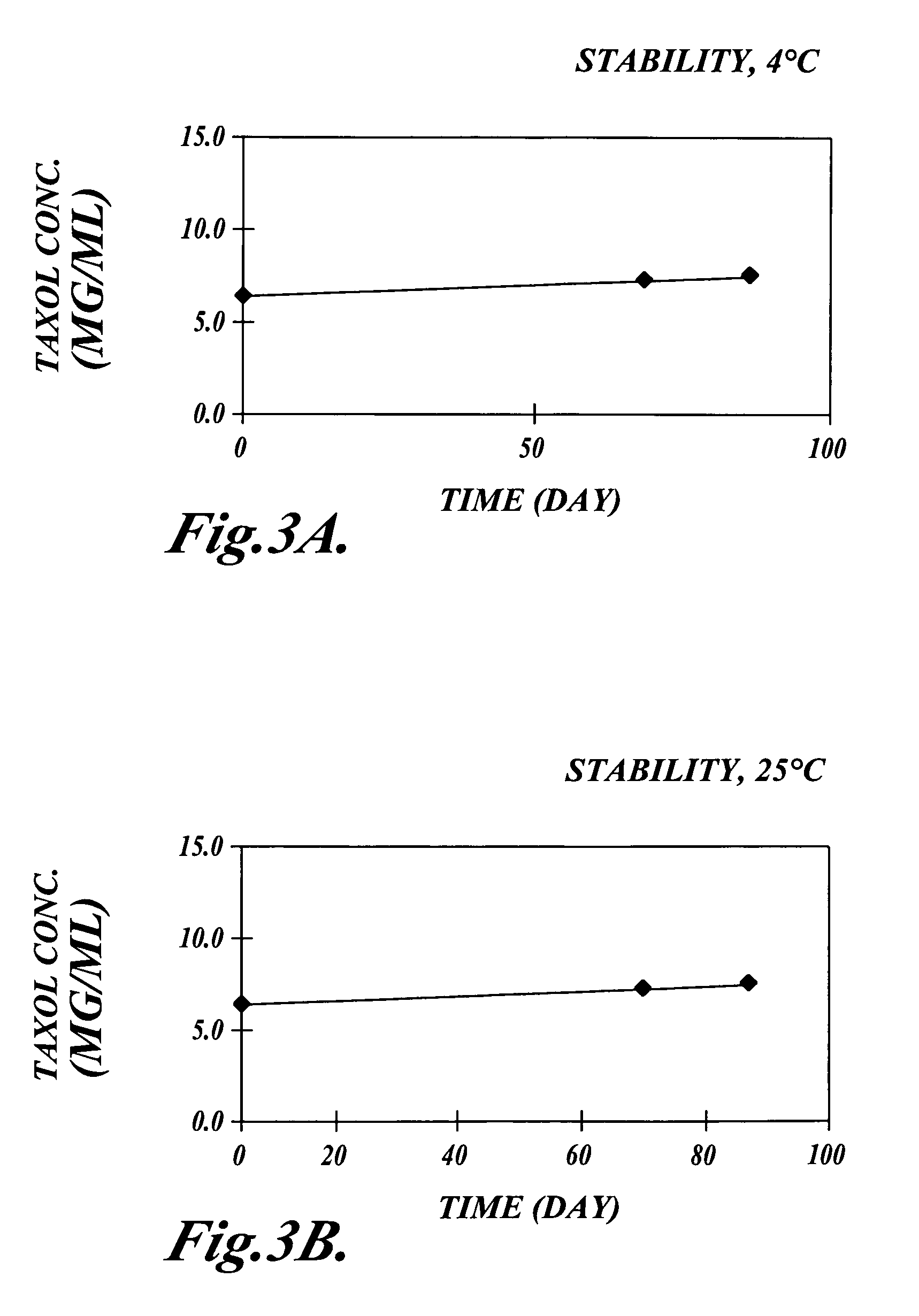
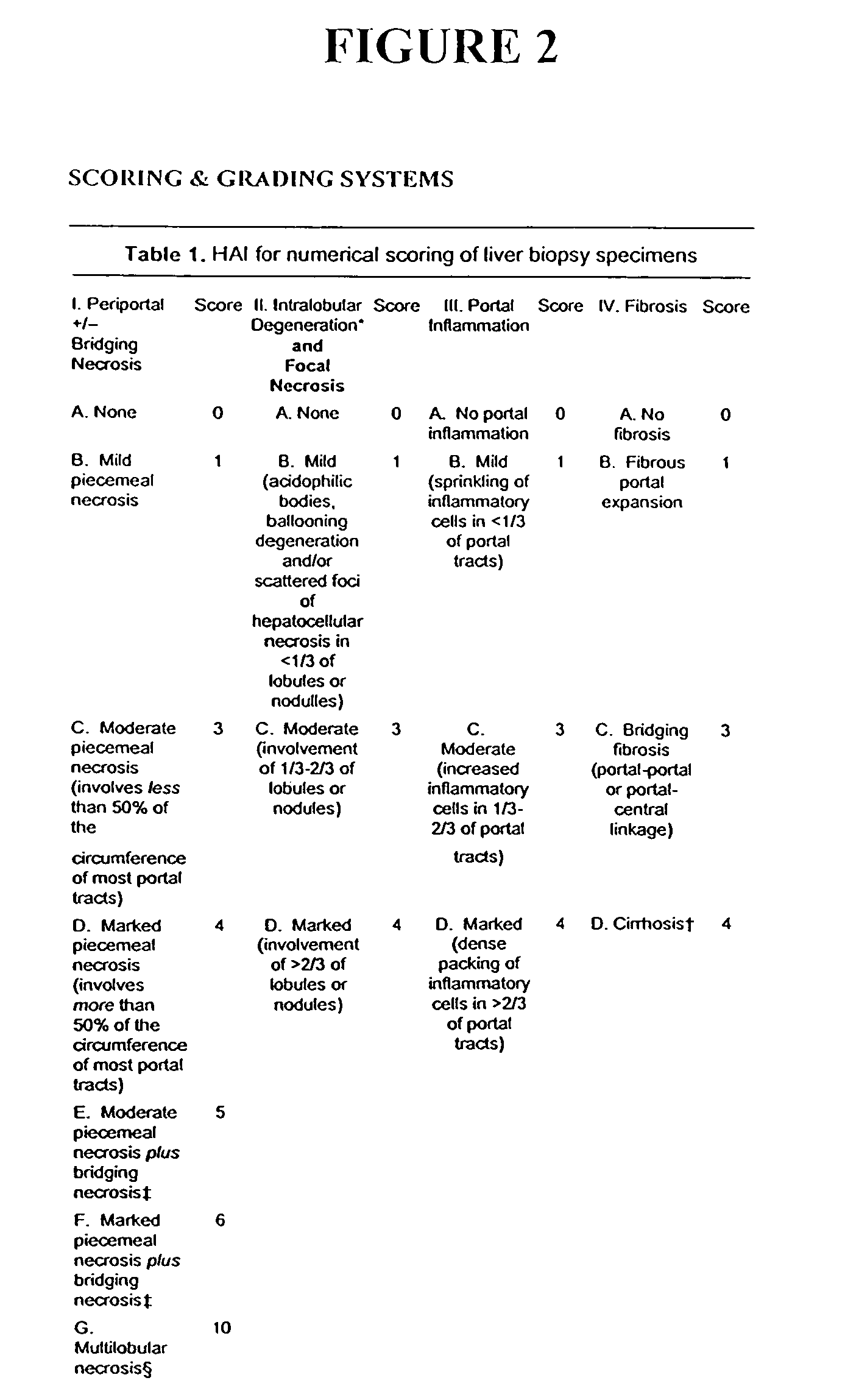
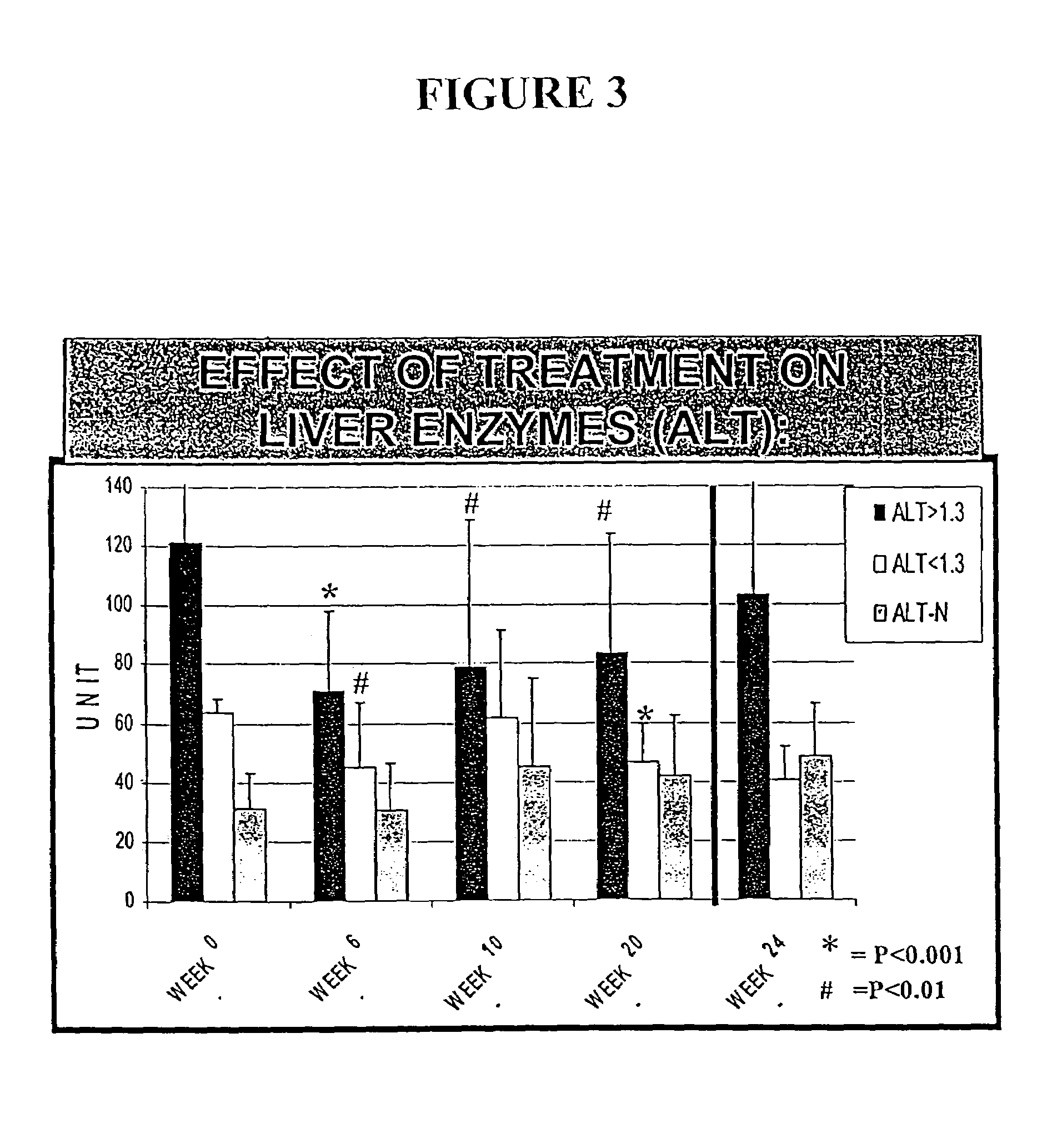
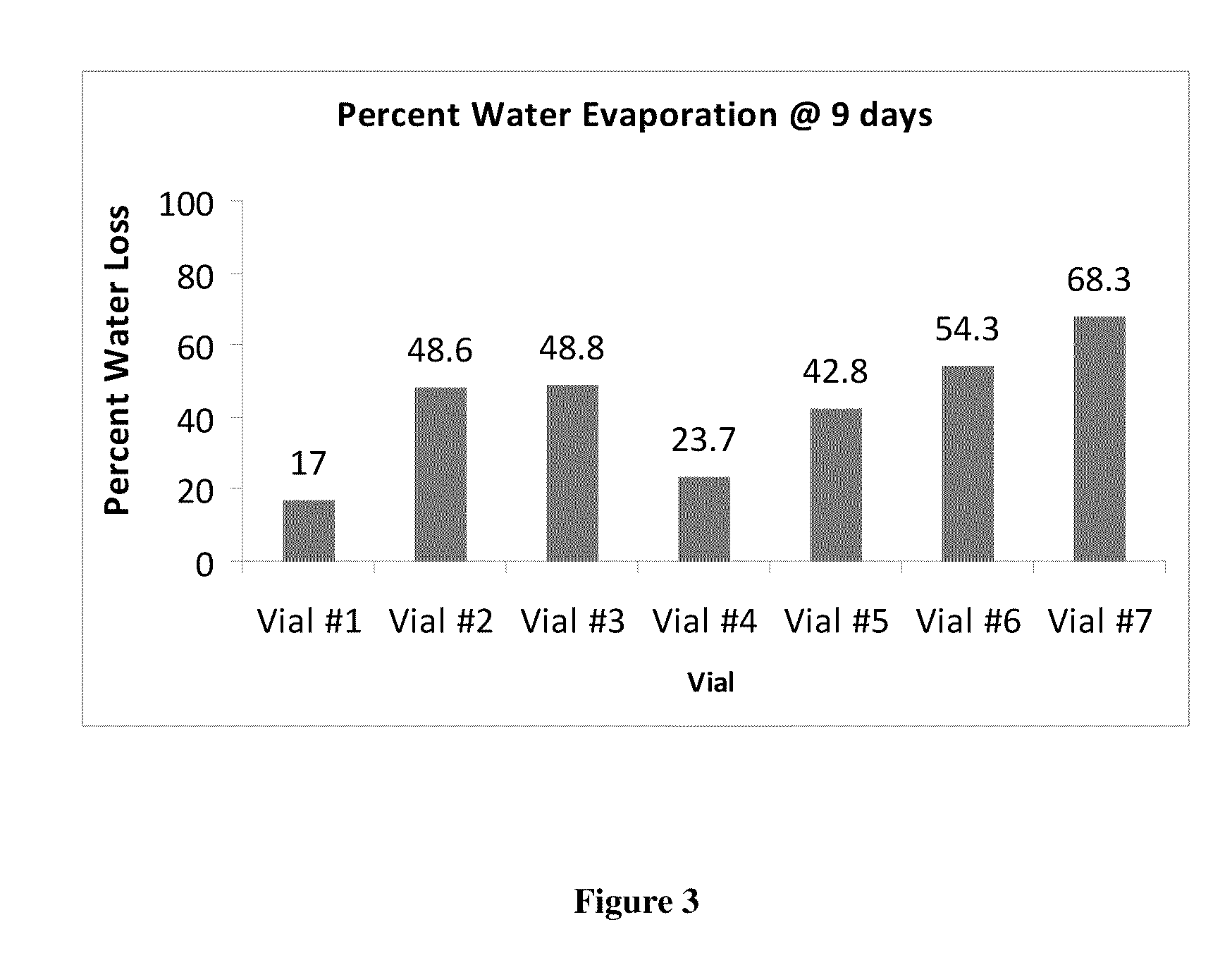
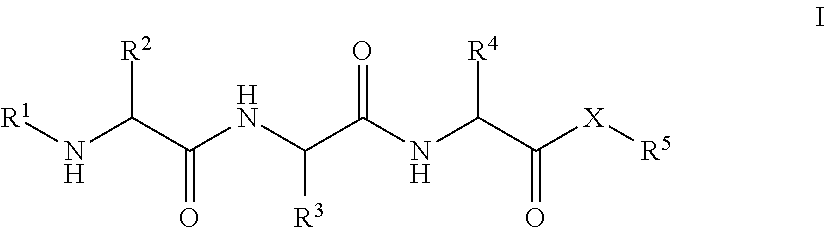
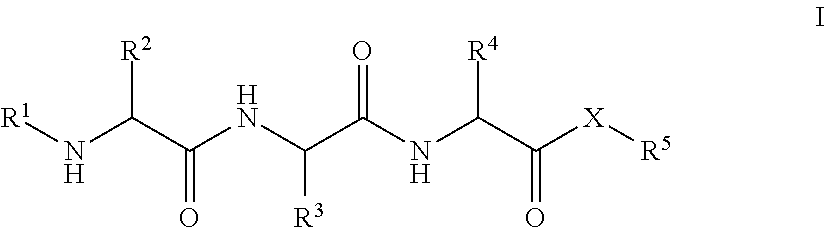
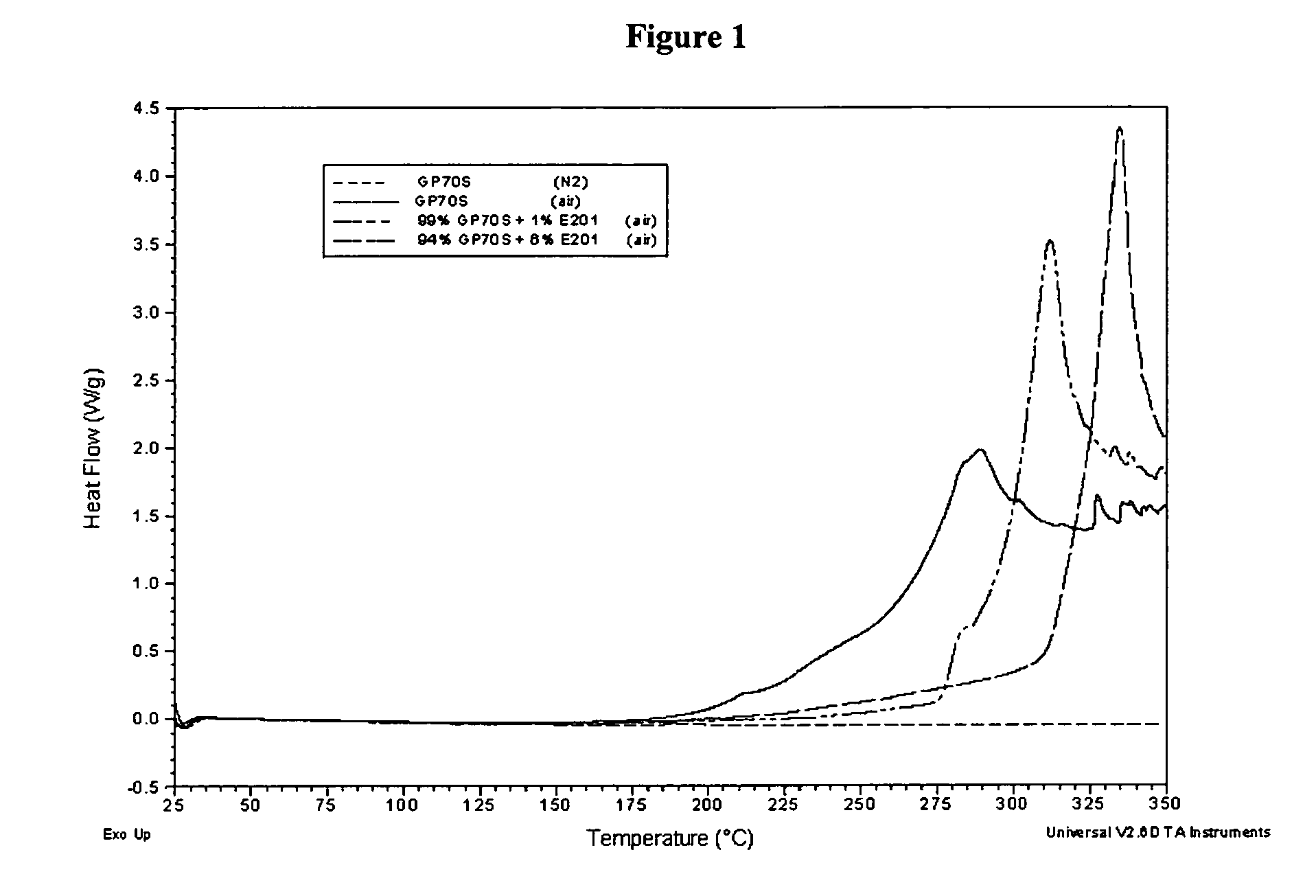
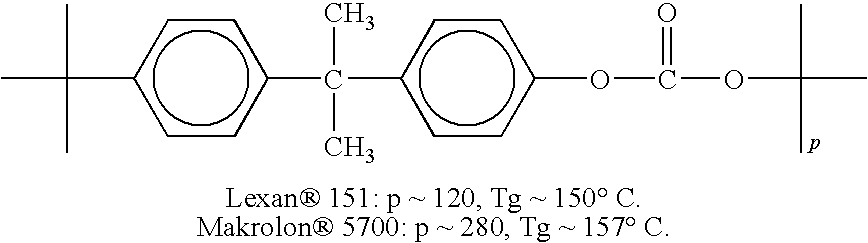
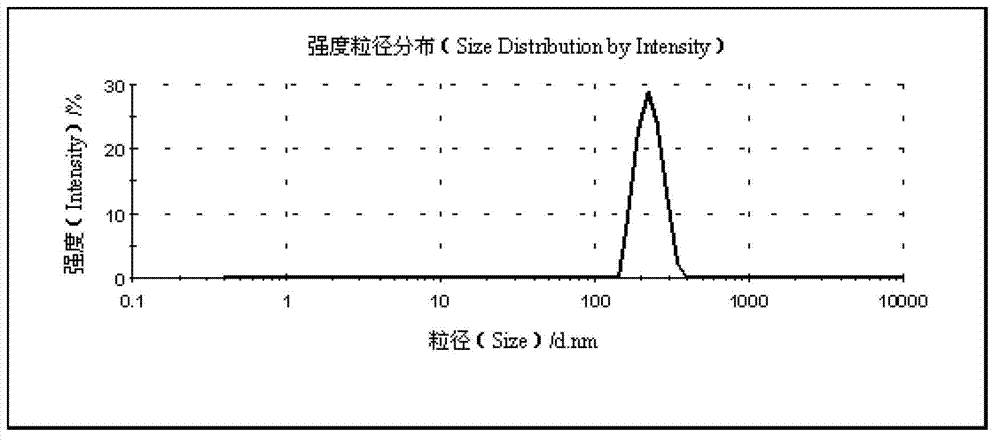
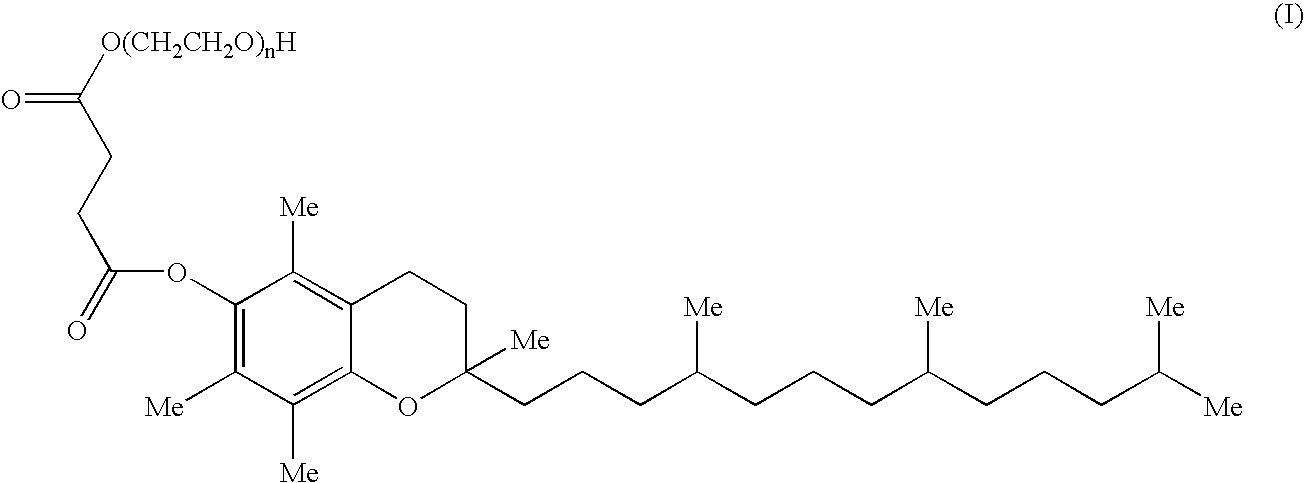
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
89 results about "Alpha-tocomonoenol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
New vitamin E isomers (gamma-tocomonoenol and alpha-tocomonoenol) in seeds, roasted seeds and roasted seed oil from the Slovenian pumpkin variety ‘ Slovenska golica’
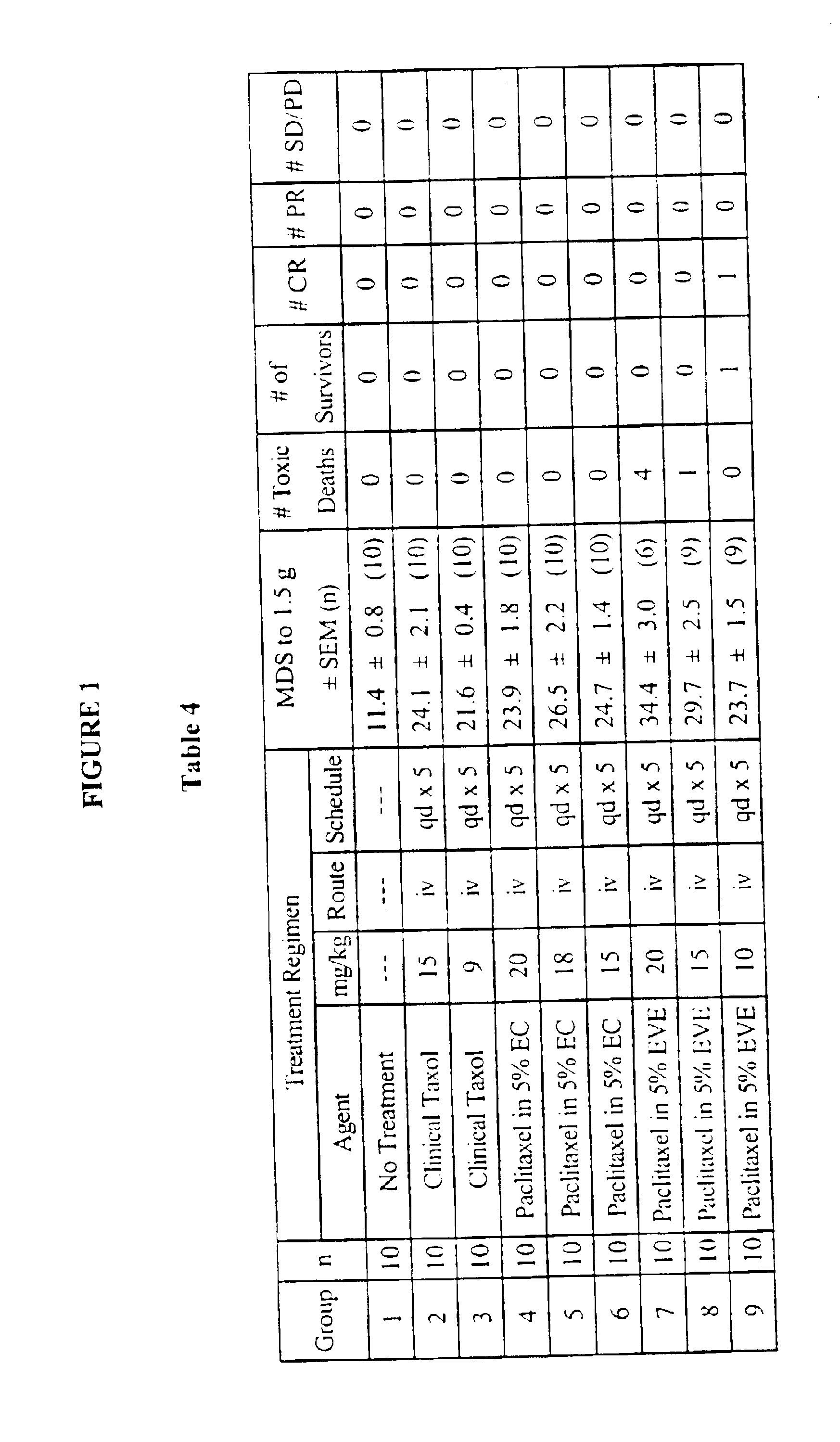
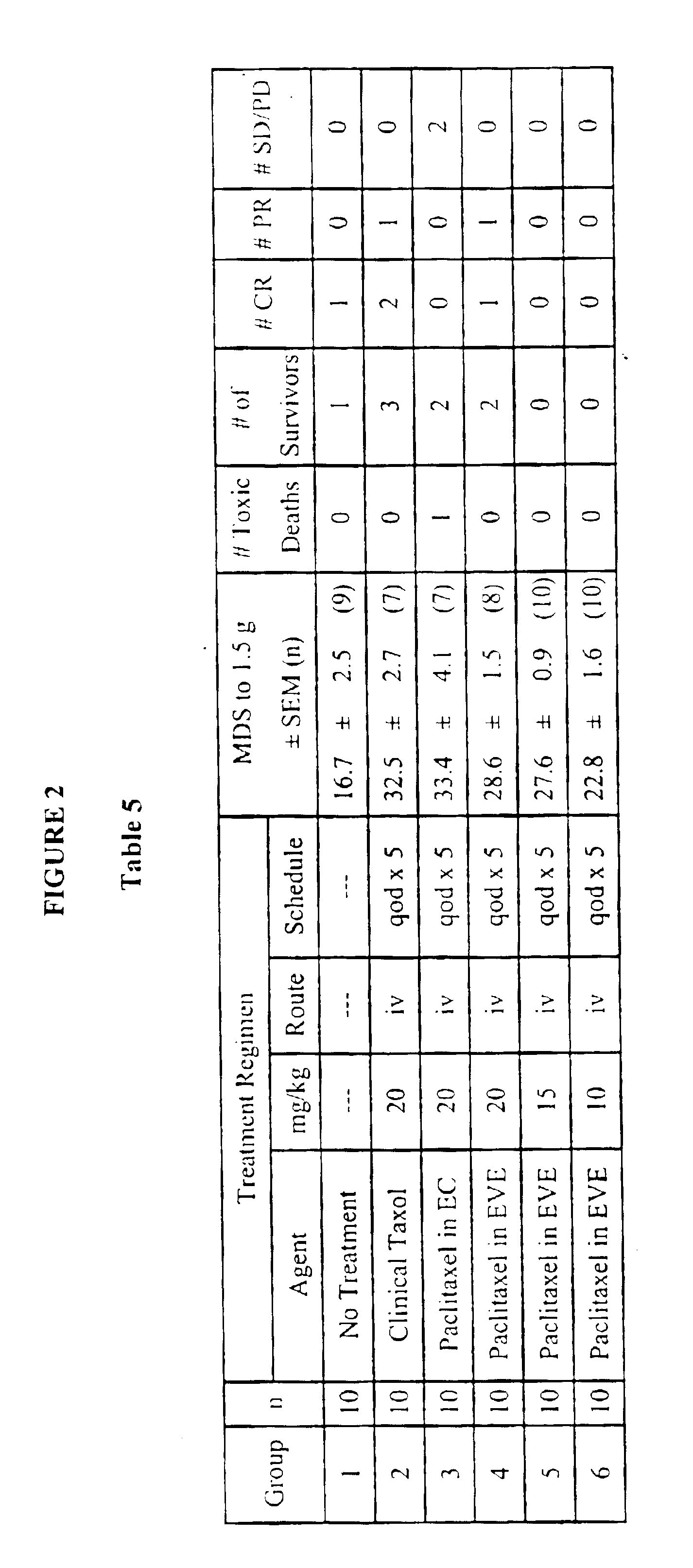
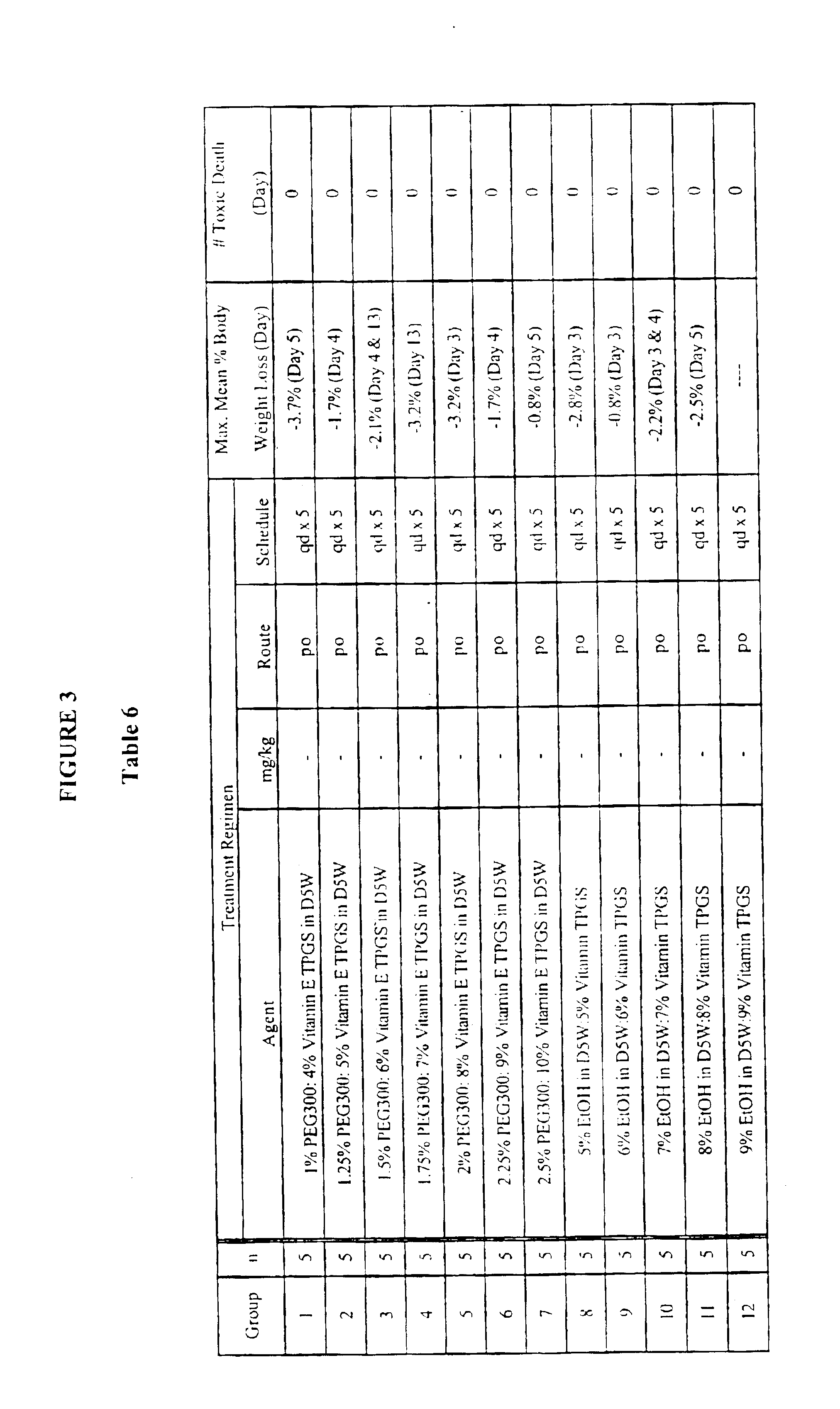
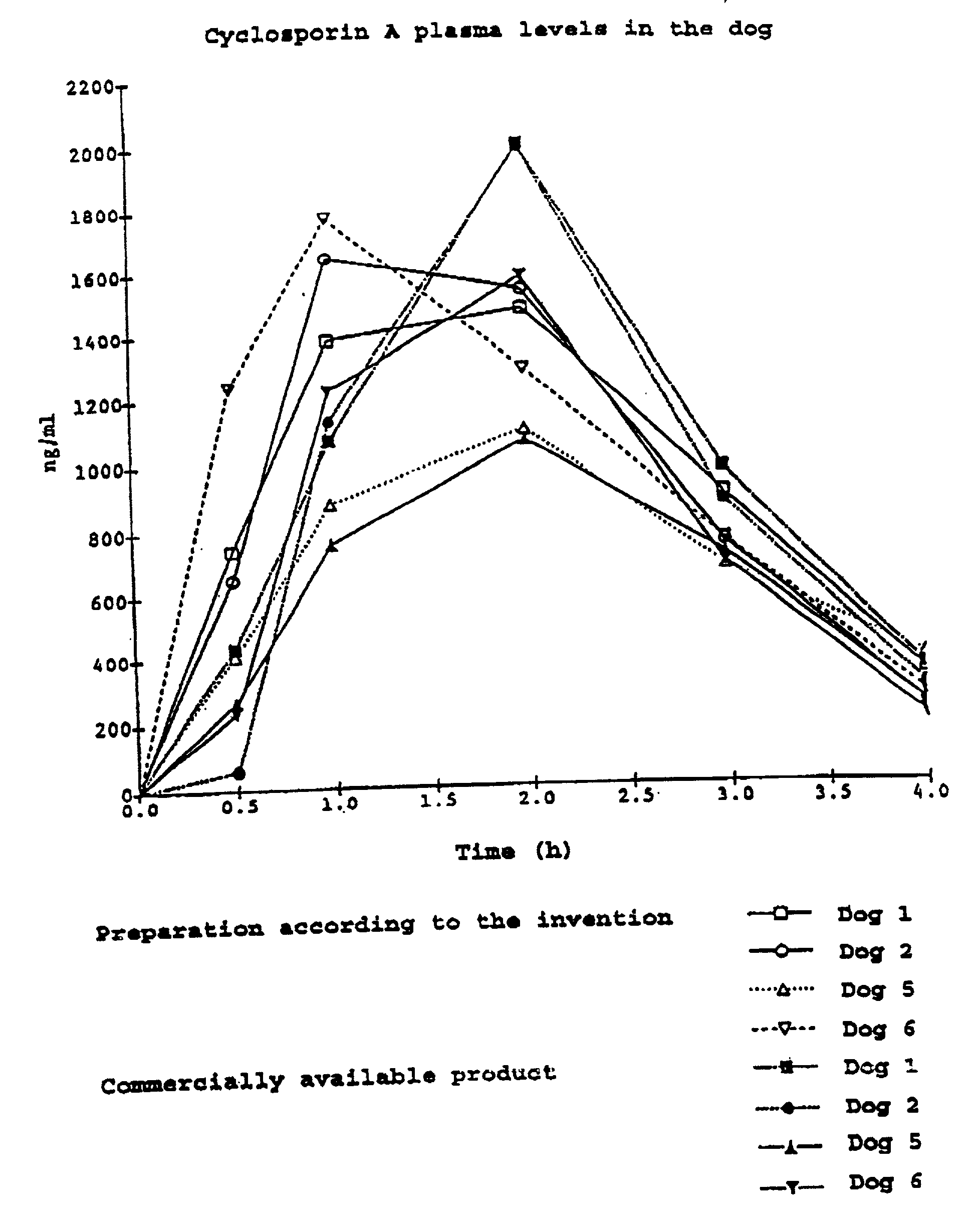
Pharmaceutical composition containing cyclosporin A
InactiveUS6022852AImprove solubilityInhibit synthesisBiocideOrganic active ingredientsAlpha-tocomonoenolTocopherol
The invention relates to a pharmaceutical composition consisting of or containing cyclosporin A and alpha -tocopherol or one of the derivatives thereof.
Owner:HEXAL AG
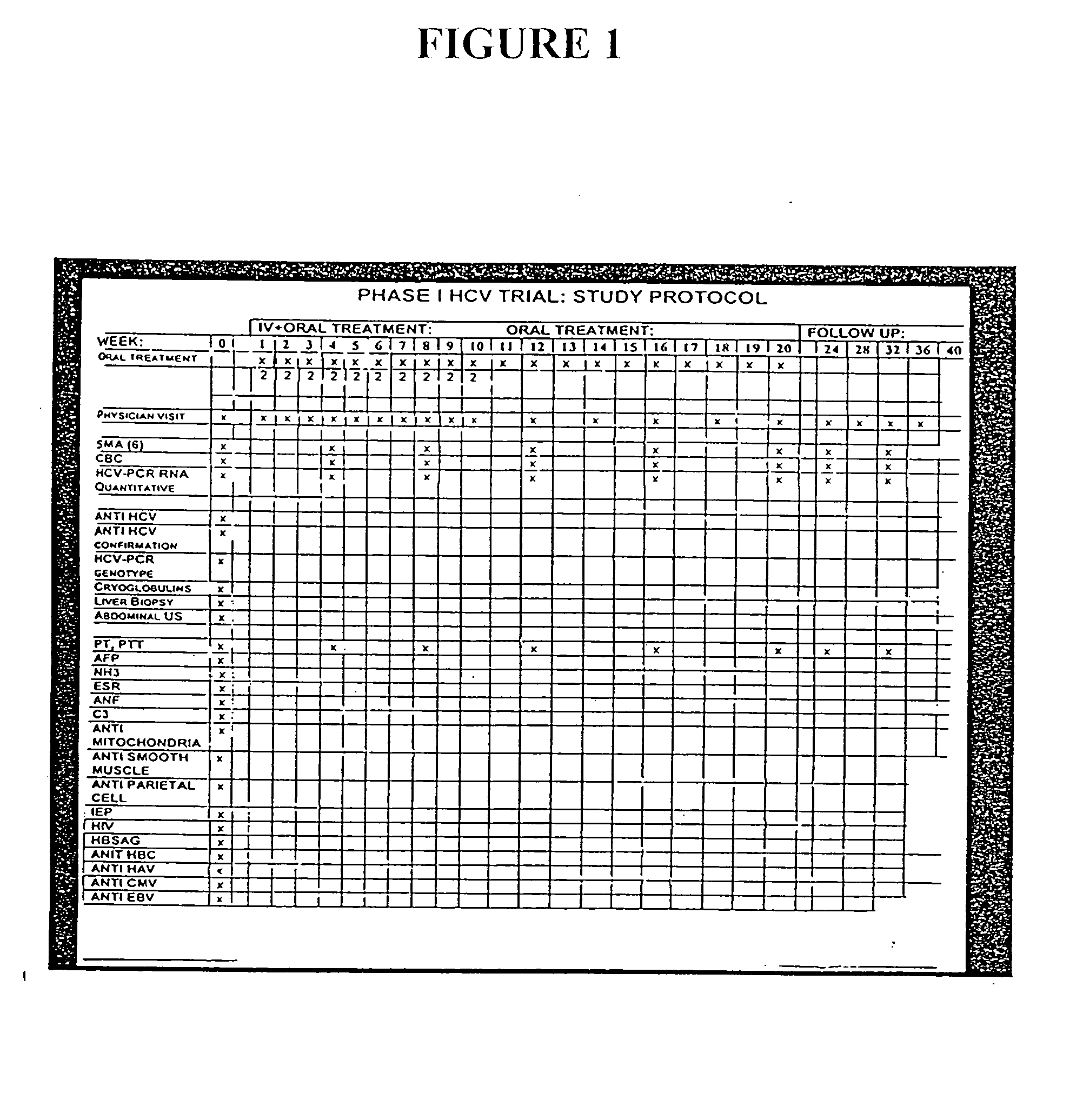
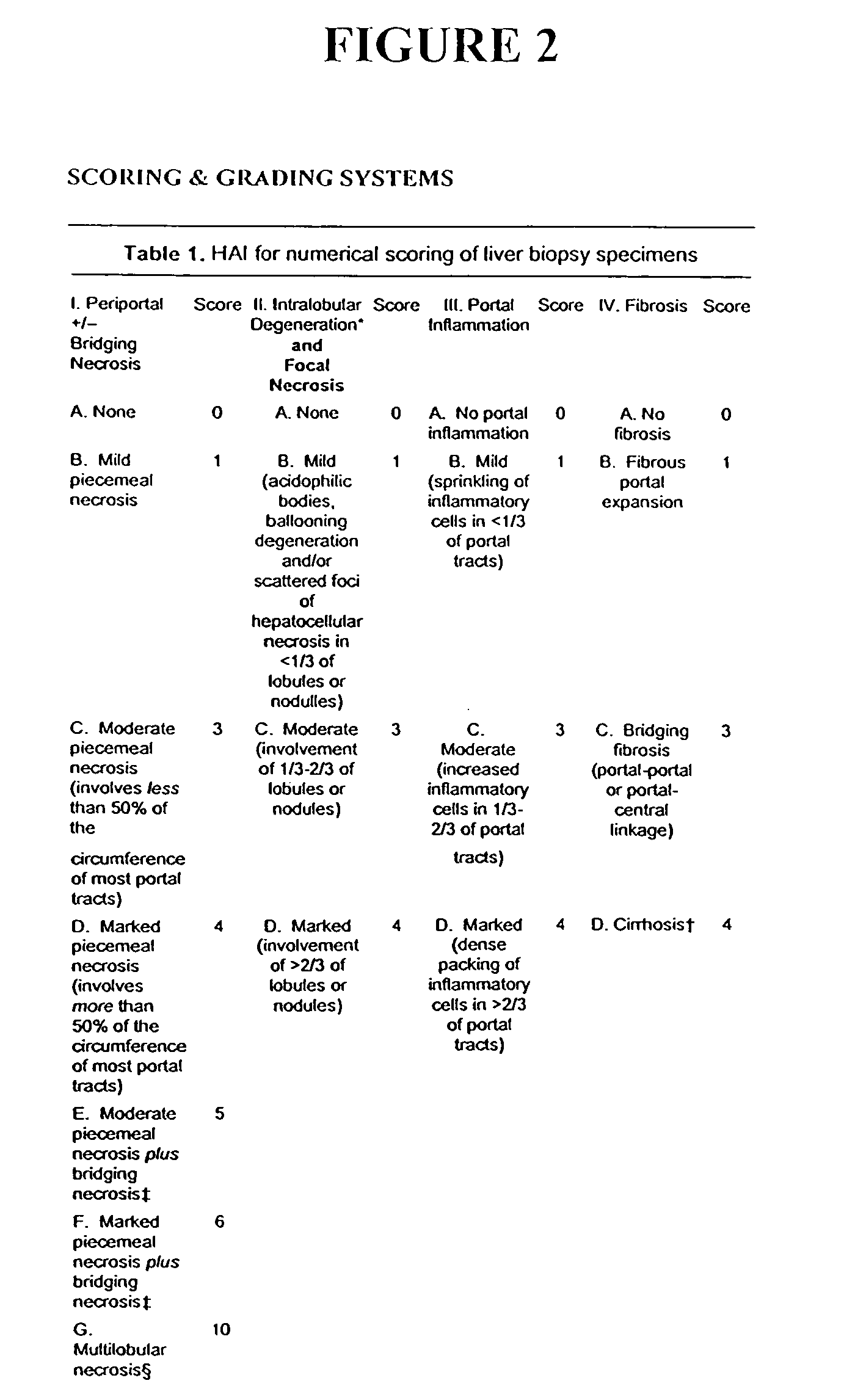
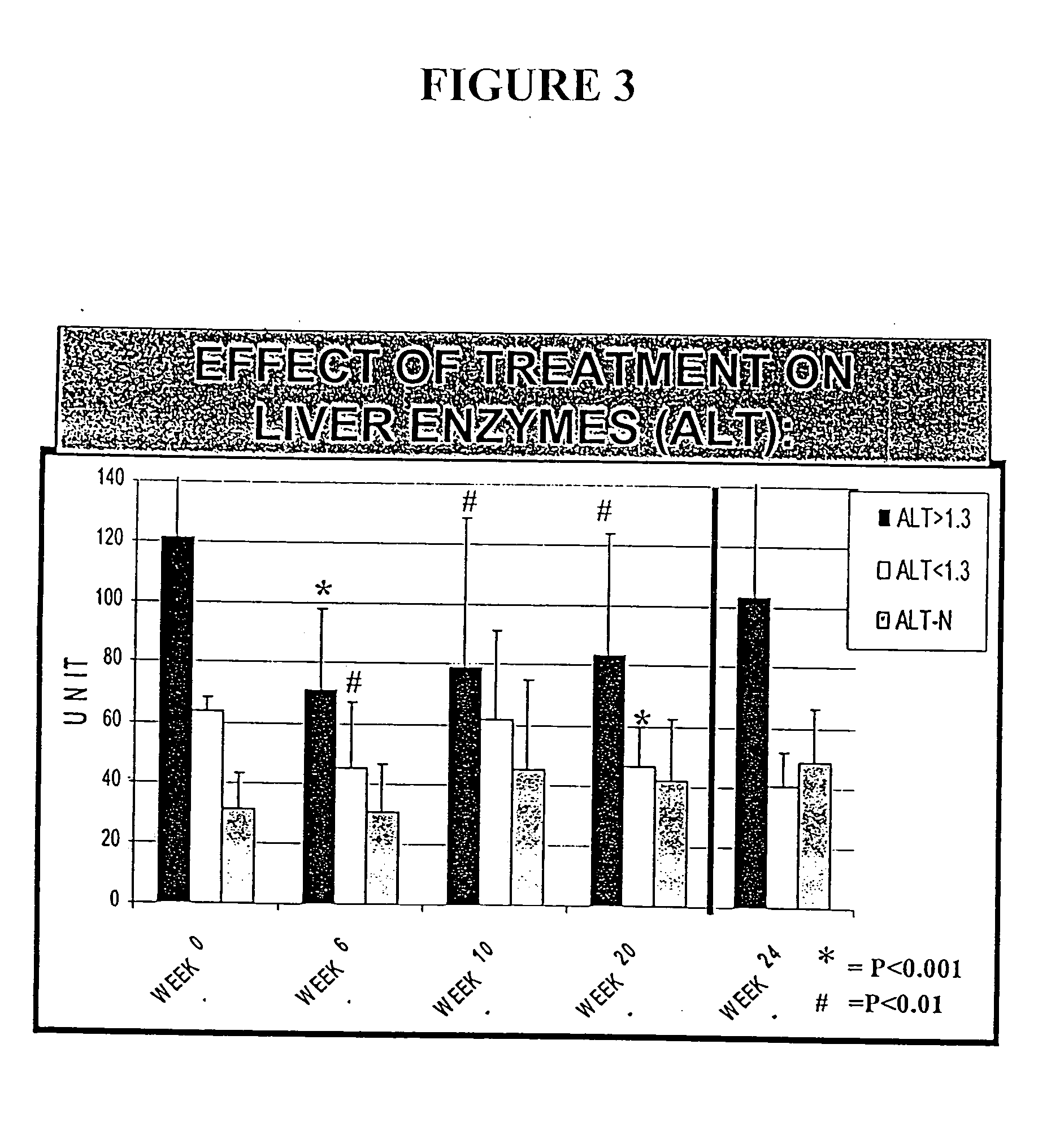
Compositions and methods useful for treating and preventing chronic liver disease, chronic HCV infection and non-alcoholic steatohepatitis
InactiveUS20050123628A1Reduce oxidative stressReduce lipid peroxidationBiocideDipeptide ingredientsChronic viral hepatitis CLipid peroxidation
The invention relates generally to compositions comprising antioxidants useful for reducing oxidative stress and lipid peroxidation, and treating chronic liver disease, chronic hepatitis C virus infection and non-alcoholic steatohepatitis. In particular, the invention relates to the preparation and oral administration of compositions comprising glycyrrhizin, schisandra, ascorbic acid, L-glutathione, silymarin, lipoic acid, and d-alpha-tocopherol. The invention also relates to the preparation and parenteral administration of compositions comprising glycyrrhizin, ascorbic acid, L-glutathione, and vitamin B-complex, preferably by infusion or intravenous injection. The invention further relates to methods of using the antioxidants, oral compositions and parenteral compositions.
Owner:ZABRECKY GEORGE
Emulsion vehicle for poorly soluble drugs
An emulsion of α-tocopherol, stabilized by biocompatible surfactants, as a vehicle or carrier for therapeutic drugs, which is substantially ethanol free and which can be administered to animals or humans via various routes is disclosed. Also included in the emulsion is PEGylated vitamin E. PEGylated α-tocopherol includes polyethylene glycol subunits attached by a succinic acid diester at the ring hydroxyl of vitamin E and serves as a primary surfactant, stabilizer and a secondary solvent in emulsions of α-tocopherol.
Owner:IGDRASOL
Emulsion vehicle for poorly soluble drugs
InactiveUS7030155B2Process stabilityEmulsion stabilizationOrganic active ingredientsBiocideEmulsionSuccinic acid
A method of making an emulsion of tocopherol incorporating a co-solvent and, stabilized by biocompatible surfactants, as a vehicle or carrier for therapeutic drugs, which is substantially ethanol free and which can be administered to animals or humans by various routes, is disclosed. Also included in the emulsion is PEGylated vitamin E. PEGylated α-tocopherol includes polyethylene glycol subunits attached by a succinic acid diester at the ring hydroxyl of vitamin E and serves as a primary surfactant, stabilizer and a secondary solvent in emulsions of α-tocopherol.
Owner:IGDRASOL
Influenza vaccine
InactiveUS20090263422A1Stimulate immune responseSsRNA viruses negative-senseViral antigen ingredientsDiseaseSterol
The present invention relates to monovalent influenza vaccine formulations and vaccination regimes for immunising against influenza disease, their use in medicine, in particular their use in augmenting immune responses to various antigens, and to methods of preparation. In particular, the invention relates to monovalent influenza immunogenic compositions comprising an influenza antigen or antigenic preparation thereof from an influenza virus strain being associated with a pandemic outbreak or having the potential to be associated with a pandemic outbreak, in combination with an oil-in-water emulsion adjuvant comprising a metabolisable oil, a sterol or a tocopherol such as alphatocopherol, and an emulsifying agent.
Owner:HANON EMMANUEL JULES +1
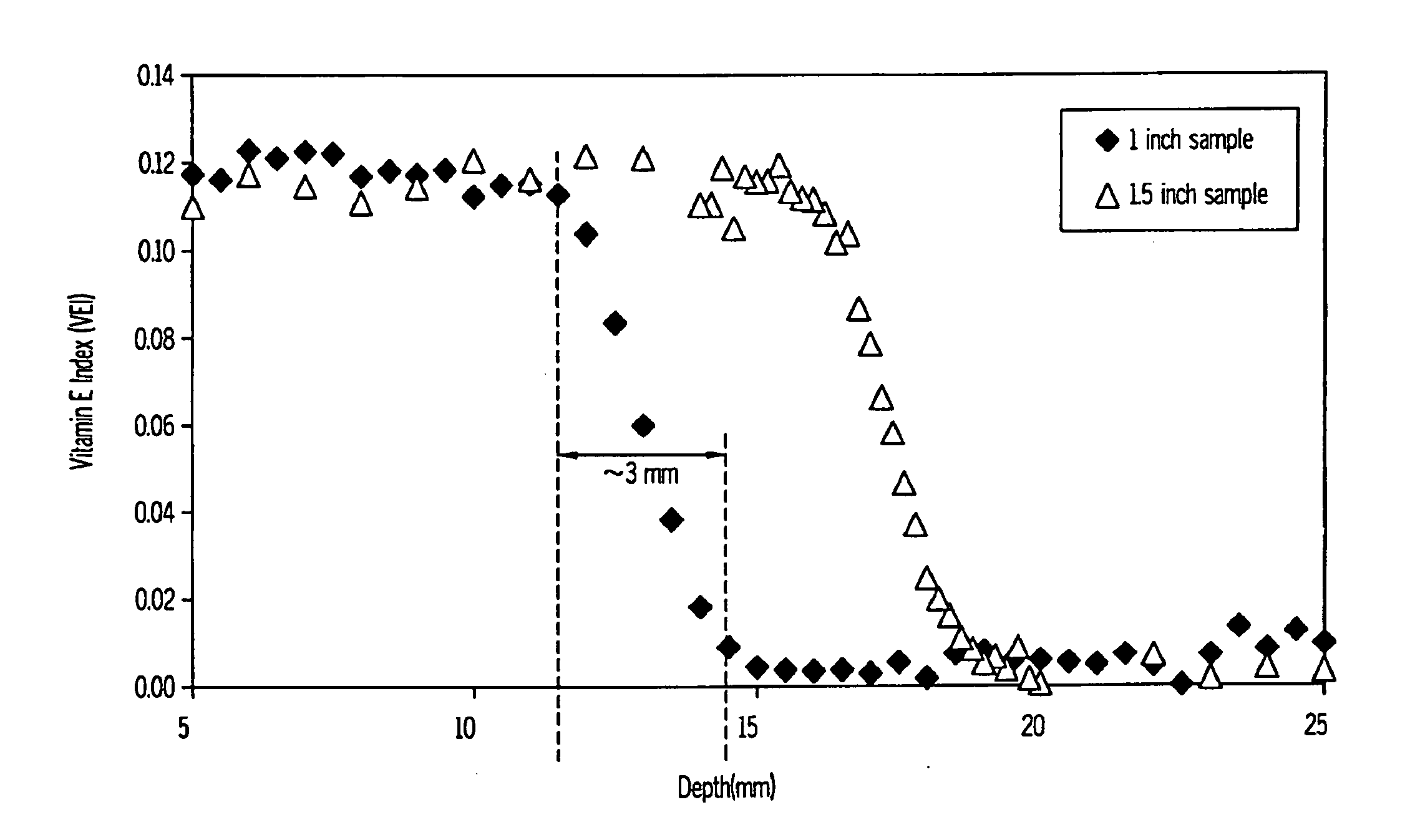
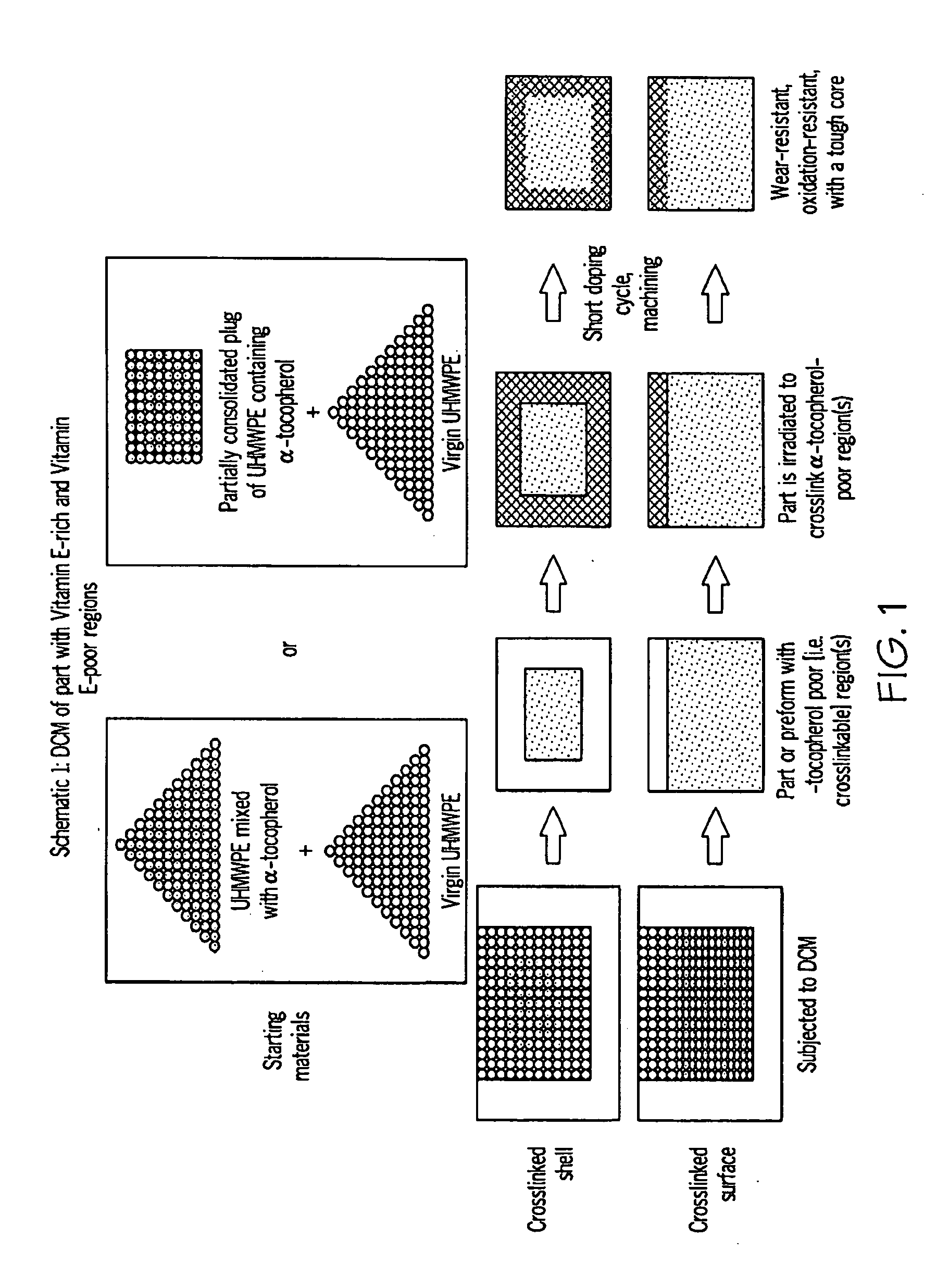
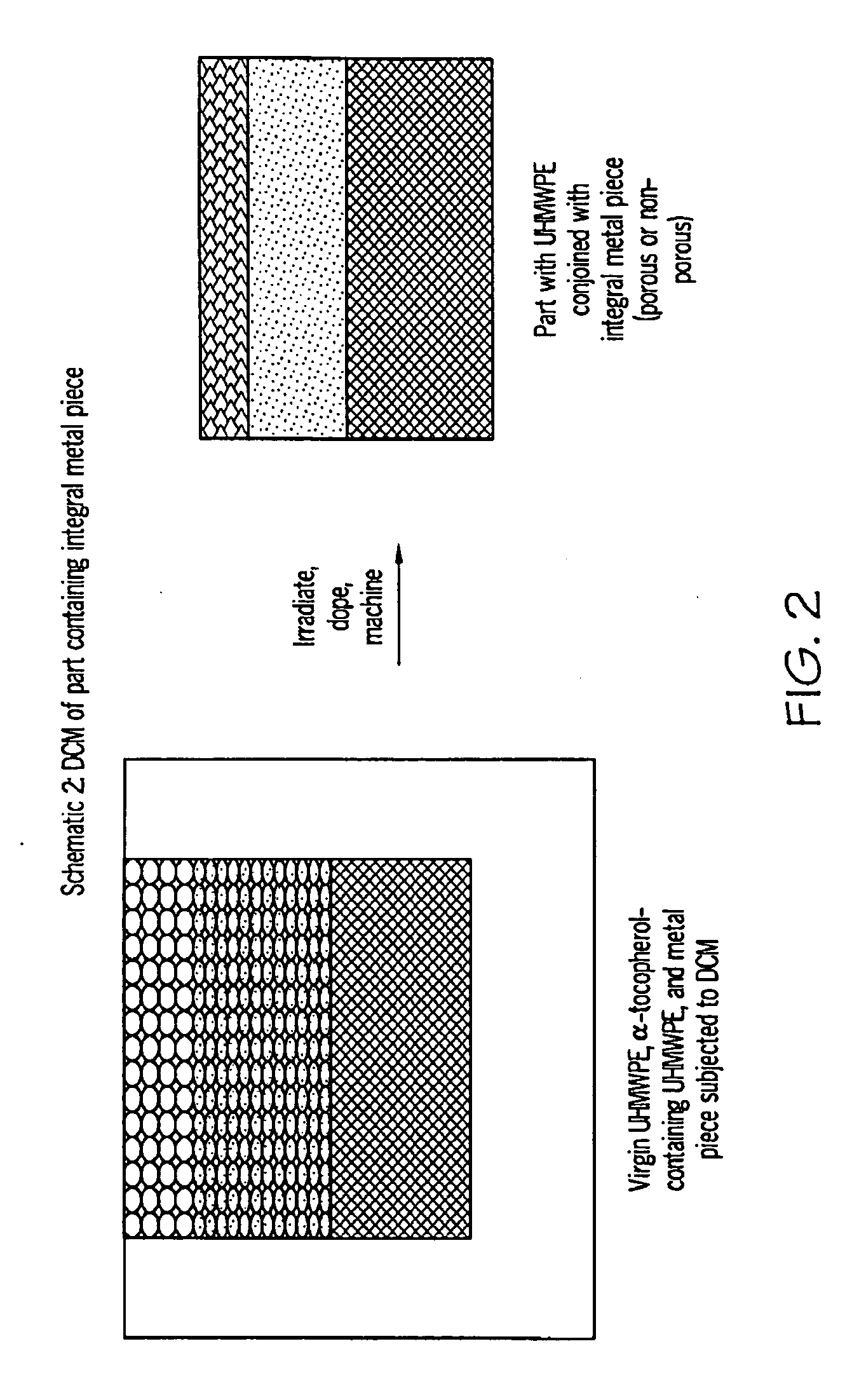
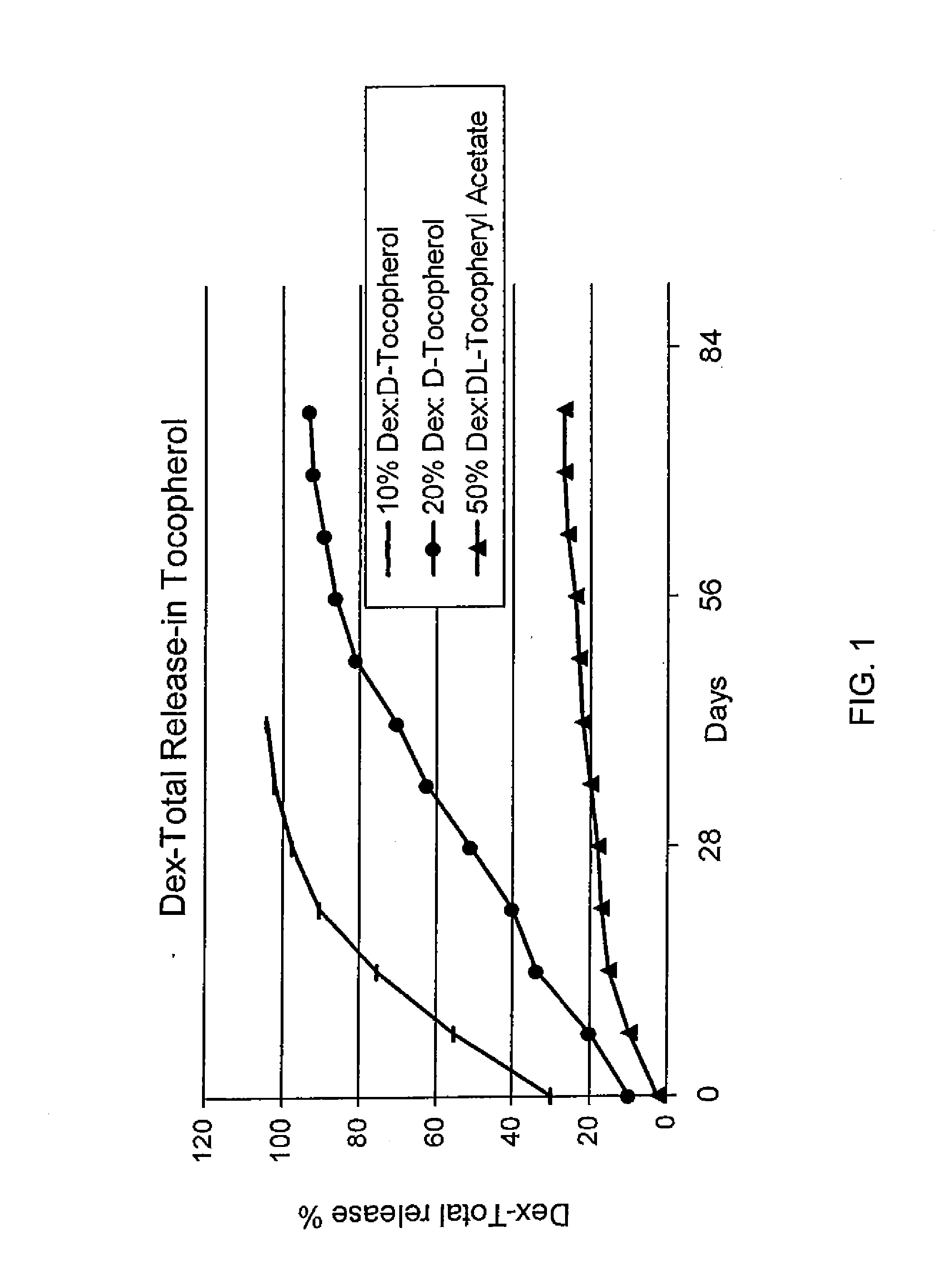
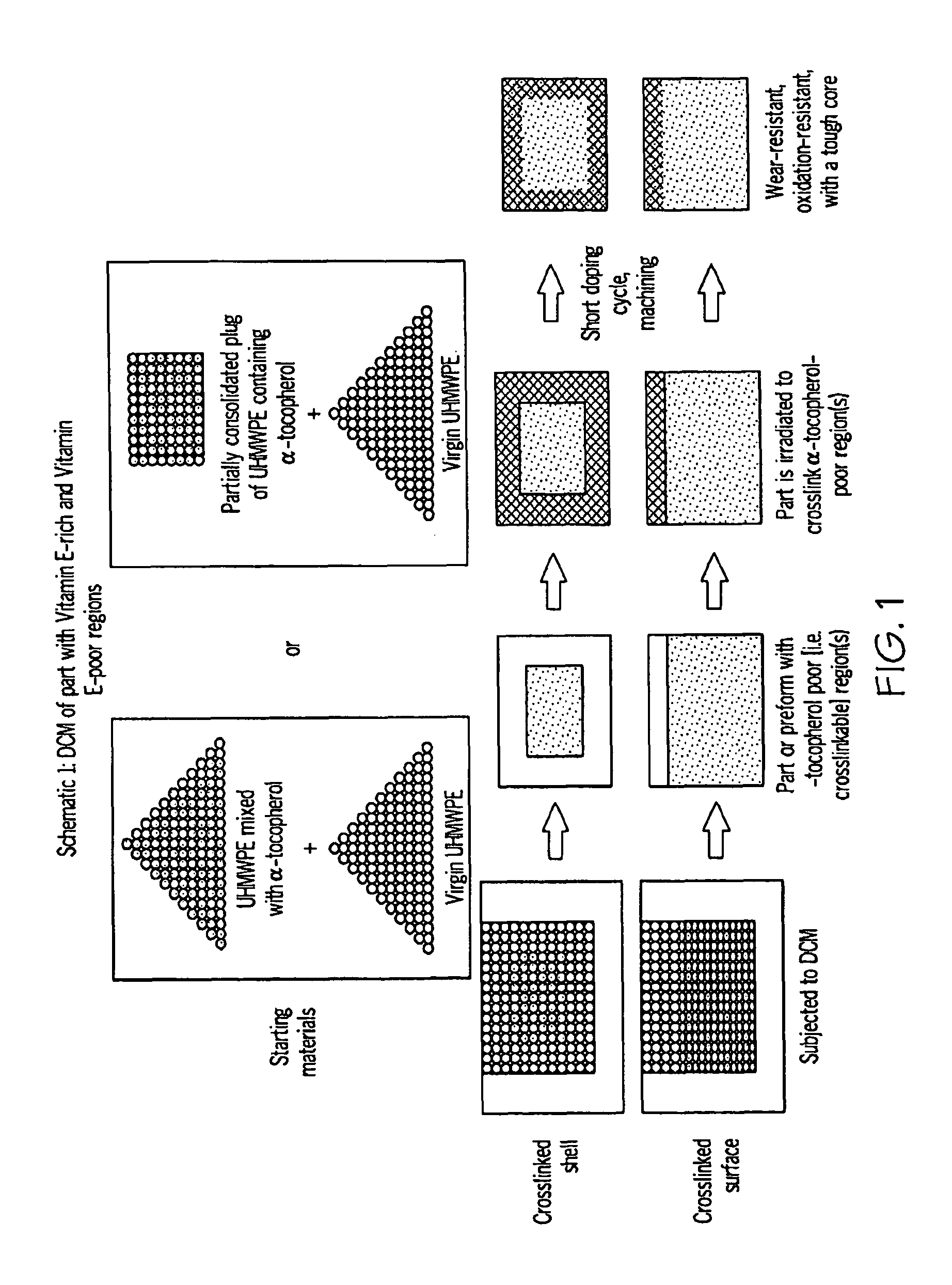
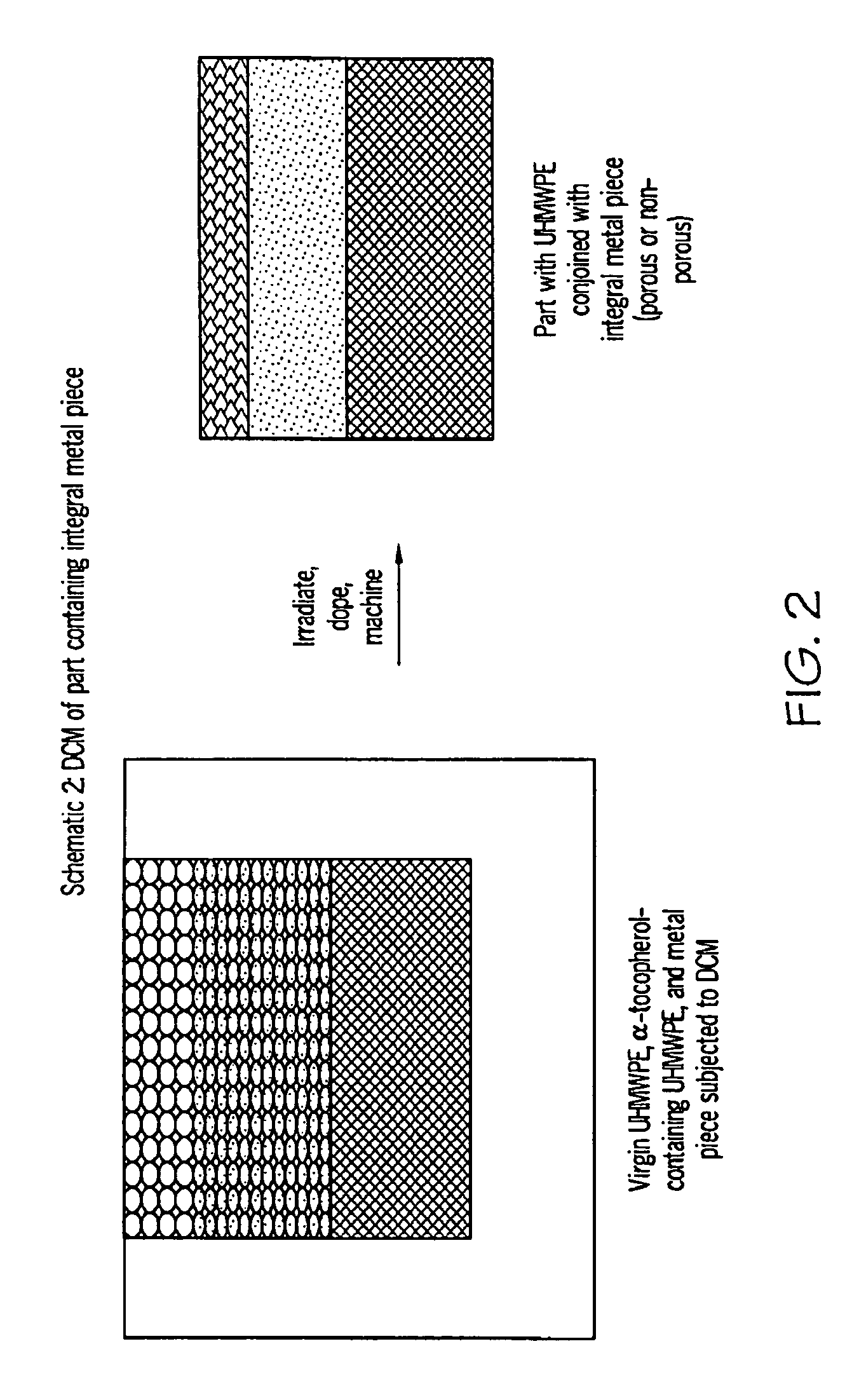
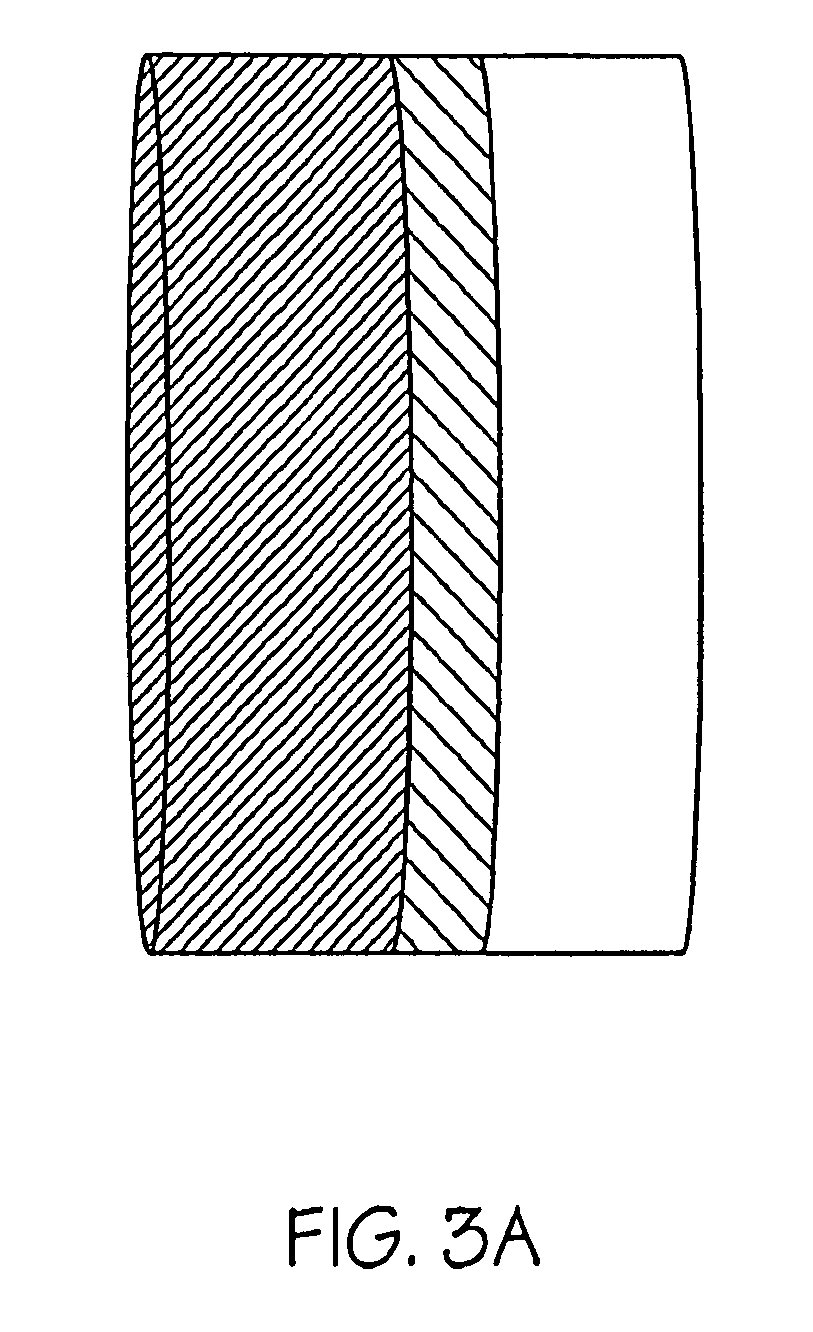
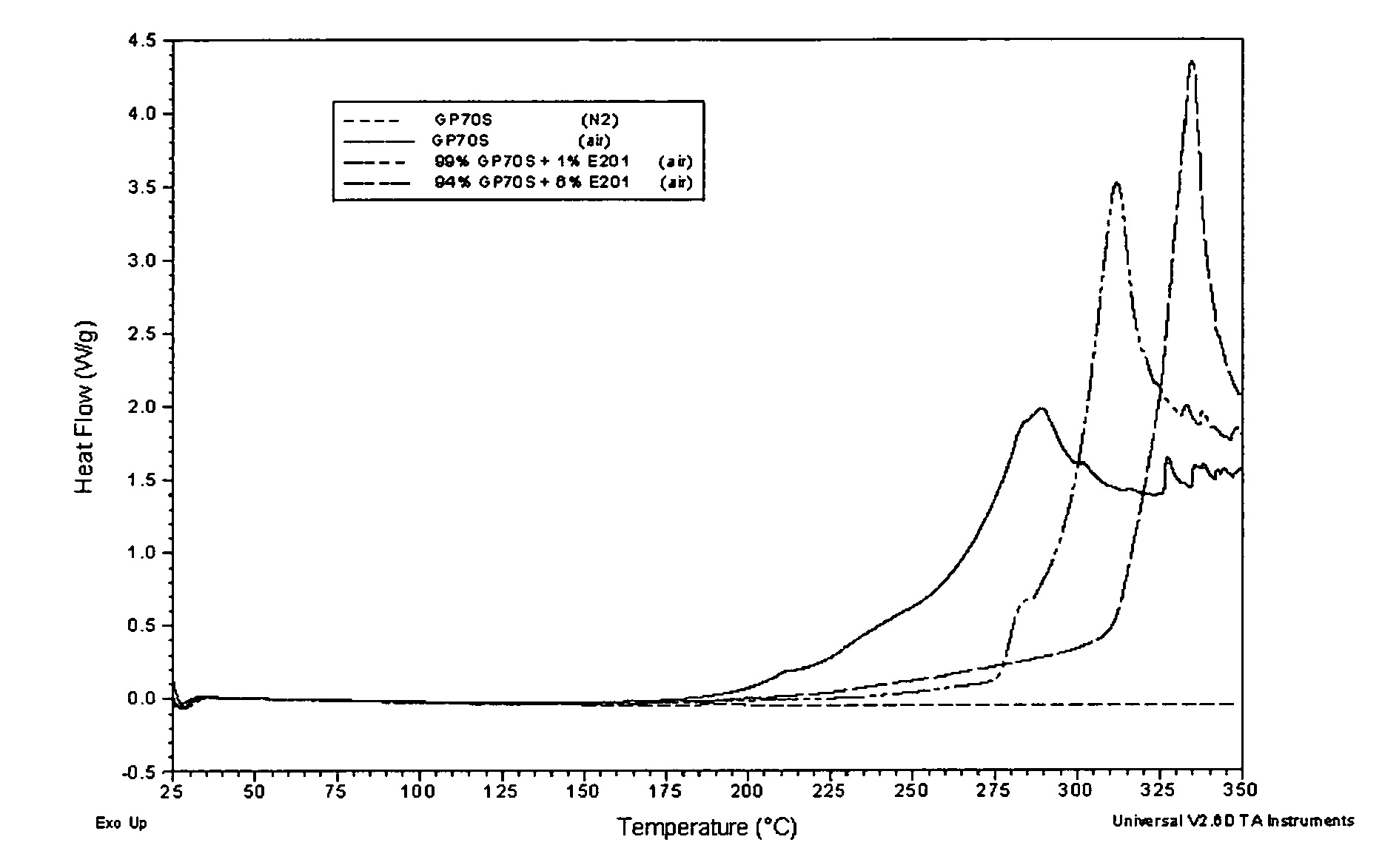
Methods for making oxidation-resistant cross-linked polymeric materials
ActiveUS20100190882A1Preventing and minimizing in vivo elution of antioxidantImpression capsSurgical adhesivesCross-linkElution
The present invention relates to methods for making cross-linked oxidation-resistant polymeric materials and preventing or minimizing in vivo elution of antioxidant from the antioxidant-containing polymeric materials. The invention also provides methods of doping polymeric materials with a spatial control of cross-linking and antioxidant distribution, for example, vitamin E (α-Tocopherol), and methods for extraction / elution of antioxidants, for example, vitamin E (α-tocopherol), from surface regions of antioxidant-containing polymeric materials, and materials used therewith also are provided.
Owner:THE GENERAL HOSPITAL CORP +1
Compositions and methods useful for treating and preventing chronic liver disease, chronic HCV infection and non-alcoholic steatohepatitis
InactiveUS7078064B2Reduce peroxidationReduce stressBiocideDipeptide ingredientsChronic viral hepatitis CLipid peroxidation
The invention relates generally to compositions comprising antioxidants useful for reducing oxidative stress and lipid peroxidation, and treating chronic liver disease, chronic hepatitis C virus infection and non-alcoholic steatohepatitis. In particular, the invention relates to the preparation and oral administration of compositions comprising glycyrrhizin, schisandra, ascorbic acid, L-glutathione, silymarin, lipoic acid, and d-alpha-tocopherol. The invention also relates to the preparation and parenteral administration of compositions comprising glycyrrhizin, ascorbic acid, L-glutathione, and vitamin B-complex, preferably by infusion or intravenous injection. The invention further relates to methods of using the antioxidants, oral compositions and parenteral compositions.
Owner:ZABRECKY GEORGE
Anticancer oral formulation
InactiveUS20100010059A1Improve oral bioavailabilityBiocideDispersion deliveryPolyethylene glycolSuccinates
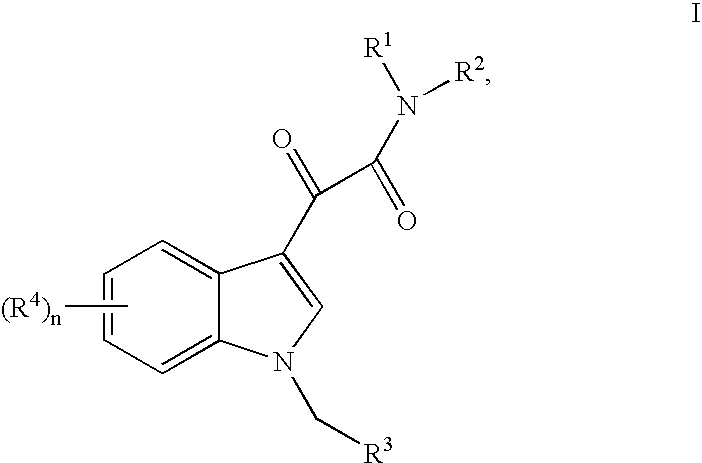
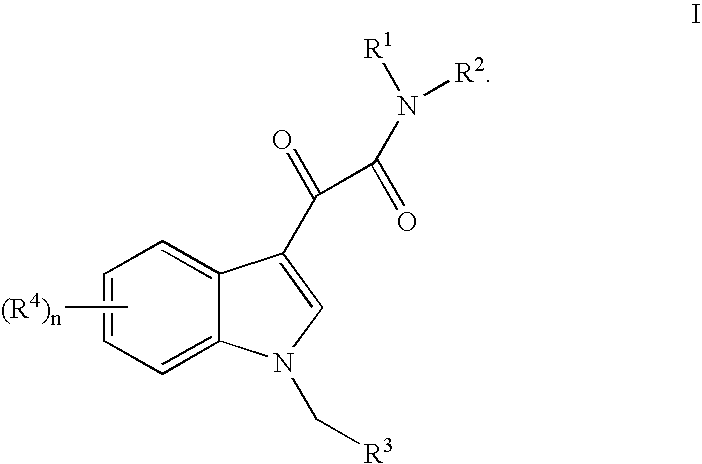
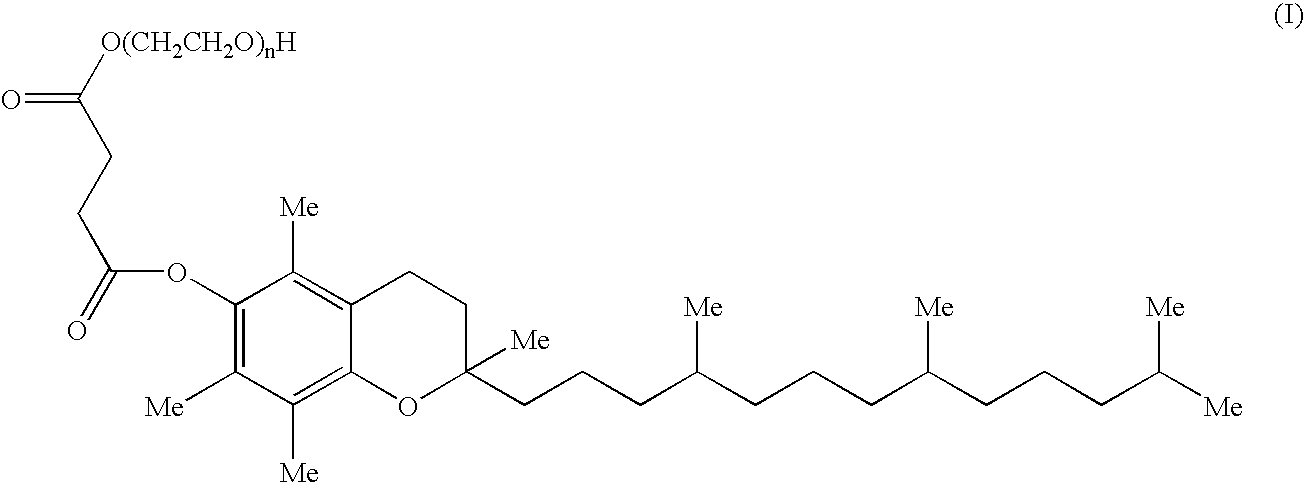
This invention relates to an oral formulation containing an effective amount of the compound of the following formula I:d-alpha-tocopheryl polyethylene glycol 1000 succinate (“TPGS”); and 2-(2-ethoxyethoxy)ethanol (“Transcutol”). R1 through R4 and n are defined herein. Also disclosed is a method of treating cancer by administering this formula to a subject orally.
Owner:NAT INST OF HEALTH REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH
Unit dosage forms for the treatment of herpes simplex
InactiveUS7351715B2Increase successSafe and effective and inexpensiveBiocidePeptide/protein ingredientsDiseaseCell membrane
The components of this invention are chosen because of their complementarity for the prevention or treatment of diseases caused by the herpes simplex virus. L-Lysine favorably increases the physiologic immunomodulation necessary for defense against this virus. Zinc improves and maintains a normal immune response. 2-Deoxy-2-D-glucose and heparin sodium alter the surface interaction between the herpes virus and the cell, preventing fusion and infectivity. N-Acetyl-L-cysteine increases glutathione levels thereby creating a thiol redox barrier to the virus at the cell membrane. Quercetin reduces intraoellular replication of the herpes virus and viral infectivity. Ascorbate, in concert with copper and D-α-tocopherol, provides an antioxidant defense against the herpes virus, which tends to lose latency during period of oxidative, free radical excess. Selenium and quercetin also participate in reducing various oxidative stresses. Together the components of this invention provide the potential for improved resistance to, improved recovery from, and a decreased frequency of recurrence of herpes simplex virus infection.
Owner:CHRONORX
Formulation to enhance antioxidant potency of Vitamin E
InactiveUS7015245B2Improve antioxidant capacityGood synergyCosmetic preparationsBiocideDiseaseOxygen radical absorbance capacity
A formulation to deliver a full-spectrum of Vitamin E isomers for improved antioxidant capacity, bioavailability, dissolution and efficacy. The formulation includes dl-α-tocopheryl acetate or dl-α-tocopheryl succinate (synthetic Vitamin E), natural Vitamin E and mixed tocopherols, such as α-, β-, γ- and δ-tocopherol, as well as four isomers (α, β, γ and δ) of tocotrienols. This formulation is designed to deliver at least 17-times the antioxidant capacity of synthetic Vitamin E (dl-α-tocopheryl acetate), and at least twice the antioxidant capacity of natural Vitamin E (d-α-tocopherol) as measured by oxygen radical absorbance capacity (ORAC) assay. The potent antioxidant capacity of this formula affords protection against oxidative damage of cell membranes, heart disease, cancer and eye and skin disease.
Owner:RICH MELVIN +2
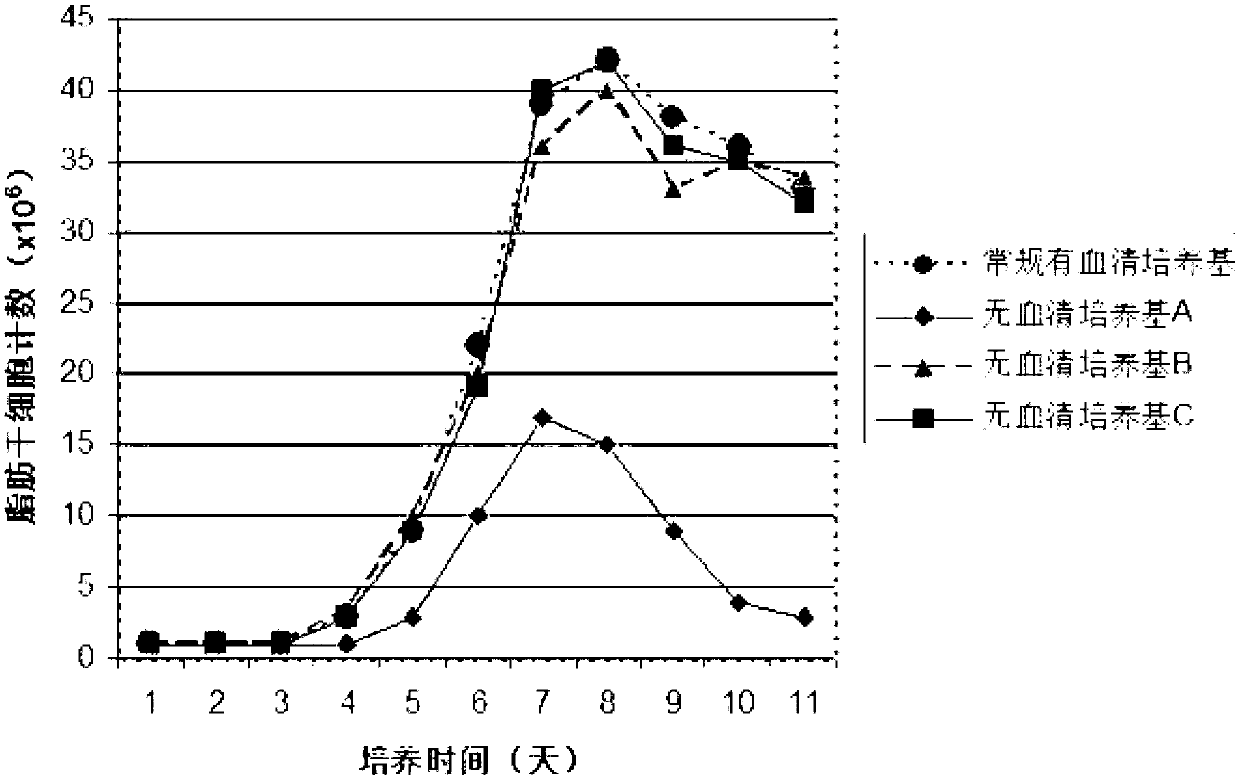
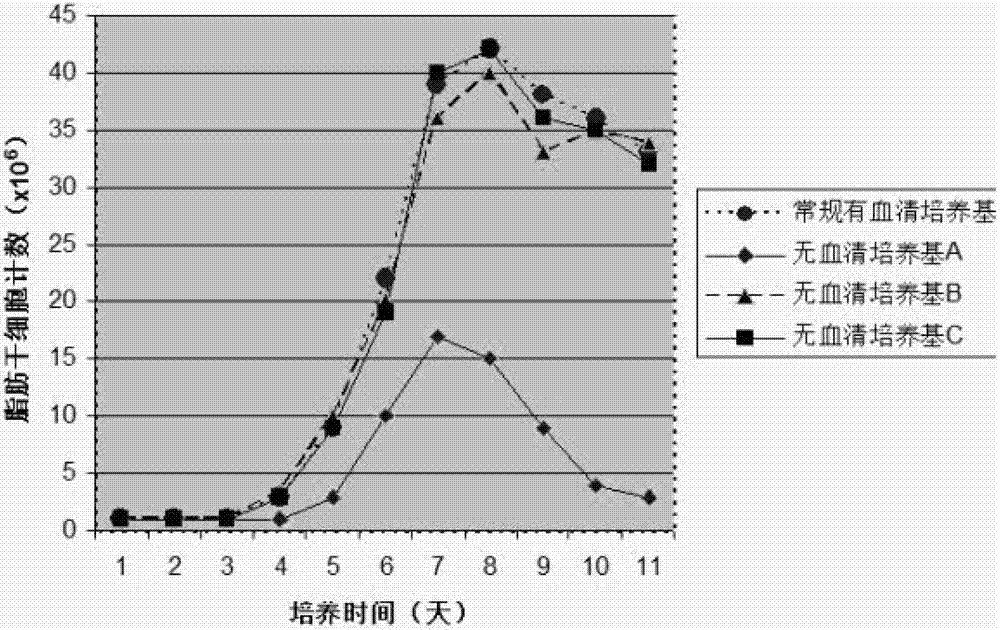
Human adipose-derived stem cell serum-free basic medium
ActiveCN102732477BOvercome riskSkeletal/connective tissue cellsMineral ascorbatesAscorbic acid 2-sulfate
The invention discloses a human adipose-derived stem cell serum-free basic medium. The medium uses a serum substitute, and is composed of a high glucose type DMEM basic medium, human serum albumin, transferrin, taurine, reduced glutathione, ceruloplasmin, L-ascorbic acid-2-sulfate, alpha-tocopherol succinate, linoleic acid, alpha-ketoglutarate and selenium. The serum-free basic medium can exempt potential threats caused by animal serum in conventional serum-containing mediums to human health, and the adipose-derived stem cells cultured by the medium is more suitable for clinical application.
Owner:JIANGSU RE STEM BIOTECH
Human adipose-derived stem cell serum-free basic medium
ActiveCN102732477AIncrease viscosityFree from mechanical damageSkeletal/connective tissue cellsAscorbic acid 2-sulfateMineral ascorbates
The invention discloses a human adipose-derived stem cell serum-free basic medium. The medium uses a serum substitute, and is composed of a high glucose type DMEM basic medium, human serum albumin, transferrin, taurine, reduced glutathione, ceruloplasmin, L-ascorbic acid-2-sulfate, alpha-tocopherol succinate, linoleic acid, alpha-ketoglutarate and selenium. The serum-free basic medium can exempt potential threats caused by animal serum in conventional serum-containing mediums to human health, and the adipose-derived stem cells cultured by the medium is more suitable for clinical application.
Owner:JIANGSU RE STEM BIOTECH
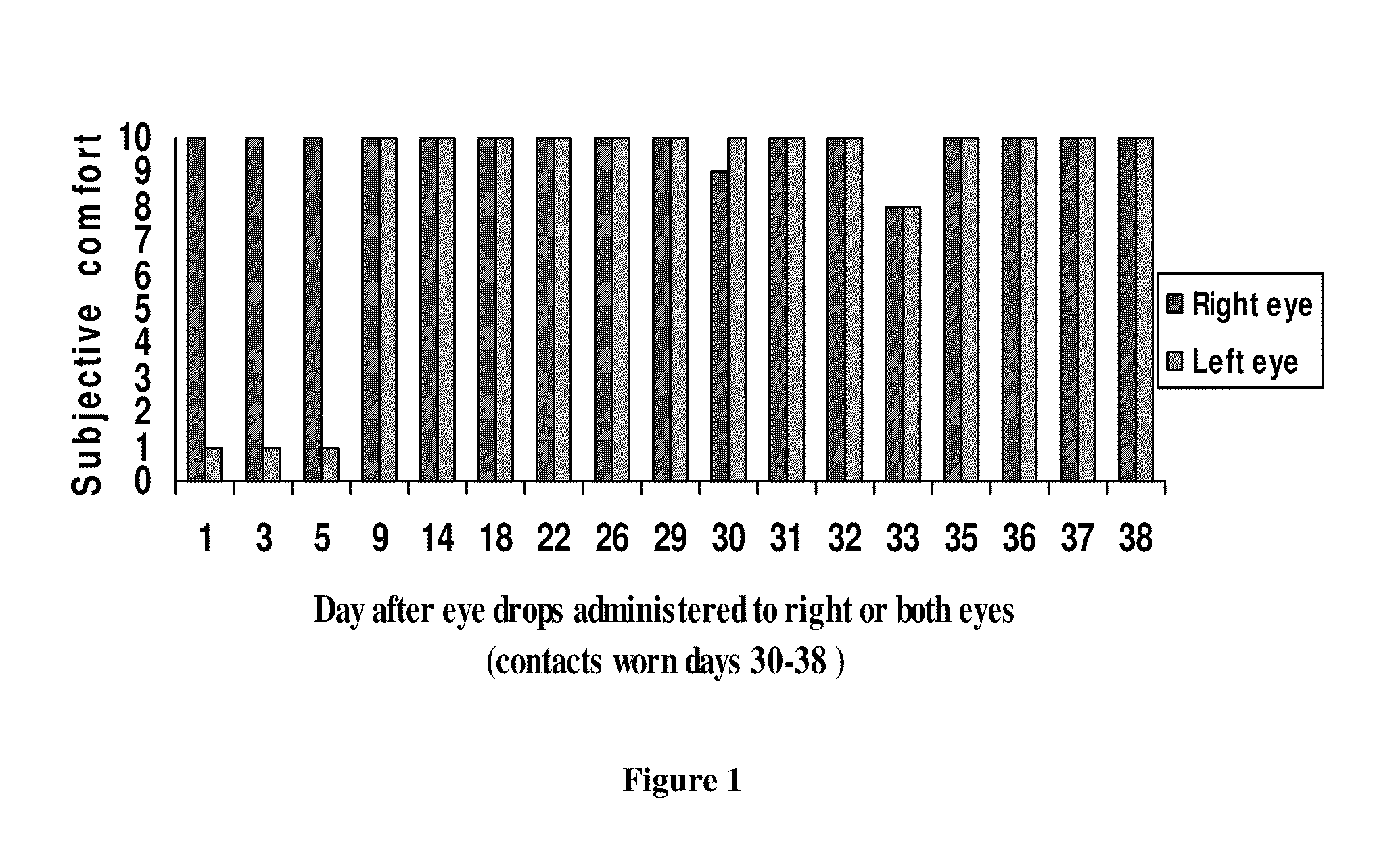
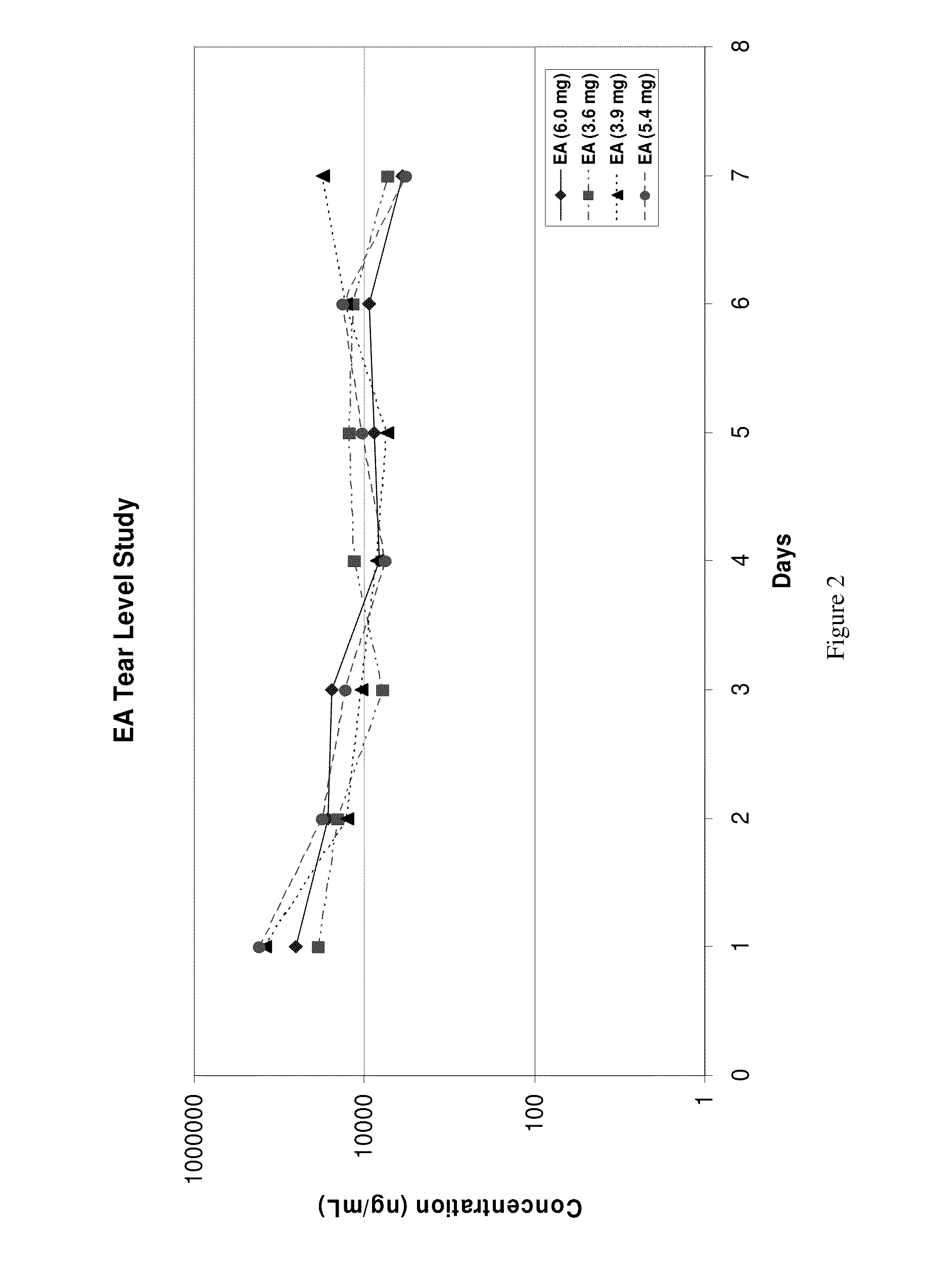
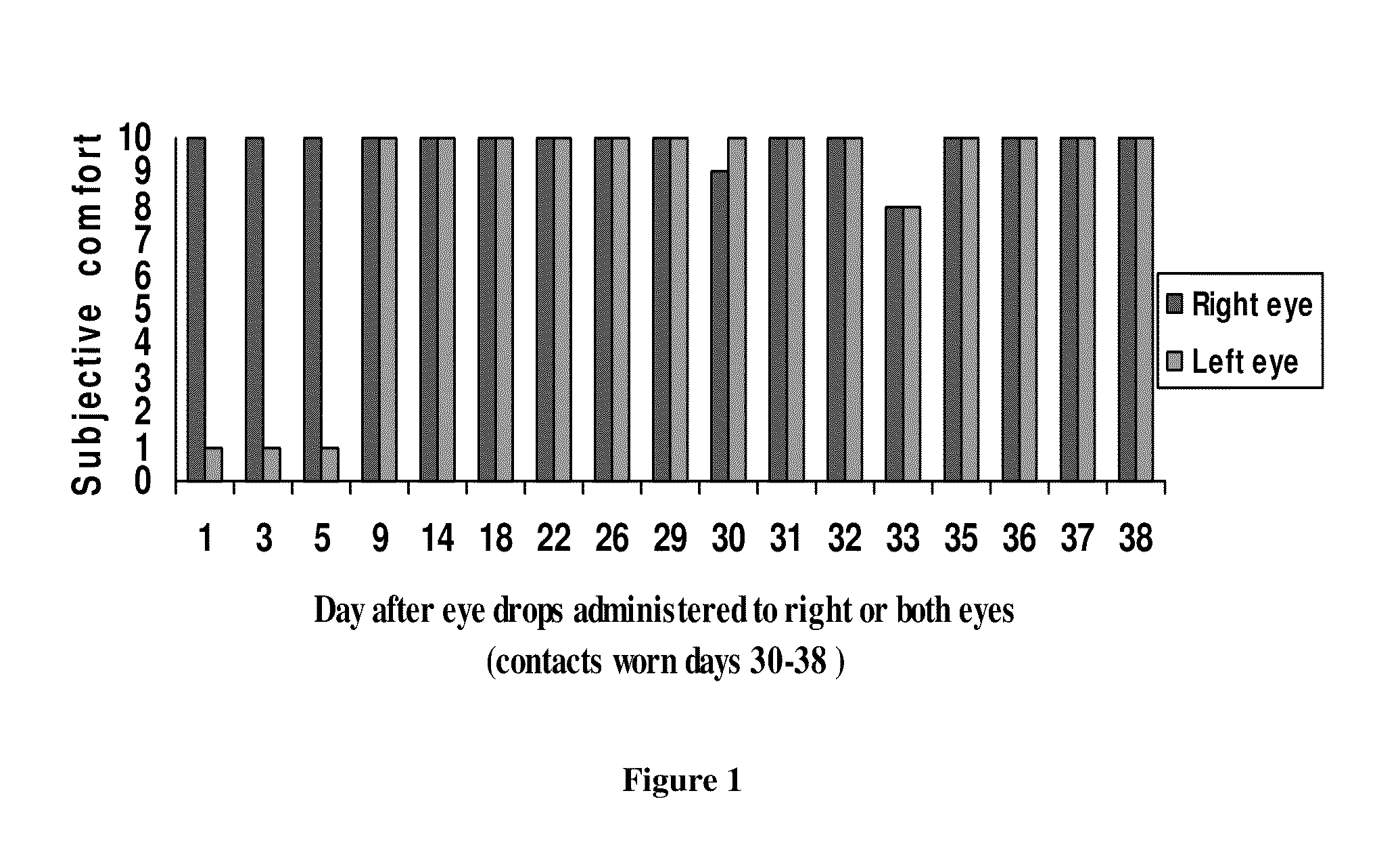
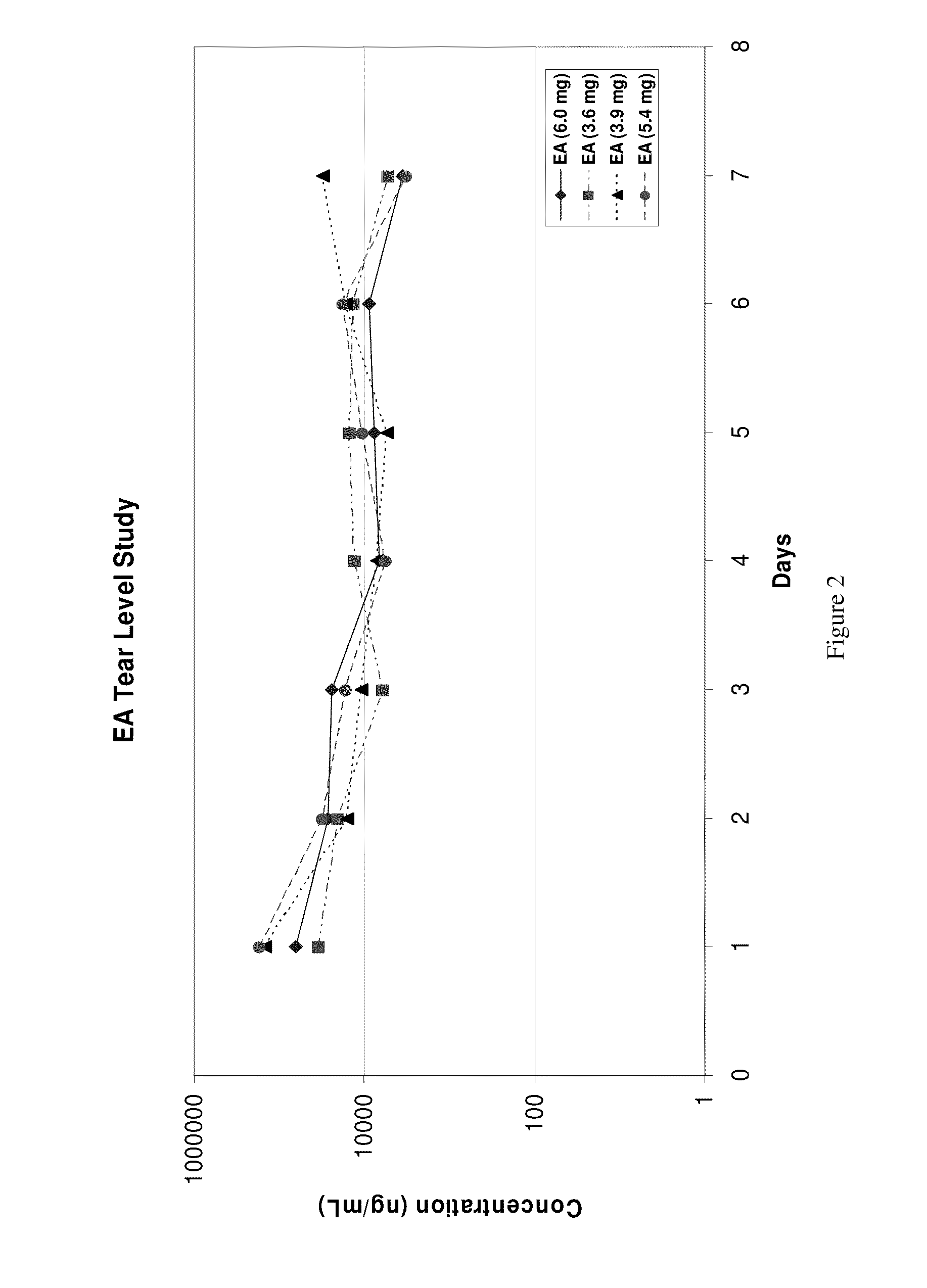
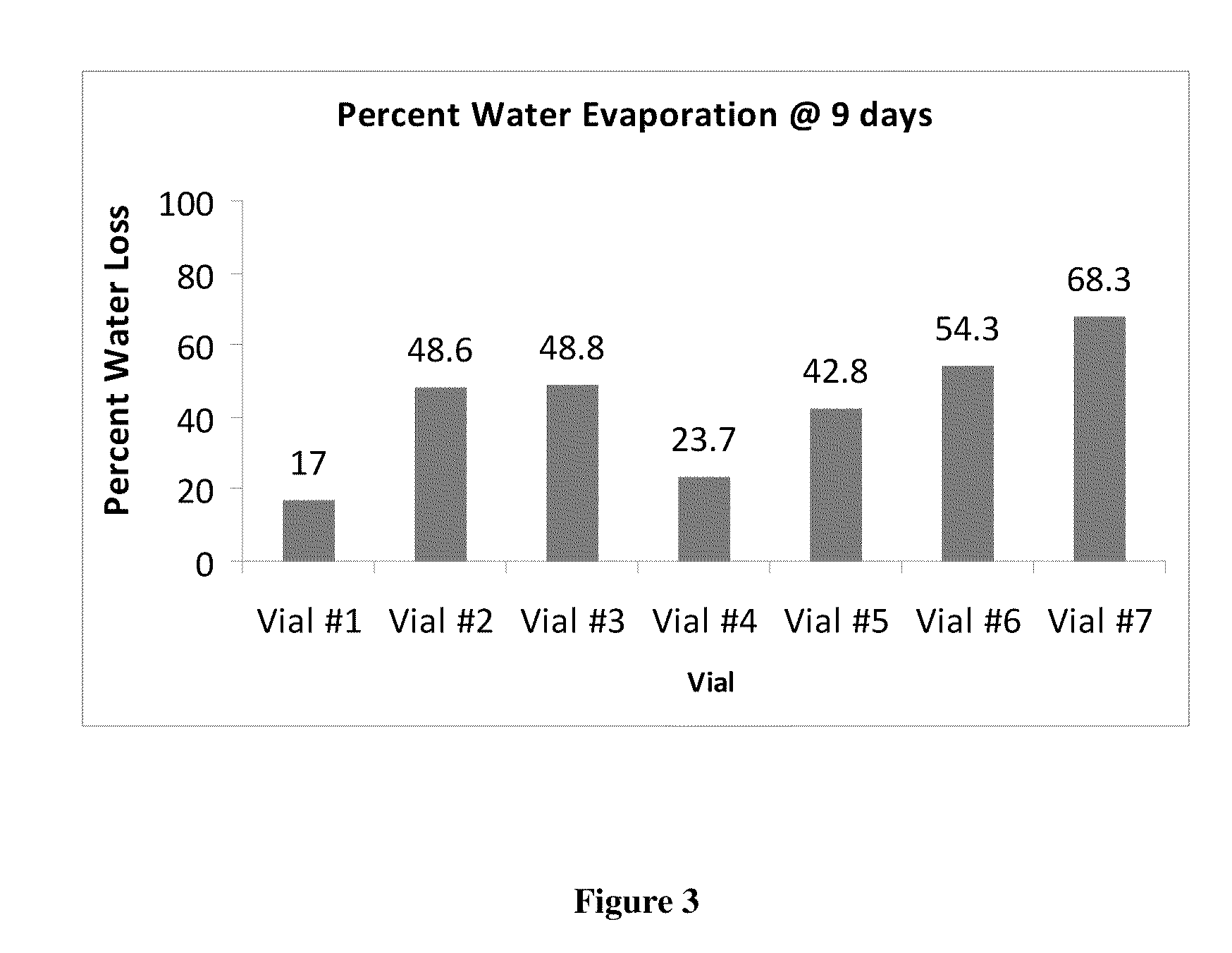
Sustained release eye drop formulations
ActiveUS20090136445A1Economical and practical and efficientEasy to produceAntibacterial agentsBiocideSolubilityIrritation
This invention provides for biocompatible, biodegradable eye drop pharmaceutical formulations useful for the treatment of ocular indications. In particular, tocopherols and their esters of low water solubility, notably α-tocopheryl acetate, are exceptional vehicles for biocompatible, nonirritating topical eye drop formulations that provide sustained release of active agents.
Owner:RAMSCOR
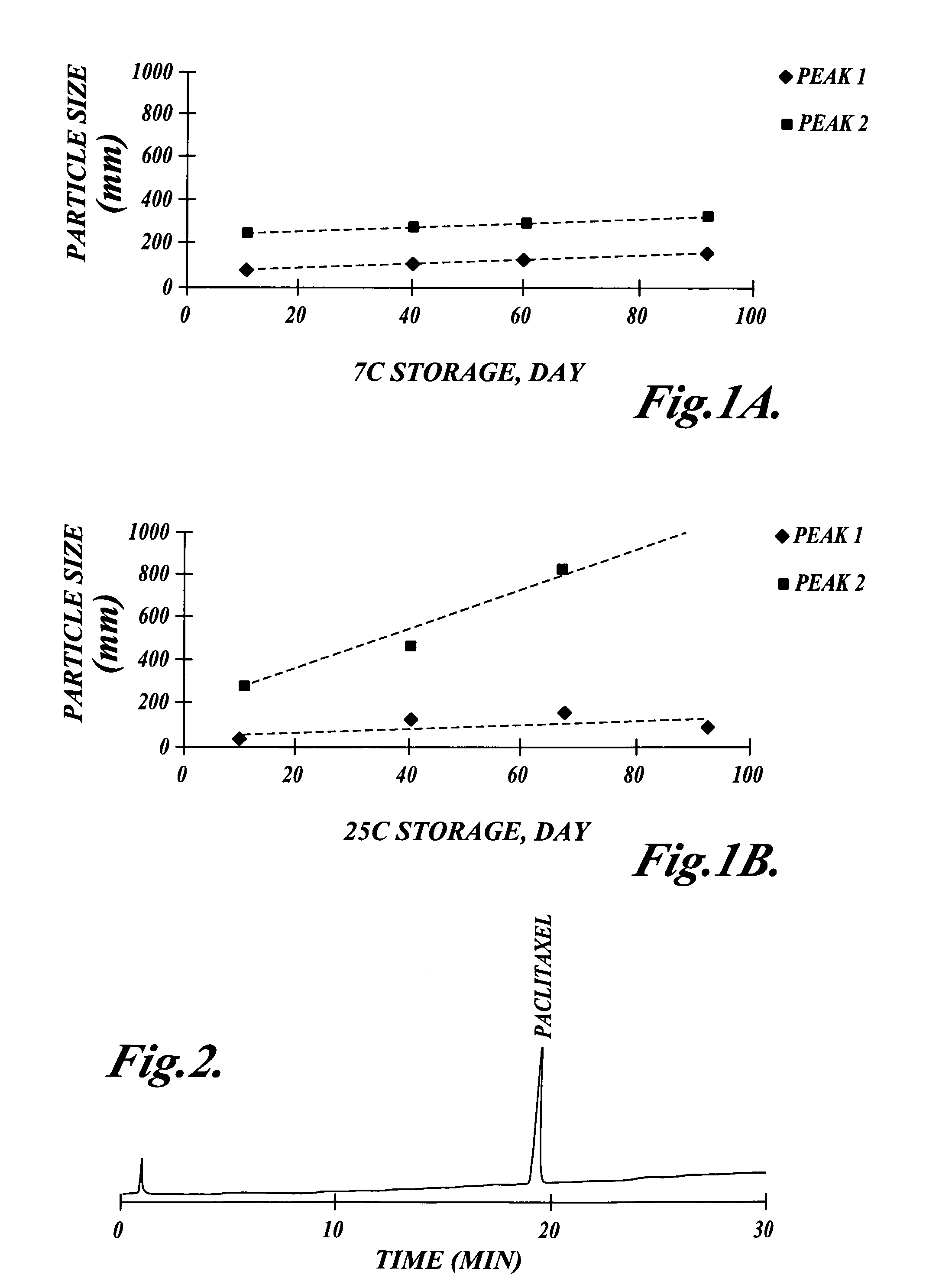
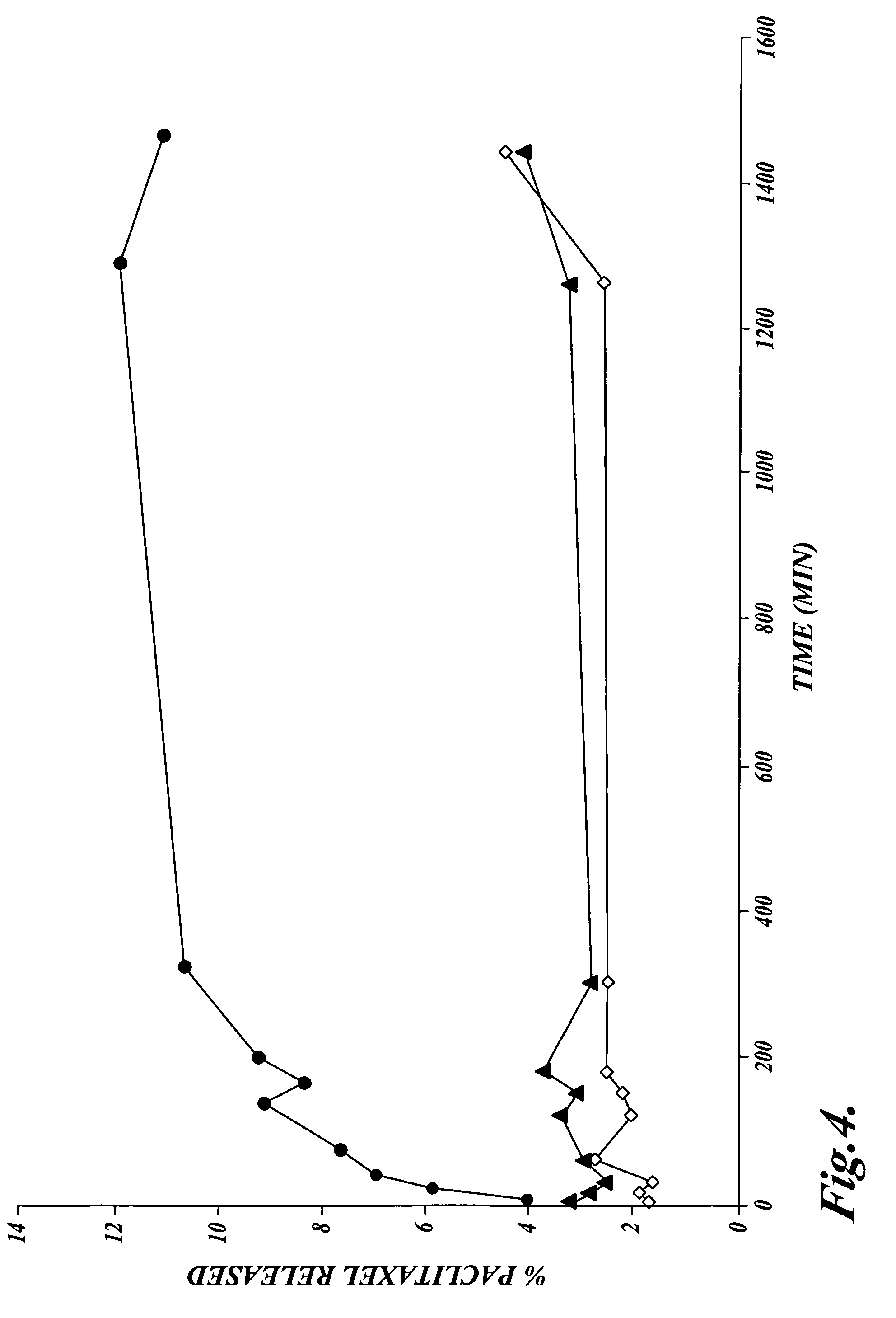
Methods for administration of paclitaxel
Methods are provided for using paclitaxel for treating diseases associated with abnormal cell proliferation and angiogenesis. In particular, methods are provided for administration of paclitaxel formulated with vitamin E derivatives such as d-alpha-tocopherol polyethylene glycol succinate to a cancer patient. By administering to a patient paclitaxel in a vehicle containing a solubilizer other than Cremophor, acute hypersensitivity caused by Cremophor can be avoided and therapeutic index of paclitaxel may also be increased through potentiation of anti-neoplastic effects by the vitamin E derivatives.
Owner:SUPERGEN
Therapeutic compositions
InactiveUS6946151B2Lower blood sugar levelsSlow onsetBiocideOrganic active ingredientsDiseaseAntioxidant
An oral composition, suitable as a hypoglycemic agent, includes an isolate from the leaves of Gymnema sylvestre, having a specified molecular weight. The isolate has a molecular weight at least about 3000 Daltons as determined by molecular weight cut-off filtration. Glucose metabolism in a human patient can be regulated by dosage forms that contain the aforesaid isolate from the leaves of Gymnema sylvestre, in combination with a non-metabolizable, water-swellable polysaccharide such as the exudate of Sterculia urens, and a water-soluble polysaccharide such as guar gum. Optionally, the present oral compositions can include a physiologically acceptable calcium source, a physiologically acceptable metal carbonate salt, a physiologically acceptable chromium salt, and / or a physiologically acceptable vanadium compound. In addition, antioxidants such as ascorbic acid, cholecalciferol, d-α-tocopherol, the carotenoids, lycopene, lutein, and the like, can be included as well. The present compositions are useful for amelioration of cholesterolemia, obesity, chronic complications of diabetes and prophylaxis for patients predisposed to the foregoing.
Owner:AYURVEDIC LIFE INT LLC
Method for preparing high-purity natural vitamin E monomers by separating mixed tocopherol
The invention discloses a method for preparing high-purity natural vitamin E monomers by separating mixed tocopherol. The method comprises the following steps of: fully dissolving the mixed tocopherol in a mobile phase solvent I so as to obtain a loading solution; adding the loading solution into a reversed-phase chromatographic column; adding a mobile phase solvent II into the chromatographic column; and performing step-by-step collection to obtain effluent I of which the purity of d-alpha-tocopherol is more than or equal to 90 percent, effluent II of which the purity of d-gamma-tocopherol is more than or equal to 90 percent and effluent III of which the purity of d-delta-tocopherol is more than or equal to 85 percent. By the method, the one-time column loading and separation of raw materials can be realized under normal pressure so as to obtain three natural vitamin E monomers. A filler in the chromatographic column can be regenerated, the used solvents can be recovered, the cost is reduced, the method is convenient to operate, and the purity is high.
Owner:ZHONGGU TIANKE TIANJIN BIOENG
Pharmaceutical preparation with cyclosporin a
InactiveUS20010014665A1Reduce nephrotoxicityReduce impactOrganic active ingredientsBiocideAlcoholVegetable oil
The invention relates to a pharmaceutical preparation which consists of or contains cyclosporin A, an emulsifying .alpha.-tocopherol derivative, an ethoxylation product of vegetable oils, fatty acids or fats as a further emulsifier and a pharmaceutically customary alcohol.
Owner:HEXAL AG
Nutritional composition for the treatment of pressure ulcers
InactiveUS6846494B1EfficacyGood curative effectBiocideHydrocarbon active ingredientsNutritional compositionAlpha-tocomonoenol
A nutritional booster composition suitable for the treatment of pressure ulcers, comprising proteins, carbohydrates and fats and, in a daily unit dose: 3-15 g of arginine or equivalents thereof, 180-840 mg of ascorbic acid equivalents, 50-400 mg of α-tocopherol equivalents, and 8-40 mg of flavonoids.
Owner:NUTRICIA
Sustained release eye drop formulations
ActiveUS20130324481A1Easy to useEfficient deliveryBiocideTetracycline active ingredientsSolubilityEye/ear drops
This invention provides for biocompatible, biodegradable eye drop pharmaceutical formulations useful for the treatment of ocular indications. In particular, tocopherols and their esters of low water solubility, notably α-tocopheryl acetate, are exceptional vehicles for biocompatible, nonirritating topical eye drop formulations that provide sustained release of active agents.
Owner:RAMSCOR
Formulation and delivery method to enhance antioxidant potency of Vitamin E
InactiveUS7012092B2Improve antioxidant capacityGood synergyOrganic active ingredientsBiocideDiseaseOxygen radical absorbance capacity
A formulation to deliver a full-spectrum of Vitamin E isomers for improved antioxidant capacity, bioavailability, dissolution and efficacy. The formulation includes dl-α-tocopheryl acetate or dl-α-tocopheryl succinate (synthetic Vitamin E), natural Vitamin E and mixed tocopherols, such as α-, β-, γ- and δ-tocopherol, as well as four isomers (α, β, γ and δ) of tocotrienols. This formulation is designed to deliver at least 17-times the antioxidant capacity of synthetic Vitamin E (dl-α-tocopheryl acetate), and at least twice the antioxidant capacity of natural Vitamin E (d-α-tocopherol) as measured by oxygen radical absorbance capacity (ORAC) assay. The potent antioxidant capacity of this formula affords protection against oxidative damage of cell membranes, heart disease, cancer and eye and skin disease.
Owner:RICH MEL +2
Compositions and Methods for Maintaining, Strengthening, Improving, or Promoting Eye Health
Compositions for maintaining, strengthening, improving, or promoting eye health comprise α-lipoic acid, ascorbic acid, and α-tocopherol, or pharmaceutical equivalents thereof. The compositions are useful to prevent, stabilize, reverse and / or treat visual acuity loss in subjects having, or being at risk of developing, an ocular disease. The compositions can further comprise one or more other antioxidants, beneficial minerals, and / or nutritional or dietary materials.
Owner:BAUSCH & LOMB INC
Methods for making oxidation-resistant cross-linked polymeric materials
The present invention relates to methods for making cross-linked oxidation-resistant polymeric materials and preventing or minimizing in vivo elution of antioxidant from the antioxidant-containing polymeric materials. The invention also provides methods of doping polymeric materials with a spatial control of cross-linking and antioxidant distribution, for example, vitamin E (α-Tocopherol), and methods for extraction / elution of antioxidants, for example, vitamin E (α-tocopherol), from surface regions of antioxidant-containing polymeric materials, and materials used therewith also are provided.
Owner:THE GENERAL HOSPITAL CORP +1
Composition and method for treating dry eye syndrome
ActiveUS8722728B2Safe, long-lasting, and relatively inexpensiveBiocideSenses disorderAcetic acidTocopheryl acetate
The present invention provides for compositions, medicaments, and methods for treating or alleviating the symptoms of dry eye syndrome or chronic dry eye. More specifically, the present embodiments provide for medicaments consisting of tocopherol or tocotrienol eyedrops. A single topical administration of tocopherol or tocotrienol eyedrops in the eyes of a subject suffering from dry eye alleviates symptoms for at least one day. In particular, the eyedrop medicament consists of α-tocopheryl acetate; α-tocopheryl acetate and about 0.5% aqueous component; or α-tocopheryl acetate, about 2.5% tocopherol emulsifier, and about 20% to about 30% aqueous excipient.
Owner:RAMSCOR
Tripeptides and derivatives thereof for cosmetic application in order to improve skin structure
The invention relates to compounds and to the cosmetically acceptable salts thereof, which correspond to general formula (I),wherein: R1 represents H, —C(O)—R6, —SO2—R6or —C(O)—XR6; R2 and R4, independent of one another, represent (CH2)n—NH2 or (CH2)3—NHC(NH)NH2; n equals 1 4; R3 represents linear or branched C1-C4 alkyl that is optionally substituted by hydroxy; R5 and R6, independent of one another, represent hydrogen, optionally substituted (C1-C24)alkyl, optionally substituted C2-C24 alkenyl, optionally substituted phenyl, optionally substituted phenyl-C1-C4 alkyl or 9-fluorenyl-methyl; X represents oxygen (—O—) or NH—; or XR5 with X═O also represents the esters of a-tocopherol, tocotrienol or retinol, with the provision that R1 and R5 do not represent hydrogen and X does not represent oxygen at the same time. The invention also relates to the production of the compounds of general formula (I) and to a cosmetically active composition that contains at least one compound of formula (I).
Owner:DSM IP ASSETS BV
Composition and method for treating dry eye syndrome
ActiveUS20100093845A1Safe, long-lasting, and relatively inexpensiveBiocideSenses disorderAcetic acidExcipient
The present invention provides for compositions, medicaments, and methods for treating or alleviating the symptoms of dry eye syndrome or chronic dry eye. More specifically, the present embodiments provide for medicaments consisting of tocopherol or tocotrienol eyedrops. A single topical administration of tocopherol or tocotrienol eyedrops in the eyes of a subject suffering from dry eye alleviates symptoms for at least one day. In particular, the eyedrop medicament consists of α-tocopheryl acetate; α-tocopheryl acetate and about 0.5% aqueous component; or α-tocopheryl acetate, about 2.5% tocopherol emulsifier, and about 20% to about 30% aqueous excipient.
Owner:RAMSCOR
Thermal receiver
A receiver element for thermal dye transfer, a print assembly including the receiver element, and a method of printing are described wherein the receiver element includes a dye-receiving layer on a support, wherein the dye-receiving layer includes a polymer, an optional release agent, and alpha-tocopherol or a derivative thereof.
Owner:KODAK ALARIS INC
Method for suppressing discoloration over time of adhesive preparation containing donepezil
InactiveUS20100048628A1Sufficient effectImprove stabilityBiocideNervous disorderPropanoic acidDonepezil
[Object] Discoloration, over time, of a donepezil-containing adhesive preparation is suppressed.[Solution] At least one species of stabilizer selected from the group consisting of ascorbic acid, a metal salt or an ester thereof, isoascorbic acid or a metal salt thereof, ethylenediamine tetraacetic acid or a metal salt thereof, 2-mercaptobenzimidazole, 3(2)-t-butyl-4-hydroxyanisole, 2,6-di-t-butyl-4-methylphenol, tetrakis[3-(3′,5′-di-t-butyl-4′-hydroxyphenyl)propionic acid]pentaerythritol, (±)-α-tocopherol, (±)-α-tocopherol acetate, rutin, hypophosphorous acid, a metal metabisulfite salt and a metal salt of hydroxymethanesulfinic acid, is blended in a pressure-sensitive adhesive layer containing a pressure-sensitive adhesive and donepezil.
Owner:NITTO DENKO CORP +1
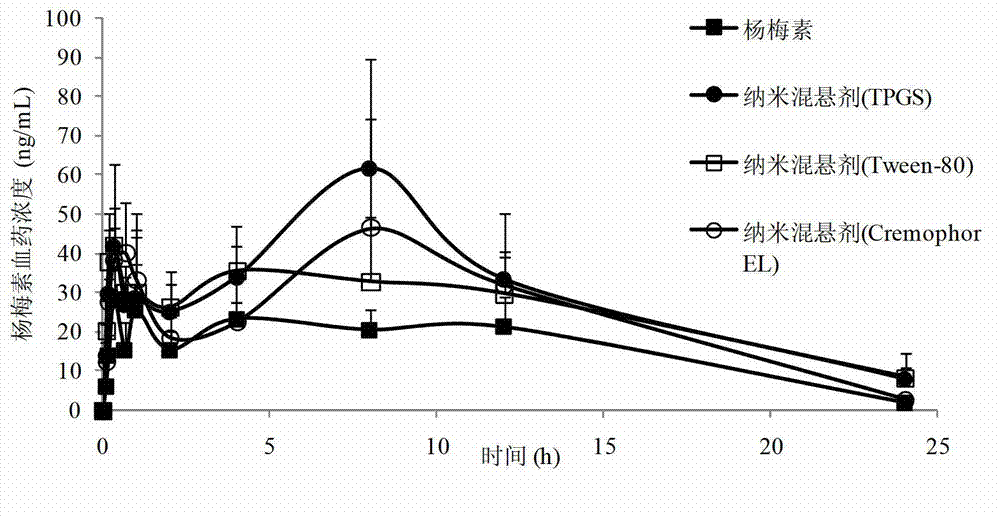
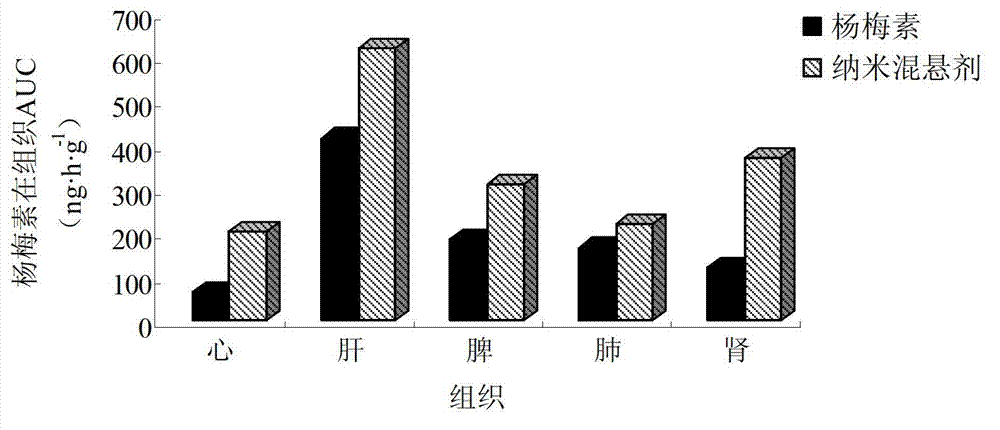
Myricetin nanosuspension and preparation method thereof
InactiveCN103083235AAvoid destructionImprove propertiesAntibacterial agentsOrganic active ingredientsSolubilityPolymer science
The invention belongs to the technical field of medicines and relates to a myricetin nanosuspension and a preparation method thereof. The myricetin nanosuspension is prepared by adopting a settling method and a high-pressure homogenization method, and the formula comprises myricetin and a stabilizer according to the weight ratio of 1: 0.25-1: 2.5. According to the preparation method provided by the invention, the formula is optimized, Tween-80, Cremophor EL and D-alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS) are screened as the stabilizer, the prepared myricetin nanosuspension has stable nature and simple composition of the formula, the preparation process is simple, convenient and feasible, and the particle size range is 100nm-300nm; and by reducing the particle size of a medicament, the solubility and the dissolution of the myricetin are obviously improved, and the oral bioavailability and the in-vivo tissue distribution of the myricetin are further improved. In addition, the myricetin nanosuspension can be freeze-dried, and proper excipients are added into the obtained freeze-dried powder for further preparing oral liquid, tablets, granules, capsules and other different oral preparations so as to facilitate clinical applications.
Owner:SHANGHAI UNIV OF T C M
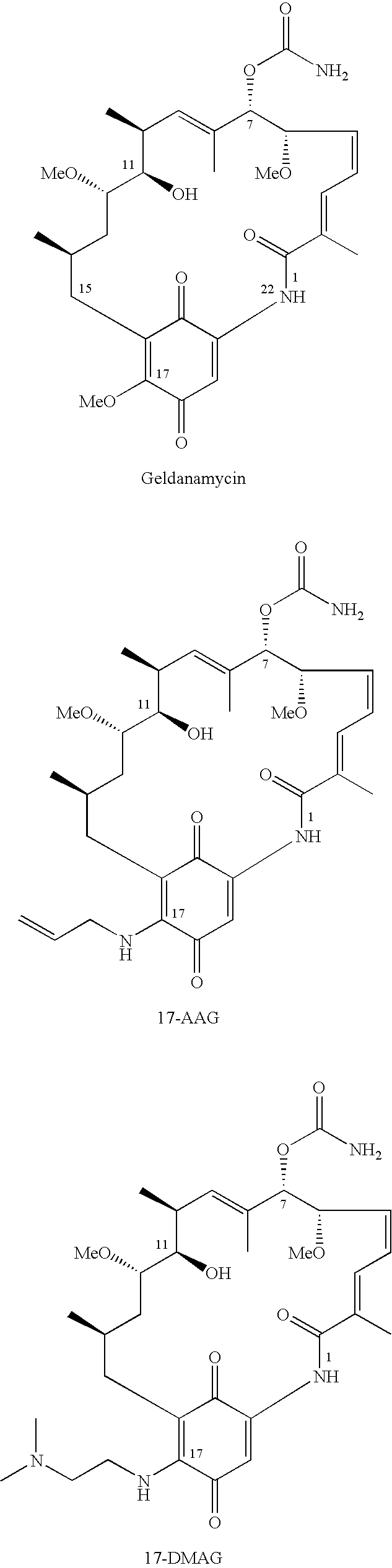
Pharmaceutical compositions comprising 17-allylamino-17-demethoxygeldanamycin
InactiveUS20070167422A1BiocideAnimal repellants17-Allylamino-17-demethoxygeldanamycinOrganic solvent
A pharmaceutical formulation comprising (a) 17-allylamino-17-demethoxy-geldanamycin; (b) an ester of d-α-tocopheryl succinate and polyethylene glycol; and (c) a pharmaceutically acceptable, water-miscible organic solvent.
Owner:KOSAN BIOSCI
Improved Neurobasal B27 culture medium, preparing method and application
The invention discloses an improved Neurobasal B27 culture medium. One liter of a Neurobasal culture solution comprises 0.09-0.11 g of biotin, 18-22 micrograms of corticosterone, 1-3 g of L-carnitine, 0.9-1.1 g of linoleic acid, 0.9-1.1 g of ethanol amine, 0.9-1.1 g of linolenic acid, 13-17 g of D(+)-galactose, 6-7 micrograms of progesterone, 15-17 g of putrescine dihydrochloride, 0.08-0.12 g of retinyl acetate, 2.2-2.8 g of thyroid-hormone-removing bovine serum albumin, 0.8-1.5 g of DL-alpha-tocopherol, 2-3 g of catalase, 0.9-1.2 g of DL-alpha-tocopheryl acetate, 0.8-1.3 g of reduced glutathione, 42-52 micrograms of lipoic acid, 2-3 g of superoxide dismutase, 4-6 g of transferring, 3-5 g of human insulin and 0.12-0.16 mg of sodium selenite. The invention further discloses a preparing method and application of the improved Neurobasal B27 culture medium. The improved Neurobasal B27 culture medium can be used for researching the influence of thyroid hormones on the process that embryonic stem cells are differentiated into nerve cells.
Owner:THE FIRST HOSPITAL OF CHINA MEDICIAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com