Myricetin nanosuspension and preparation method thereof
A nano-suspension, myricetin technology, applied in the direction of anti-toxic agents, anti-viral agents, pharmaceutical formulations, etc., can solve problems such as retention, no nanotechnology application, no preparation technology, myricetin oral absorption, etc., to improve Properties, not easy to store, and the effect of simple composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Screening of Stabilizers
[0033] Weigh Tween-801g, CremophorEL1g, TPGS1g, PVP1g, Tylosamer 1g, CremophorEL0.8g + Tylosamer 0.2g, add 200mL water to dissolve it respectively, weigh 2400mg of myricetin raw material and dissolve it in 120mL ethanol, Under the condition of 16000rpm high-shear homogenization, take 20mL of liquid medicine and add them to different water phases, continue shearing for 20min, recover the organic solvent, put the obtained suspension in a high-pressure homogenizer, and circulate it 5 times at 200bar. 5 cycles at 500 bar and 15 cycles at 800 bar.
[0034] The particle size, PdI, Zeta potential and sedimentation ratio of myricetin nanosuspensions with different stabilizers after 15 days of storage are shown in Table 1. The results showed that the particle size, PdI and stability of myricetin nanosuspensions obtained by using different stabilizers were somewhat different. The particle size of the nanosuspension prepared with PVP, Tylosamer, and Ty...
Embodiment 2
[0038] Investigation on the Ratio of Myricetin and Stabilizer
[0039]Weigh Tween-800g, Tween-800.05g, Tween-800.1g, Tween-800.2g, Tween-800.3g, Tween-800.5g, Tween-801g, add 200mL water to dissolve them respectively, Weigh 1000 mg of myricetin raw material and dissolve it in 50 mL of ethanol. Under the condition of 16000 rpm high-shear milk homogeneity, take 10 mL of liquid medicine and add it to different water phases, continue shearing for 20 min, recover the organic solvent, and place the obtained suspension in In the high-pressure homogenizer, there are 5 cycles at 200bar, 5 cycles at 500bar, and 15 cycles at 800bar.
[0040] The particle size, PdI, Zeta potential and sedimentation ratio of myricetin nanosuspensions with different ratios of stabilizers after 15 days of storage are shown in Table 2.
[0041] The results showed that when the mass ratio of myricetin to Tween-80 was 1:0 and 1:5, the particle size of the sample was too large and the stability was poor, so it ...
Embodiment 3
[0045] Weigh 0.5g of Cremophor EL, add 200mL of water to dissolve it, weigh 200mg of myricetin raw material and dissolve it in 20mL of methanol, add the drug solution into the water phase under the condition of 12000rpm high-shear milk, continue shearing for 20min, and recover the organic The solvent is obtained by placing the obtained suspension in a high-pressure homogenizer, circulating 5 times at 200 bar, 5 times at 500 bar, and 15 times at 800 bar.
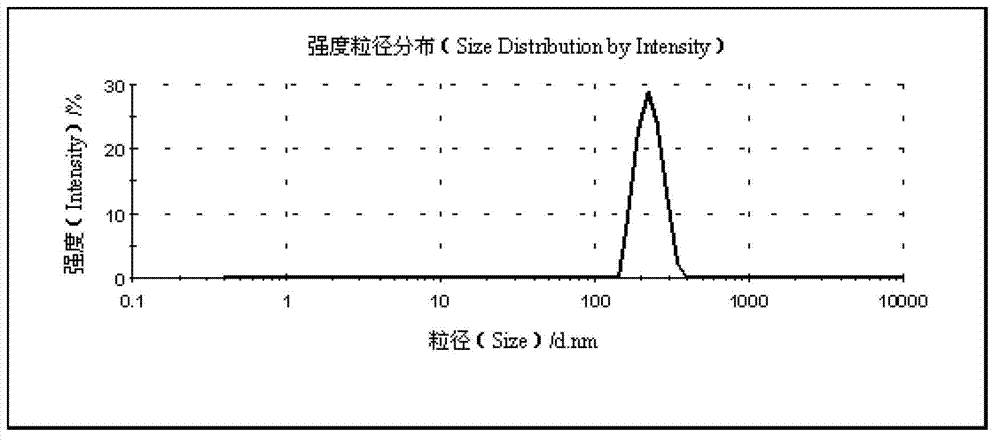
[0046] The particle size of the myricetin nanosuspension prepared by this process is 296.7nm, the PdI is 0.153, the Zeta potential is -19.6mV, and the sedimentation ratio is 80.44% after being placed for 10 days.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com